Tailings Reuse in Low-Permeability Reactive Geochemical Barriers
Abstract
1. Introduction
2. Materials
3. Methods
3.1. Experimental Conditions
3.2. Batch Tests with Adsorption and Desorption Processes
- Samples of 2 g of solid residue were placed in a plastic tube (volume of 40 cm3) and brought into contact with 20 mL of a 1 mM KNO3 solution at pH = 5.5. The samples were then agitated for 24 h to stabilize the ionic strength of the aqueous solution and the ionic charge of the particles.
- After 24 h, they were centrifuged for 10 min at 900 rpm. separating the solid phase from the liquid phase.
- At the end of step 2, the solid samples were washed in two stages: first, with Milli-Q water at pH = 5.5, and then with a dilute 0.01 mM KNO3 solution at pH = 5.5. A liquid volume of 20 mL was used for both washes. In the first stage, the samples were shaken for one hour, centrifuged at 900 rpm, and the solid phase was separated from the liquid phase. In the second stage, the samples were shaken for 24 h, centrifuged for 10 min at 900 rpm, and the solid phase was separated from the liquid phase.
- After step 3, 20 mL of solution with different concentrations of metal in each container was added to the solids.
- The solid samples in contact with the metal solutions were placed on a rotary shaker at 10 rpm and allowed to equilibrate for the following time intervals: 5, 10, 30, 60, 120, 240, 480, and 1440 min. This process was conducted to obtain the adsorption isotherm and adsorption kinetics.
- At the end of each time interval, the solution was centrifuged for 10 min at 900 rpm and then filtered through a 0.45-micron Millipore filter to separate the solid phase from the liquid phase.
- The pH of the filtered aqueous solution was measured, and the solute concentration was determined by ICP-AES.
- After step 6, the solid phase used in the adsorption process (at the points where the metal was not completely adsorbed from the solution) was contacted with a dilute aqueous solution of 0.01 mM KNO3 without metal. The samples were then placed in a shaker at 10 rpm and allowed to equilibrate for the following time intervals: 5, 10, 30, 60, 120, 240, 480, and 1140 min, to obtain the desorption isotherm and desorption kinetics.
- Steps 6 and 7 were repeated.
3.3. Transport and Flow Tests of Solutes
