Abstract
This study explores biochar-based catalysts made from hardwood (HW) and wheat straw (WS) biomass for activating persulfate (PS) in the removal of lindane and β-endosulfan from water. The effects of pyrolysis temperature, solution pH, and PS concentration were investigated. The results indicated that both feedstock and pyrolysis temperature are key factors influencing biochar composition. Biochars pyrolyzed at 700 °C exhibited higher surface areas compared to those pyrolyzed at 400 °C, suggesting more effective interactions with the target pesticides. Changes in pH had a minimal impact on pesticide removal, while increasing the PS concentration from 0.5 to 3 mM accelerated degradation. However, further increases in PS concentration slowed the degradation of both pesticides. Under optimal conditions (pH of 7.0 ± 0.2 and PS concentration of 3 mM), the HW700/PS and WS700/PS systems achieved > 90% removal of pesticides within 4 h. Quenching experiments confirmed that non-radical species (1O2), generated through persulfate activation by biochar, were the key factor in lindane degradation in both systems, supporting the catalytic role of biochar rather than mere adsorption. In the HW700/PS system, SO4•−, HO•, and 1O2 acted synergistically to enhance the degradation of β-endosulfan, whereas in the WS700/PS system, the degradation was mainly driven by SO4•− and 1O2. Notably, HW700 biochar maintained its activation efficiency during β-endosulfan degradation even after five cycles. This research offers new insights into the potential of biochar-activated PS as a green, cost-effective, and efficient method for water treatment, addressing pesticide-contaminated surface water and promoting agricultural waste recycling.
1. Introduction
Pesticides are essential in agriculture, but their misuse can harm both the environment and human health. With growing concerns about agrochemical use, preserving resources like water for future generations has become crucial. Addressing pesticide-related challenges is necessary for developing sustainable solutions that reduce environmental and public health risks [1]. Organochlorine pesticides (OCPs) are recognized for their environmental persistence, high toxicity, potential for long-range atmospheric transport, and tendency to bioaccumulate. Consequently, they have been listed as persistent organic pollutants (POPs) of global concern under the Stockholm Convention [2,3,4,5]. Representing approximately 40% of widely used pesticides, OCPs constitute a significant environmental hazard [6].
Lindane and β-endosulfan were chosen in this study as representative OCPs due to their widespread use and environmental persistence. Lindane was produced in over 600,000 tonnes globally between the 1950s and early 2000s for insect control in agriculture, including fruits, vegetables, and soil treatment. Due to its carcinogenic and teratogenic properties, the US EPA and WHO classify lindane as a significant health risk [7]. By 2010, over 50 countries banned its use in agriculture, yet lindane persists in water sources worldwide, with concentrations ranging from 0.087 to 5509 μg/L, raising concerns about public health and the environment. Additionally, stocks of lindane produced before the ban may still exist in environmental matrices and markets, prolonging its ecological impact [7,8].
Endosulfan (present as α- and β-endosulfan, originates from cyclopentadiene. Both isomers degrade slowly in soil and water to form endosulfan sulfate, which then breaks down into various byproducts, such as chloride ions and endosulfan diol [4,9,10]. Endosulfan’s extensive agricultural use has led to contamination in air, soil, and water, posing serious ecological risks [9]. Like Lindane, regulatory bans on endosulfan have been enforced in many countries [4]. Despite China’s halt on production and use in 2019, endosulfan remains in use in some developed and developing nations, pending the development of alternatives [11,12].
The growing levels of environmental pollutants emphasize the need for cost-effective, efficient removal methods. A range of physical, chemical, and biological methods has been employed to eliminate OCPs from water, including adsorption, membrane filtration, coagulation, advanced oxidation processes (AOPs), and biodegradation [1,13]. Recently, biochar (BC) has gained attention for its catalytic role in AOPs and its effectiveness in removing OCPs due to its high efficiency and availability [14,15]. Biochar, produced through pyrolysis of biomass in anaerobic conditions, has unique characteristics such as a microporous structure, abundant functional groups, large surface area, and high porosity, which make it effective for pollutant removal and environmental remediation [16]. Biochar is also a potent activator of persulfate (PS) for degrading organic pollutants, likely due to its oxygen-containing functional groups [17,18]. In contrast to traditional AOPs that produce hydroxyl radicals (HO•), sulfate radicals (SO4•−) in the BC/PS system have higher redox potential, a broader pH range, and longer half-lives [16]. Organic pollutant degradation in BC/PS systems occurs via both free radical and non-radical pathways. The free radical pathway involves biochar’s catalytic sites cleaving the O-O bond in PS, forming SO4•−, HO•, and superoxide ions (O2•−), while the non-radical pathway involves singlet oxygen (1O2), electron transfer, and surface complexes [19] (Equations (1) and (2)).
(BCsurface)--OOH + S2O82− → SO4•− + (BCsurface)--OO• + HSO4−
(BCsurface)--OH + S2O82− → SO4•− + (BCsurface)--O• + HSO4−
Studies [15,20,21,22] suggest that wood-based biomass is favored for biochar production due to its high carbon content and favorable structure. However, non-wood biomaterials, such as agricultural waste and animal manure, also have significant potential by reducing environmental waste and being converted into biochar for environmental remediation. Biochar from unmodified biomass for persulfate activation may be more sustainable, cost-effective, and eco-friendly, while modified biochar could involve additional chemical treatments, increasing costs and environmental risks. Optimizing pyrolysis conditions, such as increasing temperature or extending heating duration, enhances biochar’s physical and chemical properties, improving pollutant adsorption [23].
Despite regulatory actions, lindane and endosulfan persist in the environment due to chemical stability, historical usage, and illicit trade. While current technologies work in controlled settings, they struggle in real-world conditions. The fate of degradation byproducts and their interactions with other pollutants remain insufficiently explored. Additionally, the availability and efficiency of sustainable alternatives, especially where these pesticides are still in use, need further research. Addressing these gaps is crucial for scalable pesticide removal solutions and environmental risk mitigation.
No study has investigated using unmodified biochar for persulfate activation to remove β-endosulfan and lindane mixture from water. The role of unmodified biochar in PS activation, its mechanism, and comparisons with other materials remain unexplored. Research focused on these areas is crucial. Understanding the mechanism of PS activation by pristine biochar and comparing its efficiency with other materials is crucial for optimizing biochar-based systems for pesticide degradation in real-world conditions. This study produced biochar from wood biomass and wheat straw residues via pyrolysis, characterized it using advanced techniques, and used it to activate PS under various conditions, such as pH and PS concentration. The biochar’s stability was assessed through multiple cycles, and reactive oxygen species in the BC/PS system, along with degradation pathways, were investigated. Furthermore, the practical potential of the BC/PS system for pesticide removal from surface water was explored.
2. Materials and Methods
2.1. Chemicals and Reagents
In this study, the following chemicals were used: lindane (PESTANAL® analytical standard, Fluka, Prague, Czech Republic) and β-endosulfan (PESTANAL® analytical standard, Sigma Aldrich, St. Louis, MO, USA) as target compounds. Potassium persulfate (ACS reagent, Sigma Aldrich) was employed as the oxidizing agent. Tert-butyl alcohol (ACS reagent, Sigma Aldrich) and sodium azide (Thermo Fisher Scientific, Waltham, MA, USA) were used as radical scavengers, while sodium thiosulfate pentahydrate (ReagentPlus®, Sigma Aldrich) served as the quenching agent. Sodium hydroxide (reagent grade, Sigma Aldrich) and sulfuric acid (Sigma Aldrich) were used for pH adjustment. Methanol and hexane were obtained from J.T. Baker®, Mumbai, India. Ultrapure deionized water was utilized to prepare all synthetic aqueous matrices and working solutions. Ultrapure deionized water was obtained using the LABCONCO Water Pro RO/PS Station Type I quality and had the following characteristics: dissolved organic carbon < 0.5 mg/L and electrical conductivity of 0.055 µS/cm.
2.2. Preparation of Biochar
In this study, two types of biomasses were used: hardwood (HW) and wheat straw (WS). The wheat straw was collected from farmland in the Autonomous Province of Vojvodina, Republic of Serbia. The biomass was oven-dried at 60 °C for 24 h and then processed in a Nabertherm Furnace (Lilienthal, Germany). The samples underwent pyrolysis in an inert argon atmosphere with a flow rate of 80 L/h. The heating rate was set at 10 °C/min. After a slow pyrolysis process lasting approximately one hour, the material was allowed to cool naturally to room temperature to form biochar. The ash produced was separated by sieving, and the biochar fragments were ground using an iron pestle. Prior to the experiments, the biochar was sieved to ensure uniform particle size and stored in airtight zip-lock bags. The materials were pyrolyzed at temperatures of 400 °C and 700 °C, and the samples were labeled as HW400, HW700, WS400, and WS700.
