Optimized CO2 Modeling in Saline Aquifers: Evaluating Fluid Models and Grid Resolution for Enhanced CCS Performance
Abstract
1. Introduction
2. Comparative Analysis: Black Oil Modeling vs. Compositional Simulation
2.1. Feasibility of Black Oil Modeling for Carbon Storage in Saline Aquifers
- Phases Encountered Underground: In saline aquifers, the primary phases are supercritical CO2 (“gas”) and brine. This two-phase system simplifies the modeling process compared to reservoirs with multiple coexisting phases, making the BoM a viable approach.
- Thermodynamic Mechanisms at Play:
- Dissolution of CO2 into Brine: Under isothermal conditions, the dissolution of CO2 into brine is driven by pressure variations, typically up to 90% of the fracture pressure. Understanding how CO2 interacts with brine is critical for accurate simulation.
- Brine Solution Volume Changes: As CO2 dissolves or is released from brine, the volume of the brine changes. These swelling or shrinking effects, which depend on pressure, must be accurately captured in the model.
- Compression and Expansion of the CO2 Phase: The free CO2 phase undergoes compression and expansion with pressure changes, which must be accounted for to simulate CO2 storage and movement correctly.
- Vaporization of Brine into the “Gas” Phase: This effect is negligible in the context of saline aquifer storage and does not impact the simulation.
- Rs (Solution Gas Oil Ratio or CO2–Brine Ratio): This parameter reflects the dissolution of CO2 into brine across the expected pressure range, from initial to closure pressures.
- Brine formation volume factor (Bb): Captures changes in brine volume due to CO2 solubility, reflecting swelling or shrinking effects as a function of reservoir pressure.
- CO2 formation volume factor (Bg): Describes the compression and expansion of the free CO2 phase as pressure changes throughout the different stages of the CCS project (injection, closure, and post-closure monitoring).
2.2. Comparative Analysis
- Eclipse E300 (Slb): Eclipse E300 is a widely used compositional simulator known for its versatility and accuracy in modeling complex fluid interactions. For carbon storage applications, Eclipse E300 employs advanced Equations of State such as the Peng–Robinson (PR) or Soave–Redlich–Kwong (SRK) EoS to model the thermodynamic behavior of CO2 and reservoir fluids. It provides detailed phase behavior representation, making it highly effective for simulating CO2 injection, migration, and trapping mechanisms. Eclipse E300 also includes features to model the dissolution of CO2 in brine and its interactions with hydrocarbons, making it suitable for both saline aquifer and depleted reservoir scenarios.
- tNavigator (Rock Flow Dynamics): tNavigator is specifically designed for complex reservoir processes, including subsurface CO2 storage. It employs sophisticated thermodynamic models and algorithms to simulate phase behavior and interactions of CO2 with reservoir fluids. tNavigator is highly capable of handling multiple phases and components, allowing for precise simulations of CO2 dissolution. Furthermore, tNavigator’s compositional model incorporates temperature and pressure effects on CO2 solubility and phase behavior, making it a robust tool for CO2 storage dynamics and risk mitigation.
- CMG-GEM (Computer Modeling Group): CMG-GEM is a leading compositional simulator designed for carbon storage and unconventional reservoirs. For carbon storage applications, CMG-GEM utilizes the Peng–Robinson Equation of State (EoS) to model the thermodynamics of CO2 and reservoir fluids. One of CMG-GEM’s key features is its integration of geochemical modeling, which simulates mineral reactions and the potential for mineral trapping of CO2. While Henry’s Law serves as the primary model for CO2 dissolution in brine, CMG-GEM offers various options for modeling CO2 solubility. The selection of a specific model is user-dependent and should correspond to the CO2 stream composition, as well as the aquifer’s conditions and chemistry. This flexibility allows for tailored simulations that account for different geochemical and thermodynamic scenarios. These capabilities enable CMG-GEM to provide detailed simulations of CO2–brine interactions and the long-term stability of stored CO2. Additionally, its ability to model complex phase behavior, including CO2–hydrocarbon interactions, makes CMG-GEM suitable for both saline aquifers and depleted oil and gas fields, ensuring effective and safe long-term CO2 sequestration.
2.2.1. Simulation Setup
2.2.2. Results
3. Grid Resolution Impact Assessment
- 1-
- Quantifying Impact: This section highlights the acceptable thresholds for coarsening grids while maintaining accuracy, which is critical for large-scale simulations where computational resources are constrained.
- 2-
- Understanding Limitations: It demonstrates that while coarse grids are suitable for some applications, certain scenarios (e.g., monitoring fine-scale plume migration) require higher resolution, emphasizing the need for targeted refinement.
- 3-
- Guidance for Operators: By providing a clear understanding of grid resolution impacts, this section offers practical guidance to operators on optimizing grid design for different simulation objectives.
3.1. Coarse Model Development & Evaluation Criteria
- Fine Model (FM): 14 × 14 × 50 grid—9800 cells—z-direction grid size: 5 m; x, y grid size: 150 m.
- Coarse Model 1 (CM1): 14 × 14 × 25 grid—4900 cells—z-direction grid size: 10 m; x, y grid size: 150 m.
- Coarse Model 2 (CM2): 14 × 14 × 10 grid—1960 cells—z-direction grid size: 25 m; x, y grid size: 150 m.
- Coarse Model 3 (CM3): 14 × 14 × 7 grid—980 cells—z-direction grid size: 50 m; x, y grid size: 150 m.
- Coarse Model 4 (CM4): 12 × 12 × 50 grid—7200 cells—x, y grid size: 175 m; z-direction grid size: 5 m.
- Coarse Model 5 (CM5): 10 × 10 × 50 grid—5000 cells—x, y grid size: 210 m; z-direction grid size: 5 m.
- Coarse Model 6 (CM6): 7 × 7 × 50 grid—2450 cells—x, y grid size: 300 m; z-direction grid size: 5 m.
3.2. Vertical Upscaling—Grid Resolution Impact in the Z-Direction
3.3. Lateral Upscaling Impact (X- and Y-Directions)
4. Discussion
5. Conclusions
Author Contributions
Funding

Data Availability Statement
Conflicts of Interest
References
- International Energy Agency (IEA). Global Energy Review: CO2 Emissions in 2022. 2022. Available online: https://iea.blob.core.windows.net/assets/3c8fa115-35c4-4474-b237-1b00424c8844/CO2Emissionsin2022.pdf (accessed on 15 November 2024).
- International Energy Agency (IEA) and Cement Sustainability Initiative (CSI). Technology Roadmap: Low-Carbon Transition in the Cement Industry. 2018. Available online: https://iea.blob.core.windows.net/assets/cbaa3da1-fd61-4c2a-8719-31538f59b54f/TechnologyRoadmapLowCarbonTransitionintheCementIndustry.pdf (accessed on 15 November 2024).