3.4. Mesurement of Metal Concentration and pH
3.5. Mesurement of Mineral Composition Using X-Ray Diffraction
4. Results
4.1. Mineralogical Composotion of Mine Tailings
4.2. Batch Tests
4.3. Adsorption of Metals as a Function of pH
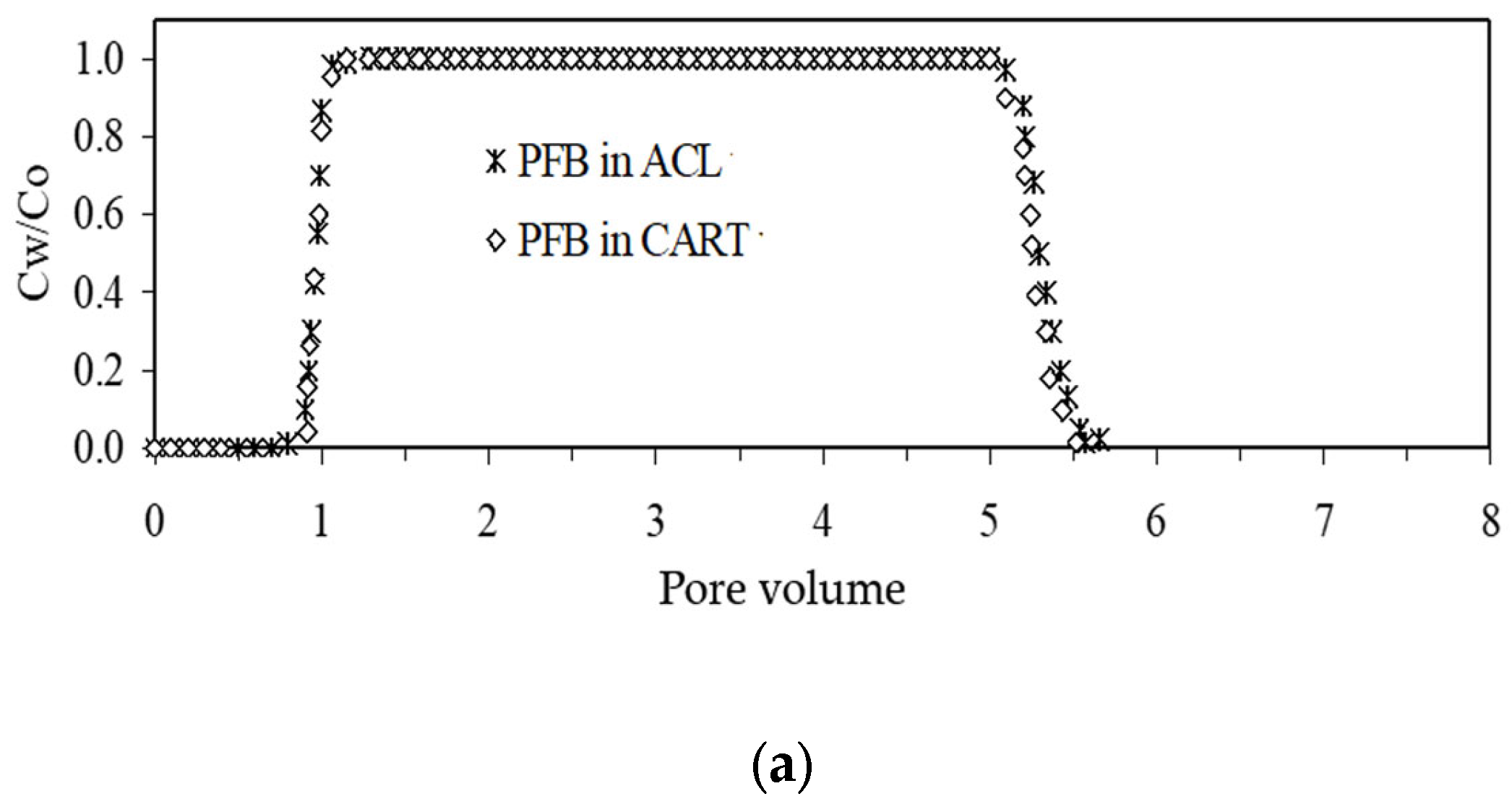
4.4. Column Solute Transport and Flow Test
4.5. Main Chemical Reactions
- Surface interaction of nickel or zinc with hydroxyl groups of hematite or goethite:
- 2.
- Precipitation of nickel hydroxide and zinc on the surface of hematite or goethite:
- 3.
- Adsorption through surface complex formation:
5. Discussion
Cost–Benefit Analysis or Feasibility Study for LPRGB Applications
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Directive (EU) 2018/850 of the European Parliament and of the Council of 30 May 2018 amending Directive 1999/31/EC on the landfill of waste. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32018L0850 (accessed on 15 January 2025).
- Rodríguez, R. Estudio Experimental de Flujo y Transporte de Cromo, Níquel y Manganeso en Residuos de la Zona Minera de Moa (Cuba): Influencia del Comportamiento Hidromecánico. Ph.D. Thesis, Universidad Politécnica de Catalunya (UPC), Barcelona, Spain, 2002. [Google Scholar]
- Rodríguez-Pacheco, R.; Candela, L.; Hidalgo, M.; Salvado, V.; Queralt, I.; Lloret, A. Evaluación de la capacidad de adsorción de dos residuos mineros para su posible uso en barreras geoquímicas reactivas de baja permeabilidad. In Proceedings of the Geo-Temas: VI Congreso Geológico de España, Zaragoza, Spain, 12–15 July 2004, ISSN 1567-5172. [Google Scholar]
- Rodríguez, R.; Candela, L. Transport of Cr(VI), Ni(II) and Mn(II) trough metallurgical waste. Batch and column experiments. In Reactive Transport in Soil and Groundwater; Nutzmann, G., Viotti, P., Aagaard, P., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 65–77. [Google Scholar]
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L. In situ remediation of Cr(VI)-contamiannted ground water. Using permeable reactive walls. Env. Sci. Tech. 1997, 31, 3348–3357. [Google Scholar] [CrossRef]
- USEPA 2021. A Community Guide to Permeable Reactive Barriers. Office of Land and Emergency Management (5203P)|EPA-542-F-21-019. 2021. Available online: www.clu-in.org (accessed on 14 January 2025).