2.3. Experimental Procedures
All experiments were performed at least duplicate, and the results are presented as mean values ± standard deviations.
Adsorption experiment. The adsorption experiment was performed in 60 mL glass vials containing of mixture of pesticides (c [lindane]0 = [β-endosulfan]0 = 100 µg/L, initial pH: 7.0 ± 0.2) and 10 mg of BC. The suspension was stirred on digital shaker (IKA® Orbital Shaker KS501 Digital, Staufen im Breisgau, Germany) at 180 rpm and maintained at 25 ± 1 °C. At specified time intervals, the samples were withdrawn and filtered through a cellulose nitrate filter paper (0.45 µm, Sartorius, Bohemia, NY, USA).
Catalytic degradation experiment. A 10 mg BC was dispersed in 50 mL of solution (0.2 g/L, c [lindane]0 = [β-endosulfan]0 = 100 µg/L, initial pH value: 7.0 ± 0.2), and the reaction was initiated by adding PS. The suspension was stirred at 180 rpm, 25 ± 1 °C. The samples was withdrawn in given intervals, also filtered through a cellulose nitrate filter paper (0.45 µm, Sartorius, Bohemia, NY, USA), and immediately quenched with 0.01 M of NaS2O3. The effects of PS concentration (0.5–9 mM) and pH values (5–9.5, adjusted with 0.1 mM NaOH and 0.1 mM H2SO4) on the mixture of pesticides degradation were investigated in BC/PS system. The characteristics of the surface water used for the investigation are summarized in the Supplementary Materials (Table S1).
The quenching tests. Different quenching agents of MeOH, TBA and NaN3 were used to determine the contributions of HO•, SO4•− and 1O2 to the mixture of pesticides catalytic degradation. The concentration of all quenching agents was 100 mM, and the test was performed under optimal conditions.
2.4. Analytical Methods
The concentrations of the target compounds were determined using gas chromatography coupled with mass spectrometry (Agilent Technologies 7890A Gas Chromatograph/5975C Mass Spectrometer, Santa Clara, CA, USA), employing a DB-5MS capillary column (30 m × 0.25 µm × 0.25 µm, J&W Scientific, Santa Clara, CA, USA) (see in the Supplementary Materials (Text S1)).
The physicochemical characteristics of biochar were assessed using a variety of methods, including elemental analysis (CHNS); Brunner Emmet Teller (BET); Fourier Transform Infrared Spectroscopy (FTIR) and scanning electron microscope (SEM). In addition to the methods mentioned above, the point of zero charge (pHpzc) of the biochar was also measured. The detailed operating parameters can be found in the Supplementary Materials (Text S2).
The pH of the samples was measured using a pH/ION 735 instrument (Model 04520006; WTW, Weilheim, Germany). The total organic matter content in surface water was evaluated by measuring the total organic carbon (TOC) using a TOC analyzer (Elementar Liqui TOC II, Model 35072028; Elementar, Langenselbold, Germany). UV absorbance at 254 nm was measured with a CINTRA 1010 spectrophotometer (GBC Scientific Equipment, Perai, Malaysia).
3. Results and Discussion
3.1. Characterization of Biochar
Elemental analysis of biomass derived from HW was provided in our study [24]. Table S2 presents the results of the elemental CHNS analysis for biochar derived from WS. All the applied biochar primarily consisted of carbon, with values ranging from 66.29% to 89.8%, highlighting carbon as the dominant component. In contrast, the hydrogen, nitrogen, and sulfur content were relatively low, suggesting that these elements are present in smaller quantities in the biochars. The carbon content of wood-derived biochar increased significantly with pyrolysis temperature, measured at 82.4 ± 1.47% at 400 °C and 89.8 ± 2.47% at 700 °C. In contrast, wheat straw biochar showed relatively stable carbon content across temperatures, with 66.70 ± 2.38% at 400 °C and 66.29 ± 2.23% at 700 °C. This suggests that wood biochar develops more condensed aromatic structures at higher temperatures compared to wheat straw biochar. These aromatic carbon frameworks are crucial for the catalytic activation of persulfate, as they facilitate electron transfer processes that lead to the generation of sulfate radicals (SO4•−). The relatively low hydrogen and oxygen content in biochar contributes to reduced polarity and increased hydrophobicity of its surface, which influences interaction with persulfate molecules and the overall stability of the catalyst. Additionally, polar functional groups, often linked to oxygen and nitrogen, can enhance persulfate adsorption and activation by providing reactive sites for electron exchange. However, higher pyrolysis temperatures reduce these groups, resulting in more chemically stable but less polar biochars, potentially affecting the balance between reactivity and durability during persulfate activation. Therefore, the elemental composition highlights the dual role of biochar’s structure and surface chemistry in catalytic efficiency, emphasizing that biochars produced under optimized pyrolysis conditions offer a favorable combination of aromaticity and functional group availability necessary for effective catalytic performance.
The results of the FTIR analysis are presented in Figure 1. Generally, the analysis reveals a reduction in functional groups as pyrolysis temperatures increase, resulting from enhanced carbonization and the removal of volatile matter [25]. The peaks of all biochars are primarily concentrated in the ranges of 3423–2851 cm−1 and 1614–746 cm−1.
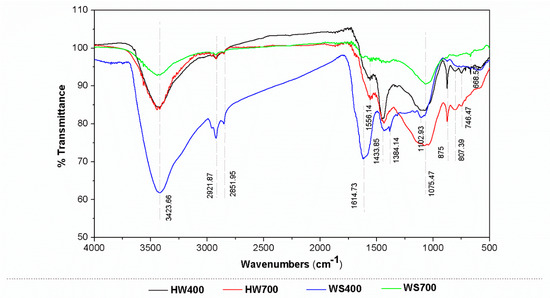
Figure 1.
FTIR analysis of biochars at different temperatures.
A peak at 3423 cm−1 in the FTIR spectra is attributed to the stretching vibration of hydroxyl groups (O-H) in alcohol and phenol structures linked to intermolecular hydrogen bonding. Hydroxyl groups are prevalent in biomass, primarily derived from glucose molecules at different positions in cellulose and hemicellulose, as well as from phenolic groups in lignin [26]. However, there is a decrease in the O-H absorption peak in the WS700 at higher temperatures due to the loss of hydrogen and oxygen. The peaks around 2921 and 2851 cm−1 are associated with the stretching vibration of the C-H bond and -CH2 groups, which originate from compounds in biomass with C-H chains, such as fatty acids and fatty alcohols [26]. The peaks of HW700 and WS700 were less intense after pyrolysis at 700 °C compared to those at lower temperatures. This can be attributed to the higher pyrolysis temperature, which caused depolymerization and the breakdown of alkyl chains in the biochar, resulting in its stabilization. The band peaks in the range 1614–1434 cm−1 signal presence of benzene rings or aromatics compounds and confirm the presence of alkenes, which are associated with C=C bonds [26,27,28]. With the increase in temperature, the C=O peak gradually decreased, while the C=C peak became more pronounced, suggesting that higher temperatures enhanced the aromatization of biochar [29]. Only in WS400 was a peak observed at 1384 cm−1 corresponds to the asymmetric and symmetric C-O vibration bands of the carboxylic acid group on the surface of the biochar. Additionally, peaks were observed at 1102 cm−1 and 1075 cm−1, likely caused by C-O-C symmetrical stretching, which is characteristic of cellulose and hemicelluloses [30]. Vibrational bands below 900 cm−1 are primarily attributed to the C–H bending vibrations of heteroaromatic and aromatic compounds, showed low intensity because it might be associated with π-electron bond [25,27,28]. The FTIR analysis results show the presence of functional groups associated with cellulose, hemicellulose, and lignin in the wood and wheat straw biomass.
The impact of temperature on the morphology of biochars was examined using SEM to analyze both biochar. The SEM images of the prepared WS400 and WS700 biochars are shown on Figure 2. Additionally, the SEM analysis of hardwood biochar is also explained in our study [24]. The surface of wheat straw biomass was smooth with uniformly distributed pores. In the longitudinal section, a well-organized duct-like structure was observed, reflecting the regular cellular architecture of wheat straw. In contrast, the longitudinal section of WS700 displayed an irregular, fragmented appearance, indicating the onset of structural breakdown. This deterioration is primarily attributed to the collapse of the carbon skeleton at elevated pyrolysis temperatures. Similar observations were reported by [31]. Overall, the results demonstrated that both the type of biomass feedstock and the pyrolysis temperature play a crucial role in determining the morphology of the resulting biochars [31,32,33]. These morphological changes, especially the increased fragmentation and porosity observed in WS700, contribute to enhanced catalytic activity. The more developed and accessible surface area facilitates the interaction between the biochar and persulfate molecules, promoting the generation of reactive species and improving the degradation efficiency of pesticides. Therefore, the SEM morphology provides visual evidence of the structural evolution of biochars and supports the observed differences in catalytic performance.