- Intergovernmental Panel on Climate Change (IPCC). Sixth Assessment Report (AR6)—Working Group III: Mitigation of Climate Change. 2022. Available online: https://www.ipcc.ch/report/ar6/wg3/downloads/report/IPCC_AR6_WGIII_FullReport.pdf (accessed on 15 November 2024).
- European Commission. A New Circular Economy Action Plan: For a Cleaner and More Competitive Europe. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52020DC0098 (accessed on 15 November 2024).
- Global CCS Institute. Global Status of CCS 2021. 2021. Available online: https://www.globalccsinstitute.com/wp-content/uploads/2023/01/Global-Status-of-CCS-2021-Global-CCS-Institute-1121-1-1.pdf (accessed on 15 November 2024).
- Benson, S.M.; Cole, D.R. CO2 Sequestration in Deep Sedimentary Formations. Elements 2008, 4, 325–331. [Google Scholar] [CrossRef]
- Ji, X.; Zhu, C. CO2 Storage in Deep Saline Aquifers. In Novel Materials for Carbon Dioxide Mitigation Technology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 299–332. [Google Scholar] [CrossRef]
- Hannis, S.; Lu, J.; Chadwick, A.; Hovorka, S.; Kirk, K.; Romanak, K.; Pearce, J. CO2 Storage in Depleted or Depleting Oil and Gas Fields: What can We Learn from Existing Projects? Energy Procedia 2017, 114, 5680–5690. [Google Scholar] [CrossRef]
- Gassara, O.; Estublier, A.; Garcia, B.; Noirez, S.; Cerepi, A.; Loisy, C.; Le Roux, O.; Petit, A.; Rossi, L.; Kennedy, S.; et al. The Aquifer-CO2Leak project: Numerical modeling for the design of a CO2 injection experiment in the saturated zone of the Saint-Emilion (France) site. Int. J. Greenh. Gas Control. 2021, 104, 103196. [Google Scholar] [CrossRef]
