From Structure to Efficiency: Unveiling the Role of Calcination Temperature in Nb2O5-Based DSSCs
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Morphological and Structural Characterization
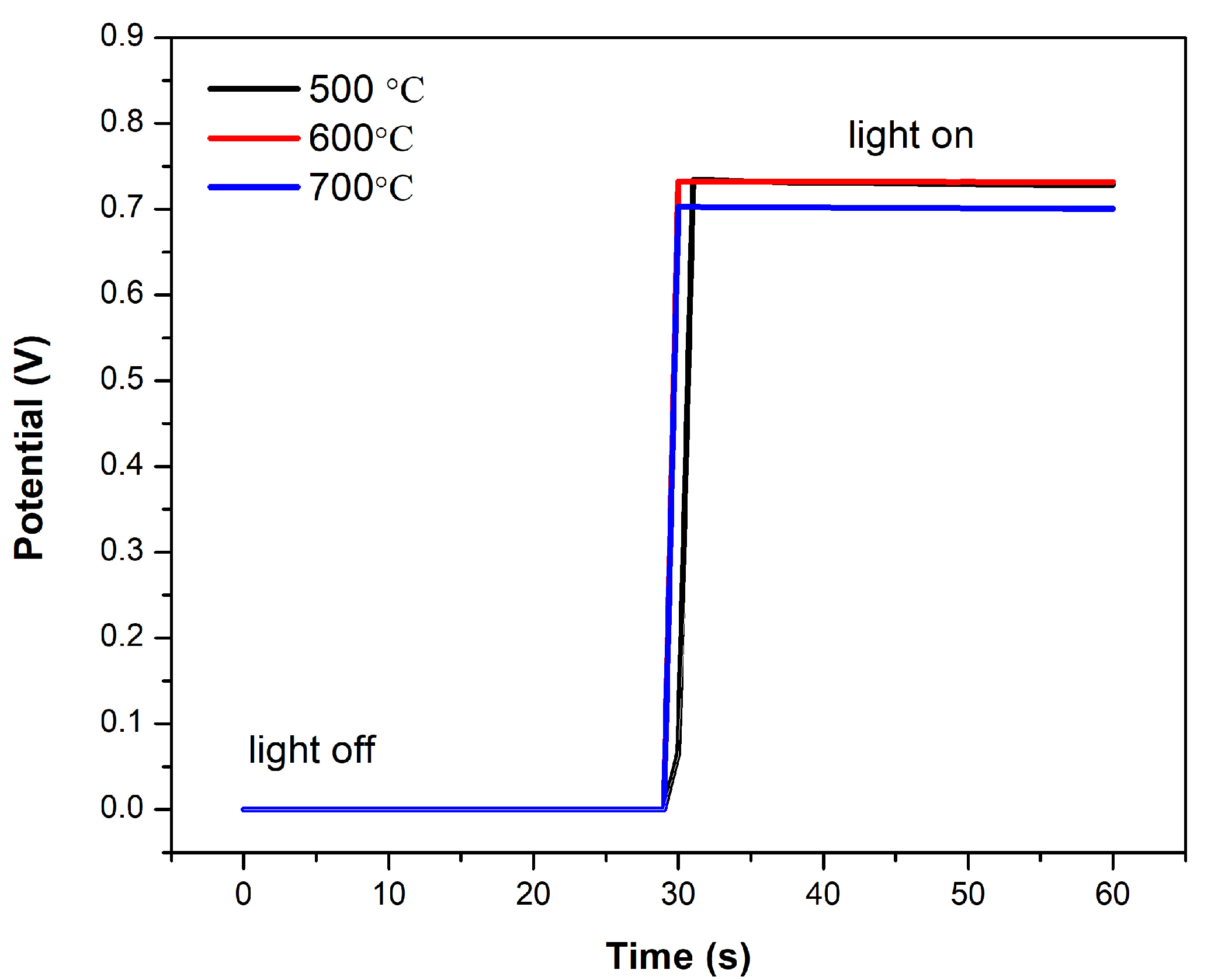
3.2. Electrochemical Device Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCE | Photoconversion Energy Efficiency |
| jsc | Short-Circuit Current |
| Voc | Open-Circuit Potential |
| FF | Fill Factor |
| DSSC | Dye-Sensitized Solar Cell |
| FTO | Fluorine-Doped Tin Oxide |
| Pin | Incident Power |
| AM | Air Mass |
| EIS | Electrochemical Impedance Spectroscopy |
References
- Kraidy, A.F.; Yapi, A.S.; Saint-Gregoire, P.; Vaillant-Roca, L.; Eke, S.; Mouangue, R.; Jamali, A.; Gagou, Y. Enhancement of Natural Dye-Sensitized Solar Cell Efficiency Through TiO2 Hombikat UV100 and TiO2 P25 Photoanode Optimization. Processes 2024, 12, 2481. [Google Scholar] [CrossRef]
- Wazzan, N. Structural Engineering of π-Linker Aromaticity in Anthanthrene-Based Dyes with D–π–A Configuration: DFT Investigation to Enhance Charge Transfer in DSSCs. Processes 2025, 13, 418. [Google Scholar] [CrossRef]
- Tractz, G.T.; Viomar, A.; Dias, B.V.; De Lima, C.A.; Banczek, E.P.; Da Cunha, M.T.; Antunes, S.R.M.; Rodrigues, P.R.P. Recombination study of dye sensitized solar cells with natural extracts. J. Braz. Chem. Soc. 2019, 30, 371–378. [Google Scholar] [CrossRef]
- Tractz, T.; Staciaki, F.; Regina, S.; Antunes, M.; Banczek, P.; Taras, M.; Rog, P. Nb2O5 synthesis and characterization by Pechini method to the application as electron transport material in a solar device. Solar Energy 2021, 216, 1–6. [Google Scholar] [CrossRef]
- Larsson, L.F.G.; Arielo, G.; Maia, R.G.T.T.; Cristina, D.; Oliszeski, S.; Helleis, R. Application of Zinc Oxide in Hybrid Solar Cells Using a P3HT and P3OT Polymer Junction as Charge Carrier. Mater. Res. 2019, 22, e20180820. [Google Scholar] [CrossRef]
- Tractz, T.; Viomar, A.; Dias, B.V.; Rodrigues, P.R.P. Photoelectrochemical Behavior of the Cell FTO/TiO2/CeO2/N719 Obtained from the Pechini and Precipitation of Cerium Oxide Methods. J. Electron. Mater. 2018, 47, 5556–5563. [Google Scholar] [CrossRef]