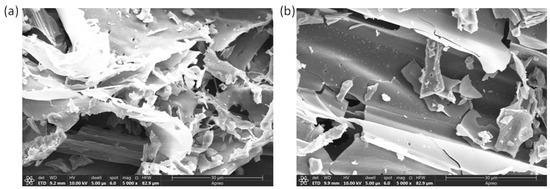
Figure 2.
SEM images of WS400 (a) and WS700 (b) obtained at the magnification of 5000×.
The higher pyrolysis temperature led to a richer porous structure and increased formation of volatile substances, resulting in WS700 having a significantly greater specific surface area (SSA) of 80.8 m2/g compared to WS400, which had 3.68 m2/g. Additionally, as demonstrated in our previous study [24], the notably higher SSA of HW700 (284 m2/g) indicates its potential as a more efficient sorbent for organic pollutants, offering enhanced accessibility to active adsorption sites.
3.2. Adsorption of Pesticides onto Biochar
The kinetic curve for the adsorption of the pesticide mixture on all the applied biochars is shown in Figure 3. The adsorption of pesticides by all biochars increased rapidly during the first six hours, reaching equilibrium within 24 h. At a pyrolysis temperature of 400 °C, the removal efficiency of lindane was approximately 38–42% for all biochars, while for endosulfan, it was up to 78% in WS400. However, when the pyrolysis temperature was increased to 700 °C, the removal efficiency improved to 56% for lindane and 84% for β-endosulfan. These results demonstrate that biochar produced at the higher temperature of 700 °C is more effective at removing the pesticide mixture and has a higher adsorption capacity for these pesticides. The experimental results best fitted the pseudo-second-order kinetic model (R2 = 0.920–0.998) compared to the pseudo-first-order (R2 = 0.149–0.852) and Elovich models (R2 = 0.769–0.953) (Table S3 in Supplementary Materials). The obtained results suggest that chemisorption may be the dominant mechanism in the adsorption process, with the main limiting factor being the interaction between the pesticides and the active sorption sites on the surface of biochars.
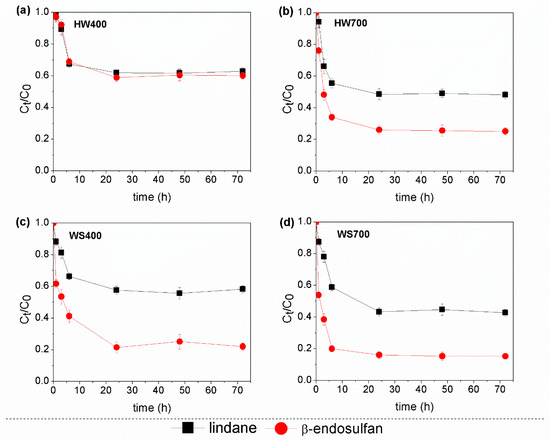
Figure 3.
Adsorption kinetics of the pesticide mixture on (a,b) hardwood and (c,d) wheat straw biochars.
3.3. Catalytic Degradation of Pesticides
Effect of PS concentration. Degradation experiments were carried out on a mixture of pesticides to evaluate the catalytic effectiveness of biochar derived from different biomasses. The results demonstrated that PS alone was not effective in oxidizing pesticides (<6%) within 48 h, suggesting that PS had not significantly impact on pesticides oxidation (Figures S1 and S2). Additionally, our review paper emphasizes that the use of PS or peroxymonosulfate (PMS) alone led to poor degradation and ineffective removal of various pesticide and intermediate compounds [14]. These include compounds from the following groups: triazines and triazoles, neonicotinoids, sulfonylureas, organochlorines and other chlorinated compounds, and benzene ring intermediates. In contrast, the simultaneous addition of biochar and PS led to varying degrees of improved removal of target compounds. The results indicate that when a combination of biochar and persulfate was used, equilibrium was reached after 4 h, which is significantly faster than the adsorption process on biochar alone. The degradation of pesticides consistently increased as the PS concentration rose from 0.5 to 3 mM. The removal efficiency of lindane rapidly up from 47% to 92% in HW700/PS, while in the HW400/PS system it moved in the range from 36% to 62% (Figure 4). On the other hand, the results in Figure 5 show that HW700 and WS700 had better effects in the removal of β-endosulfan, with the degree of degradation ranging from 61% to 94% in the HW700/PS system and 60–88% in the WS700/PS system, respectively. Endosulfan removal efficiency varied between 51% and 70% in the HW400/PS system and between 44% and 64% in the WS400/PS system.
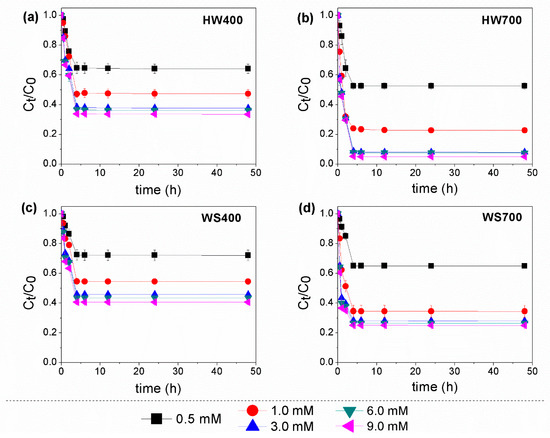
Figure 4.
Effect of PS concentration on the degradation of lindane using: (a) HW400/PS; (b) HW700/PS; (c) WS400/PS; and (d) WS700/PS. Experimental conditions: [lindane]0 = 100 µg/L; [catalyst]0 = 0.2 g/L; [PS]0 = 0.5–9 mM; pH 7.0 ± 0.2.
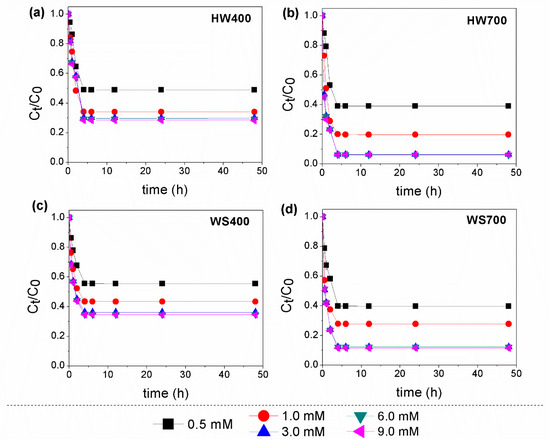
Figure 5.
Effect of PS concentration on the degradation of β-endosulfan using: (a) HW400/PS; (b) HW700/PS; (c) WS400/PS; and (d) WS700/PS. Experimental conditions: [β-endosulfan]0 = 100 µg/L; [catalyst]0 = 0.2 g/L; [PS]0 = 0.5–9 mM; pH 7.0 ± 0.2.
Overall, HW700 was able to almost completely degrade both lindane and β-endosulfan at PS dose of 3 mM, highlighting its structural advantage in catalytic activation. However, when the PS concentration was further increased from 3 to 9 mM, the improvement in performance was minimal. These results suggest that the initial concentration of PS is crucial for the oxidation of organic pollutants through SO4•−, as it directly influences the equilibrium concentration of SO4•− in degradation systems. The enhancement of degradation performance was observed with the increase in PS, as the availability of PS ions led to the generation of more sulfate and hydroxyl radicals [34]. However, very high concentrations may deplete SO4•−, slowing down the degradation of the target pollutant due to the scavenging effect [35]. This phenomenon was explained in Equations (3)–(5) [34,36]. The SO4•− or HO• radicals were converted into the less oxidative peroxydisulfate radical (S2O8•−), leading to a decrease in degradation performance [37].
SO4•− + S2O82− → S2O8•− + SO42−
SO4•− + SO4•− → S2O82−
HO• + S2O82− → S2O8•− + OH−
The results above suggest that increasing the PS concentration within a certain range was beneficial for the degradation of both pesticides. In summary, an optimized system with 0.2 g/L of catalyst obtained at pyrolysis temperature of 700 °C and 3 mM of oxidant for removing pesticides in a synthetic water matrix is recommended, with a reaction time of 4 h. This is consistent with the results of Huang et al. (2022) [38], where a novel N-doped biochar-loaded nanoscale zero-valent iron (nZVI) composite (Fe@N-BC) was used and evaluated for PS activation to remove lindane. To optimize the Fe@N2-BC900/PS for oxidation of lindane, they assessed various dosages of PS (2–6 mM), finding that as the PS dosage increased from 3 to 6 mM, the degradation kinetics improved slightly. Therefore, under optimal conditions (1.0 g/L catalyst, 4 mM PS, and an initial concentration of lindane 10 mg/L), they achieved complete removal of lindane by applying the Fe@N2-BC900/PS system within 60 min.