- Ismail, I.; Gaganis, V. Carbon Capture, Utilization, and Storage in Saline Aquifers: Subsurface Policies, Development Plans, Well Control Strategies and Optimization Approaches—A Review. Clean Technol. 2023, 5, 609–637. [Google Scholar] [CrossRef]
- Pruess, K.; García, J.; Kovscek, T.; Oldenburg, C.; Rutqvist, J.; Steefel, C.; Xu, T. Code intercomparison builds confidence in numerical simulation models for geologic disposal of CO2. Energy 2004, 29, 1431–1444. [Google Scholar] [CrossRef]
- Class, H.; Ebigbo, A.; Helmig, R.; Dahle, H.K.; Nordbotten, J.M.; Celia, M.A.; Audigane, P.; Darcis, M.; Ennis-King, J.; Fan, Y.; et al. A benchmark study on problems related to CO2 storage in geologic formations. Comput. Geosci. 2009, 13, 409–434. [Google Scholar] [CrossRef]
- Ismail, I.; Fotias, S.P.; Avgoulas, D.; Gaganis, V. Integrated Black Oil Modeling for Efficient Simulation and Optimization of Carbon Storage in Saline Aquifers. Energies 2024, 17, 1914. [Google Scholar] [CrossRef]
- Aziz, K.; Settari, A. Petroleum Reservoir Simulation; Elseviers: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Peaceman, D.W. Fundamentals of Numerical Reservoir Simulation; Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Blair, L.M.; Quinn, J.A. Measurement of Small Density Differences: Solutions of Slightly Soluble Gases. Rev. Sci. Instrum. 1968, 39, 75–77. [Google Scholar] [CrossRef]
- Sayegh, S.G.; Najman, J. Phase behavior measurements of CO2-SO2-brine mixtures. Can. J. Chem. Eng. 1987, 65, 314–320. [Google Scholar] [CrossRef]
- Ennis-King, J.; Paterson, L. Role of Convective Mixing in the Long-Term Storage of Carbon Dioxide in Deep Saline Formations. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 5–8 October 2003. [Google Scholar] [CrossRef]