- Lü, X.; Mou, X.; Wu, J.; Zhang, D.; Zhang, L.; Huang, F.; Xu, F.; Huang, S. Improved-Performance Dye-Sensitized solar cells using Nb-Doped TiO2 electrodes: Efficient electron Injection and transfer. Adv. Funct. Mater. 2010, 20, 509–515. [Google Scholar] [CrossRef]
- Hossain, M.I.; Aissa, B.; Khandakar, A.; Thomas, K.; Rahman, A.; Mansour, S. Exploring the optical and morphological properties of metal oxide thin films produced via reactive electron beam evaporation. Cogent Eng. 2024, 11, 2338144. [Google Scholar] [CrossRef]
- Hossain, M.I.; Alharbi, F.H.; Tabet, N. Copper oxide as inorganic hole transport material for lead halide perovskite based solar cells. Sol. Energy 2015, 120, 370–380. [Google Scholar] [CrossRef]
- Haque, M.M.; Mahjabin, S.; Abdullah, H.B.; Akhtaruzzaman, M.; Almohamadi, H.; Islam, M.A.; Hossain, M.I.; Ibrahim, M.A.; Chelvanathan, P. Exploring the theoretical potential of tungsten oxide (WOx) as a universal electron transport layer (ETL) for various perovskite solar cells through interfacial energy band alignment modulation. J. Phys. Chem. Solids 2025, 196, 112324. [Google Scholar] [CrossRef]
- Hossain, M.I.; Al Kubaisi, G.; Aïssa, B.; Mansour, S. Probing the hydrophilic behaviour of e-beam evaporated silica thin films for PV-soiling application. Mater. Sci. Technol. 2022, 38, 753–759. [Google Scholar] [CrossRef]
- Hossain, M.I.; Chelvanathan, P.; Aissa, B.; Khandakar, A.; Rahman, A.; Mansour, S. Enhanced perovskite solar cells performance with TiOx and SnOx thin films as electron transport layers. Sci. Rep. 2025, 15, 7709. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.I.; Tong, Y.; Shetty, A.; Mansour, S. Probing the degradation pathways in perovskite solar cells. Sol. Energy 2023, 265, 112128. [Google Scholar] [CrossRef]
- Hossain, M.I.; Chelvanathan, P.; Khandakar, A.; Thomas, K.; Rahman, A.; Mansour, S. Enhanced efficiency of bifacial perovskite solar cells using computational study. Sci. Rep. 2024, 14, 12984. [Google Scholar] [CrossRef]