Effect of pH. It is well known that solution pH has a significant impact on AOPs by affecting the generation of ROS and the charge on the catalyst surface [39,40]. Therefore, to achieve effective oxidative degradation, it is important to consider the dissociation constants of the target pollutant as well as the catalyst’s point of zero charge [14]. To investigate this, the degradation of lindane and β-endosulfan by HW700- and WS700-activated PS at different pH values was examined (Figure 6). The pesticides removal in HW700/PS system efficiency increases slightly with pH, exceeding 90% when the pH reaches 7 and 9.5. An exception is observed in the WS700/PS system, where a slightly lower removal efficiency was noted during Lindane degradation, with values between 67% and 72% at pH levels ranging from 5 to 9. The relatively slow removal of pesticides under acidic conditions may be attributed to the fact that an excess of H+ ions can neutralize SO4•− and HO• (Equations (6) and (7)) [41]. Additionally, a high concentration of H+ can stabilize PS, further inhibiting PS activation and ultimately reducing pesticide degradation.
SO4•− + H+ + e− → HSO4•−
HO• + H+ + e− → H2O
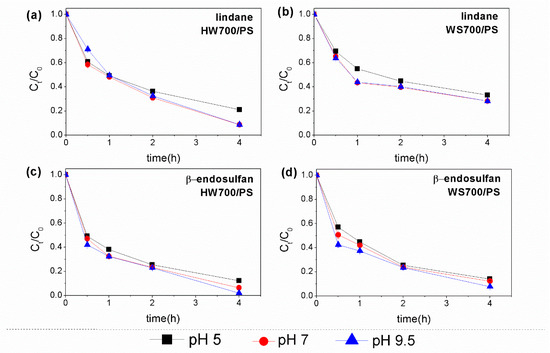
Figure 6.
Effect of pH values on the degradation of (a,b) lindane and (c,d) β-endosulfan using HW700/PS and WS700/PS systems. Experimental conditions: [lindane]0 = [β-endosulfan]0 = 100 µg/L; [catalyst]0 = 0.2 g/L; [PS]0 = 3 mM.
On the other hand, in this study, the pHpzc values of HW700 and WS700 were approximately 6.29 and 7.16, respectively (Figure S3). It can be inferred that when the pH is below the pHpzc, the biochar surface becomes positively charged, while it becomes negatively charged at pH values above the pHpzc [42]. Therefore, it can be said that both catalysts are generally negatively charged at a pH between 7 and 9.5. As lindane is a non-ionic compound that does not easily ionize in water, it predominantly exists as a neutral molecule within the examined pH range. The absence of electrostatic attraction or repulsion between the catalysts and lindane explains why changes in pH do not significantly affect lindane removal by the applied catalysts. Additionally, Huang et al. (2022) [38] report that the Fe@N-BC catalyst was effective for lindane degradation across a broad pH range. For β-endosulfan, at the tested pH, the endosulfan moieties are negatively charged (pKa = −5.5) [43]. Also, the limited impact of pH on endosulfan removal is likely due to the interplay between the biochar surface charge, non-electrostatic interactions, and the unique properties of both the biochar and endosulfan molecules, which facilitate effective adsorption and removal within the pH range of 5–9. Overall, the experimental results indicated that the degradation of pesticides using both catalytic systems was not significantly influenced by the initial pH values. As a result, the catalysts demonstrate a wide pH tolerance range, making them highly beneficial for practical applications.
A review of the available literature established that biochar-based catalysts demonstrated stable performance across a broad pH range of 3–10 [39,44,45,46,47]. However, the effectiveness of certain catalysts significantly decreased under alkaline conditions (pH > 9) [48], as well as in strongly alkaline and acidic conditions (3 < pH < 9) [49,50]. Acidic conditions lead to protonation of the catalyst’s surface [50] which promotes the leaching of metal ions from the catalyst. The decomposition of oxidants is influenced by pH, with acidic conditions favoring the breakdown of persulfate (PS) and the production of sulfate and hydroxyl radicals [34,46]. However, an excess of protons can convert these reactive species, HO• and SO4•−, into less reactive forms such as H2O and HSO4−, reducing their effectiveness [39]. On the other hand, in strongly alkaline environments, the degradation rate of some pollutants decreases, likely due to enhanced electrostatic repulsion between the catalyst surface and PS/PMS molecules [46]. Furthermore, electrostatic repulsion between certain pesticides (e.g., triclosan, 2,4-D) and the biochar catalyst can limit their interaction, thereby lowering catalytic efficiency [39,50,51].
3.4. Identifying the Primary Radical Species
According to previous research, the removal of pollutants using persulfate-based AOPs primarily depends on both free radical and non-radical pathways [52]. The free radical pathway mainly involves HO• and SO4•−, whereas the non-radical pathway is primarily driven by singlet oxygen (1O2) [53,54]. To identify the primary reactive radicals species in the HW700/PS and WS700/PS systems, quenching experiments were carried out with different scavengers, such as MeOH, TBA, and NaN3. MeOH was able to capture both SO4•− and HO• radicals concurrently, whereas TBA exhibited a stronger reactivity with HO•, as indicated by their respective rate constants (Equations (8)–(11)) [55,56]. While NaN3 was employed as a quencher for 1O2 with the reaction rate constants of ~108 M−1s−1 [51,55].
MeOH + SO4•− → products (k = 3.2 × 106 M−1 s−1)
MeOH + HO• → products (k = 9.7 × 108 M−1 s−1)
TBA + SO4•− → products (k = 4.0 − 9.1 × 105 M−1 s−1)
TBA+ HO• → products (k = 3.8 − 7.6 × 108 M−1 s−1)
As shown in Figure 7, the addition of MeOH and TBA to the reaction system did not affect the extent of lindane degradation. This indicates that only small amounts of SO4•− and HO• were produced in HW700/PS and WS700/PS systems and that these were not the primary radical species involved in lindane degradation. In contrast, significant inhibition of lindane degradation was observed with the addition of NaN3. Lindane degradation decreased by 29% in the HW700/PS and by 25% in the WS700/PS system. This observation suggests that 1O2 play crucial roles in the lindane degradation catalyzed by HW700/PS and WS700/PS. Similar results were obtained by Huang et al. (2022) [38], who suggested that during the degradation of lindane using the Fe@N2-BC900/PS system, O2•− and 1O2 were the dominant active species. In the HW700/PS system, the presence of TBA resulted in a 20% decrease in β-endosulfan removal efficiency. On the other hand, the introduction of MeOH led to a 40% reduction in removal efficiency. Furthermore, the addition of NaN3 caused a 22% decline in removal efficiency (Figure 8a). These results indicate that the degradation of β-endosulfan in the HW700/PS system is influenced by HO•, SO4•−, and 1O2. The addition of TBA in the WS700/PS system had no effect on the reduction in β-endosulfan degradation. This implies that HO• was not the primary species responsible for the degradation of β-endosulfan (Figure 8b). However, the addition of MeOH led to a 24% decrease in β-endosulfan degradation efficiency, suggesting that the SO4•− radical is involved in the reaction. NaN3 was able to moderately reduce the degradation of β-endosulfan by 16%. The results indicate that the degradation of β-endosulfan in both systems involved contributions from both free radical and non-radical pathways, with the free radical mechanism playing a more prominent role. The HW700/PS system showed a higher free radical contribution than the WS700/PS system, likely due to the more stable carbon structure of HW700. The different contributions of 1O2 between the systems may be due to variations in carbonyl group content, which primarily drives the non-radical pathway [54].
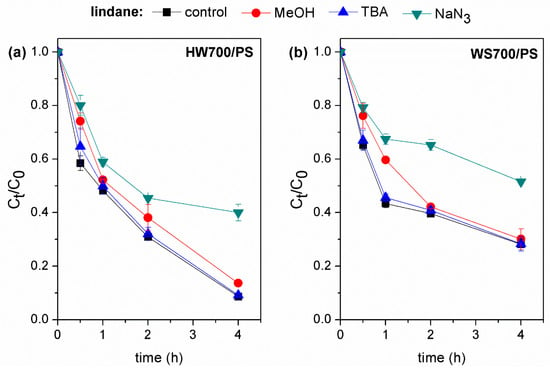
Figure 7.
Catalytic degradation of lindane by (a) HW700/PS and (b) WS700/PS systems with different scavengers. Experimental conditions: [lindane]0 = 100 µg/L; [catalyst]0 = 0.2 g/L; [PS]0 = 3 mM; [MeOH]0 = [TBA]0 = [NaN3]0 = 100 mM; pH 7.0 ± 0.2.
