Towards Circularity in Agriculture: A Case of Bioactive Compound Recovery from Sea Buckthorn Residual Leaves and Twigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Bioactive Compounds from Leaves and Twigs
2.1.1. Materials
2.1.2. Proximate Composition Analysis of Sea Buckthorn Biomass
2.1.3. Preparation of Sea Buckthorn Biomass Extracts
2.1.4. Determination of Proanthocyanidins (PACs) by the Bate-Smith Assay
2.1.5. Total Phenolic Content (TPC) by Folin−Ciocalteu Assay
2.1.6. Assessment of Antioxidant Activity
2.1.7. Statistical Analyses
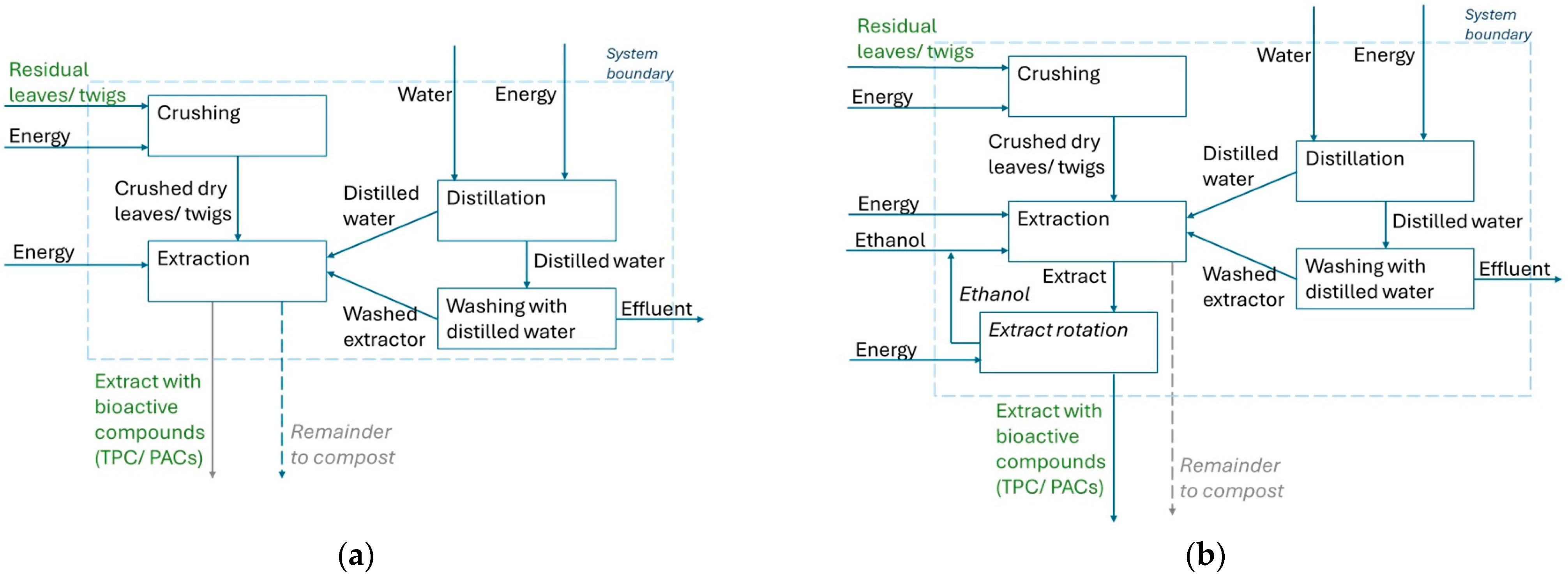
2.2. Environmental Impact Assessment of the Performed Extractions
- Definition of the goal and scope of the study, and identification of a functional unit and system boundaries;
- Life-cycle inventory analysis;
- Life-cycle impact assessment and interpretation.