- Raba-Páez, A.M.; Suarez-Ballesteros, D.N.; Martínez-Zambrano, J.J.; Rojas-Sarmiento, H.A.; Rincón-Joya, M. Pechini method used in the obtention of semiconductor nanoparticles based niobium. Dyna 2015, 82, 52–58. [Google Scholar] [CrossRef]
- Guimaraes, R.R.; Parussulo, A.L.A.; Araki, K. Impact of nanoparticles preparation method on the synergic effect in anatase/rutile mixtures. Electrochim. Acta 2016, 222, 1378–1386. [Google Scholar] [CrossRef]
- Guimaraes, R.R.; Parussulo, A.L.A.; Toma, H.E.; Araki, K. New tunable ruthenium complex dyes for TiO2 solar cells. Inorganica Chim. Acta 2013, 404, 23–28. [Google Scholar] [CrossRef]
- Tractz, G.T.; Antunes, S.R.M.; Rodrigues, P.R.P. Nb/TiO2 oxides: A study of synthesis and electron transport mechanism as an ETL in a solar device. J. Photochem. Photobiol. A Chem. 2023, 444, 114899. [Google Scholar] [CrossRef]
- Maia, P.R.P.R.G.A.R.; Larsson, L.F.G.; Viomar, A.; Maia, E.C.R.; de Santana, H. Aperfeiçoamento da produção de partículas de óxido de zinco para aplicação em células solares. Cerâmica 2016, 62, 91–97. [Google Scholar] [CrossRef][Green Version]
- Maia, G.A.R.; Larsson, L.F.G.; Viomar, A.; Matos, L.A.C.; Antunes, S.R.M.; Maia, E.C.R.; Oliveira, M.F.; Cunha, M.T.; Rodrigues, P.R.P. Influence of zinc oxide morphology in hybrid solar cells of poly(3-octylthiophene). J. Mater. Sci. Mater. Electron. 2016, 27, 8271–8278. [Google Scholar] [CrossRef]
- Vanlaeke, P.; Swinnen, A.; Haeldermans, I.; Vanhoyland, G.; Aernouts, T.; Cheyns, D.; Deibel, C.; D’Haen, J.; Heremans, P.; Poortmans, J.; et al. P3HT/PCBM bulk heterojunction solar cells: Relation between morphology and electro-optical characteristics. Sol. Energy Mater. Sol. Cells 2006, 90, 2150–2158. [Google Scholar] [CrossRef]