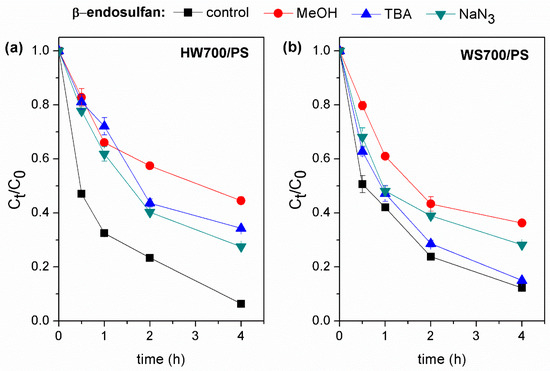
Figure 8.
Catalytic degradation of β-endosulfan by (a) HW700/PS and (b) WS700/PS systems with different scavengers. Experimental conditions: [lindane]0 = 100 µg/L; [catalyst]0 = 0.2 g/L; [PS]0 = 3 mM; [MeOH]0 = [TBA]0 = [NaN3]0 = 100 mM; pH 7.0 ± 0.2.
3.5. Stability and Potential for Reusability of Catalyst
The stability of the applied biochars is essential for their practical use. The catalytic activity of the HW700/PS and WS700/PS systems for both pesticides degradation was evaluated over five consecutive cycles. After each cycle, BC was separated, rinsed with deionized water, and dried. Lindane removal efficiency decreased by approximately 15% after four run in the HW700/PS system (Figure 9). In contrast, when using WS700 biochar, the removal efficiency of lindane dropped significantly to 43% after the third cycle compared to the first run (Figure 10). The decrease in degradation efficiency of lindane with each run could be due to biochar particles being lost from the system during sampling. Additionally, fouling of active sites by adsorbed pesticide molecules and degradation products, possible structural changes in the biochar surface, and aggregation of biochar particles during reuse may also contribute to performance decline. Further investigation is required to clarify the relative impact of these factors. Furthermore, the presence of lindane molecules and their degradation products in subsequent cycles may interfere with the active sites, leading to reduced degradation performance. The removal efficiency of β-endosulfan in the first and fifth runs were similar, indicating the high stability of the prepared of HW700 biochar. However, when WS700 was used, the β-endosulfan removal efficiency declined by approximately 20–26% after the fourth and fifth runs (Figure 10). This exceptional stability of HW700 during removal of β-endosulfan can be attributed to the straightforward yet effective preparation method of HW700, involving high-temperature pyrolysis without any additional chemical modifications like impregnation. Moreover, this stability aligns with the consistent performance observed under different pH conditions.
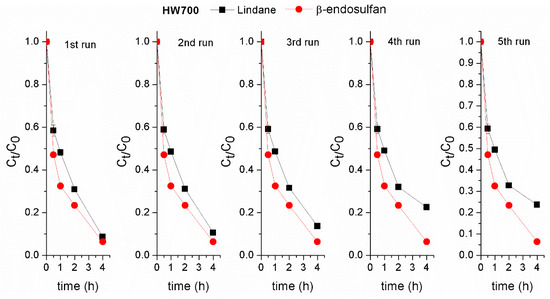
Figure 9.
Assessment of the stability of HW700 biochar in the degradation of lindane and β-endosulfan across successive experiments. Experimental conditions: [lindane]0 = [β-endosulfan]0 = 100 µg/L; [catalyst]0 = 0.2 g/L; [PS]0 = 3 mM; pH 7.0 ± 0.2.
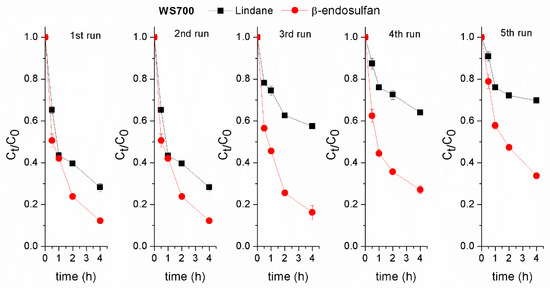
Figure 10.
Assessment of the stability of the prepared WS700 biochar in the degradation of lindane and β-endosulfan across successive experiments. Experimental conditions: [lindane]0 = [β-endosulfan]0 = 100 µg/L; [catalyst]0 = 0.2 g/L; [PS]0 = 3 mM; pH 7.0 ± 0.2.
3.6. Possible Mechanism Involved in PS Activation and Pesticides Removal
The combination of adsorption by biochar and oxidative degradation by activated persulfate creates a synergistic effect. Biochar not only adsorbs the pesticides but also helps in the activation of persulfate, making the overall process more efficient in removing the contaminants [57]. The analysis above indicated that the activation mechanisms of PS were closely linked to the catalyst’s properties (Section 3.4). The adsorption of pesticides onto biochar derived from HW and WS biomass involves several types of interactions between the biochar surface and the target compounds (Figure 11). These interactions are primarily physical and chemical, influencing the biochar’s ability to adsorb these compounds. The biochar surface, with its oxygenated functional groups, engages with the pesticides via Van der Waals forces, which are weak and distance-dependent. These interactions play a key role in the initial adsorption of the pesticides. Biochar surfaces contain functional groups like hydroxyl, carboxyl, and phenolic groups. These functional groups can form hydrogen bonds with pesticide molecules, thereby enhancing the pesticides’ retention on the biochar surface. Lindane and endosulfan both feature aromatic structures—lindane has a benzene-like ring as part of a cyclohexane structure, with each carbon atom bonded to a chlorine atom, while endosulfan has a cyclodiene structure. The aromatic components of these pesticides can interact through π–π interactions with the aromatic carbon structures on the biochar surface, which strengthens the adsorption of the compounds. Additionally, both pesticides are hydrophobic compounds, meaning they repel water. Biochar, especially when sourced from HW or WS, possesses hydrophobic characteristics due to its carbon-rich, aromatic structure. This promotes strong hydrophobic interactions, causing the pesticides to be attracted to the hydrophobic regions of the biochar surface, thereby increasing their adsorption.
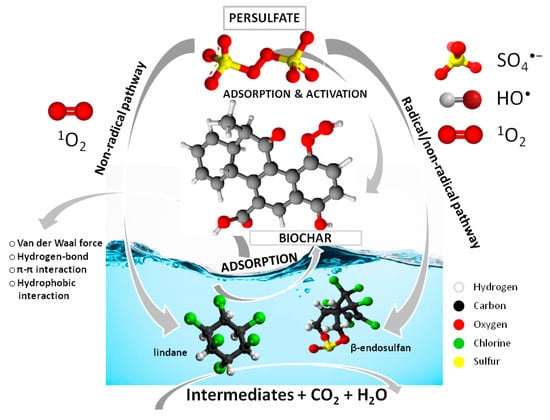
Figure 11.
Proposed mechanism scheme.
On the other hand, it can be concluded that pesticide degradation primarily occurs through a combination of free radical and non-free radical pathways. Initially, the adsorption of PS on the biochar surface is facilitated by electrostatic interactions and hydrogen bonding between the PS molecules (typically in the form of persulfate anions) and the surface functional groups. After PS is adsorbed onto the biochar surface, it can be activated by the biochar’s surface sites. This process produces highly reactive SO4•− radicals (Equations (1) and (2)), which then react with H2O to generate another reactive species, HO• (Equation (12)) [58]. Furthermore, the peroxide bond in PS could undergo cleavage to produce O2•−, which can subsequently generate 1O2 through reactions with H+, SO4•−, or water (Equations (13)–(15)) [59]. Moreover, certain oxygen-containing functional groups (e.g., -C-OH and –C=O) on the biochar surface can also activate PS, leading to the production of HO•, SO4•−, and 1O2 [60].
SO4•− + H2O → HO• + SO42− + H+
2O2•− + 2H+ → 1O2 + H2O2
2H2O + 2O2•− → 1O2 + 2OH− + H2O2
O2•− + SO4•− → 1O2 + SO42−
In summary, singlet oxygen (1O2) and active functional groups played a key role exclusively through non-radical pathways in the oxidative degradation of lindane. In contrast, the degradation of β-endosulfan involved a combined effect of the free radical pathway induced by ROS, along with non-radical pathways driven by 1O2 and active functional groups, resulting in its breakdown into smaller molecules such as CO2 and H2O.
3.7. Practical Application of Catalyst in Surface Water Treatment
Biochar materials are more affordable than other metal or synthetic carbon materials since they primarily come from natural sources and often use waste products. Their low cost and environmental benefits make biochar an attractive option for industrial production and practical applications. Additionally, the applicability of biochar is an important factor to consider [61].
