Full-Scale Pore Structure and Gas Adsorption Characteristics of the Medium-Rank Coals from Qinshui Basin, North China
Abstract
1. Introduction
2. Samples and Experiments
2.1. Samples
2.2. Methodology
2.2.1. Methane Isothermal Adsorption
2.2.2. Low-Temperature Carbon Dioxide Adsorption, Liquid Nitrogen Adsorption, and Mercury Intrusion Experiments
3. Result
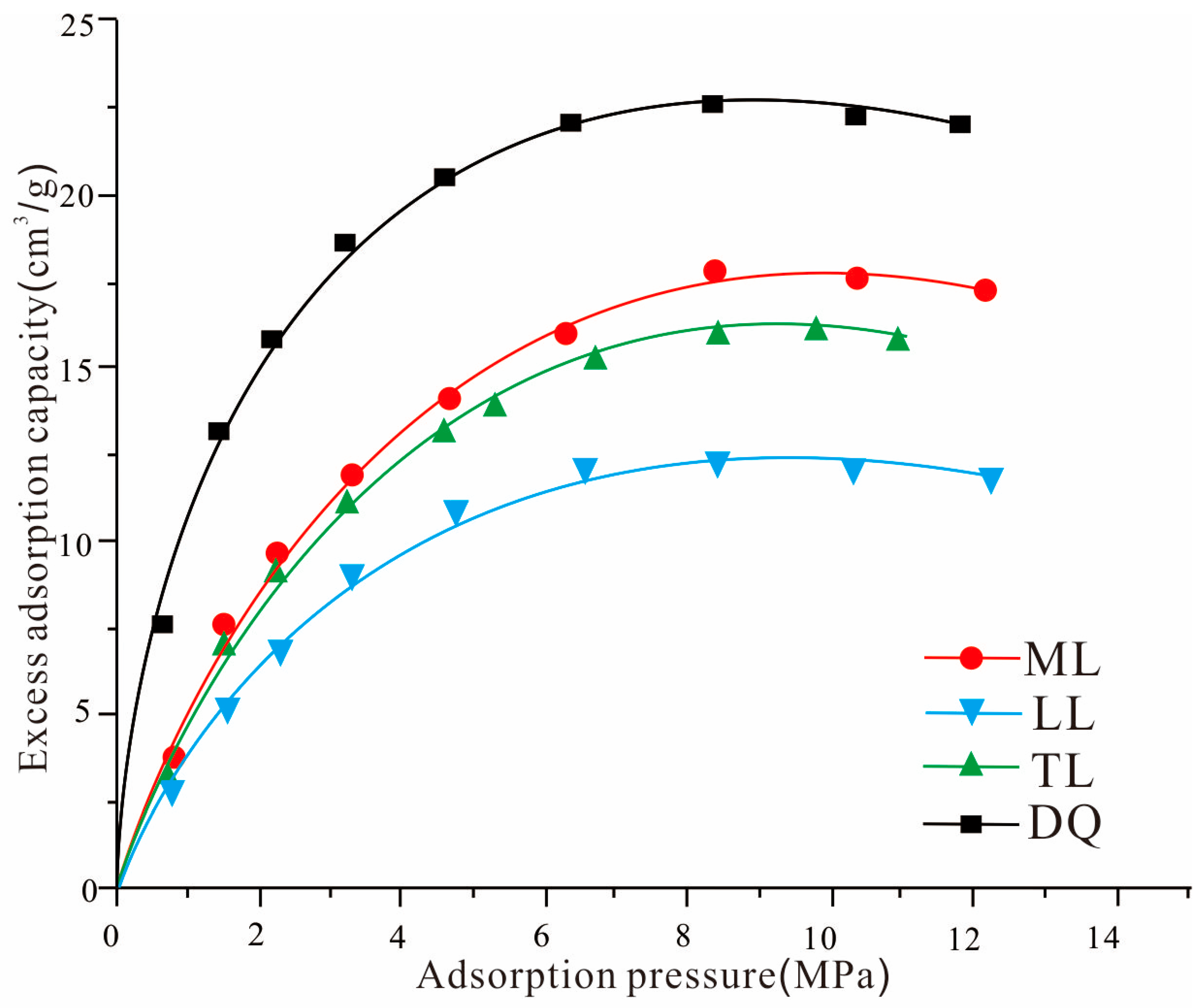
3.1. Gas Isothermal Adsorption
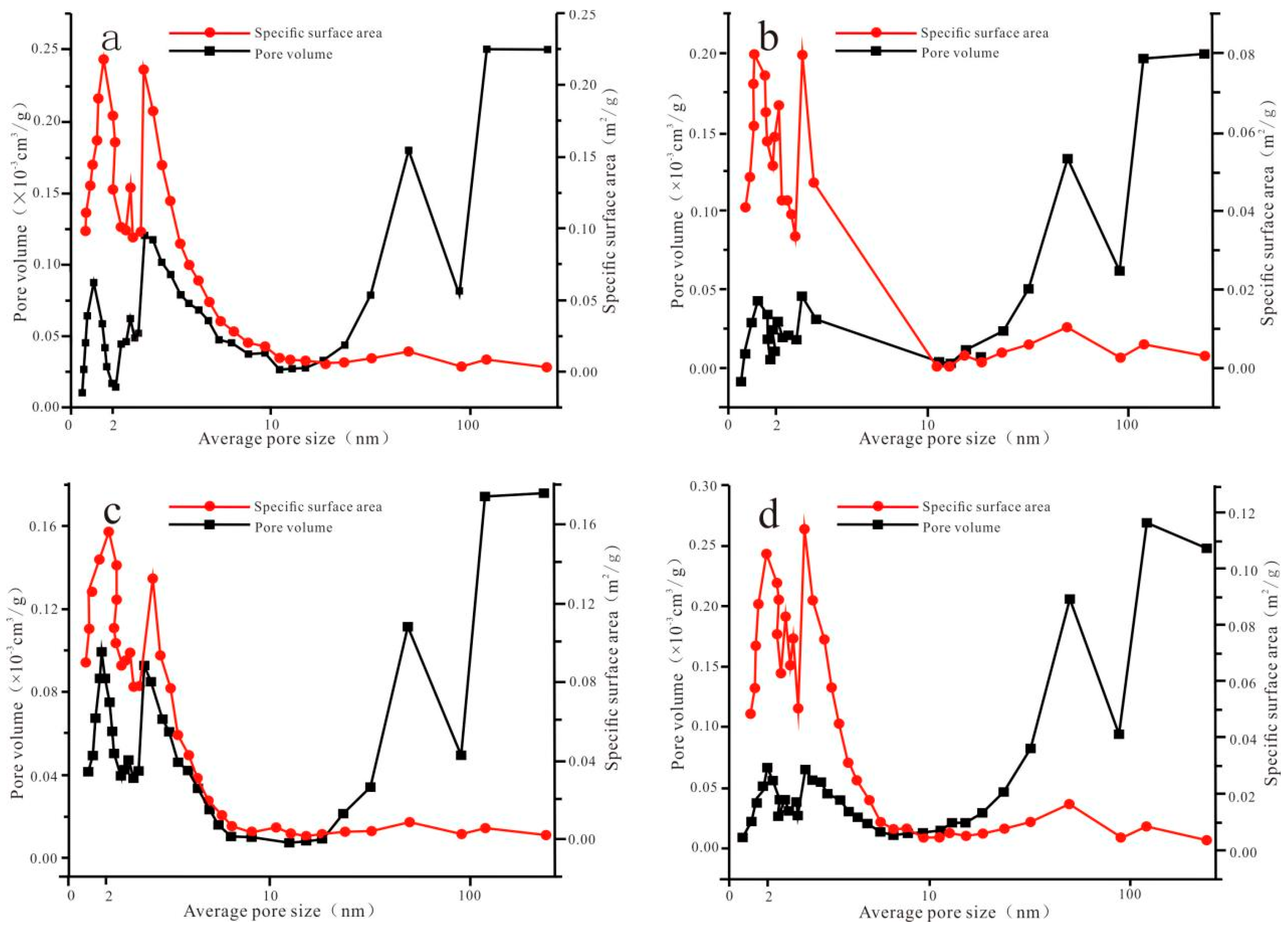
3.2. Pore Volume and Specific Surface Area