- FRTR 2025. Federal Remediation Technologies Roundtable. Available online: https://www.frtr.gov/matrix-2019/Permeable-Reactive-Barriers/ (accessed on 14 January 2025).
- DIN 38414-S4; German Standard Methods for the Examination of Water, Waste Water and Sludge; Sludge and Sediments (Group S); Determination of Leachability by Water (S 4). Determination of Leachability by Water. Institutfür Normung: Berlin, Germany, 1984.
- Robles-Arenas, V.M.; Rodríguez, R.; García, C.; Manteca, J.I.; Candela, L. Sulphide-mining impacts in the physical environment: Sierra de Cartagena-La Unión (SE Spain) case study. Environ. Geol. 2006, 51, 57–64. [Google Scholar] [CrossRef]
- Alcolea, A. Geoavailability of Ni, Cu, Zn, As, Cd, and Pb in th, e Sierra de Cartagena-La Unión (SE Spain). Doctoral Thesis, Universidad Politécnica de Cartagena, Departamento de Ingeniería Minera, Geológica y Cartográfica, Cartagena, Murcia, Spain, 2015. [Google Scholar]
- Rodríguez-Pacheco, R.; García, G.; Caparrós-Ríos, A.V.; Robles-Arenas, V.; García-García, C.; Millán, R.; Pérez-Sanz, A.; Alcolea-Rubio, L.A. Mineralogy, Geochemistry and Environmental Hazards of Different Types of Mining Waste from a Former Mediterranean Metal Mining Area. Land 2023, 12, 499. [Google Scholar] [CrossRef]
- Selim, H.M.; Amacher, C. Reactivity and Transport of Heavy Metals in Soils; Lewis Publishers: New York, NY, USA, 1997. [Google Scholar]
- Aris, R. On the dispersion of linear kinematic waves. Proc. R. Soc. Lond. Ser. A. 1958, 245, 268–277. [Google Scholar]