3. Results
3.1. Results of Extraction of Bioactive Compounds
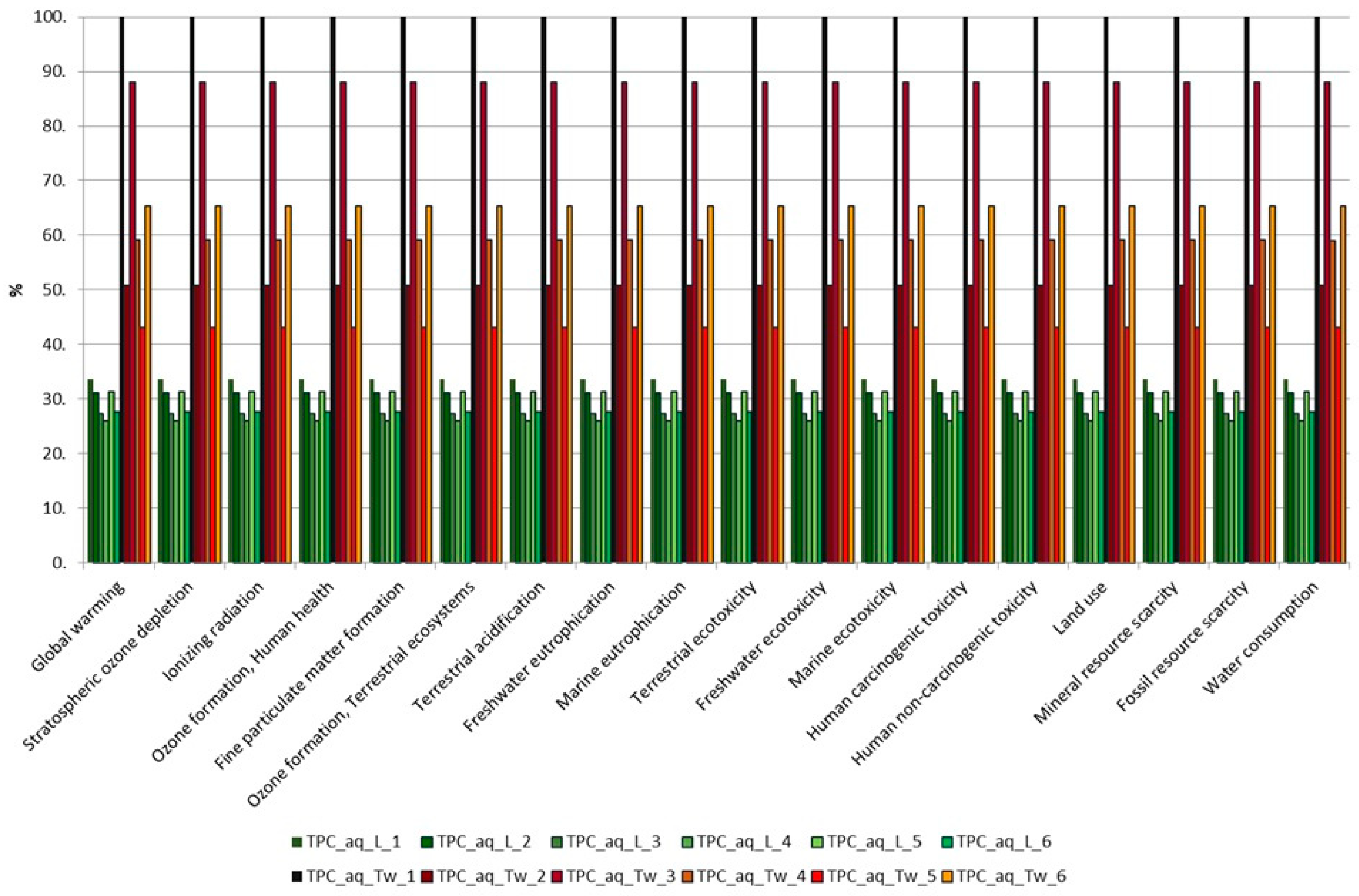
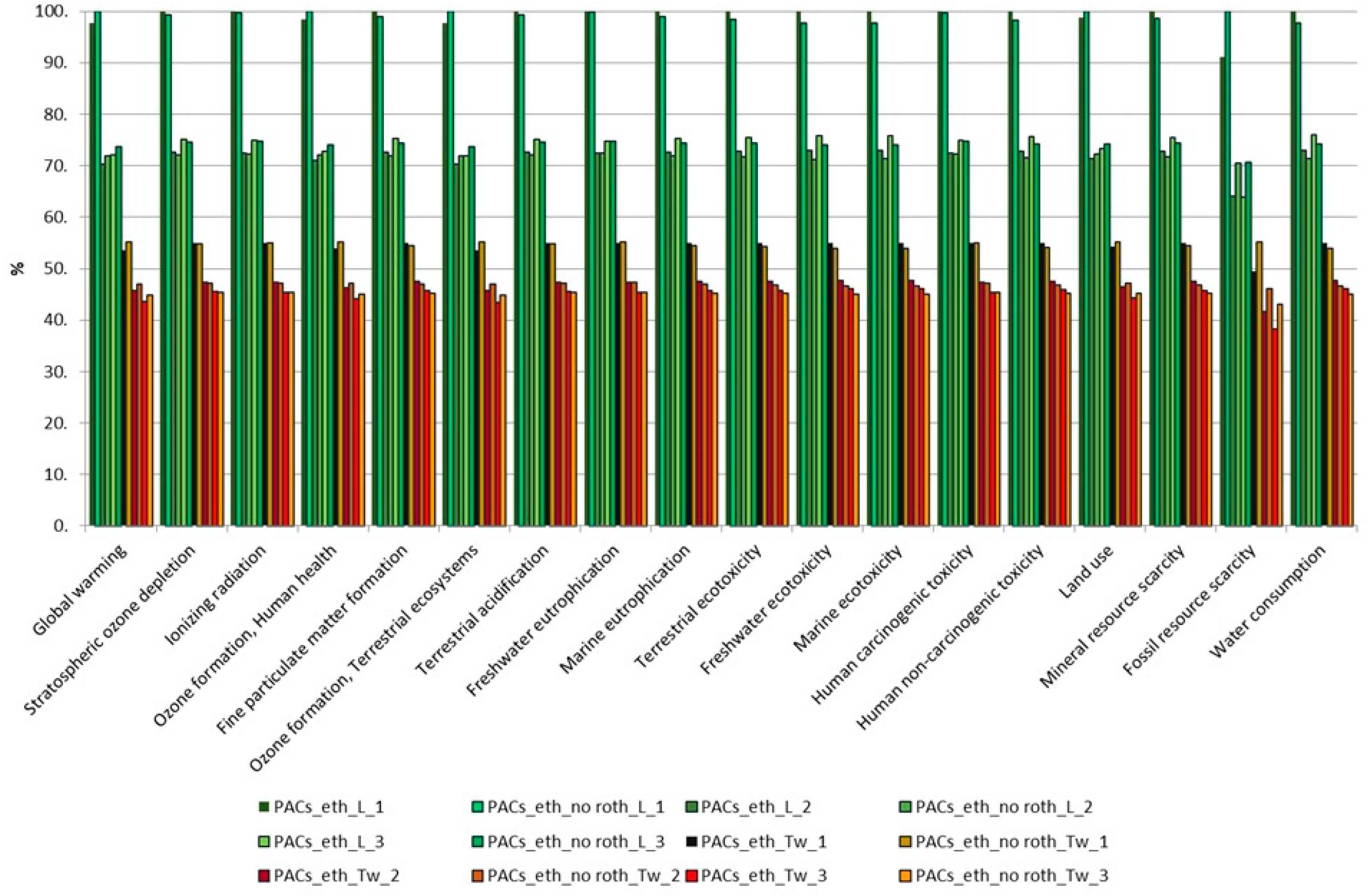
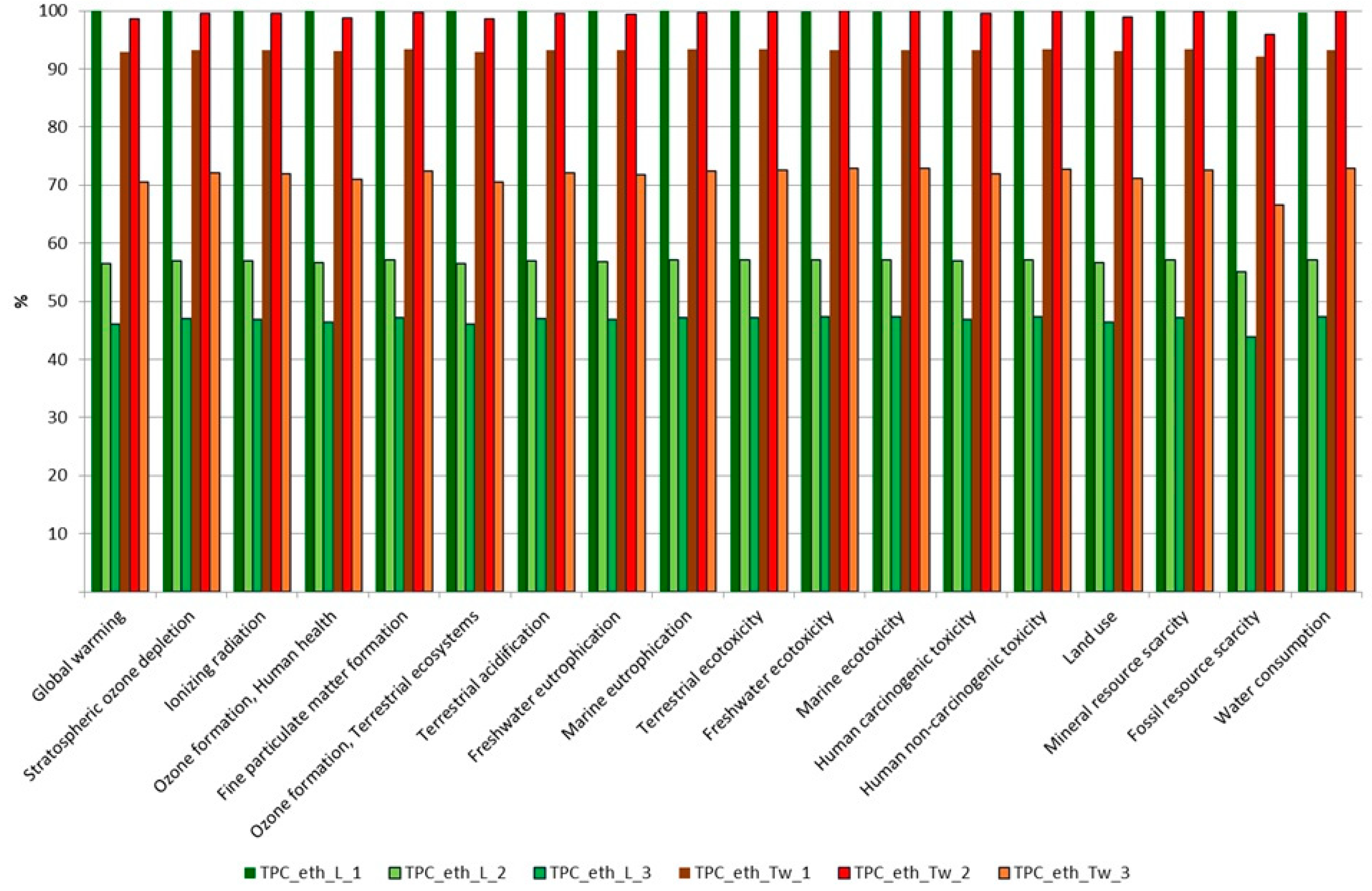
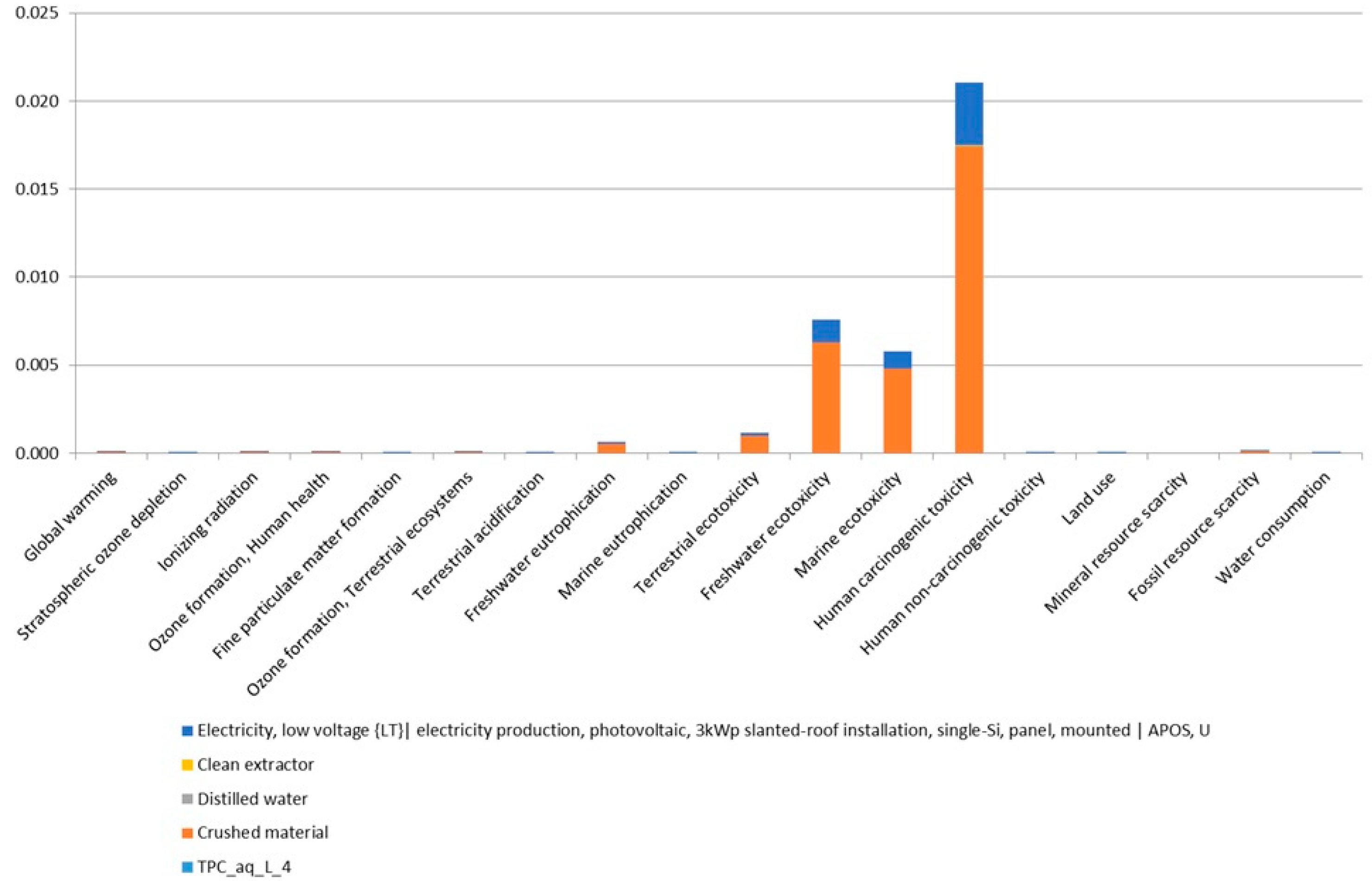
3.2. Comparison of Extractions Based on Their Environmental Impact Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LCA | Life-cycle assessment |
| PACs | Proanthocyanidins |
| TPC | Total Phenolic Content |
References
- United Nations Department of Economic and Social Affairs (UN/DESA). Circular Agriculture for Sustainable Rural Development. In Policy Brief No. 105; United Nations: New York, NY, USA, 2021; Available online: https://desapublications.un.org/policy-briefs/undesa-policy-brief-105-circular-agriculture-sustainable-rural-development (accessed on 15 April 2025).