- Jarzynska, K.; Ciura, K.; Gao, X.J.; Mikolajczyk, A.; Gao, X.; Puzyn, T. Understanding the zeta potential of nanomaterials through predictive nanoinformatics. Nano Today 2025, 64, 102783. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Malaika, A.; Ptaszynska, K.; Pereira, M.F.R.; Figueiredo, J.L. Impact of thermal treatment of Nb2O5 on its performance in glucose dehydration to 5-hydroxymethylfurfural in water. Nanomaterials 2020, 10, 1685. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Sathasivam, S.; Williamson, B.A.D.; Althabaiti, S.A.; Obaid, A.Y.; Basahel, S.N.; Mokhtar, M.; Scanlon, D.O.; Carmalt, C.J.; Parkin, I.P. Chemical Vapor Deposition Synthesis and Optical Properties of Nb2O5 Thin Films with Hybrid Functional Theoretical Insight into the Band Structure and Band Gaps. ACS Appl. Mater. Interfaces 2017, 9, 18031–18038. [Google Scholar] [CrossRef]
- Sultana, R.; Islam, K.; Chakraborty, S. Tuning Optical and Electrochemical Properties of Nb2O5 Thin Films via WO3 Doping. Trans. Electr. Electron. Mater. 2025, 26, 48–59. [Google Scholar] [CrossRef]
- Chaiworn, P.; Teerananattapong, W.; Reunchan, P.; Tubtimtae, A.; Wongrat, E. Effect of tungsten oxide concentration on structural, morphological, compositional, dispersion energy, and linear/nonlinear optical properties of niobium pentoxide thin films. Opt. Mater. 2025, 161, 116807. [Google Scholar] [CrossRef]
- Longo, C.; De Paoli, M.A. Dye-Sensitized Solar Cells: A Successful Combination of Materials. J. Braz. Chem. Soc. 2003, 14, 889–901. [Google Scholar] [CrossRef]
- Wu, J.J.; Chen, G.R.; Yang, H.H.; Ku, C.H.; Lai, J.Y. Effects of dye adsorption on the electron transport properties in ZnO-nanowire dye-sensitized solar cells. Appl. Phys. Lett. 2007, 90, 2007–2009. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, J.H.; Kim, H.B.; Park, K.H.; Lee, J.W.; Cho, S.Y. Adsorption and kinetics properties of TiO2 photoelecrode using natural paprika oleoresin (Capsicum annuum L.) dye for dye-sensitized solar cells. Int. J. Electrochem. Sci. 2018, 13, 3935–3947. [Google Scholar] [CrossRef]
- Ramasamy, E.; Lee, J. Ordered mesoporous SnO2-based photoanodes for high-performance dye-sensitized solar cells. J. Phys. Chem. C 2010, 114, 22032–22037. [Google Scholar] [CrossRef]
- Valerio, T.; Arielo, G.; Maia, R.; Fernanda, L. Study of the Nb2O5 Insertion in ZnO to Dye-sensitized Solar Cells 2. Material and Methods. Mater. Res. 2019, 22, 1–5. [Google Scholar] [CrossRef]