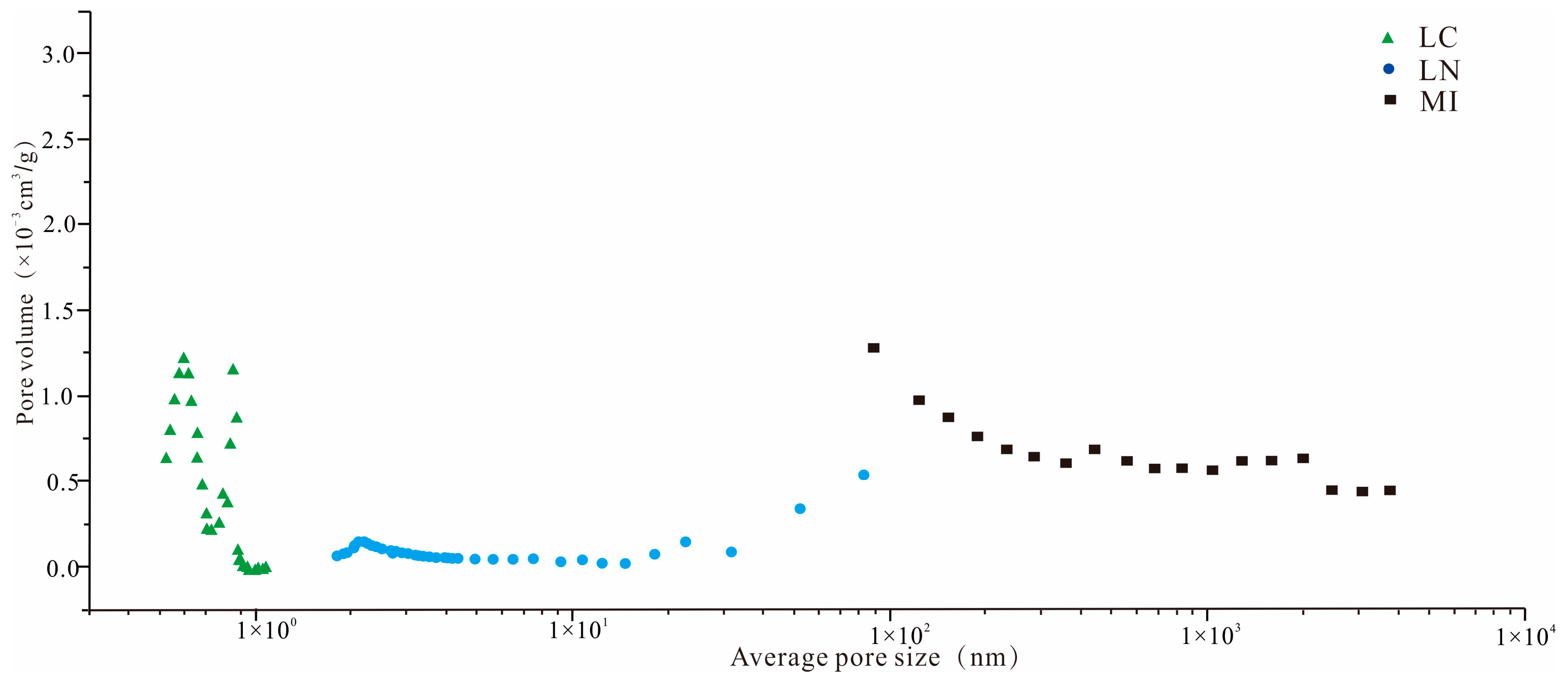
3.3. Pore Types
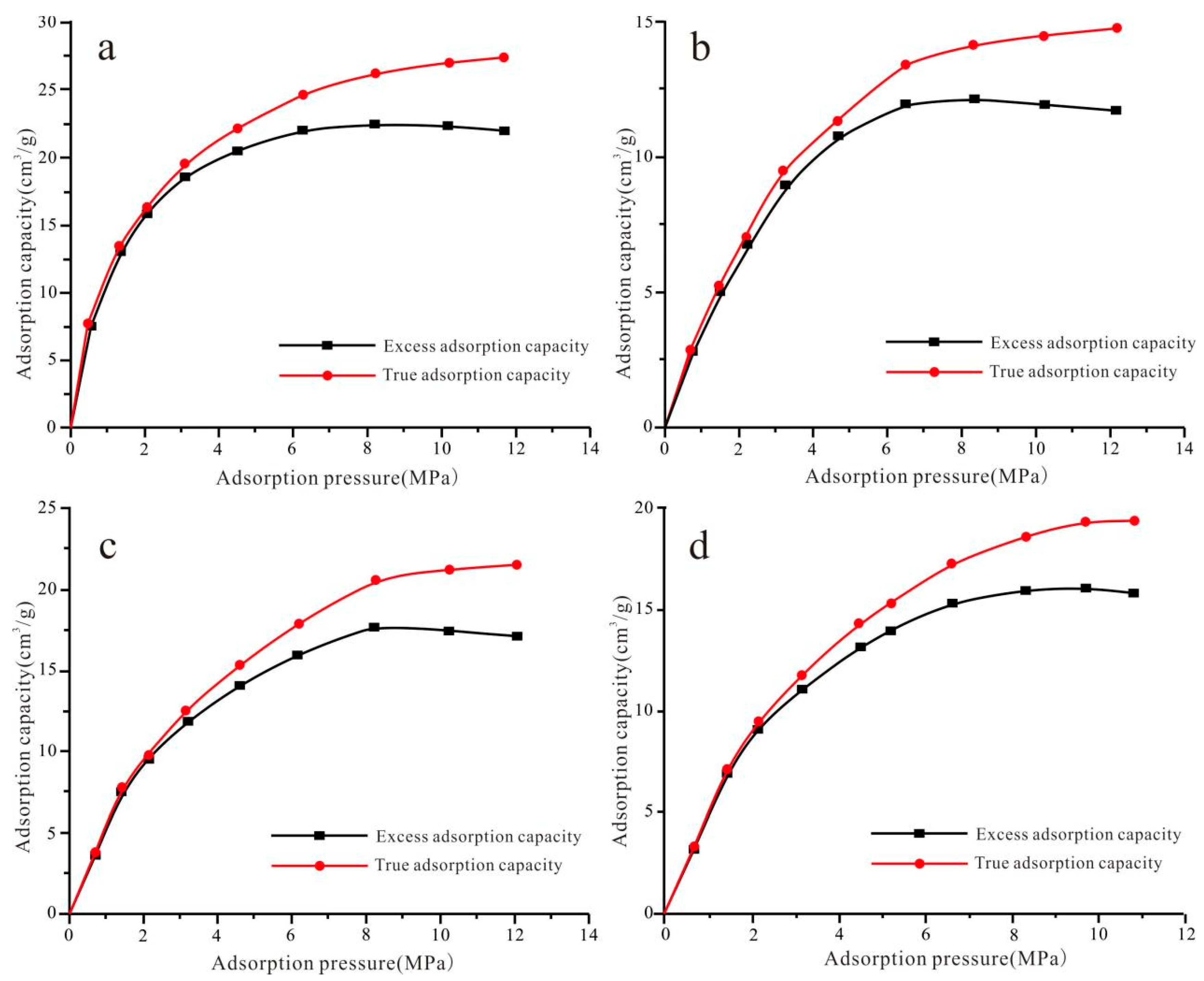
3.4. True Adsorption Versus Excess Adsorption
4. Discussions
4.1. Pore Structures
4.1.1. Full-Scale Pore Structure Characterization
4.1.2. Relationship Between Pore Volume, Specific Surface Area, and Pore Size
4.1.3. Characterization of Full-Scale Pore Structure
4.2. Adsorption Curve Fitting Based on Improved D-R Adsorption Modeling
4.3. Factors Affecting Gas Adsorption
4.3.1. Effect of Coal Composition on Gas Adsorption
4.3.2. Effect of Pore Characteristics on Gas Adsorption
5. Conclusions
- (1)
- The full-scale pore structure characterization of coal through CO2–liquid nitrogen–mercury intrusion series experiments yielded that the pore volume of coal was dominated by the composition of seepage pores, and the specific surface area was dominated by the provision of absorption pores. And, the adsorption data of gas in the medium-rank sample conditions were well fitted by the improved D-R isothermal adsorption model, and the fitting errors were all within 1%.