- Šalaševičienė, A.; Šarkinas, A.; Makštutienė, N. Circular Economy in Agriculture: The Case for Bioactive Compounds from Sea Buckthorn By-products. Environ. Res. Eng. Manag. 2025, 81, 5–18. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.T.; Tran, V.T. Circular Agriculture: A General Review of Theories, Practices, and Policy Recommendations. Vietnam J. Agric. Sci. 2024, 7, 2173–2184. [Google Scholar] [CrossRef]
- Mei, D.; Ma, X.; Fu, F.; Cao, F. Research status and development prospects of sea buckthorn (Hippophae rhamnoides L.) resources in China. Forests 2023, 14, 2461. [Google Scholar] [CrossRef]
- Ruan, C.-J.; Rumpunen, K.; Nybom, H. Advances in Improvement of Quality and Resistance in a Multipurpose Crop: Sea Buckthorn. Crit. Rev. Biotechnol. 2013, 33, 126–144. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Z.; Liao, X. Bioactive Compounds, Health Benefits and Functional Food Products of Sea Buckthorn: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6761–6782. [Google Scholar] [CrossRef]
- Skalski, B.; Kontek, B.; Olas, B. Anti-Platelet Properties of Phenolic Extracts from the Leaves and Twigs of Elaeagnus rhamnoides (L.) A. Nelson. Molecules 2019, 24, 3620. [Google Scholar] [CrossRef]
- Jasniewska, A.; Diowksz, A. Wide Spectrum of Active Compounds in Sea Buckthorn (Hippophae rhamnoides) for Disease Prevention and Food Production. Antioxidants 2021, 10, 1279. [Google Scholar] [CrossRef]
- Nybom, H.; Ruan, C.; Rumpunen, K. The Systematics, Reproductive Biology, Biochemistry, and Breeding of Sea Buckthorn—A Review. Genes 2023, 14, 2120. [Google Scholar] [CrossRef]
- Ma, X.; Yang, W.; Kallio, H.; Yang, B. Health Promoting Properties and Sensory Characteristics of Phytochemicals in Berries and Leaves of Sea Buckthorn (Hippophaë rhamnoides). Crit. Rev. Food Sci. Nutr. 2022, 62, 3798–3816. [Google Scholar] [CrossRef]