- Hassanzadeh, H.; Pooladi-Darvish, M.; Keith, D.W. Modelling of Convective Mixing in CO2 Storage. J. Can. Pet. Technol. 2005, 44, PETSOC-05-10-04. [Google Scholar] [CrossRef]
- Coats, K.H. An Equation of State Compositional Model. Soc. Pet. Eng. J. 1980, 20, 363–376. [Google Scholar] [CrossRef]
- Young, L.C.; Stephenson, R.E. A Generalized Compositional Approach for Reservoir Simulation. Soc. Pet. Eng. J. 1983, 23, 727–742. [Google Scholar] [CrossRef]
- Yang, Z.-L.; Yu, H.-Y.; Chen, Z.-W.; Cheng, S.-Q.; Su, J.-Z. A compositional model for CO2 flooding including CO2 equilibria between water and oil using the Peng–Robinson equation of state with the Wong–Sandler mixing rule. Pet Sci. 2019, 16, 874–889. [Google Scholar] [CrossRef]
- Li, H.; Durlofsky, L.J. Ensemble level upscaling for compositional flow simulation. Comput. Geosci. 2016, 20, 525–540. [Google Scholar] [CrossRef]
- He, C.; Durlofsky, L.J. Structured flow-based gridding and upscaling for modeling subsurface flow. Adv. Water Resour. 2006, 29, 1876–1892. [Google Scholar] [CrossRef]
- Ringrose, P.; Bentley, M. Reservoir Model Design; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Pickup, G.E.; Kiatsakulphan, M.; Mills, J.R. Analysis of Grid Resolution for Simulations of CO2 Storage in Deep Saline Aquifers. In Proceedings of the ECMOR XII—12th European Conference on the Mathematics of Oil Recovery, Oxford, UK, 6–9 September 2010. [Google Scholar] [CrossRef]
- Rabinovich, A.; Itthisawatpan, K.; Durlofsky, L.J. Upscaling of CO2 injection into brine with capillary heterogeneity effects. J. Pet. Sci. Eng. 2015, 134, 60–75. [Google Scholar] [CrossRef]