- BOE, 30 de abril de 1986. Criterios relativos a los límites de vertidos. Available online: https://www.boe.es/eli/es/rd/1986/04/11/849/con (accessed on 30 January 2025).
- Sparks, D.L. Ion exchange processes. In Chapter 6: Environmental Soil Chemistry; Academic Press: New York, NY, USA, 1995; pp. 141–158. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry Groundwater and Pollution; A.A. Balkema: Rotterdam, The Netherlands, 1993. [Google Scholar]
- Anderson, M.A.; Rubin, A.J. Adsorption of inorganics at soil-liquid interfaces. Soil Sci. 1982, 133, 257–258. [Google Scholar] [CrossRef]
- Tan, K.H. Electrochemical properties of solid constituents. In Chapter 6. Environmental Soil Science; Dekker, M., Ed.; INC.: New York, NY, USA, 1994. [Google Scholar]
- McKenzie, R.M. The adsorption of lead and other heavy metals on oxides of manganese and iron. Aust. J. Soil Res. 1980, 18, 61–73. [Google Scholar] [CrossRef]
- Stollenwerk, K.G. Geochemical interactions between constituents in acidic groundwater and alluvium in an aquifer near Globe, Arizona. Appl. Geochem. 1994, 9, 353–369. [Google Scholar] [CrossRef]
- Payne, T.E.; Lumpkin, G.R.; Waite, T.D. UraniumVI adsorption on model minerals: Controlling factors and surface complexation modeling. In Adsorption of Metals by Geomedia; Jenne, E.A., Ed.; Academic Press: New York, NY, USA, 1998; Volume 583, pp. 75–97. [Google Scholar] [CrossRef]
- Wang, W.Z.; Brusseau, M.L.; Artiola, J. Nonequilibrium and Nonlinear Sorption during Transport of Cadmium, Nickel, and Strontium through Subsurface Soils. In Adsorption of Metals by Geomedia; Jenne, E.A., Ed.; Academic Press: New York, NY, USA, 1998; Volume 583, pp. 427–443. [Google Scholar] [CrossRef]