- Shi, S.; Wu, R.; Han, Z.; Sun, Y.; Li, P.; Ren, F.; Shang, N. Prospects of sea buckthorn (Hippophae rhamnoides L.) polysaccharides: Preparation, structural characterization, and bioactivities diversity. Food Sci. Hum. Wellness 2025, 14, 9250001. [Google Scholar] [CrossRef]
- Janceva, S.; Andersone, A.; Lauberte, L.; Bikovens, O.; Nikolajeva, V.; Jashina, L.; Zaharova, N.; Telysheva, G.; Senkovs, M.; Rieksts, G.; et al. Sea Buckthorn (Hippophae rhamnoides) Waste Biomass after Harvesting as a Source of Valuable Biologically Active Compounds with Nutraceutical and Antibacterial Potential. Plants 2022, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Giurescu, I.; Șesan, T.-E.; Badju, S.; Lupu, C.; Oancea, F. Preparation of Compost from Sea Buckthorn Branches by Using a Multipurpose Trichoderma Strain. Sci. Bull. Ser. F Biotechnol. 2023, XXVII, 97–104. Available online: https://biotechnologyjournal.usamv.ro/index.php/scientific-papers/current/8-administrative/617-scientific-bulletin-series-f-biotechnologies-vol-xxvii-no-1-2023 (accessed on 1 June 2025).
