Study on the Analysis of Toluene Degradation via Microwave Plasma Based on Density Functional Theory Calculations
Abstract
1. Introduction
2. Model Descriptions and Computational Methods
2.1. Model Descriptions
2.2. Computational Methods
3. Results and Discussion
3.1. The Initial Step for Toluene Degradation
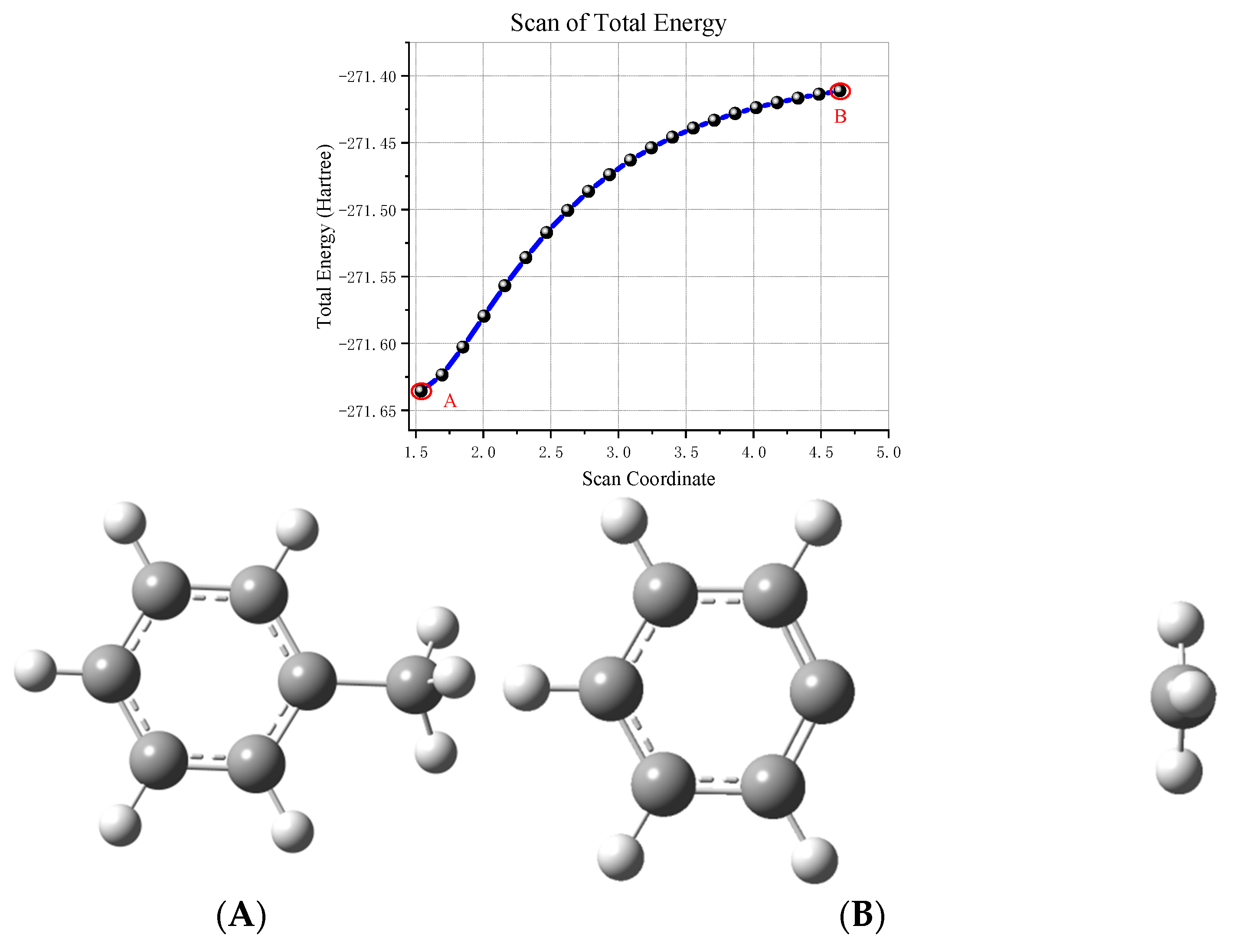
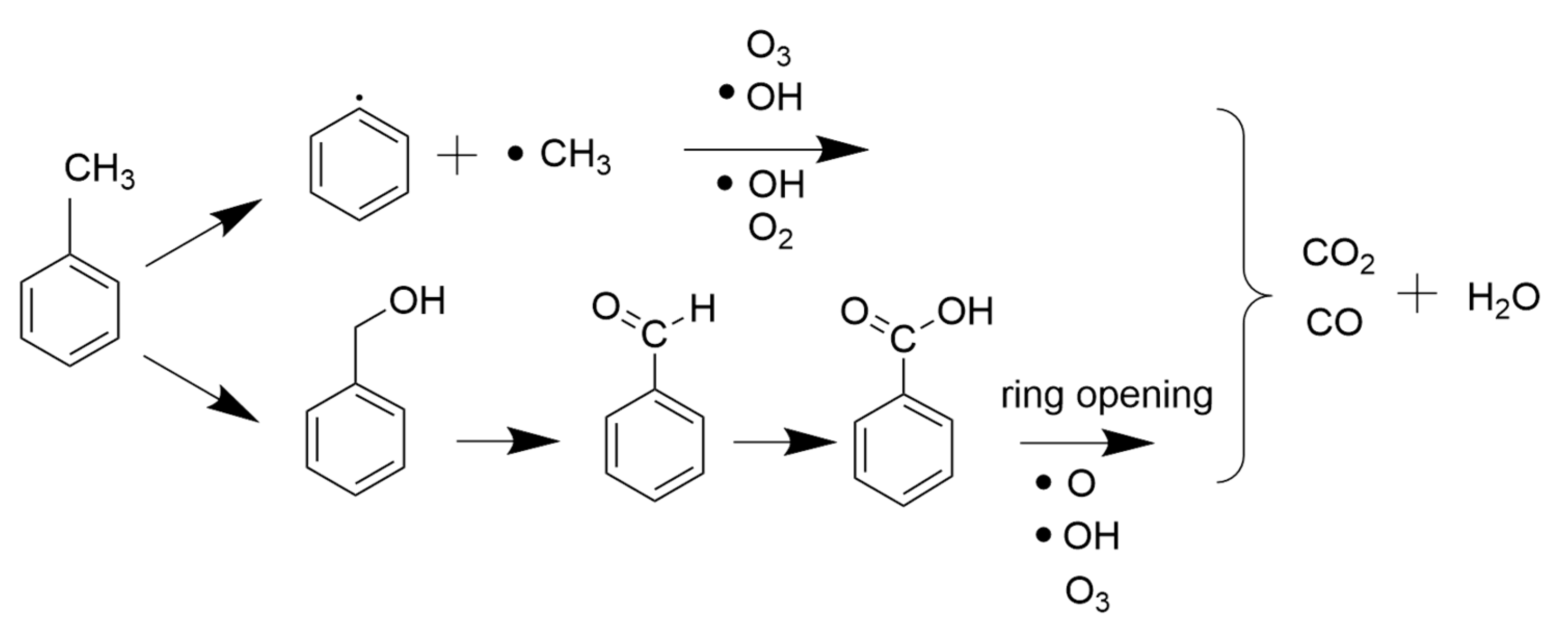
Direct Degradation Reaction of Toluene
- (1)