- Poulsen, I.F.; Bruun, H.C. Soil sorption of nickel in presence of citrate or arginine. Water Air Pollut. 2000, 120, 249–259. [Google Scholar] [CrossRef]
- Buchter, B.; Davidoff, B.; Amacher, M.C.; Hinz, C.; Iskandar, I.K.; Selim, H.M. Correlation of Freundlich Kd and n retention parameters with soils and elements. Soil Sci. 1989, 148, 370–379. [Google Scholar] [CrossRef]
- Smith, K.S.; Ranville, J.F.; Plumlee, G.S.; Macalady, D.L. Predictive double layer modeling of metal sorption in mine drainage systems. In Chapter 24: Adsorption of metals by Geomedia; Academic Press: New York, NY, USA, 1998. [Google Scholar] [CrossRef]

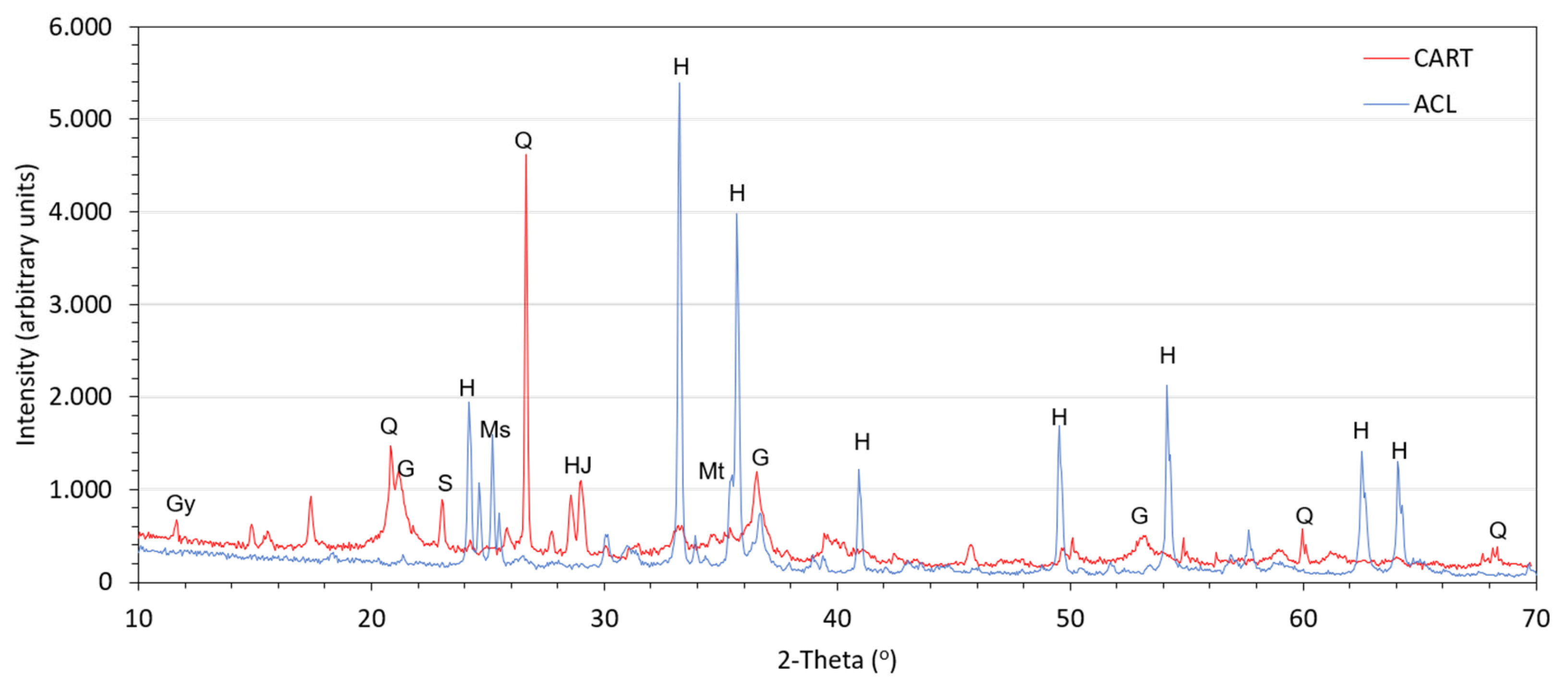
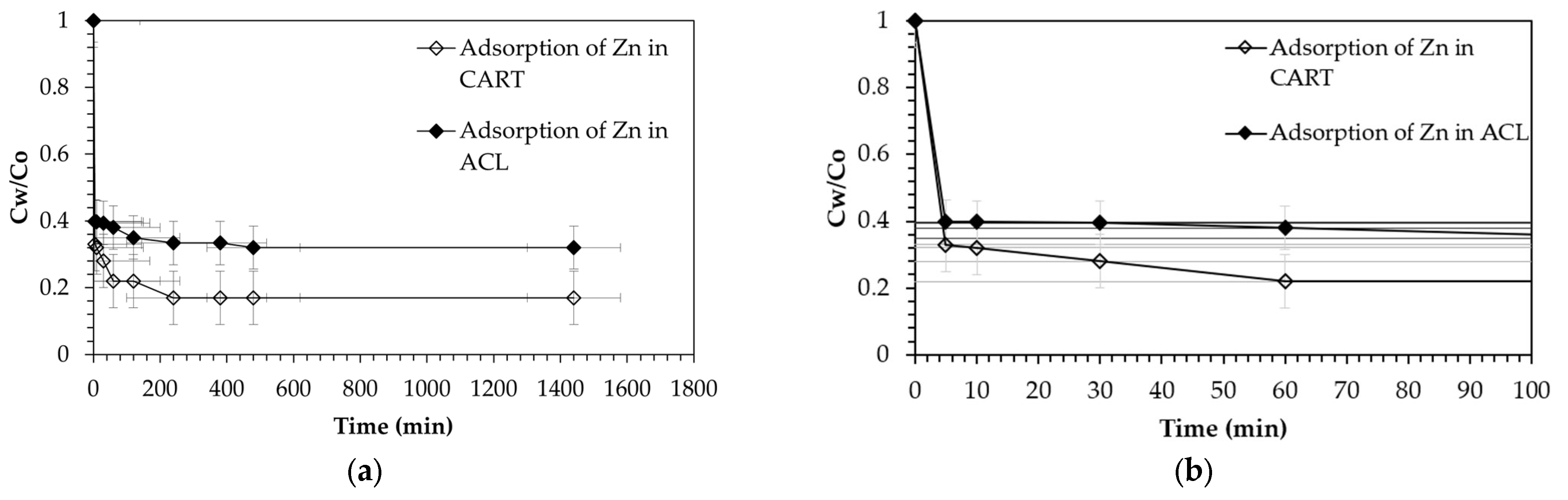
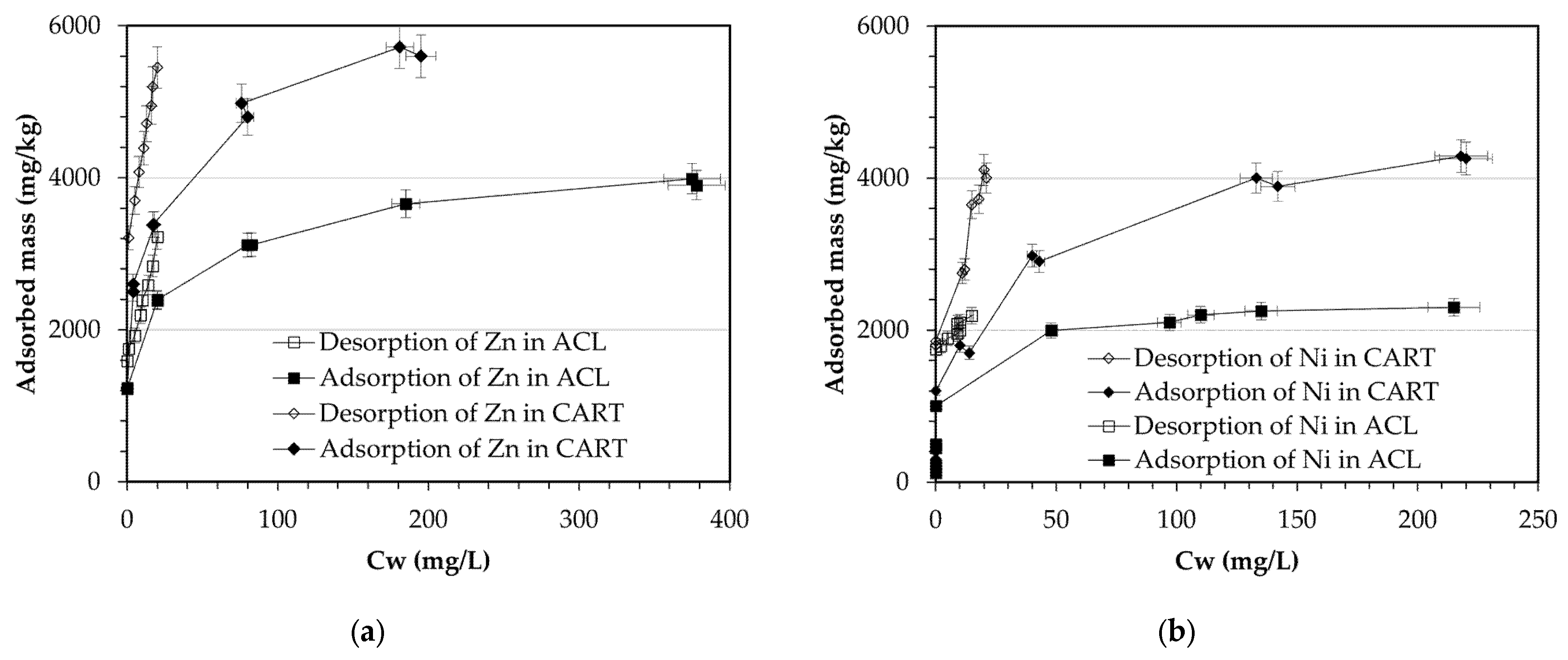
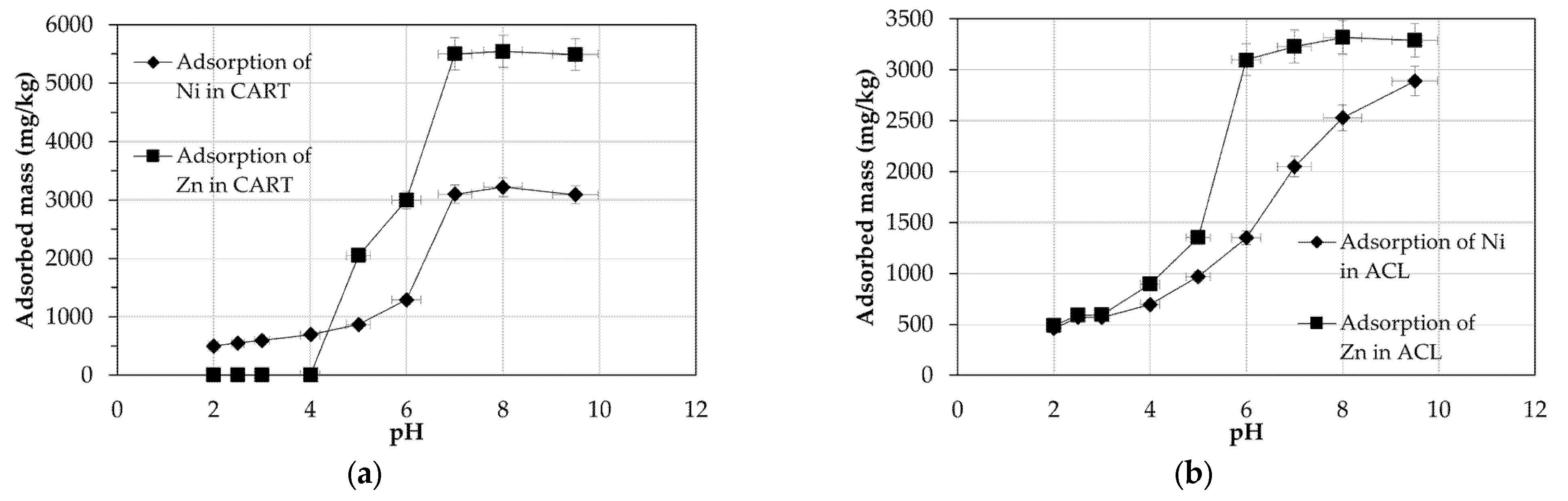

| Parameter | ACL | CART | Parameter | ACL | CART |
|---|---|---|---|---|---|
| Sand < 2 mm (%) | 10 | 36 | Organic matter (%) | 4.6 | 4.4 |
| Silt < 0.063 mm (%) | 70 | 52 | Fe2O3 (%) | 43.6 | 35.8 |
| Clay < 0.002 mm (%) | 20 | 12 | SiO2 (%) | 4.7 | 30.4 |
| Approximate effective surface (m2/g) | 82 | 69 | MnO (%) | 0.5 | 1.1 |
| pH (ratio 1:2.5) | 6.45 | 7.88 | CaO (%) | 0.1 | 5.0 |
| Electric conductivity (mS/cm) (1:2.5) | 670 | 956 | Al2O3 (%) | 5.1 | 11.1 |
| Hydraulic conductivity | 3 × 10−9 | 2 × 10−9 | MgO (%) | 16.2 | 3.8 |
| CEC (meq/100g solid) | 10.0 | 18.7 | Amorphous Fe (%) | 4.5 | 6.3 |
| Class of Waste | Type of Waste Deposit | Saturated Permeability (m/s) | Thickness of the Impermeable Layer (m) |