- (2)
- The maximum adsorption capacity of coal samples was positively correlated with Ro,max, a specular group, and a fixed carbon content, negatively correlated with the content of an inert group, minerals, ash, and volatile matter, and the macroscopic coal characteristics controlled the adsorption capacity of coals mainly by controlling the degree of pore development.
- (3)
- The mode of gas adsorption in coal pores varies with pressure. Under the influence of the adsorption potential and the interaction force between gas molecules, gas adsorption occurs mainly in a single layer on the pore surface under low-pressure conditions; under high-pressure conditions, multilayer adsorption is enhanced and pore filling is increased. Due to the control effect of the adsorption potential on the adsorption process, the filling order of gas in pores of different scales is different. With the increase in pressure, gas molecules preferentially filled in the absorption pores, followed by adsorption pore filling.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, W.; Hu, X.; Xie, J.; Zhao, Y. An intelligent gel designed to control the spontaneous combustion of coal: Fire prevention and extinguishing properties. Fuel 2017, 210, 826–835. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, X.; Cheng, W.; Lu, W. Influence of synthetic conditions on the performance of melamine–phenol–formaldehyde resin microcapsules. High Perform. Polym. 2019, 31, 197–210. [Google Scholar] [CrossRef]
- Fan, T.; Zhou, G.; Wang, J. Preparation and characterization of a wetting-agglomeration-based hybrid coal dust suppressant. Process Saf. Environ. Prot. 2018, 113, 282–291. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, X.; Tan, Y.; Zhang, H.; Huang, W.; Li, Q. A Study of Rockburst Hazard Evaluation Method in Coal Mine. Shock Vib. 2016, 16, 8740868. [Google Scholar] [CrossRef]
- Ye, J. China’s CBM exploration and production and associated technological advancements: A review and reflections. Coal Geol. Explor. 2025, 53, 114–127, (In Chinese with an English abstract). [Google Scholar]
- Zhang, Y.; Zhu, G.; Zheng, Q.; Zhang, X.; Hu, Q. Distribution characteristics of coalbed methane resources in China and recommendations for exploration research. Unconv. Oil Gas 2022, 9, 1–8, 45, (In Chinese with an English abstract). [Google Scholar]
- Zhou, D.; Chen, G.; Zhao, S.; Shen, B.; Liu, Z.; Ye, J.; Chen, X.; Zhang, J. Distribution Characteristics and Exploration and Development Prospects of Coalbed Methane Resources in Sinopec. Coal Sci. Technol. 2025, 53, 19–30, (In Chinese with an English abstract). [Google Scholar]
- Xu, H.; Tang, D.; Tao, S.; Li, S.; Tang, S.; Chen, S.; Zong, P.; Dong, Y. Differences in geological conditions of deep and shallow coalbed methane andtheir formation mechanisms. Coal Geol. Explor. 2024, 52, 33–39, (In Chinese with an English abstract). [Google Scholar]
- Xu, T.; Li, Z.; Nino, R.; Louk, A.K.; Wang, Z.; Song, D. Temperature-dependent diffusion process of methane through dry crushed coal. J. Nat. Gas Sci. Eng. 2015, 22, 609–617. [Google Scholar]
- Wang, Z.; Xu, T.; Yue, G.; Kang, B.; Xie, C.; Li, X. Physical simulation of temperature influence on gas sorption and kinetics in coal: Benefits of temperature under 273.15 K. Fuel 2015, 158, 207–216. [Google Scholar] [CrossRef]