- Čulina, P.; Repajić, M.; Elez Garofulić, I.; Dragović-Uzelac, V.; Pedisić, S. Evaluation of Polyphenolic Profile and Antioxidant Activity of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) Leaf and Berry Extracts Obtained via Optimized Microwave-Assisted and Accelerated Solvent Extraction. Processes 2024, 12, 126. [Google Scholar] [CrossRef]
- Čulina, P.; Balbino, S.; Vitali Čepo, D.; Golub, N.; Elez Garofulić, I.; Dragović-Uzelac, V.; You, L.; Pedisić, S. Stability of Fatty Acids, Tocopherols, and Carotenoids of Sea Buckthorn Oil Encapsulated by Spray Drying Using Different Carrier Materials. Appl. Sci. 2025, 15, 1194. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Chen, X.; Zhang, J.; Zhang, Y. Dynamic changes in flavonoid, phenolic, and polysaccharide contents in leaves and fruits of sea buckthorn during the growing season in southeastern Tibet plateau. Sci. Hortic. 2022, 307, 111497. [Google Scholar] [CrossRef]
- Sanwal, N.; Mishra, S.; Sharma, N.; Sahu, J.K.; Raut, P.K.; Naik, S.N. Evaluation of the phytoconstituents and bioactivity potentials of Sea buckthorn (Hippophae sp.) leaves using GC-MS, HPLC-PDA and ICP-MS: A gender-based comprehensive metabolic profiling. J. Food Meas. Charact. 2023, 17, 2767–2781. [Google Scholar] [CrossRef]
- Żuchowski, J. Phytochemistry and pharmacology of sea buckthorn (Elaeagnus rhamnoides; syn. Hippophae rhamnoides): Progress from 2010 to 2021. Phytochem. Rev. 2022, 22, 3–33. [Google Scholar] [CrossRef]
- Guo, Z.; Cheng, J.; Zheng, L.; Xu, W.; Xie, Y. Mechanochemical-Assisted Extraction and Hepatoprotective Activity Research of Flavonoids from Sea Buckthorn (Hippophaë rhamnoides L.) Pomaces. Molecules 2021, 26, 7615. [Google Scholar] [CrossRef]
- Naviglio, D. Naviglio’s principle and presentation of an innovative Solid–Liquid extraction technology: Extractor Naviglio®. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 1997. [Google Scholar]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters: Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2017.
- Megazyme. Total Dietary Fiber Assay Procedure. 2017. Available online: https://d1kkimny8vk5e2.cloudfront.net/documents/Assay_Protocol/K-TDFR-200A_DATA.pdf (accessed on 15 February 2024).
- BS EN 15621:2017; Animal Feeding Stuffs: Methods of Sampling and Analysis. Determination of Calcium, Sodium, Phosphorus, Magnesium, Potassium, Sulphur, Iron, Zinc, Copper, Manganese and Cobalt After Pressure Digestion by ICP-AES. European Standards s.r.o.: Pilsen, Czech Republic, 2017.
- Bate-Smith, E. Astringent tannins of the leaves of Geranium species. Phytochemistry 1981, 20, 211–216. [Google Scholar] [CrossRef]
- Cáceres-Mella, A.; Peña-Neira, Á.; Narváez-Bastias, J.; Jara-Campos, C.; López-Solís, R.; Canals, J.M. Comparison of analytical methods for measuring proanthocyanidins in wines and their relationship with perceived astringency. Int. J. Food Sci. Technol. 2013, 48, 2588–2594. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- ISO 14040:2006/Amd 1:2020; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 1 February 2025).
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/38498.html (accessed on 1 February 2025).