- Toluene cracking reaction
- (2)
- Oxidation reaction of toluene
3.2. The Subsequent Degradation of Toluene
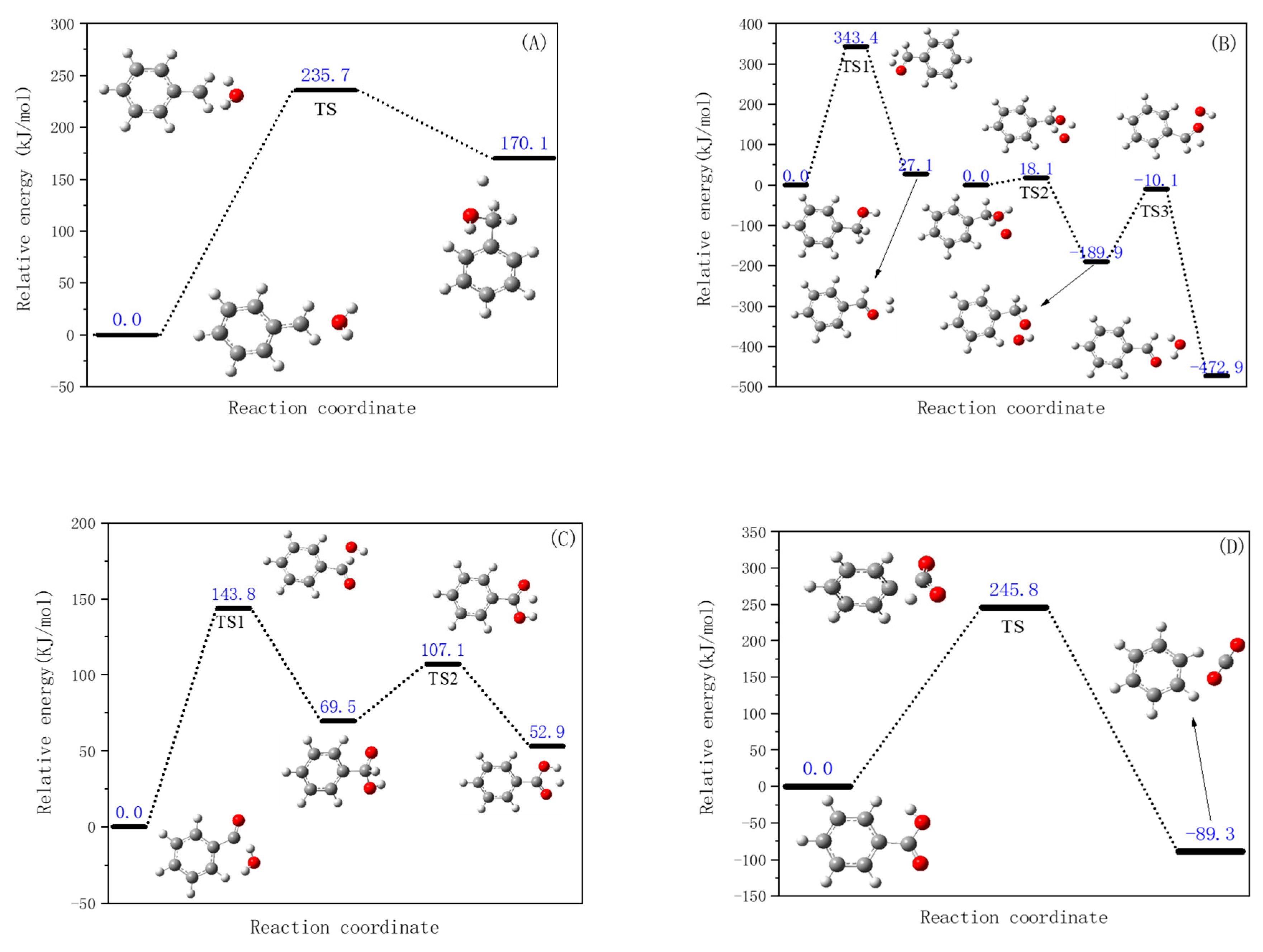