To evaluate the adaptability of the BC/PS system in real water matrix, catalytic degradation reactions were performed using surface water sample. The performance of the HW700/PS and WS700/PS systems in pesticide degradation in surface water was assessed under optimal conditions (4 h at pH 7, PS concentration of 3 mM, and catalyst dose of 0.2 g/L). The HW700/PS system effectively removed approximately 90% of both pesticides from surface water under optimal conditions. However, when WS700 biochar was combined with persulfate, the degradation rates achieved were 45% for lindane and 70% for endosulfan (Figure 12). The reduced efficiency of the WS700/PS system during pesticide degradation can be attributed to the fact that naturally occurring components in water, such as organic matter and inorganic ions (e.g., HCO3−/CO32−, Cl−, H2PO4−/HPO42−), exhibit a stronger inhibitory effect compared to the BW700/PS process. Namely, these components can interact with the generated radicals, reducing the number of free radicals available to react with the target compounds [62]. Based on the obtained results and the cost-effectiveness of biochar, it is confirmed that the HW700/PS system may be promising for practical water purification.
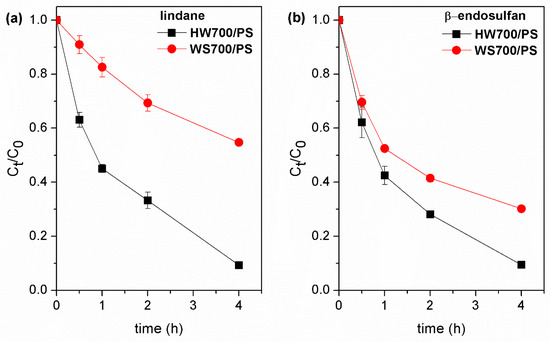
Figure 12.
Practical application of catalytic degradation for (a) lindane and (b) β-endosulfan in surface water.
Several studies have shown that the degradation of atrazine, metolachlor, triclosan, and pesticide intermediates is notably hindered in wastewater, necessitating extended reaction times. The high concentrations of organic and inorganic compounds likely quenched reactive radicals and blocked catalyst active sites, reducing free radical generation compared to reactions without interfering ions. However, the inhibitory effects of HCO3−/CO32− are less pronounced in natural waters compared to wastewater [34,51,63,64].
4. Conclusions
In conclusion, this study successfully developed a novel biochar catalyst derived from hardwood (HW) and wheat straw (WS) biomass, demonstrating remarkable catalytic efficiency in activating persulfate (PS) for the degradation of organochlorine pesticides (OCPs), particularly β-endosulfan and lindane. The biochar, produced via pyrolysis under controlled conditions, offers a promising, cost-effective, and environmentally sustainable alternative for pesticide removal from water. The use of agricultural waste for biochar synthesis not only addresses the pressing issue of waste disposal but also contributes to environmental sustainability by providing a functional catalyst for water treatment.
This study highlighted the significant impact of pyrolysis temperature on the catalytic performance of biochar. Biochars pyrolyzed at 700 °C showed superior pesticide degradation efficiency, achieving over 90% removal of pollutants under optimal conditions. These findings underscore the importance of pyrolysis conditions in tailoring biochar’s physical and chemical properties for specific environmental applications. The high removal efficiency achieved within 4 h of reaction under the optimal conditions demonstrates the potential for biochar-based systems to be employed in real-world water treatment scenarios.
Additionally, this study provided valuable insights into the degradation pathways of lindane and β-endosulfan using the biochar/PS system. The radical quenching experiments revealed that the non-radical (1O2) pathway was predominantly responsible for lindane degradation, while β-endosulfan removal in the HW700/PS system was driven by a combination of sulfate radicals (SO4•−), singlet oxygen (1O2), and hydroxyl radicals (HO•). This detailed understanding of the degradation mechanisms is crucial for optimizing biochar-based catalytic systems and further enhancing their performance for pesticide degradation in complex environmental matrices.
The practical applicability of biochar in water treatment was further demonstrated by the excellent repeatability observed in the HW700 catalyst, which consistently removed β-endosulfan from water over multiple cycles. This indicates the potential for reusing biochar in long-term water treatment processes, reducing operational costs and increasing the sustainability of the treatment systems. Compared to WS700, HW700 showed slightly higher performance, making it a more favorable candidate for practical water treatment applications.
In summary, this research contributes to the growing body of knowledge on the use of biochar in environmental remediation, providing a novel approach for the activation of persulfate in pesticide removal. The findings suggest that biochar derived from agricultural waste biomass, especially at higher pyrolysis temperatures, can be an efficient and sustainable solution for removing persistent organic pollutants from water. Future research should focus on further optimizing biochar properties, exploring its use in diverse environmental conditions, and investigating its potential in treating a broader range of pollutants, thus paving the way for more scalable and cost-effective water treatment technologies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13061856/s1, Table S1: Characteristics of the surface water; Text S1: The detailed parameter settings of GC/MS system; Text S2: Characterization of biochar; Table S2: Analysis of the elemental composition of the applied biochar; Table S3: Sorption kinetics models fitting parameters; Figure S1: Effect of PS alone on the degradation of lindane. Experimental conditions: [lindane]0 = 100 µg/L; [PS]0 = 0.5–9 mM; pH 7.0 ± 0.2; Figure S2: Effect of PS alone on the degradation of β-endosulfan. Experimental conditions: [β-endosulfan]0 = 100 µg/L; [PS]0 = 0.5–9 mM; pH 7.0 ± 0.2; Figure S3: Determination of the zero point charge (pHzpc) for (a) hardwood and (b) wheat straw biomass. References [65,66,67,68,69,70] are cited in the supplementary materials.
Author Contributions
T.S., writing—original draft, conceptualization, methodology, visualization, data curation, and software. T.M.S., investigation, formal analysis, data curation, methodology, visualization, and writing—review and editing. T.A., writing—review and editing, formal analysis, data curation, and visualization. J.A., writing—review and editing, validation, and visualization. N.Đ., writing—review and editing, investigation, and visualization. S.M., writing—review and editing, validation, and visualization. J.M.J., writing—review and editing, visualization, validation, and methodology. J.B., project administration, supervision, funding acquisition, resources, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Fund of the Republic of Serbia Program PROMIS2023, #10810, EnviroChar project.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research was supported by the Science Fund of the Republic of Serbia, #10810, Sustainable solutions in environmental chemistry: exploring biochar potential–EnviroChar.
Conflicts of Interest
The authors declare that they have no financial conflicts of interest or personal relationships that could have influenced the work presented in this paper.