- Ismail, I.; Fotias, S.P.; Tartaras, E.; Stefatos, A.; Gaganis, V. Impact of Grid Resolution on Accuracy of In-Situ CO2 Modeling for Improved CCS Project Design and Implementation. In Proceedings of the Mediterranean Offshore Conference, Alexandria, Egypt, 20–22 October 2024. [Google Scholar] [CrossRef]
- Mo, S.; Akervoll, I. Modeling Long-Term CO2 Storage in Aquifer with a Black-Oil Reservoir Simulator. In Proceedings of the SPE/EPA/DOE Exploration and Production Environmental Conference, Galveston, TX, USA, 7–9 March 2005. [Google Scholar] [CrossRef]
- Hassanzadeh, H.; Pooladi-Darvish, M.; Elsharkawy, A.M.; Keith, D.W.; Leonenko, Y. Predicting PVT data for CO2–brine mixtures for black-oil simulation of CO2 geological storage. Int. J. Greenh. Gas Control. 2008, 2, 65–77. [Google Scholar] [CrossRef]
- Pour, S.S.; Pickup, G.E.; Mackay, E.J.; Heinemann, N. Flow Simulation of CO2 Storage in Saline Aquifers Using Black Oil Simulator. In Proceedings of the Carbon Management Technology Conference, Orlando, FL, USA, 7–9 February 2012. [Google Scholar] [CrossRef]
- Spycher, N.; Pruess, K. CO2-H2O mixtures in the geological sequestration of CO2. II. Partitioning in chloride brines at 12–100 °C and up to 600 bar. Geochim. Cosmochim Acta 2005, 69, 3309–3320. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Kumar, A. A Simulation Study of Carbon Sequestration in Deep Saline Aquifers. Master’s Thesis, The University of Texas at Austin, Austin, TX, USA, 2004. [Google Scholar]
- Spycher, N.; Pruess, K.; Ennis-King, J. CO2-H2O mixtures in the geological sequestration of CO2. I. Assessment and calculation of mutual solubilities from 12 to 100 °C and up to 600 bar. Geochim. Cosmochim. Acta 2003, 67, 3015–3031. [Google Scholar] [CrossRef]