- Busch, A.; Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal Geol. 2011, 87, 49–71. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Yuan, C.; Tao, Q. Thermodynamic analysis of high-pressure gas adsorption on coal-based activated carbon. Fuel 2018, 230, 172–184. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, T. New insights from supercritical methane adsorption in coal: Gas resource estimation, thermodynamics, and engineering application. Energy Fuels 2018, 32, 5001–5009. [Google Scholar] [CrossRef]
- Bae, J.S.; Bhatia, S.K. High-pressure adsorption of methane and carbon dioxide on coal. Energy Fuels 2006, 20, 2599–2607. [Google Scholar] [CrossRef]
- Okolo, G.N.; Everson, R.C.; Neomagus, H.W.; Sakurovs, R.; Grigore, M.; Bunt, J.R. The carbon dioxide, methane and nitrogen high-pressure sorption properties of South African bituminous coals. Int. J. Coal Geol. 2019, 209, 40–53. [Google Scholar] [CrossRef]
- Sang, S.; Zhu, Y.; Zhang, J.; Zhang, X.; Tang, J. Soild-Gas interaction mechanism of Coal-adsorbed Gas(II)—Physical process and theoretical model of Coal-adsored Gas. Nat. Gas Ind. 2005, 25, 16–18+21, (In Chinese with an English abstract). [Google Scholar]
- Crosdale, P.J.; Beamish, B.B.; Valix, M. Coalbed gas sorption related to coal composition. Int. J. Coal Geol. 1998, 35, 147–158. [Google Scholar] [CrossRef]
- Stuart, D.; Richard, S.; Steve, W. Supercritical gas sorption on moist coals. Int. J. Coal Geol. 2008, 74, 203–214. [Google Scholar]
- Han, S.; Sang, S.; Liang, J. High pressure gas adsorption of medium and high-rank coal in southern Qinshui basin. Coal Geol. Explor. 2018, 46, 13–25. [Google Scholar]
- Wang, C.; Li, S. Pore structure characteristics of low-rank coal and theirinfluence on gas adsorption. China Saf. Sci. J. 2015, 25, 133–138, (In Chinese with an English abstract). [Google Scholar]
- Monsalvo, M.A.; Shapiro, A.A. Study of high-pressure adsorption from supercritical fluids by the potential theory. Fluid Phase Equilibria 2009, 283, 56–64. [Google Scholar] [CrossRef]
- Monsalvo, M.A.; Shapiro, A.A. Modeling adsorption of binary and ternary mixtures on microporous media. Fluid Phase Equilibria 2007, 254, 91–100. [Google Scholar] [CrossRef]
- Dundar, E.; Zacharia, R.; Chhine, R.; Benard, P. Modified potential theory for modeling supercritical gas adsorption. Int. J. Hydrogen Energy 2012, 37, 9137–9147. [Google Scholar] [CrossRef]
- Sang, S.; Zhu, Y.; Zhang, S.; Zhang, J.; Tang, J. Soild-Gas interaction mechanism of Coal-adsorbed Gas(I)―Coal pore structure and Solid-Gas interaction. Nat. Gas Ind. 2005, 1, 13–15, (In Chinese with an English abstract). [Google Scholar]
- Tunio, S.Q.; Ismail, M.S. Effect of coal rank and porosity on the optimization of ECBM recovery. Asian J. Appl. Sci. 2014, 7, 158–168. [Google Scholar] [CrossRef]
- Keshavarz, A.; Sakurovs, R.; Grigore, M.; Sayyafzadeh, M. Effect of maceral composition and coal rank on gas diffusion in Australian coals. Int. J. Coal Geol. 2017, 173, 65–75. [Google Scholar] [CrossRef]
- Xu, F.; Xiao, Z.; Chen, D.; Yan, X.; Wu, N.; Li, X.; Miao, Y. Current status and development direction of coalbed methane exploration technology in China. Coal Sci. Technol. 2019, 47, 205–215, (In Chinese with an English abstract). [Google Scholar]
- GB/T 6948-2008; Method of Determining Microscopically the Reflectance of Vitrinite in Coal. Standards Press of China: Beijing, China, 2008.
- GB/T8899-2013; Determination of Maceral Group Composition and Minerals in Coal. Standards Press of China: Beijing, China, 2013.
- GB/T212-2008; Proximate Analysis of Coal. Standards Press of China: Beijing, China, 2008.
- GB/T19560-2008; Experimental Method of High-Pressure Isothermal Adsorption to Coal. Standards Press of China: Beijing, China, 2008.