References
- da Silva Júnior, A.H.; Silva de Oliveira, C.R.; Lear, T.W.; Mapossa, A.B.; Fiates, J.; de Souza, A.U.; de Arruda Guelli Ulson de Souza, S.M.; da Silva, A. Organochlorine pesticides remediation techniques: Technological perspective and opportunities. J. Hazard. Mater. Lett. 2024, 5, 100098. [Google Scholar] [CrossRef]
- Asefa, E.M.; Mergia, M.T.; Mengistu, D.A.; Damtew, Y.T.; Dugusa, F.F.; Tessema, R.A.; Enoe, J.; Ober, J.; Teklu, B.M.; Woldemariam, E.D. Organochlorine pesticides in Ethiopian waters: Implications for environmental and human health. Toxicol. Rep. 2024, 12, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Dong, F.; Pan, X.; Wu, X.; Xu, J.; Zheng, Y. Quantitative screening of organophosphorus and organochlorine pesticides in water and soil using comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. J. Chromatogr. Open. 2024, 5, 100140. [Google Scholar] [CrossRef]
- Wu, Y.; Lv, Y.; Jin, J.; Fan, Y.; Li, R.; Bao, J.; Jiang, J.; Han, J.; Wang, Y. Kinetics of and solvent effects on photodegradation of hexachlorobenzene (HCB) and endosulfan (endosulfan I and endosulfan II) in solution. Emerg. Contam. 2024, 10, 100379. [Google Scholar] [CrossRef]
- Duro, A.; Goudou, F.; Minofar, B.; Carmenate-Rodriguez, C.; Gaspard, S.; Jauregui-Haza, U. A computational study of adsorption on activated carbons containing basic oxygenated surface groups of two priority organochlorine pesticides from water. Colloids Surf. A Physicochem. Eng. Asp. 2025, 704, 135449. [Google Scholar] [CrossRef]
- Carolin, F.C.; Kamalesh, T.; Kumar, P.S.; Rangasamy, G. An insights of organochlorine pesticides categories, properties, eco-toxicity and new developments in bioremediation process. Environ. Pollut. 2023, 333, 122114. [Google Scholar] [CrossRef]
- Keshu; Rani, M.; Shanker, U. Synthesis and characterization of novel guar gum based waste material derived nanocomposite for effective removal of hexabromocyclododecane and lindane. Int. J. Biol. Macromol. 2024, 268, 131535. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, S.; Silvestri, D.; Hrabak, P.; Padil, V.V.T.; Torres-Mendieta, R.; Wacławek, M.; Černík, M.; Dionysiou, D.D. Chemical oxidation and reduction of hexachlorocyclohexanes: A review. Water Res. 2019, 162, 302–319. [Google Scholar] [CrossRef]
- Mudhoo, A.; Bhatnagar, A.; Rantalankila, M.; Srivastava, V.; Sillanpää, M. Endosulfan removal through bioremediation, photocatalytic degradation, adsorption and membrane separation processes: A review. Chem. Eng. J. 2019, 360, 912–928. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Govarthanan, M.; Mohan, K.; Ganesan, A.R.; Yusoff, A.R.M.; Gu, F.L. Persistence, toxicological effect and ecological issues of endosulfan–A review. J. Hazard. Mater. 2021, 416, 125779. [Google Scholar] [CrossRef]
- Guo, L.; Gu, C.; Huang, T.; Gao, H.; Zhao, Y.; Mao, X.; Ma, J. Signatures of Indian endosulfan usage in China’s environment. Chemosphere 2022, 306, 135644. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-I.; Kim, J.-E. Uptake of endosulfan isomers from soils by leafy vegetable lettuce: A comparative study between model-predicted and field-experimented results. Sci. Total Environ. 2022, 844, 157056. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Keshu; Shanker, U. Green construction of biochar@NiFe2O4 nanocomposite for highly efficient photocatalytic remediation of pesticides from agriculture wastewater. Chemosphere 2024, 352, 141337. [Google Scholar] [CrossRef] [PubMed]
- Molnar Jazić, J.; Gross, A.; Glaser, B.; Agbaba, J.; Simetić, T.; Nikić, J.; Maletić, S. Boosting advanced oxidation processes by biochar-based catalysts to mitigate pesticides and their metabolites in water treatment: A meta-analysis. J. Environ. Chem. Eng. 2024, 12, 114260. [Google Scholar] [CrossRef]
- Song, Q.; Kong, F.; Liu, B.-F.; Song, X.; Ren, H.-Y. Biochar-based composites for removing chlorinated organic pollutants: Applications, mechanisms, and perspectives. Environ. Sci. Ecotechnology 2024, 21, 100420. [Google Scholar] [CrossRef]
- Istiqomah, N.A.; Jung, D.; Khim, J. Biochar-based persulfate activation: Rate constant prediction, key variables identification, and system optimization. J. Water Process Eng. 2024, 65, 105839. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Jiang, D.; Gao, Y.; Chen, Y.; Wang, W.; Cao, B.; Tao, Y.; Wang, L.; Zhang, Y. Removal of atrazine by biochar-supported zero-valent iron catalyzed persulfate oxidation: Reactivity, radical production and transformation pathway. Environ. Res. 2020, 184, 109260. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Luo, Z.; Zheng, S.; Zheng, Q.; Wu, B. Effective degradation of ofloxacin using nano-zero-valent iron activated persulfate supported by the reusable porous gel microsphere. Sep. Purif. Technol. 2024, 331, 125634. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Li, C.; Guo, J.; Sun, C. Zero-valent iron loaded on N-doped biochar fabricated by one-step pyrolysis of K2FeO4 and coffee grounds as a persulfate activator for Bisphenol A degradation. Process Saf. Environ. Prot. 2023, 170, 328–338. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar production techniques utilizing biomass waste-derived materials and environmental applications–A review. J. Hazard. Mater. Adv. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Boraah, N.; Chakma, S.; Kaushal, P. Optimum features of wood-based biochars: A characterization study. J. Environ. Chem. Eng. 2023, 11, 109976. [Google Scholar] [CrossRef]
- Patel, M.R.; Panwar, N.L. Biochar from agricultural crop residues: Environmental, production, and life cycle assessment overview. Resour. Conserv. Recycl. Adv. 2023, 19, 200173. [Google Scholar] [CrossRef]
- Xin, Z.; Tong, J.; Wang, J.; Ruan, C.; Lyu, J.; Shi, J. Research progress on activated persulfate by biochar: Soil and water environment remediation, mechanism exploration and simulation calculation. Chem. Eng. J. 2024, 493, 152718. [Google Scholar] [CrossRef]
- Mutić, S.; Anojčić, J.; Đukanović, N.; Apostolović, T.; Simetić, T.; Petrović, J.; Beljin, J. Exploring wood-derived biochar potential for electrochemical sensing of fungicides mancozeb and maneb in environmental water samples. Talanta 2025, 287, 127648. [Google Scholar] [CrossRef]
- Manjunath, B.; Ouellet-Plamondon, C.M.; Das, B.B.; Rao, S.; Bhojaraju, C.; Rao, M. Areca nut husk biochar as a sustainable carbonaceous filler for cement: Pyrolysis temperature and its effect on characterization, strength, and hydration. Ind. Crops Prod. 2024, 222, 119883. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Lu, C.; Ruan, M.; Wen, Y.; Wang, S.; Song, Y.; Li, L.; Zhou, L.; Jiang, H.; et al. High-throughput prediction of stalk cellulose and hemicellulose content in maize using machine learning and Fourier transform infrared spectroscopy. Bioresour. Technol. 2024, 413, 131531. [Google Scholar] [CrossRef]
- Ge, L.; Yao, L.; Wang, Y.; Zuo, M.; Liu, Y.; Wu, K.; Zhang, W.; Xu, C. The preparation, layered characterization and potential applications of corncob biochar. J. Anal. Appl. Pyrol. 2024, 183, 106808. [Google Scholar] [CrossRef]
- Rambhatla, N.; Panicker, T.F.; Mishra, R.K.; Manjeshwar, S.K.; Sharma, A. Biomass pyrolysis for biochar production: Study of kinetics parameters and effect of temperature on biochar yield and its physicochemical properties. Results Eng. 2025, 25, 103679. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Z.; Shao, J.; Luo, S.; Yu, D. Novel soybean dregs biochar concrete: Characterization and evaluation of the mechanical properties and microstructure. Constr. Build. Mater. 2025, 458, 139512. [Google Scholar] [CrossRef]
- Geetha, T.; Smitha, J.K.; Sebastian, M.; Litty, M.I.; Joseph, B.; Joseph, J.; Nisha, T.S. Synthesis and characterization of nano iron oxide biochar composite for efficient removal of crystal violet from water. Heliyon 2024, 10, e39450. [Google Scholar] [CrossRef]
- Chai, B.; Xiao, T.; Xiao, E.; Du, S.; Yang, S.; Yin, H.; Dang, Z.; Pan, K. Enhancing microplastics removal from soils using wheat straw and cow dung-derived biochars. J. Clean. Prod. 2024, 470, 143288. [Google Scholar] [CrossRef]
- Shan, R.; Han, J.; Gu, J.; Yuan, H.; Luo, B.; Chen, Y. A review of recent developments in catalytic applications of biochar-based materials. Resour. Conserv. Recycl. 2020, 162, 105036. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, B.; Chen, M.; Wu, P.; Lee, X.; Xing, Y. Invasive plants as potential sustainable feedstocks for biochar production and multiple applications: A review. Resour. Conserv. Recycl. 2021, 164, 1052024. [Google Scholar] [CrossRef]
- El-Bestawy, E.A.; Gaber, M.; Shokry, H.; Samy, M. Effective degradation of atrazine by spinach-derived biochar via persulfate activation system: Process optimization, mechanism, degradation pathway and application in real wastewater. Environ. Res. 2023, 229, 115987. [Google Scholar] [CrossRef]