|---|---|---|---|
| CIII | hazardous waste | 1.0 × 10−9 | 5.0 |
| CII | non-hazardous waste | 1.0 × 10−9 | 1.0 |
| CI | inert (non-reactive waste | 1.0 × 10−7 | 1.0 |
| Parameter | Units | ACL | CART | Parameter | Units | ACL | CART |
|---|---|---|---|---|---|---|---|
| Length (L) | cm | 10.00 | 10.00 | Apparent density (ρh) | g/cm3 | 2.11 | 2.12 |
| Diameter (Φ) | cm | 1.60 | 1.60 | Dry density (ρh) | g/cm3 | 1.44 | 1.53 |
| Total volume (V) | cm3 | 20.11 | 20.11 | Pore Volume (Vp) | cm3 | 12.22 | 12.9 |
| Blind volume, (Vm) | cm3 | 0.121 | 0.112 | Porosity (n) | cm3/cm3 | 0.61 | 0.64 |
| Tailings mass (M) | g | 30.66 | 30.70 | Flow velocity (v) | cm/h | 1.2 | 1.2 |
| Volumetric water content (θ) | cm3/cm3 | 0.61 | 0.64 | Particle density (ρs) | g/cm3 | 3.97 | 3.85 |
| Tailings | Metal | N | Kd | n | r2 | log(σa) | σn | N | Kd | r2 | σa | σb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adsorption | Desorption | |||||||||||
| CART | Zn(II) | 14 | 2347 | 0.15 | 0.97 | 0.02 | 0.01 | 14 | 119.06 | 0.99 | 2.87 | 36.92 |
| Ni(II) | 13 | 1194 | 0.24 | 0.91 | 0.03 | 0.04 | 14 | 109.5 | 0.96 | 8.76 | 126.03 | |
| ACL | Zn(II) | 14 | 1962 | 0.11 | 0.97 | 0.01 | 0.006 | 14 | 76.24 | 0.98 | 4.01 | 46.9 |
| Ni(II) | 9 | 1351 | 0.101 | 0.91 | 0.03 | 0.02 | 9 | 30.27 | 0.91 | 3.83 | 33.6 | |
| Tailings | pHCo | pHa | pHd | PVi | PVd | v (m/s) | Co (mg/l) | Cwf (mg/l) | Sin (mg) | Srec (%) | Sret (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CART. | Zn(II) | 5.67 | 7.30 | 7.32 | 124 | 156 | 2.8 × 10−6 | 416 | 0.01 | 665.43 | 80.5 | 19.5 |