- Spycher, N.; Pruess, K. A Phase-Partitioning Model for CO2–Brine Mixtures at Elevated Temperatures and Pressures: Application to CO2-Enhanced Geothermal Systems. Transp. Porous Media 2010, 82, 173–196. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R.; Zhu, C.; Chou, I.-M. An improved model for the calculation of CO2 solubility in aqueous solutions containing Na+, K+, Ca2+, Mg2+, Cl−, and SO42−. Mar. Chem. 2006, 98, 131–139. [Google Scholar] [CrossRef]
- Rowe, A.M.; Chou, J.C.S. Pressure-volume-temperature-concentration relation of aqueous sodium chloride solutions. J. Chem. Eng. Data 1970, 15, 61–66. [Google Scholar] [CrossRef]
- Kestin, J.; Khalifa, H.E.; Correics, R.J. Tables of the Dynamic and Kinematic Viscosity of Aqueous NaCl Solution in the Temperature Range 20-150 C and the Pressure Range 0.1–35 MPa. J. Phys. Chem. Ref. Data 1981, 10, 71–88. [Google Scholar] [CrossRef]
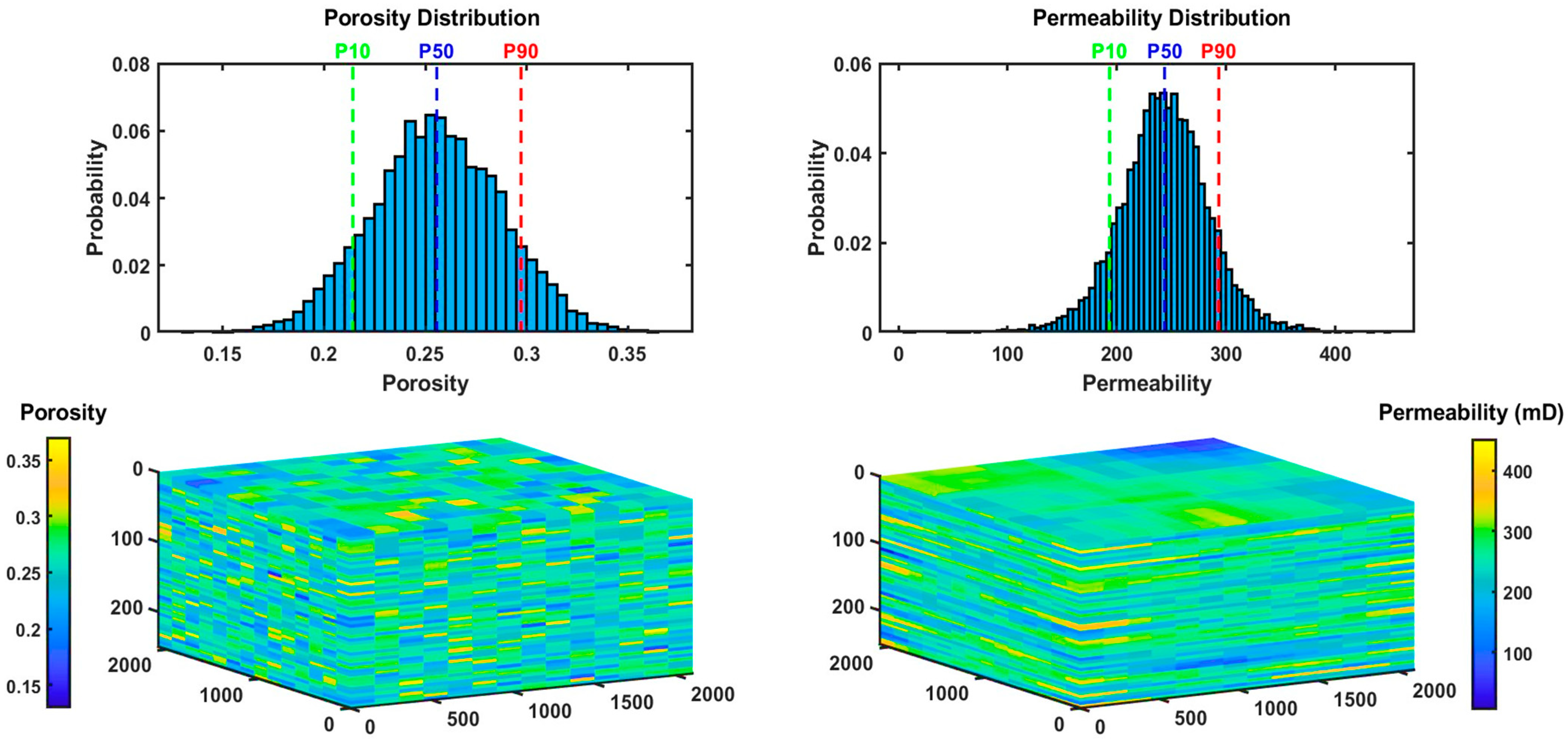

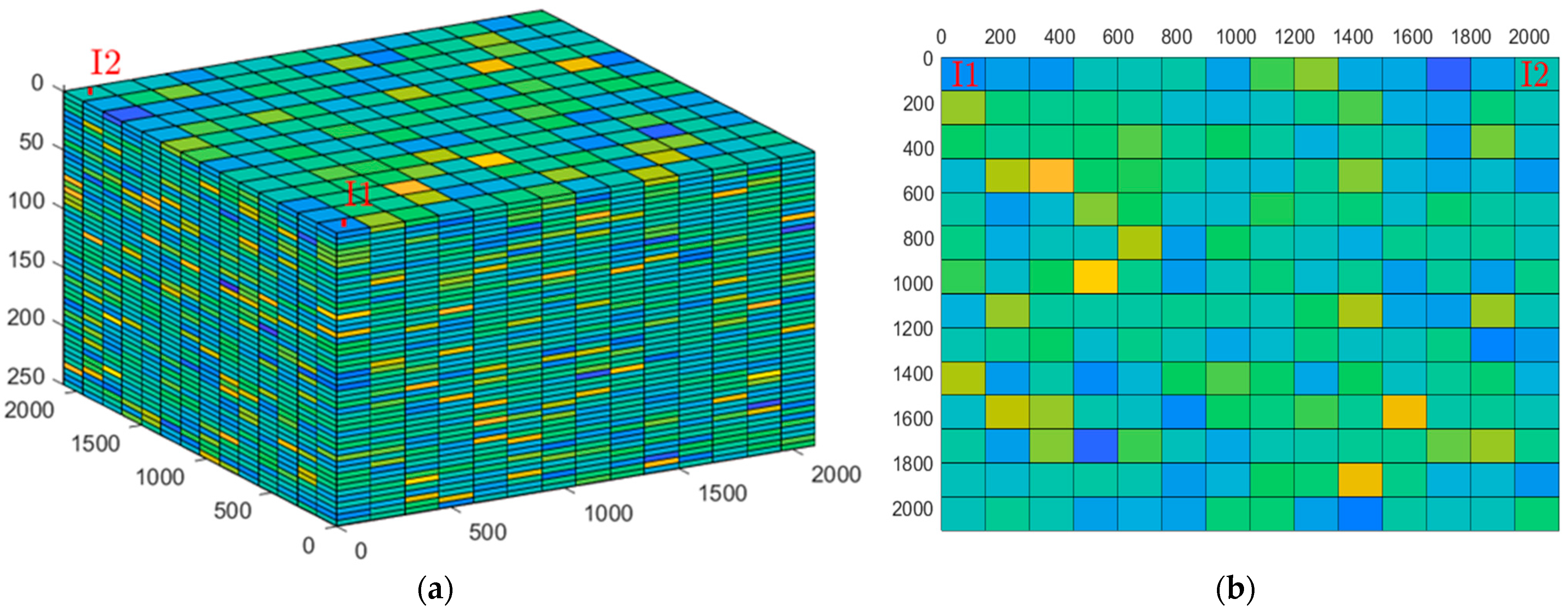

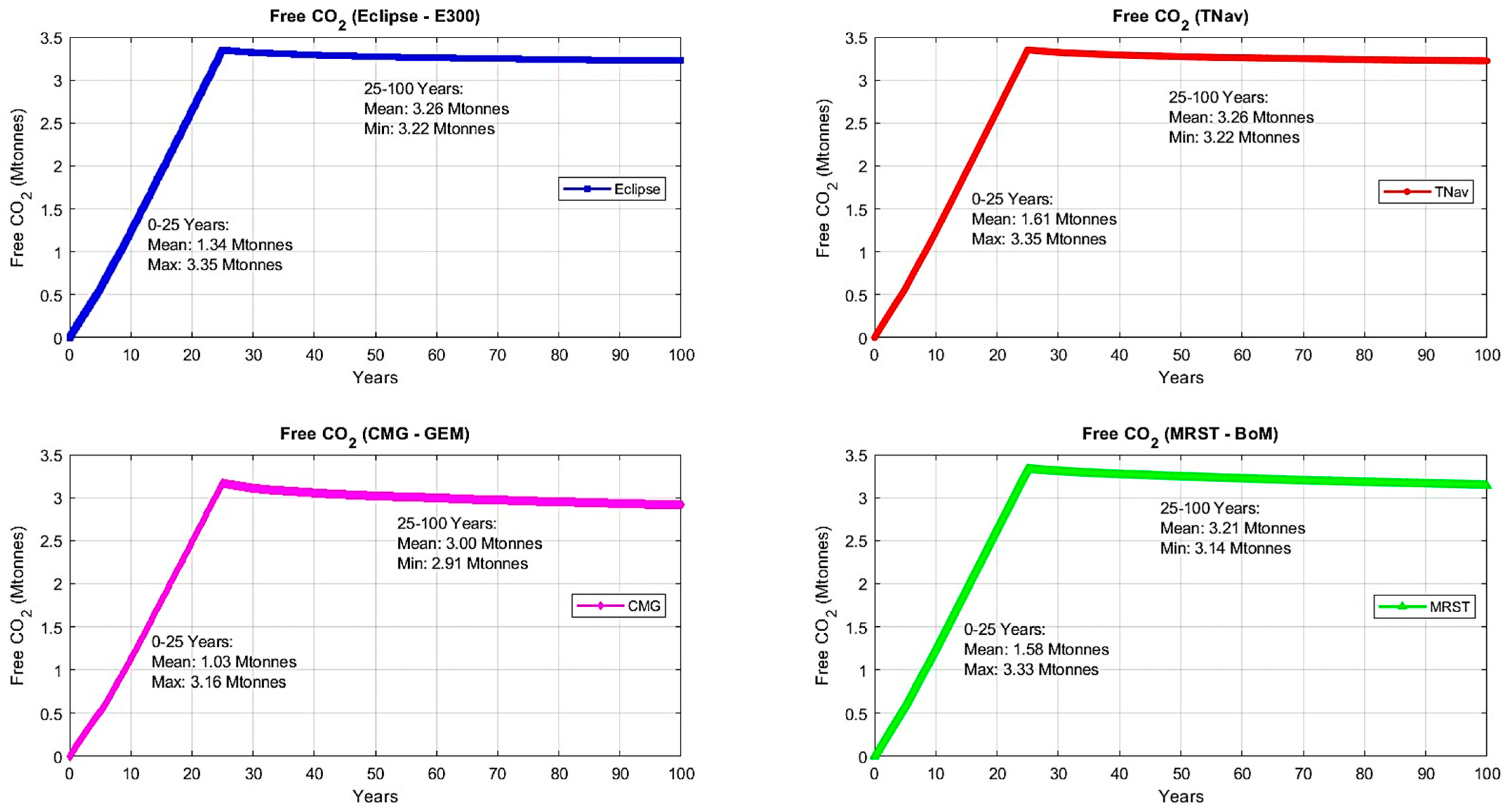

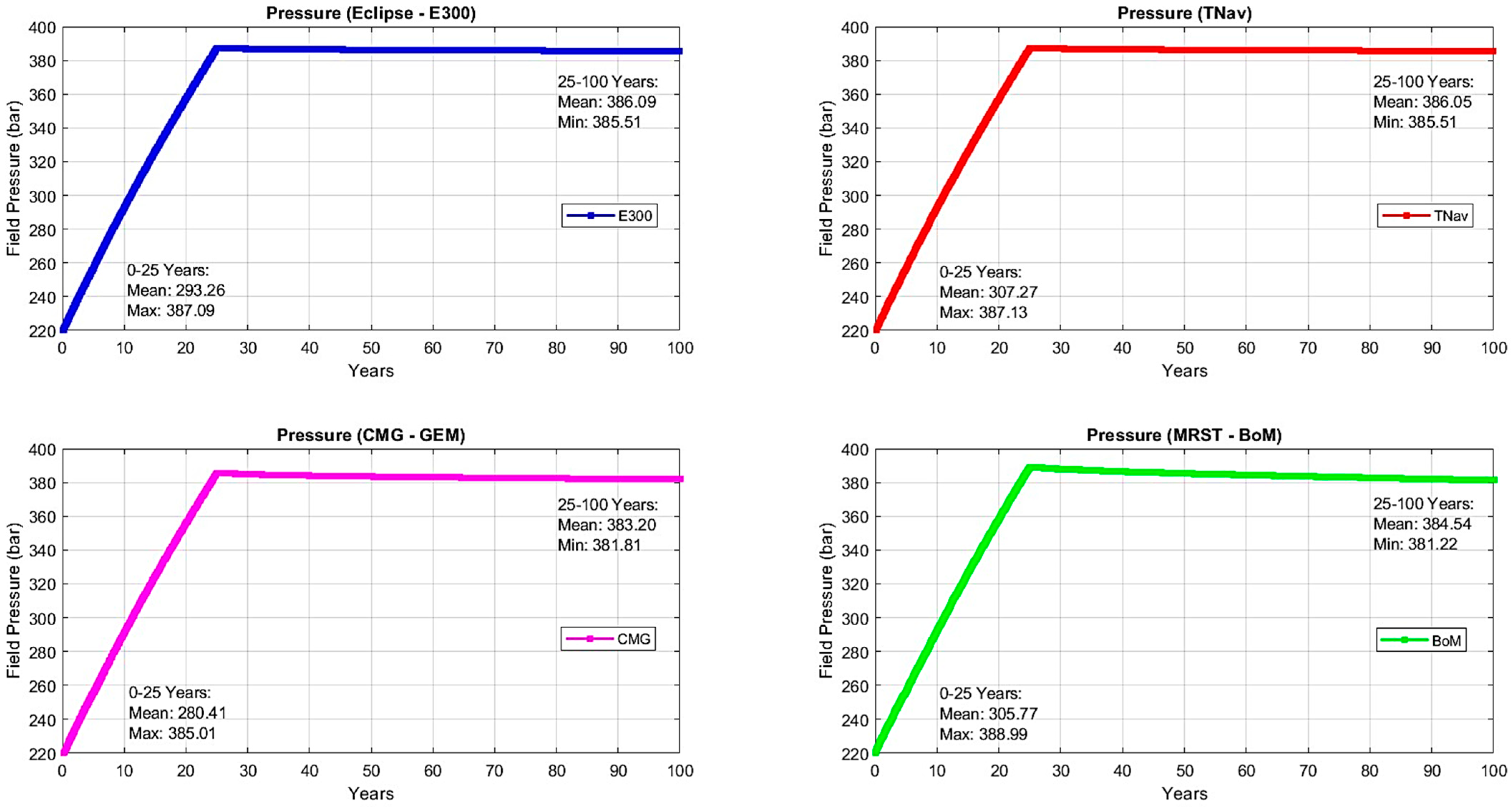
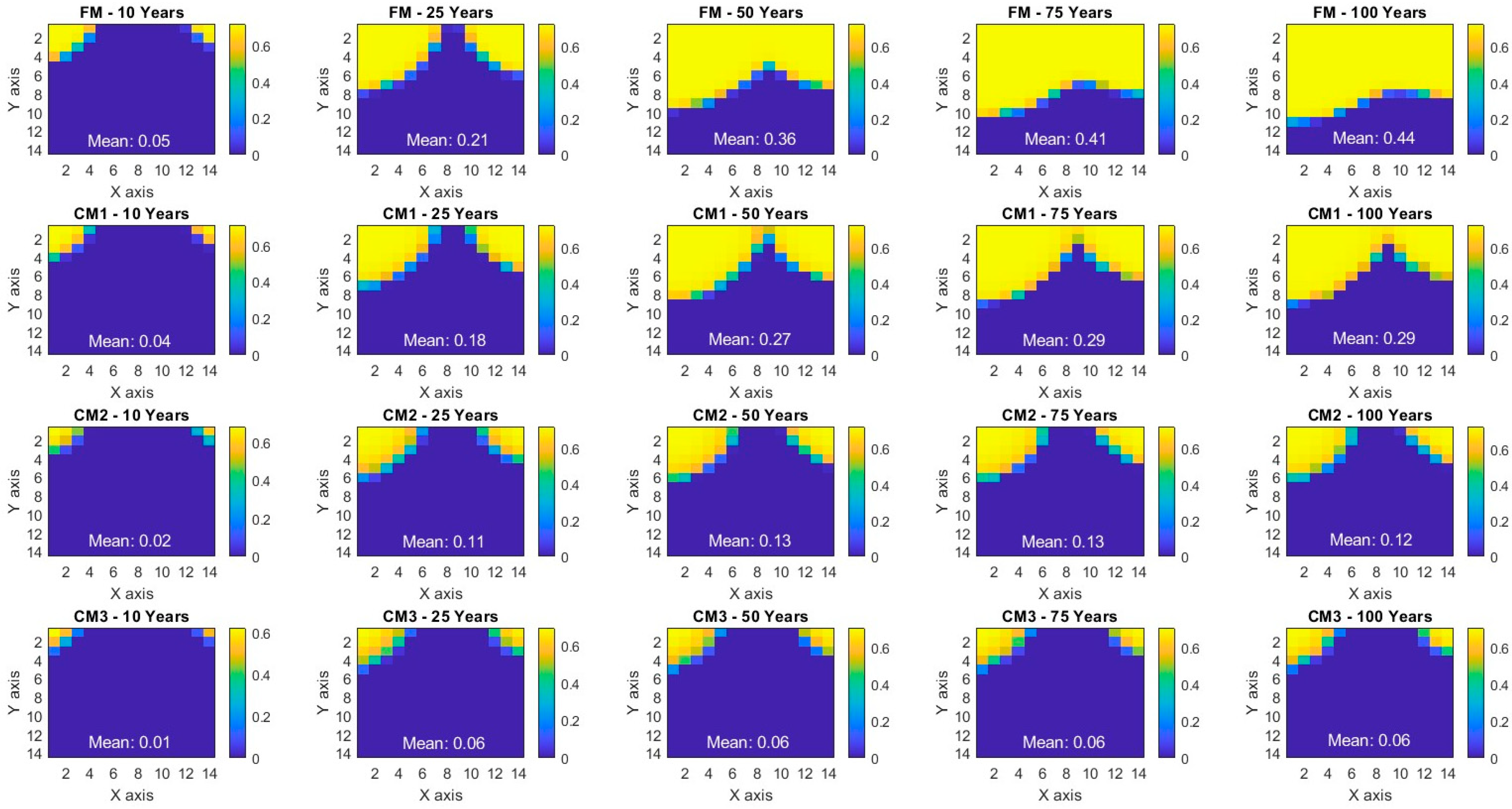
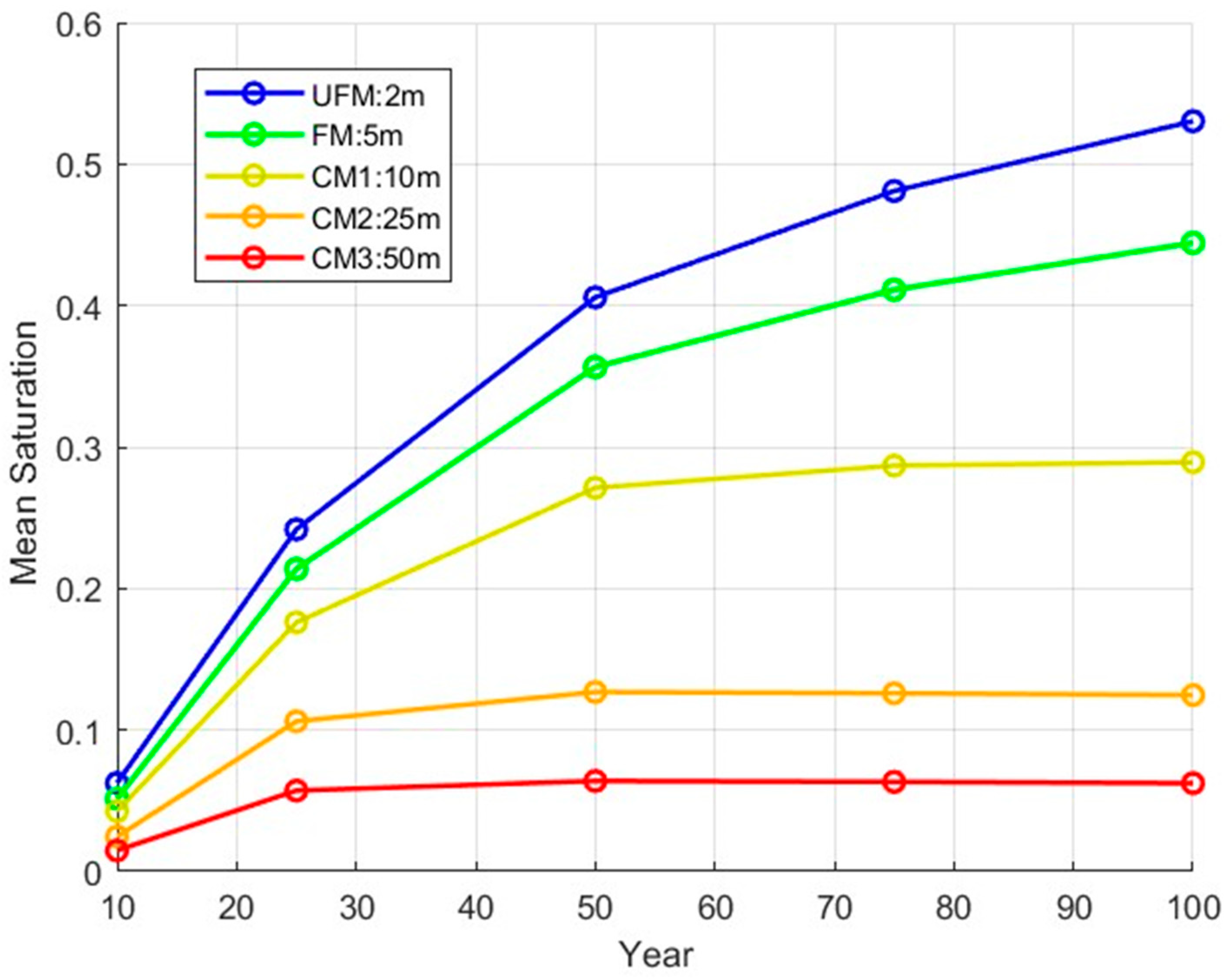
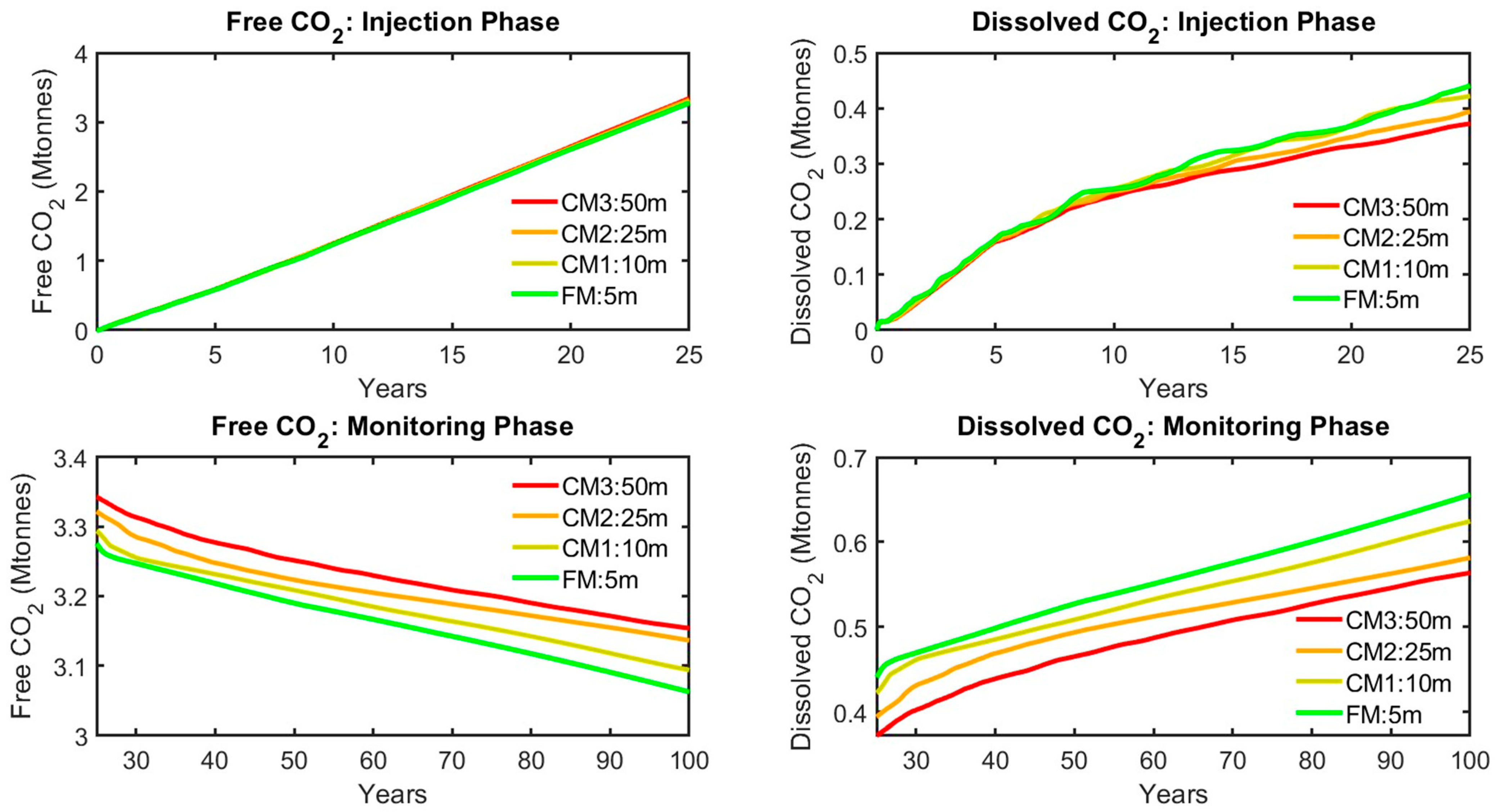
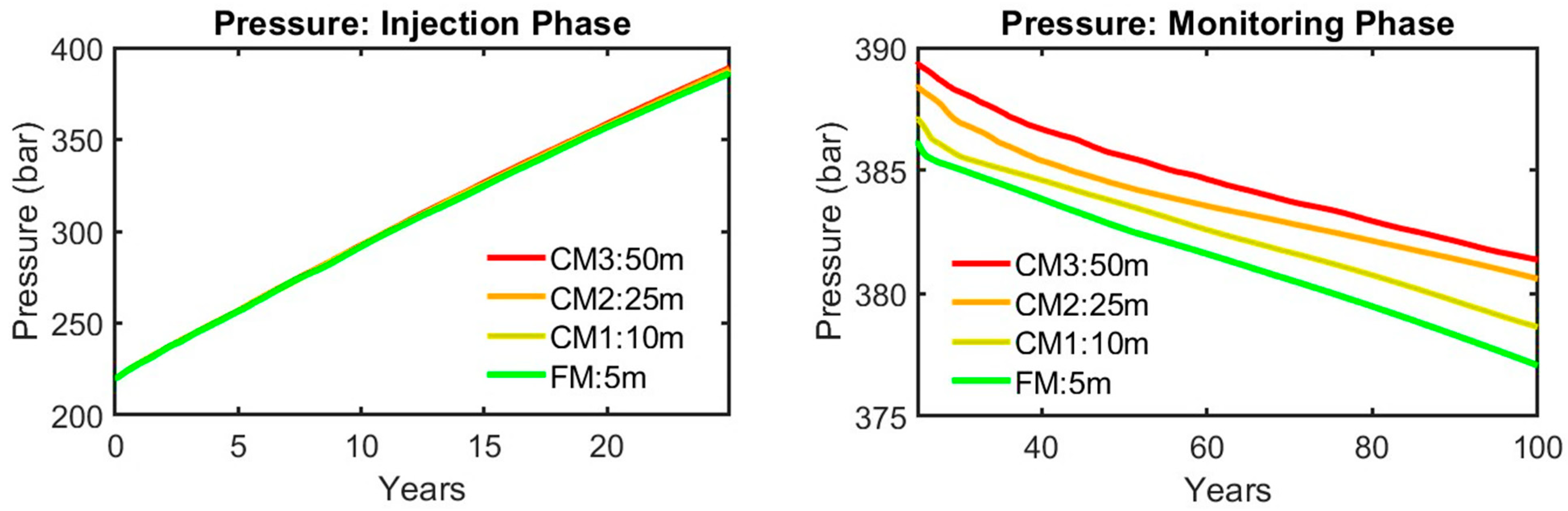



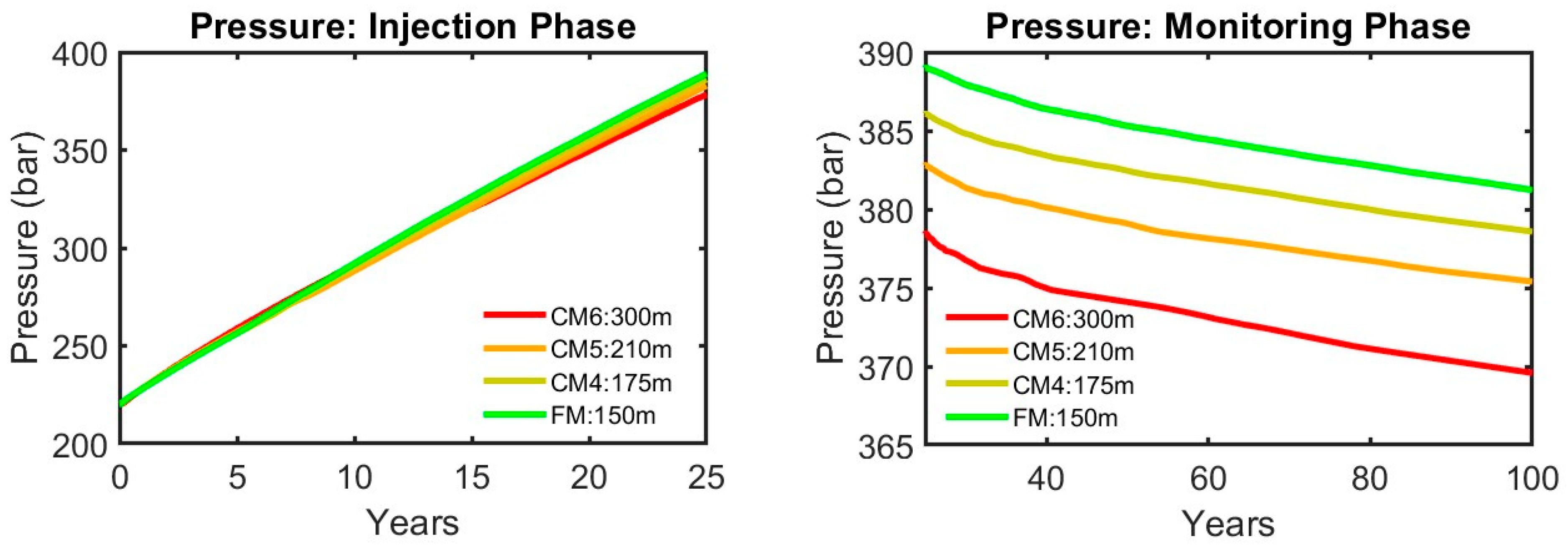
| Injection Phase | Monitoring Phase | Average (Injection Phase and CCS Timeline) | |||||
|---|---|---|---|---|---|---|---|
| Model | 10 Years | 25 Years | 50 Years | 75 Years | 100 Years | T1–T25 | T1–T100 |
| E300 | 2.01% | 4.13% | 4.18% | 4.94% | 5.50% | 3.07% | 4.15% |
| CMG | 0.52% | 2.38% | 4.56% | 4.57% | 4.81% | 1.45% | 3.37% |
| TNav | 3.08% | 3.41% | 3.60% | 4.38% | 4.94% | 3.24% | 3.88% |
| Model Name | Grid Dimensions | Number of Cells | Z-Direction Grid Size | X-/Y-Direction Grid Size |
|---|---|---|---|---|
| Fine Model (FM) | 14 × 14 × 50 | 9800 | 5 m | 150 m |