- Sun, Y.; Wang, Y.; Guo, J.; Zhao, Y. Bioactive Compounds from Sea Buckthorn. In Sea Buckthorn; Tian, J., Fang, H., Chen, S., Wei, C., Wei, X., Eds.; Springer: Singapore, 2025. [Google Scholar] [CrossRef]
- He, Q.; Yang, K.; Wu, X.; Zhang, C.; He, C.; Xiao, P. Phenolic compounds, antioxidant activity and sensory evaluation of sea buckthorn (Hippophae rhamnoides L.) leaf tea. Food Sci. Nutr. 2022, 11, 1212–1222. [Google Scholar] [CrossRef]
- Sudirman, S.; Wardana, A.K.; Herpandı, H.; Widiastuti, I.; Sarı, D.I.; Janna, M. Antioxidant activity of polyphenol compounds extracted from Nypa fruticans Wurmb. (Nipa palm) fruit husk with different ethanol concentration. Int. J. Second. Metab. 2024, 11, 355–363. [Google Scholar] [CrossRef]
- Sedraoui, S.; Badr, A.; Barba, M.G.M.; Doyen, A.; Tabka, Z.; Desjardins, Y. Optimization of the Ultrahigh-Pressure–Assisted Extraction of Phenolic Compounds and Antioxidant Activity from Palm Dates (Phoenix dactylifera L.). Food Anal. Methods 2020, 13, 1556–1569. [Google Scholar] [CrossRef]
- Andersone, A.; Janceva, S.; Lauberte, L.; Ramata-Stunda, A.; Nikolajeva, V.; Zaharova, N.; Rieksts, G.; Telysheva, G. Anti-Inflammatory, Anti-Bacterial, and Anti-Fungal Activity of Oligomeric Proanthocyanidins and Extracts Obtained from Lignocellulosic Agricultural Waste. Molecules 2023, 28, 863. [Google Scholar] [CrossRef]
- Asofiei, I.; Calinescu, I.; Trifan, A.; Gavrila, A.I. A Semi-Continuous Process For Polyphenols Extraction From Sea Buckthorn Leaves. Sci. Rep. 2019, 9, 12044. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Zhu, L.; Geng, Z.; Liu, X.; Cheng, Z.; Zhao, M.; Zhang, Q.; Yang, X. Sea Buckthorn Pretreatment, Drying, and Processing of High-Quality Products: Current Status and Trends. Foods 2023, 12, 4255. [Google Scholar] [CrossRef]

| Sample | Extraction Cycles | Cycle Duration, min |
|---|---|---|
| Leaves extract_1 Twigs extract_1 | 20 | 10 |
| Leaves extract_2 Twigs extract_2 | 20 | 5 |
| Leaves extract_3 Twigs extract_3 | 20 | 2 |
| Leaves extract_4 Twigs extract_4 | 60 | 10 |
| Leaves extract_5 Twigs extract_5 | 60 | 5 |
| Leaves extract_6 Twigs extract_6 | 60 | 2 |
| Sample | Extraction Cycles | Cycle Duration, min | Ethanol conc., % |
|---|---|---|---|
| Leaves extract_4-1 Twigs extract_4-1 | 60 | 10 | 70 |
| Leaves extract_4-2 Twigs extract_4-2 | 60 | 10 | 60 |
| Leaves extract_4-3 Twigs extract_4-3 | 60 | 10 | 50 |
| Parameters | Sea Buckthorn Leaves | Sea Buckthorn Twigs |
|---|---|---|
| Moisture content, % | 5.37 ± 0.23 | 5.87 ± 0.21 |
| Crude protein content, % | 15.40 ± 0.57 | 13.20 ± 0.39 |
| Fat content, % | 7.30 ± 0.36 | 3.60 ± 0.19 |
| of which: | ||
| saturated fatty acids, % | 2.49 ± 0.11 | 0.91 ± 0.06 |
| monounsaturated fatty acids, % | 0.37 ± 0.04 | 0.67 ± 0.05 |
| polyunsaturated fatty acids, % | 4.45 ± 0.07 | 2.02 ± 0.05 |
| omega-3 fatty acids, % | 3.37 ± 0.03 | 0.80 ± 0.04 |
| omega-6 fatty acids, % | 1.08 ± 0.09 | 1.17 ± 0.05 |
| omega-9 fatty acids, % | 0.30 ± 0.02 | 0.61 ± 0.04 |
| Carbohydrates, % | 27.85 ± 1.51 | 15.79 ± 0.74 |