| Ni(II) | 5.70 | 7.28 | 7.26 | 111 | 169 | 2.8 × 10−6 | 416 | 0.02 | 595.67 | 90.3 | 9.7 | |
| ACL | Zn(II) | 5.67 | 6.84 | 6.90 | 102 | 178 | 2.8 × 10−6 | 416 | 0.021 | 518.52 | 86 | 14 |
| Ni(II) | 5.70 | 6.81 | 6.92 | 93 | 187 | 2.8 × 10−6 | 416 | 0.03 | 472.77 | 96 | 4 |
| Mineral | Formula | [18] pH in KNO3 Solution | pH [19] (p. 163) | pH [17] (p. 154) | pH [16] (p. 134) | Specific Surface, Se (m2/g) |
|---|---|---|---|---|---|---|
| Hematite | α-Fe2O 3 | 8.5 | 8.5 | 6.7 | 85 [20]; 22 [18] | |
| Goethite | α-FeOOH | 3.2 | 7.3 | 75 [20], 28–91 [18] | ||
| Maghemite | 6.8 | 6.7 | ||||
| Magnetite | 6.2 [20] | 85 [20] | ||||
| Amorphous aluminum | Al(OH)3 | 8.3 | 5.0 | |||
| AlOOH | 9.2 | |||||
| Amorphous iron | Fe(OH)3 | 8.5 | 8.5 | 8.5 | ||
| Ferrihydrite | Fe5OH8 4H2O | 8.1 | 8.1 | 600 [21] | ||
| Gibbsite | α-Al(OH)3 | 7.1 | 4.8 | 5 | 18–47 [18] | |
| Quartz | SiO2 | 2 | 2.9 | 2.0 | ||
| Calcined magnesite | δ-MgO | 4.6 | 12.4 | |||
| Mn minerals | β-MnO2 | 7.3 | ||||
| δ-MnO2 | 1.5 | |||||
| γ-MnO2 | 5.6 | 6 [21] | 2.8 | 85 [21] |
| Reference | Place | Soil/Tailings | Ni(II) | Zn(II) | ||||
|---|---|---|---|---|---|---|---|---|
| pH | CEC | n | Kd | n | Kd | |||
| [23] | Canada | Hayhook | 7.5 | 6.3 | 0.373 | 161.9 | ||
| [24] | Denmark | Ronhave | 7.0 | 10.9 | 0.775 | 95.4 | ||
| [20] | Louisiana | Alligator | 4.8 | 30.2 | 0.939 | 37.8 | 1.011 | 28.1 |
| [20] | New Mexico | Calciorthid | 8.5 | 14.7 | 0.504 | 206.0 | 0.51 | 420 |
| [20] | S. Carolina | Cecil | 5.7 | 2.0 | 0.688 | 6.8 | 0.724 | 11.2 |