- Valerio, T.L.; Tractz, G.; Maia, G.A.R.; Banczek, E.D.P.; Rodrigues, P.R.P. Minimizing of charge recombination by Nb2O5 addition in dye-sensitized solar cells. Opt. Mater. 2020, 109, 110310. [Google Scholar] [CrossRef]
- de Lima, A.A.; Tractz, G.T.; Macedo, A.G.; Thomazi, F.; Rodrigues, P.R.P.; Dartora, C.A. Electrochemical investigation of PEDOT:PSS and Nb2O5 composites as counter electrodes for dye-sensitized solar cells. Opt. Mater. 2022, 132, 112763. [Google Scholar] [CrossRef]
- Yang, J.H.; Bark, C.W.; Kim, K.H.; Choi, H.W. Characteristics of the dye-sensitized solar cells using TiO2 nanotubes treated with TiCl4. Materials 2014, 7, 3522–3532. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Jonh Wiley & Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Liberatore, M.; Decker, F.; Burtone, L.; Zardetto, V.; Brown, T.M.; Reale, A.; Di Carlo, A. Using EIS for diagnosis of dye-sensitized solar cells performance. J. Appl. Electrochem. 2009, 39, 2291–2295. [Google Scholar] [CrossRef]
- Gratzel, M.; Wang, Q.; Moser, J.E. Electrochemical impedance spectroscopic analysis of dye-sensitized solar cells. J. Phys. Chem. B 2005, 109, 14945–14953. [Google Scholar] [CrossRef]
- Tractz, G.T.; Dias, B.V.; Banczek, E.P.; da Cunha, M.T.; Alves, G.J.T.; Rodrigues, P.R.P. Dye Sensitized Solar Cells (CSSC): Perspectives, Materials, Functioning and Characterization Techniques. Rev. Virtual De. Química 2020, 12, 748–774. [Google Scholar] [CrossRef]
- Bisquert, J.; Fabregat-santiago, F. Electron Lifetime in Dye-sensitized solar cells: Theory and Interpretation of Measurements. J. Phys. Chem. C 2009, 113, 17278–17290. [Google Scholar] [CrossRef]
- Camargo Matheus, A.P.; Gonçalves Larsson, L.F.; Tractz, G.T.; Pinto Rodrigues, P.R. The TiO2/electrolyte interface in DSSCs: An electrochemical study of the insertion of BTAH, BZM, and indole. Opt. Mater. 2025, 159, 116637. [Google Scholar] [CrossRef]