- Stephen, B.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Wood, J.M.; Burgis, S.E.; Aquino, S.D.; Freeman, M. Nanopore-Structure Analysis and Permeability Predictions for a Tight Gas Siltstone Reservoir by Use of Low-Pressure Adsorption and Mercury-Intrusion Techniques. SPE Reserv. Eval. Eng. 2012, 15, 648–661. [Google Scholar] [CrossRef]
- Gan, H.; Nandi, S.P.; Walker, P.L., Jr. Nature of the porosity in American coals. Fuel 1972, 5, 272–277. [Google Scholar] [CrossRef]
- Walker, P.L., Jr.; Verma, S.K.; Rivera-Utrilla, J.; Davis, A. Densities, porosities and surface areas of coal macerals as measured by their interaction with gases, vapours and liquids. Fuel 1988, 67, 1615–1623. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Fu, X.; Qin, Y.; Zhang, W.; Wei, C.; Zhou, R. Study on fractal classification and natural classification of coal pores based on coalbed methane transportation. Sci. Bull. 2005, 50, 51–55. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, D.; Pan, Z.; Yao, Y.; Li, J.; Qiu, Y. Pore structure and its impact on CH4 adsorption capacity and flow capability of bituminous and subbituminous coals from Northeast China. Fuel 2013, 103, 258–268. [Google Scholar] [CrossRef]
- Chen, P.; Tang, X. The research on the adsorption of nitrogen in low temperature and micro-pore properties in coal. J. China Coal Soc. 2001, 26, 552–556. [Google Scholar]
- Yang, Z.; Qin, Y.; Gao, D.; Chen, R. Differences between apparent and true adsorption quantity of coalbed methane under supercritical conditions and their geological significance. Nat. Gas Ind. 2011, 31, 13–16. [Google Scholar]
- Sircar, S. Gibbsian Surface excess for gas adsorption revisited. Ind. Eng. Chem. Res. 1999, 38, 3670–3682. [Google Scholar] [CrossRef]
- Sakurovs, R.; Day, S.; Weir, S.; Duffy, G. Application of a modified Dubinin-Radushkevich equation to adsorption of gases by coals under supercritical conditions. Energy Fuels 2007, 21, 992–997. [Google Scholar] [CrossRef]
- Wang, K.; Qiao, P.; Wang, Z.; Liu, X.; Li, Y. Multiple scale pore size characterization of coal based on carbon dioxide and liquid nitrogen adsorption, high-pressure mercury intrusion and low field nuclear magnetic resonance. China Min. Mag. 2017, 26, 146–152. [Google Scholar]
- Huo, Z.; Xi, Z.; Guo, J.; Wang, D. Pore characteristics of tectonic coal based on nitrogen adsorption and SEM. Coal Eng. 2024, 56, 149–156, (In Chinese with an English abstract). [Google Scholar]
- Wang, R.; Feng, H. Applicability of mercury intrusion method and nitrogen adsorption method in characterizingpore structure of high-rank coal. Oil Drill. Prod. Technol. 2024, 46, 112–118, (In Chinese with an English abstract). [Google Scholar]