| [20] | Hawaii | Kula | 5.9 | 22.5 | 0.738 | 110.0 | 0.724 | 238 |
| [20] | Louisiana | Lafitte | 3.9 | 26.9 | 0.903 | 50.1 | 0.891 | 20.1 |
| [20] | Hawaii | Molakai | 6.0 | 11.0 | 0.720 | 44.9 | 0.675 | 80.4 |
| [20] | Louisiana | Norwood | 6.9 | 4.1 | 0.661 | 20.9 | 0.515 | 42.1 |
| [20] | Louisiana | Olivier | 6.6 | 8.6 | 0.646 | 50.5 | 0.625 | 89.1 |
| [20] | Florida | Unnamed | 4.3 | 2.7 | 0.836 | 3.4 | ||
| [20] | Lowa | Webster | 7.6 | 48.1 | 0.748 | 3.4 | 0.697 | 774 |
| [20] | N. Hampshire | Windsor | 5.3 | 2.0 | 0.741 | 8.4 | 0.792 | 9.68 |
| this study | Moa, Cuba | ACL | 6.5 | 10.0 | 0.101 | 1351 | 0.11 | 1962 |
| this study | Cartagena, Spain | CART | 7.88 | 18.7 | 0.24 | 1194 | 0.15 | 2347 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Pacheco, R.; Butlanska, J.; Oliva-González, A.O. Tailings Reuse in Low-Permeability Reactive Geochemical Barriers. Processes 2025, 13, 1870. https://doi.org/10.3390/pr13061870
Rodríguez-Pacheco R, Butlanska J, Oliva-González AO. Tailings Reuse in Low-Permeability Reactive Geochemical Barriers. Processes. 2025; 13(6):1870. https://doi.org/10.3390/pr13061870
Chicago/Turabian StyleRodríguez-Pacheco, Roberto, Joanna Butlanska, and Aldo Onel Oliva-González. 2025. "Tailings Reuse in Low-Permeability Reactive Geochemical Barriers" Processes 13, no. 6: 1870. https://doi.org/10.3390/pr13061870
APA StyleRodríguez-Pacheco, R., Butlanska, J., & Oliva-González, A. O. (2025). Tailings Reuse in Low-Permeability Reactive Geochemical Barriers. Processes, 13(6), 1870. https://doi.org/10.3390/pr13061870