| Coarse Model (CM1) | 14 × 14 × 25 | 4900 | 10 m | 150 m |
| Coarse Model 2 (CM2) | 14 × 14 × 10 | 1960 | 25 m | 150 m |
| Coarse Model 3 (CM3) | 14 × 14 × 7 | 980 | 50 m | 150 m |
| Coarse Model 4 (CM4) | 12 × 12 × 50 | 7200 | 5 m | 175 m |
| Coarse Model 5 (CM5) | 10 × 10 × 50 | 5000 | 5 m | 210 m |
| Coarse Model 6 (CM6) | 7 × 7 × 50 | 2450 | 5 m | 300 m |
| Injection Phase | Monitoring Phase | Average (Injection Phase and CCS Timeline) | |||||
|---|---|---|---|---|---|---|---|
| Model | 10 Years | 25 Years | 50 Years | 75 Years | 100 Years | T1–T25 | T1–T100 |
| CM1 | 17.23% | 17.64% | 23.95% | 30.23% | 34.9% | 17.44% | 24.79% |
| CM2 | 52.02% | 50.36% | 64.42% | 69.36% | 71.94% | 51.19% | 61.62% |
| CM3 | 71.18% | 73.26% | 82.06% | 84.6% | 85.96% | 72.22% | 79.41% |
| Injection Phase | Monitoring Phase | Average (Injection Phase and CCS Timeline) | |||||
|---|---|---|---|---|---|---|---|
| Model | 10 Years | 25 Years | 50 Years | 75 Years | 100 Years | T1–T25 | T1–T100 |
| CM4 | 37.74% | 9.15% | 5.26% | 4.8% | 4.96% | 23.44% | 12.38% |
| CM5 | 56.57% | 23.95% | 13.75% | 13.22% | 13.62% | 40.26% | 24.22% |
| CM6 | 100% | 65.59% | 49% | 46.4% | 47.57% | 82.79% | 61.71% |
| Criteria | Black Oil Model (BoM) | Compositional Models (e.g., GEM) |
|---|---|---|
| CO2 phase treatment | CO2 treated as pseudo-oil or pseudo-gas; simplified representation | Full thermodynamic equilibrium between phases using EoS |
| Dissolution handling | Indirect (via tuning/solubility model); no explicit mass transfer between phases | Explicit solubility trapping governed by EoS & equilibrium constraints |
| Impurity effects | Cannot capture non-ideal behavior from impurities unless thermodynamic model is tuned | Captures multi-component, non-ideal phase behavior if EoS is appropriately tuned |