- Li, N.; Liu, H.; Sang, S. Comparative study on pore size distribution in coal based on mercury intrusion-low temperature liquid nitrogen combined pore and nuclear magnetic resonance analysis. Saf. Coal Mines 2024, 55, 1–9, (In Chinese with an English abstract). [Google Scholar]
- Li, Y.; Zhang, Y.; Zhang, L.; Hou, J. Characterization on pore structure of tectonic coals based on the method of mercury intrusion, carbon dioxide adsorption and nitrogen adsorption. J. China Coal Soc. 2019, 44, 1188–1196, (In Chinese with an English abstract). [Google Scholar]
- Levine, J.R. Model Study of the Influence of Matrix Shrinkage on Absolute Permeability of Coal Bed Reservoirs; Geological Society Special Publication: London, UK, 1996; Volume 109, pp. 197–212. [Google Scholar]
- Robertson, E.P.; Christiansen, R.L. Modeling laboratory permeability in coal using sorption-induced-strain. SPE Reserv. Eval. Eng. 2007, 10, 260–269. [Google Scholar] [CrossRef]
- Pan, Z.; Connell, L.D. A theoretical model for gas adsorption-induced coal swelling. Int. J. Coal Geol. 2007, 69, 243–252. [Google Scholar] [CrossRef]
- Su, X.; Zhang, L.; Lin, X. Influence of Coal rank on Coal adsorption capacity. Nat. Gas Ind. 2005, 25, 19–21. [Google Scholar]
- Li, Z.; Yao, Y.; Zhou, H.; Bai, X. Study on Coal and Rock Maceral Composition Affected to Methane Adsorption Capacity. Coal Sci. Technol. 2012, 40, 125–128, (In Chinese with an English abstract). [Google Scholar]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K.; Airey, D.; Weniger, P. Estimation of gas adsorption capacity in coal: A Review and an analytical study. Int. J. Coal Prep. Util. 2012, 32, 25–55. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D. Adsorption Characteristics of Coal Reservoirs in North China and lts Influencing Factors. J. China Univ. Min. Technol. 2007, 36, 308–314, (In Chinese with an English abstract). [Google Scholar]
- Polanyi, M. The Potential Theory of Adsorption: Authority in science has its uses and its dangers. Science 1963, 141, 1010–1013. [Google Scholar] [CrossRef] [PubMed]





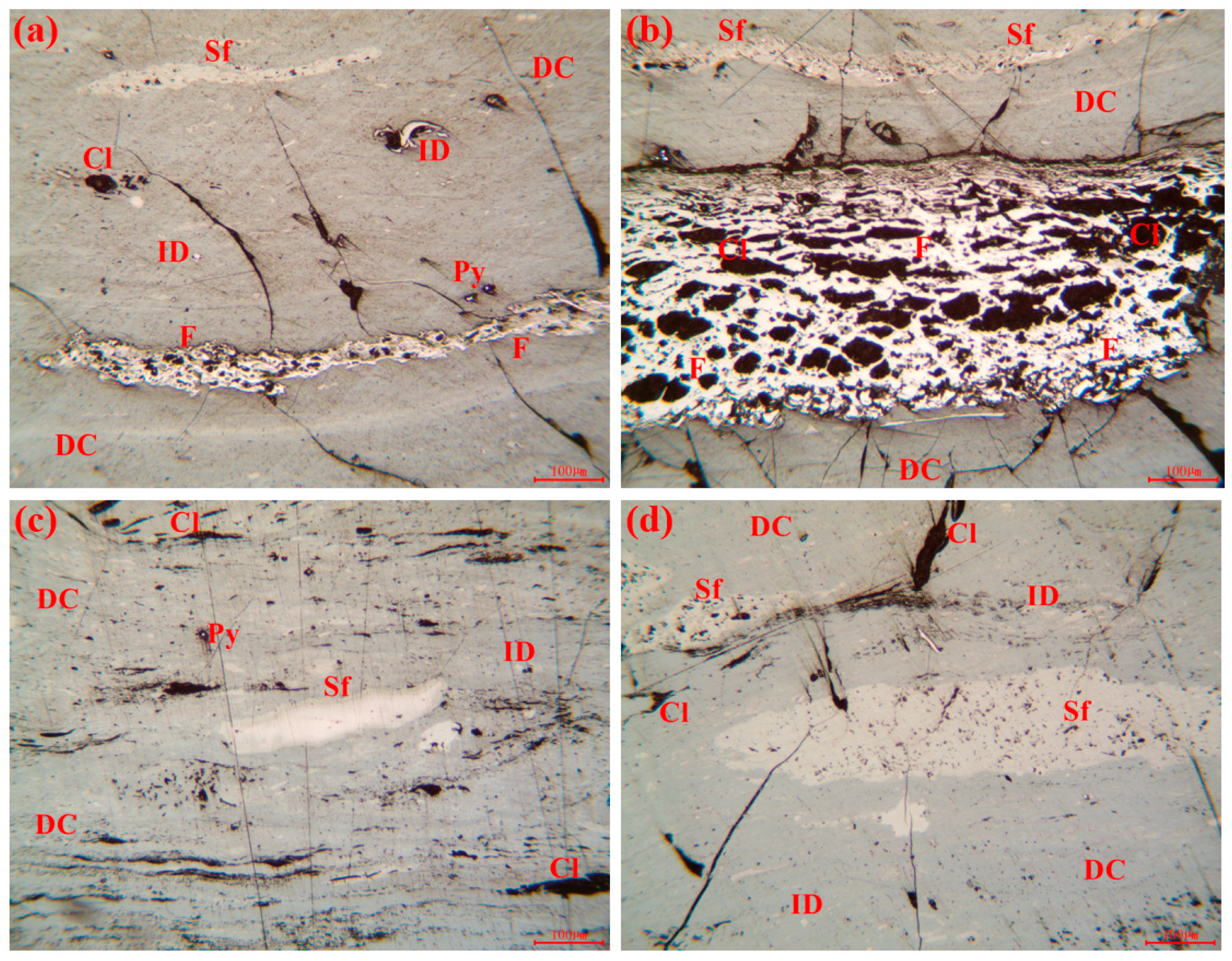

| Sample | Sample Location | Burial Depth | Coal Seam |
|---|---|---|---|
| LL | Liulin Mine, Liulin | 600–800 m | mainly mining No.4 coal seams |
| TL | Tunlan Mine, Gujiao | about 400–500 m | mainly mining No.2/3 coal seams |
| ML | Malan Mine, Gujiao | about 700–800 m | mining No.2/8 coal seams |
| DQ | Dongqu Mine, Gujiao | more than 800 m | mainly mining No.8 coal seams |