| Total sugar content, % | 2.94 ± 0.09 | 5.90 ± 0.08 |
| of which: | ||
| fructose, % | 0.34 ± 0.11 | 1.79 ± 0.07 |
| glucose, % | 1.28 ± 0.09 | 1.89 ± 0.08 |
| sucrose, % | 1.34 ± 0.07 | 2.22 ± 0.12 |
| Dietary fiber content, % | 38.73 ± 1.05 | 59.60 ± 0.78 |
| of which: | ||
| soluble | 3.51 ± 0.16 | 3.90 ± 0.17 |
| insoluble | 35.22 ± 0.98 | 55.70 ± 1.62 |
| Mineral content, % | 5.35 ± 0.16 | 1.94 ± 0.09 |
| of which: | ||
| potassium (K), mg/100 g | 66.29 ± 2.91 | 75.98 ± 2.81 |
| magnesium (Mg), mg/100 g | 59.04 ± 2.78 | 20.96 ± 1.05 |
| calcium (Ca), mg/100 g | 353.05 ± 5.45 | 68.08 ± 2.78 |
| Sample | Amount of PACs, mgCE/g | Amount of TPC, mgGAE/g | Antioxidant Activity, % |
|---|---|---|---|
| Leaves extract_1 | 12.36 ± 0.16 b | 41.94 ± 0.17 f | 86.08 ± 0.09 d |
| Twigs extract_1 | 10.30 ± 0.08 a | 14.20 ± 0.05 a | 88.98 ± 0.12 g |
| Leaves extract_2 | 17.30 ± 0.21 d | 44.05 ± 0.14 g | 86.57 ± 0.11 e |
| Twigs extract_2 | 36.13 ± 0.21 i | 27.09 ± 0.06 d | 89.70 ± 0.05 h |
| Leaves extract_3 | 22.18 ± 0.19 f | 49.24 ± 0.93 i | 84.20 ± 0.03 c |
| Twigs extract_3 | 14.80 ± 0.09 c | 15.33 ± 0.20 b | 90.37 ± 0.11 i |
| Leaves extract_4 | 27.58 ± 0.31 a | 61.22 ± 0.26 j | 81.97 ± 0.06 a |
| Twigs extract_4 | 31.12 ± 0.16 h | 27.06 ± 0.48 d | 87.71 ± 0.14 f |
| Leaves extract_5 | 19.63 ± 0.35 e | 46.47 ± 0.08 h | 82.95 ± 0.12 b |
| Twigs extract_5 | 52.79 ± 0.21 j | 33.96 ± 0.12 e | 89.74 ± 0.12 h |
| Leaves extract_6 | 21.78 ± 0.16 f | 49.92 ± 0.09 i | 85.97 ± 0.11 d |
| Twigs extract_6 | 25.13 ± 0.15 g | 21.17 ± 0.22 c | 89.81 ± 0.13 h |
| Sample | Amount of PACs, mgCE/g | Amount of TPC, mgGAE/g | Antioxidant Activity, % |
|---|---|---|---|
| Leaves extract_4-1 | 29.88 ± 0.21 a | 37.17 ± 0.24 a | 85.09 ± 0.61 b |
| Twigs extract_4-1 | 54.43 ± 0.13 c | 39.90 ± 0.21 b | 90.31 ± 0.42 c |
| Leaves extract_4-2 | 40.85 ± 0.52 b | 64.72 ± 0.87 d | 89.97 ± 0.17 c |
| Twigs extract_4-2 | 62.80 ± 0.31 d | 37.15 ± 0.25 a | 77.46 ± 0.36 a |
| Leaves extract_4-3 | 39.19 ± 0.31 b | 77.89 ± 0.81 e | 85.70 ± 0.51 b |
| Twigs extract_4-3 | 64.72 ± 0.36 e | 50.79 ± 0.31 c | 89.56 ± 0.55 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almonaitytė, K.; Kruopienė, J. Towards Circularity in Agriculture: A Case of Bioactive Compound Recovery from Sea Buckthorn Residual Leaves and Twigs. Processes 2025, 13, 1884. https://doi.org/10.3390/pr13061884
Almonaitytė K, Kruopienė J. Towards Circularity in Agriculture: A Case of Bioactive Compound Recovery from Sea Buckthorn Residual Leaves and Twigs. Processes. 2025; 13(6):1884. https://doi.org/10.3390/pr13061884
Chicago/Turabian StyleAlmonaitytė, Karolina, and Jolita Kruopienė. 2025. "Towards Circularity in Agriculture: A Case of Bioactive Compound Recovery from Sea Buckthorn Residual Leaves and Twigs" Processes 13, no. 6: 1884. https://doi.org/10.3390/pr13061884
APA StyleAlmonaitytė, K., & Kruopienė, J. (2025). Towards Circularity in Agriculture: A Case of Bioactive Compound Recovery from Sea Buckthorn Residual Leaves and Twigs. Processes, 13(6), 1884. https://doi.org/10.3390/pr13061884