- Hayat, W.; Zhang, Y.; Hussain, I.; Huang, S.; Du, X. Comparison of radical and non-radical activated persulfate systems for the degradation of imidacloprid in water. Ecotoxicol. Environ. Saf. 2020, 188, 109891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Huang, D.; Zhang, Y.; El Houda Bouroubi, N.; Chen, P.; Ganbold, N.; Hea, P.; Liu, J.; Fang, Y.; Gana, M.; et al. Natural mineral-derived Fe/Mn-BC as efficient peroxydisulfate activator for 2,4-dichlorophenol removal from wastewater: Performance and sustainable catalytic mechanism. J. Environ. Manage. 2023, 335, 117540. [Google Scholar] [CrossRef]
- Miserli, K.; Kogola, D.; Paraschoudi, I.; Konstantinou, I. Activation of persulfate by biochar for the degradation of phenolic compounds in aqueous systems. Chem. Eng. J. Adv. 2022, 9, 100201. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, P.; Wang, C.; Tang, J.; Sun, H. Enhancement of persulfate activation by Fe-biochar composites: Synergism of Fe and N-doped biochar. Appl. Catal. B Environ. 2022, 303, 120926. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, Y.; Guo, N.; Yang, Q. Heterogeneous activation of peroxymonosulfate by Co3O4 loaded biochar for efficient degradation of 2,4-dichlorophenoxyacetic acid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127152. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Z.; Li, H.; Zheng, K.; Luo, J.; Liu, H.; Li, Y.; Ai, L.; Wang, J.; Song, Y.; et al. MFe2O4/biochar composites in persulfate-advanced oxidation process for antibiotic treatment: A mini review. J. Water Process Eng. 2024, 68, 106535. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, H.; Zhong, Z.; Wang, A.; Xiang, M.; Xu, S.; Dong, C.; Chang, Z. Fe3O4 loaded on ball milling biochar enhanced bisphenol a removal by activating persulfate: Performance and activating mechanism. J. Environ. Manage. 2022, 319, 115661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, H.; Zhang, C.; Dawolo, E.H.; Chen, B.; Ding, N. Degradation mechanism of metronidazole using persulfate activated by boron/copper doped biochar derived from Chlorella vulgaris. Process Saf. Environ. Prot. 2024, 191, 1394–1406. [Google Scholar] [CrossRef]
- Luttah, I.; Onunga, D.O.; Shikuku, V.O.; Otieno, B.; Kowenje, C.O. Removal of endosulfan from water by municipal waste incineration fly ash-based geopolymers: Adsorption kinetics, isotherms, and thermodynamics. Front. Environ. Chem. 2023, 4, 1164372. [Google Scholar] [CrossRef]
- He, L.; Shi, Y.; Chen, Y.; Shen, S.; Xue, J.; Ma, Y.; Zheng, L.; Wu, L.; Zhang, Z.; Yang, L. Iron-manganese oxide loaded sludge biochar as a novel periodate activator for thiacloprid efficient degradation over a wide pH range. Sep. Purif. Technol. 2022, 288, 120703. [Google Scholar] [CrossRef]
- Jiang, Q.; Jiang, S.; Li, H.; Zhang, R.; Jiang, Z.; Zhang, Y. A stable biochar supported S-nZVI to activate persulfate for effective dichlorination of atrazine. Chem. Eng. J. 2022, 431, 133937. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Z.; Chen, X.; Xi, S.; Cui, K.; Li, J.; Dong, D.; Wu, F.; Wu, Z. Efficient degradation of thiamethoxam pesticide in water by iron and manganese oxide composite biochar activated persulfate. Chem. Eng. J. 2023, 473, 145051. [Google Scholar] [CrossRef]
- Yu, B.; Man, Y.; Wang, P.; Wu, C.; Xie, J.; Wang, W.; Jiang, H.; Zhang, L.; Zhang, Y.; Mao, L.; et al. Catalytic degradation of dimethomorph by nitrogen-doped rice husk biochar. Ecotoxicol. Environ. Saf. 2023, 257, 114908. [Google Scholar] [CrossRef]
- Liu, W.; Lu, Y.; Dong, Y.; Jin, Q.; Lin, H. A critical review on reliability of quenching experiment in advanced oxidation processes. Chem. Eng. J. 2023, 466, 143161. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Ding, D.; Cai, T. From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor. Appl. Catal. B Environ. 2019, 254, 312–320. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, Y.; Liu, J.; Yang, Z.; Yang, Q. Highly efficient activation of peroxymonosulfate by cobalt ferrite anchored in P-doped activated carbon for degradation of 2,4-D: Adsorption and electron transfer mechanism. J. Colloid Interface Sci. 2023, 642, 757–770. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater. Chem. Eng. J. 2019, 356, 350–358. [Google Scholar] [CrossRef]
- Lin, D.; Fu, Y.; Li, X.; Wang, L.; Hou, M.; Hu, D.; Li, Q.; Zhang, Z.; Xu, C.; Qui, S.; et al. Application of persulfate-based oxidation processes to address diverse sustainability challenges: A critical review. J. Hazard. Mater. 2022, 440, 129722. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wen, Y.; Liu, M.; Su, L.; Wang, Y.; Li, S.; Zhong, M.; Zhou, Z.; Zhou, N. Simultaneous removal of organic inorganic composite contaminants by in situ double modified biochar: Performance and mechanisms. J. Taiwan Inst. Chem. Eng. 2022, 139, 104523. [Google Scholar] [CrossRef]
- Dai, L.-Y.; Li, B.; Xu, H.-Y.; Wang, W.-S.; Zhang, S.-Q.; Xu, Y.; Qi, S.-Y.; He, X.-L.; Jin, L.-G. Magnetic nanoreactor Fe3O4@HNTs as heterogeneous Fenton-like catalyst for acid fuchsin degradation: Efficiency, kinetics and mechanism. J. Phys. Chem. Sol. 2023, 180, 111445. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Jiang, S.; Li, H.; Zhang, R.; Qu, J.; Zhang, S.; Han, W. One-step synthesis of biochar supported nZVI composites for highly efficient activating persulfate to oxidatively degrade atrazine. Chem. Eng. J. 2021, 420, 129868. [Google Scholar] [CrossRef]
- Simetić, T.; Nikić, J.; Kuč, M.; Tamindžija, D.; Tubić, A.; Agbaba, J.; Molnar Jazić, J. New Insight into the Degradation of Sunscreen Agents in Water Treatment Using UV-Driven Advanced Oxidation Processes. Processes 2024, 12, 1156. [Google Scholar] [CrossRef]
- Wu, L.; Gao, Y.; Qiu, S.; Hu, Z.; Liu, C.; Yue, C.; Zhou, J. Efficient oxidative remediation of polycyclic aromatic hydrocarbons (PAHs)-contaminated soil: A thorough comprehension of Fe-loaded biochar activated persulfate. Chemosphere 2024, 368, 143699. [Google Scholar] [CrossRef]
- Qian, W.; Deng, Y.-L.; Liu, X.-L.; Liu, H.; Ye, M.-Y.; Li, Y.-Y.; Zhang, Y.-Z.; Diao, Z.-H.; Liang, J.-L. Degradation of ofloxacin by activation of persulfate with metal-N co-doped modified peanut shell biochar: The key role of cobalt doping. J. Water Process Eng. 2025, 70, 106967. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Li, Y.; Wang, C.; Kuang, S.; Ren, P.; Xie, B. Persulfate oxidation of tetracycline, antibiotic resistant bacteria, and resistance genes activated by Fe doped biochar catalysts: Synergy of radical and non-radical processes. Chem. Eng. J. 2023, 464, 142558. [Google Scholar] [CrossRef]
- Pi, Z.; Li, X.; Wang, D.; Xu, Q.; Tao, Z.; Huang, X.; Yao, F.; Wu, Y.; He, L.; Yang, Q. Persulfate activation by oxidation biochar supported magnetite particles for tetracycline removal: Performance and degradation pathway. J. Clean. Prod. 2019, 235, 1103–1115. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Tang, L.; Wang, J.; Yu, J.; Zhang, H.; Yu, M.; Zou, J.; Xie, Q. Egg shell biochar-based green catalysts for the removal of organic pollutants by activating persulfate. Sci. Total Environ. 2020, 745, 141095. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kang, Z.; Xu, G.; Wang, J.; Yu, Y. Degradation of bensulfuron methyl by nitrogen/boron codoped biochar activated peroxydisulfate at lower temperature. J. Clean. Prod. 2023, 402, 136816. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Z.; Ifthikar, J.; Shi, L.; Du, Y.; Zhu, J.; Xi, S.; Chen, Z.; Chen, Z. Treatment of refractory contaminants by sludge-derived biochar/persulfate system via both adsorption and advanced oxidation process. Chemosphere 2017, 185, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Pan, J.; Tian, K.; Qin, J.; Qing, T.; Zhang, J. Efficient degradation of p chlorophenol by N,S-codoped biochar activated perxymonosulfate. Process Saf. Environ. Prot. 2023, 169, 437–446. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington DC, USA, 2012; ISBN 978-087553-013-0. [Google Scholar]
- Šolić, M.; Maletić, S.; Kragulj Isakovski, M.; Nikić, J.; Watson, M.; Kónya, Z.; Tricković, J. Comparing the Adsorption Performance of Multiwalled Carbon Nanotubes Oxidized by Varying Degrees for Removal of Low Levels of Copper, Nickel and Chromium (VI) from Aqueous Solutions. Water 2020, 12, 723. [Google Scholar] [CrossRef]
- SRPS EN ISO 6878; Water Quality—Determination of Phosphorus—Spectrometric Method with Ammonium Molybdate. Institute for Standardization: Belgrade, Serbia, 2008.
- SRPS H.Z.1.111; Pure Chemicals—Ammonium Acetate—Measurement of pH Value—Potentiometric Method. Institute for Standardization: Belgrade, Serbia, 1987.
- SRPS ISO H.ZI.184; Water Testing—Determination of Ammonia Content—Method Using Nessler’s Reagent. Institute for Standardization: Belgrade, Serbia, 1974.
- US EPA. Nitrogen, Kjeldahl, Total (Colorimetric, Titrimetric, Potentiometric); Method 351.3; US EPA: Washington, DC, USA, 1978.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).