3.2.1. Direct Degradation Reaction of Organic Intermediates
- (1)
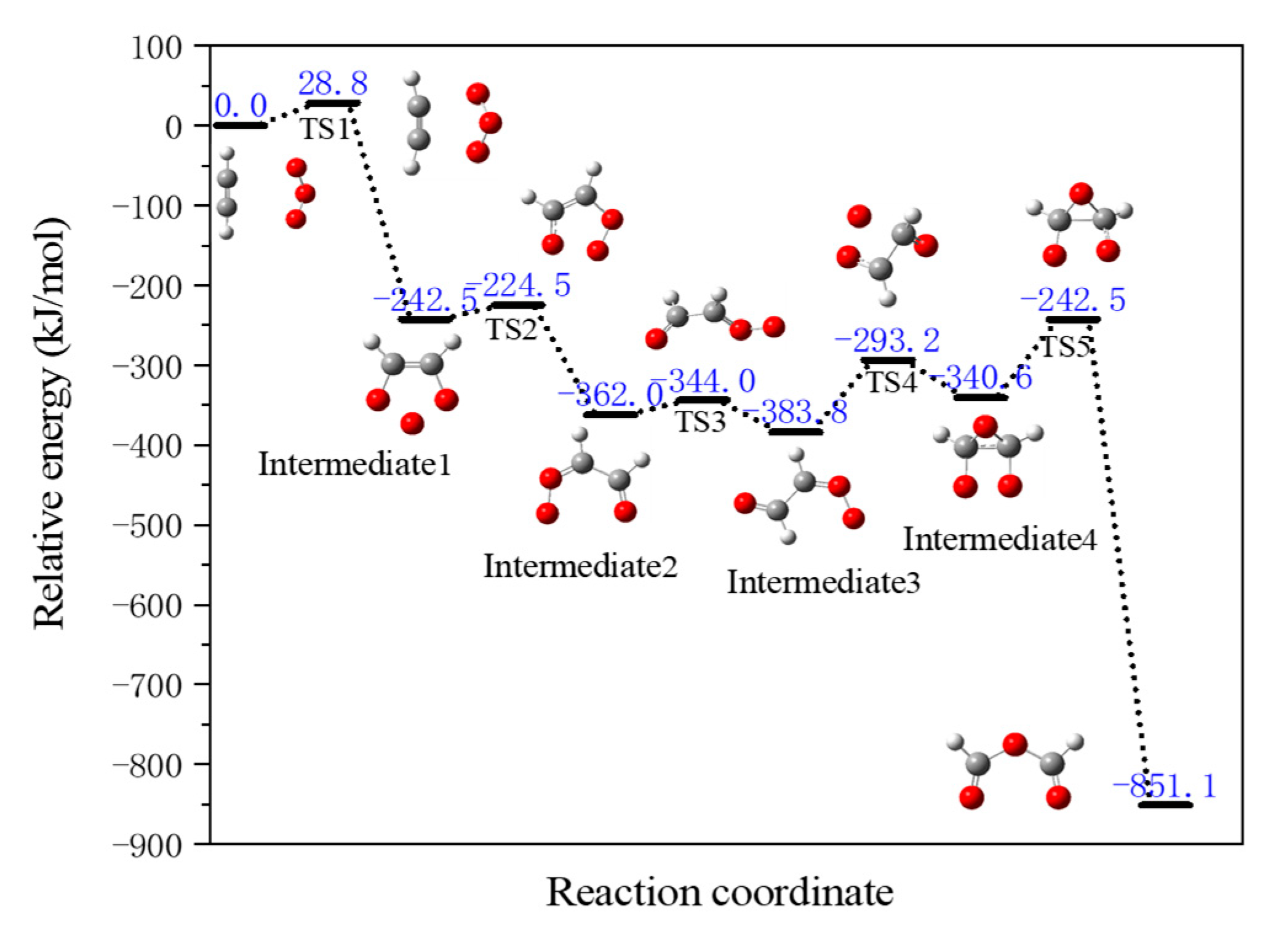
- Cleavage reaction of the benzene ring