| Computational cost | Low; suitable for large-scale screening runs and prospecting tasks | High; especially with multiple components and fine resolution |
| Data requirements | Moderate; fewer fluid-specific inputs | High; requires PVT data, EOS tuning, and phase-behavior characterization |
| Regulatory use—early stage | Acceptable, with caveats and sensitivity analyses (e.g., DoE) | Preferred but not always required (case specific) |
| Regulatory use—liability transfer | Insufficient for mass accounting, dissolution tracking, and MRV compliance | Essential for regulatory compliance and long-term liability transfer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, I.; Fotias, S.P.; Pissas, S.; Gaganis, V. Optimized CO2 Modeling in Saline Aquifers: Evaluating Fluid Models and Grid Resolution for Enhanced CCS Performance. Processes 2025, 13, 1901. https://doi.org/10.3390/pr13061901
Ismail I, Fotias SP, Pissas S, Gaganis V. Optimized CO2 Modeling in Saline Aquifers: Evaluating Fluid Models and Grid Resolution for Enhanced CCS Performance. Processes. 2025; 13(6):1901. https://doi.org/10.3390/pr13061901
Chicago/Turabian StyleIsmail, Ismail, Sofianos Panagiotis Fotias, Spyridon Pissas, and Vassilis Gaganis. 2025. "Optimized CO2 Modeling in Saline Aquifers: Evaluating Fluid Models and Grid Resolution for Enhanced CCS Performance" Processes 13, no. 6: 1901. https://doi.org/10.3390/pr13061901
APA StyleIsmail, I., Fotias, S. P., Pissas, S., & Gaganis, V. (2025). Optimized CO2 Modeling in Saline Aquifers: Evaluating Fluid Models and Grid Resolution for Enhanced CCS Performance. Processes, 13(6), 1901. https://doi.org/10.3390/pr13061901