| Sample | Ro,max (%) | Maceral and Mineral (%) | Proximate Analysis (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Vitrinite | Inertinite | Exinite | Mineral | Mad | Ad | Vdaf | FCad | ||
| LL | 1.40 | 76.14 | 12.09 | 11.77 | 6.21 | 0.31 | 21.42 | 16.85 | 61.42 |
| TL | 1.93 | 74.54 | 8.92 | 16.54 | 16.54 | 0.23 | 29.76 | 12.57 | 56.44 |
| ML | 1.28 | 84.66 | 6.20 | 9.14 | 7.36 | 0.40 | 22.04 | 14.22 | 63.34 |
| DQ | 1.89 | 91.83 | 3.85 | 4.32 | 5.13 | 0.33 | 18.65 | 7.30 | 73.72 |
| LC | LN | LN and MI | Full-Scale Pore Structure | ||
|---|---|---|---|---|---|
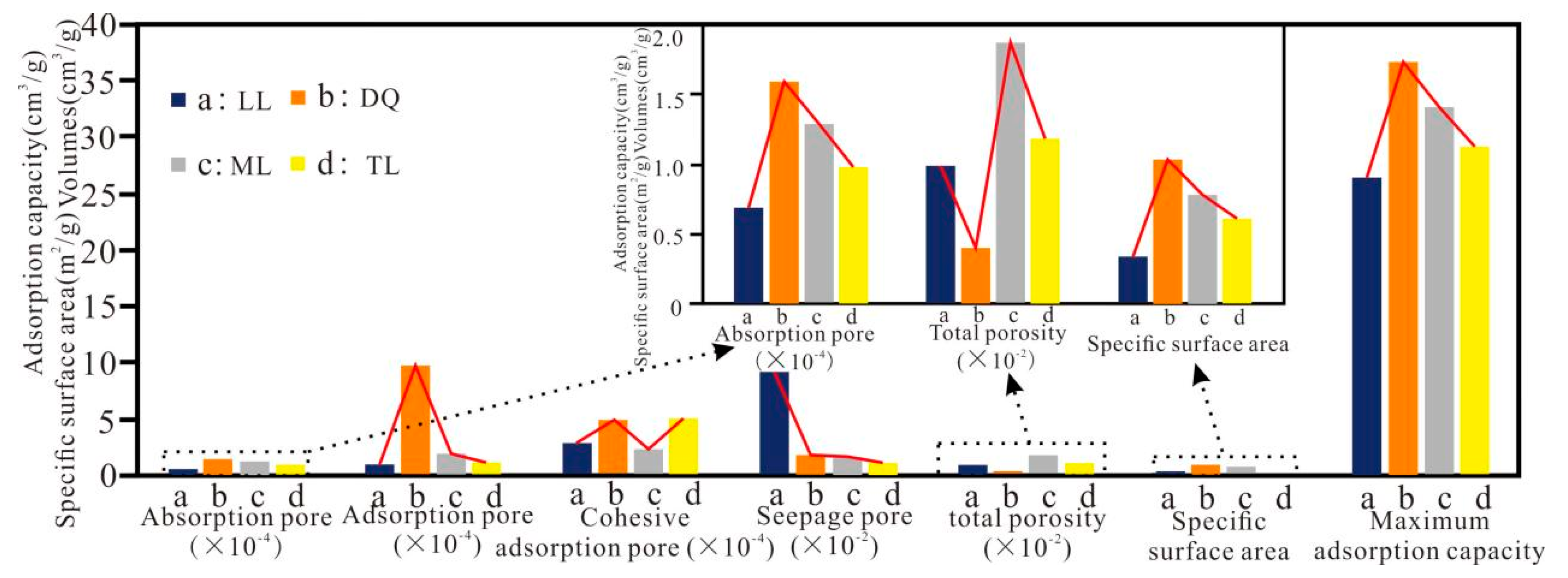
| Sample | Absorption pore (10−3 cm3/g) | Adsorption pore (10−3 cm3/g) | Coalescence–adsorption pore (10−3 cm3/g) | Seepage pore (10−3 cm3/g) | Total pore volume (10−3 cm3/g) |
| LL | 0.07 | 0.12 | 0.30 | 9.43 | 9.92 |
| DQ | 0.16 | 0.99 | 0.50 | 2.04 | 3.69 |
| ML | 0.13 | 0.22 | 0.26 | 18.69 | 19.30 |
| TL | 0.10 | 0.13 | 0.53 | 10.75 | 11.51 |
| LC | LN | Full-Scale Pore Structure | |||
|---|---|---|---|---|---|
| Sample | Absorption pore surface area (m2/g) | Adsorption Pore specific surface area (m2/g) | Coalescence–adsorption specific surface area (m2/g) | Liquid nitrogen specific surface area (m2/g) | Pore total specific surface Area (m2/g) |
| LL | 53.97 | 0.31 | 0.04 | 0.35 | 54.32 |
| DQ | 69.32 | 0.99 | 0.06 | 1.05 | 70.37 |
| ML | 79.91 | 0.76 | 0.02 | 0.78 | 80.69 |
| TL | 110.62 | 0.61 | 0.01 | 0.62 | 111.24 |
| Sample Number | n0 (cm3/g) | D | k | R2 |
|---|---|---|---|---|
| TL | 29.195 | 0.094 | 0.032 | 0.997 |
| DQ | 36.435 | 0.085 | 0.030 | 0.999 |
| LL | 26.461 | 0.100 | 0.053 | 0.996 |
| ML | 32.622 | 0.099 | 0.032 | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; He, S.; Qiu, F.; Cai, Y.; Wei, H.; Li, B. Full-Scale Pore Structure and Gas Adsorption Characteristics of the Medium-Rank Coals from Qinshui Basin, North China. Processes 2025, 13, 1862. https://doi.org/10.3390/pr13061862
Hu Y, He S, Qiu F, Cai Y, Wei H, Li B. Full-Scale Pore Structure and Gas Adsorption Characteristics of the Medium-Rank Coals from Qinshui Basin, North China. Processes. 2025; 13(6):1862. https://doi.org/10.3390/pr13061862
Chicago/Turabian StyleHu, Yingchun, Shan He, Feng Qiu, Yidong Cai, Haipeng Wei, and Bin Li. 2025. "Full-Scale Pore Structure and Gas Adsorption Characteristics of the Medium-Rank Coals from Qinshui Basin, North China" Processes 13, no. 6: 1862. https://doi.org/10.3390/pr13061862
APA StyleHu, Y., He, S., Qiu, F., Cai, Y., Wei, H., & Li, B. (2025). Full-Scale Pore Structure and Gas Adsorption Characteristics of the Medium-Rank Coals from Qinshui Basin, North China. Processes, 13(6), 1862. https://doi.org/10.3390/pr13061862