- Rigueira, R.F.; Tractz, G.T.; Barancelli, D.A. Fabrication of a dye sensitized solar cell using TiO2 sensitized with a mono-carbonyl curcumin analog. J. Iran. Chem. Soc. 2024, 21, 2095–2101. [Google Scholar] [CrossRef]
- Bisquert, J.; Fabregat-Santiago, F. Impedance Spectroscopy: A General Introduction and Application to Dye-Sensitized Solar Cells, 1st ed.; EPFL Press: Lausanne, Switzerland, 2010. [Google Scholar]

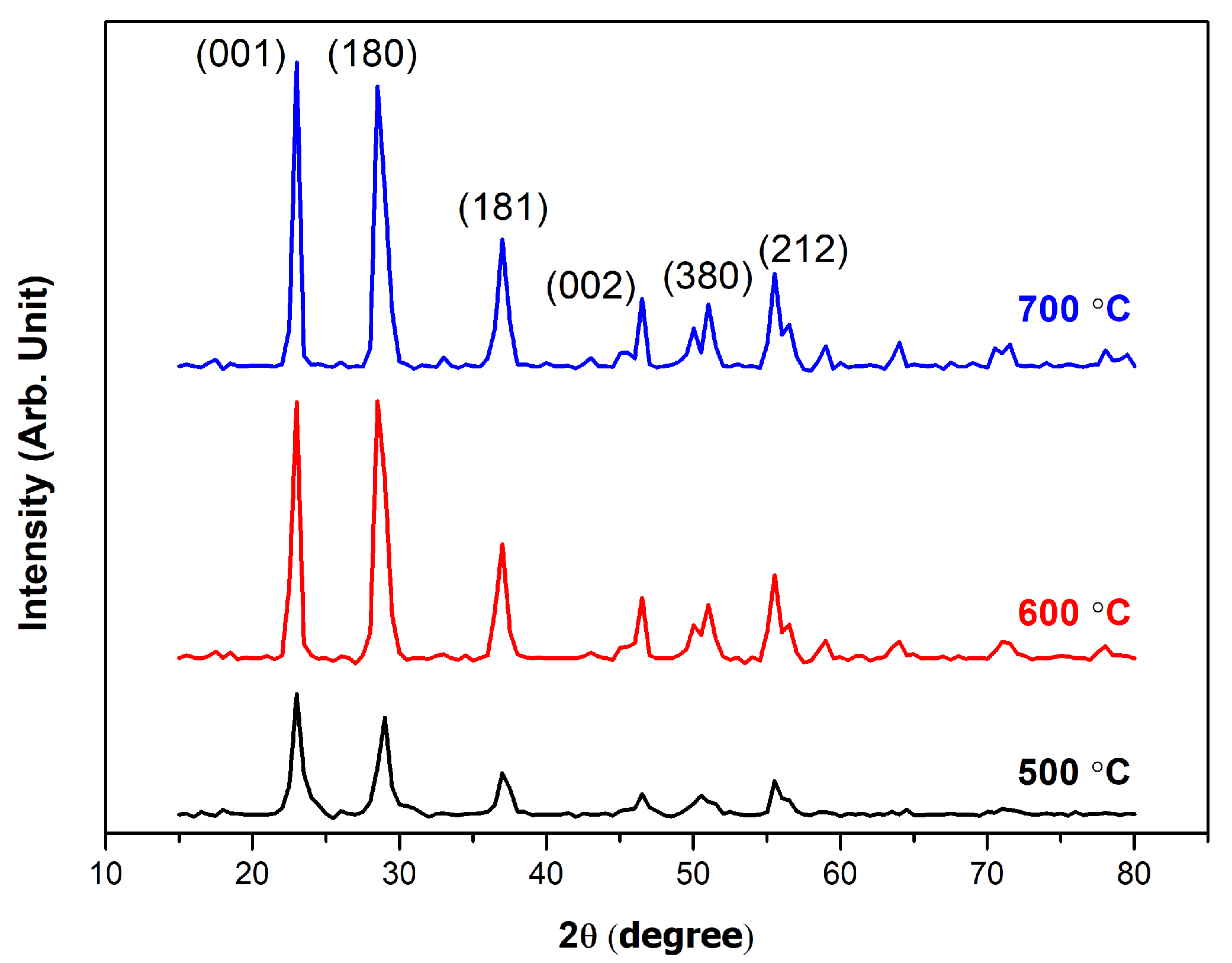


| Temperature (°C) | Particle Size Diameter (nm) | Zeta Potential (mV) |
|---|---|---|
| 500 | 827.63 ± 26 | −25.53 ± 2.84 |
| 600 | 642.17 ± 37 | −8.27 ± 0.32 |
| 700 | 1022.57 ± 46.8 | −20.43 ± 1.33 |
| Temperature (°C) | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|
| 500 | 3.19 | 0.738 | 0.481 | 1.13 |
| 600 | 4.23 | 0.694 | 0.474 | 1.39 |
| 700 | 1.36 | 0.751 | 0.536 | 0.55 |
| Temperature (°C) | RS (Ω) | RCT (Ω) | RCE (Ω) |
|---|---|---|---|
| 500 | 41.9 | 305.0 | 84.7 |
| 600 | 13.5 | 66.4 | 349.0 |
| 700 | 6.57 | 736.0 | 246.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davi, R.L.C.; Almeida, E.A.d.; Peron, A.P.; Banczek, E.d.P.; Junior, O.V.; Tractz, G.T. From Structure to Efficiency: Unveiling the Role of Calcination Temperature in Nb2O5-Based DSSCs. Processes 2025, 13, 1857. https://doi.org/10.3390/pr13061857
Davi RLC, Almeida EAd, Peron AP, Banczek EdP, Junior OV, Tractz GT. From Structure to Efficiency: Unveiling the Role of Calcination Temperature in Nb2O5-Based DSSCs. Processes. 2025; 13(6):1857. https://doi.org/10.3390/pr13061857
Chicago/Turabian StyleDavi, Ronald Luiz Castiglioni, Edson Araujo de Almeida, Ana Paula Peron, Everson do Prado Banczek, Osvaldo Valarini Junior, and Gideã Taques Tractz. 2025. "From Structure to Efficiency: Unveiling the Role of Calcination Temperature in Nb2O5-Based DSSCs" Processes 13, no. 6: 1857. https://doi.org/10.3390/pr13061857
APA StyleDavi, R. L. C., Almeida, E. A. d., Peron, A. P., Banczek, E. d. P., Junior, O. V., & Tractz, G. T. (2025). From Structure to Efficiency: Unveiling the Role of Calcination Temperature in Nb2O5-Based DSSCs. Processes, 13(6), 1857. https://doi.org/10.3390/pr13061857