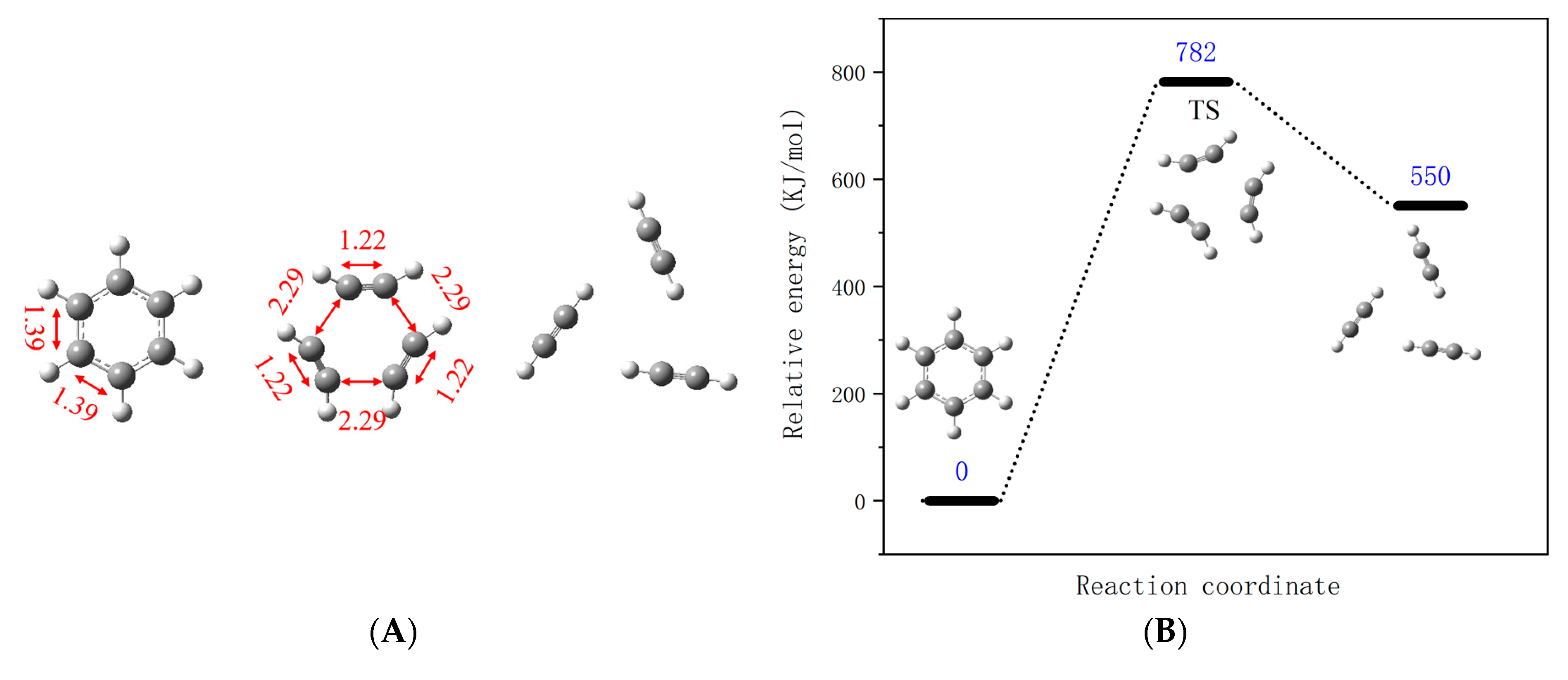
3.2.2. Free Radical Oxidation Reaction
- (1)
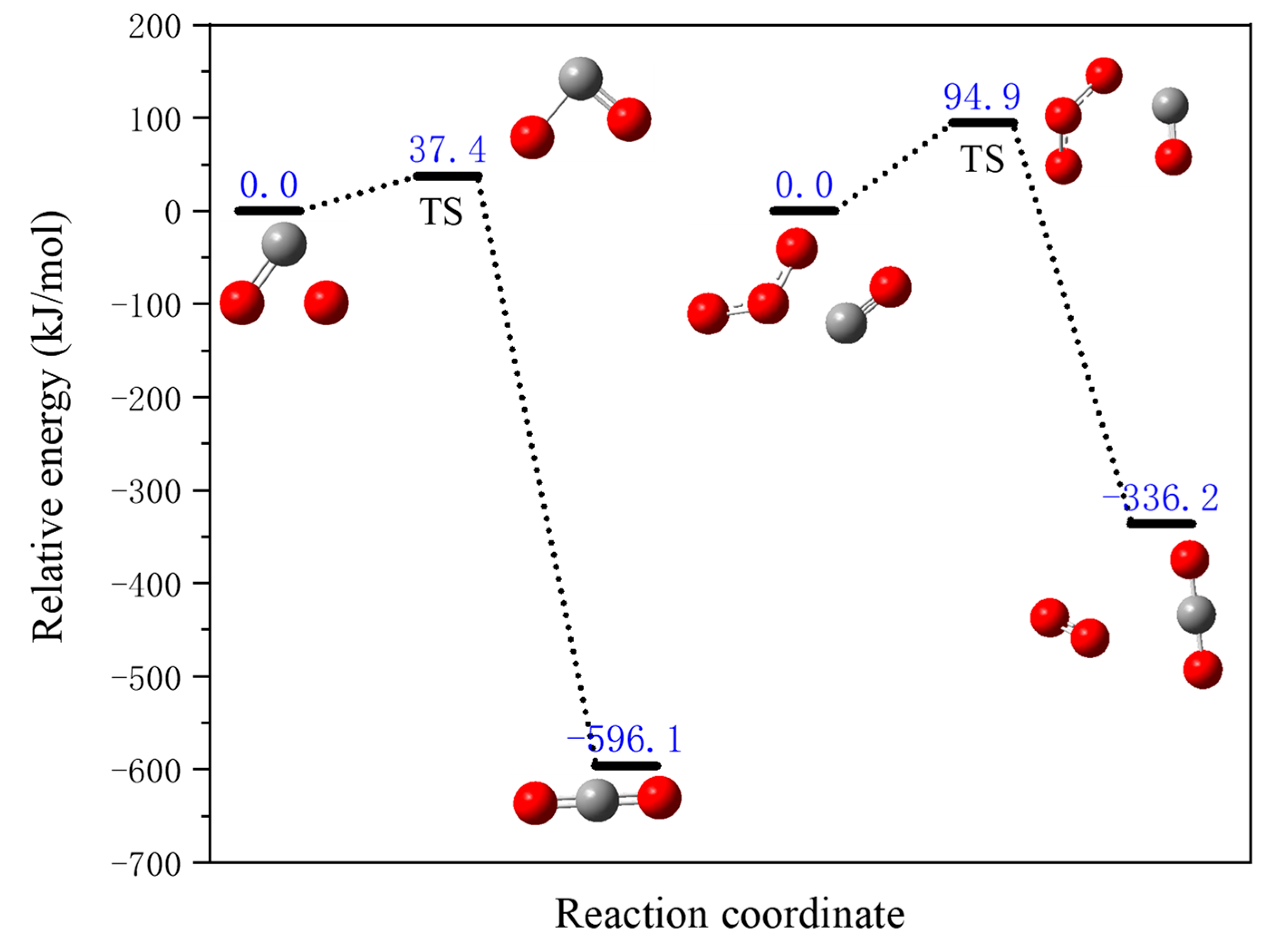
- Acetylene oxidation reaction
- (1)
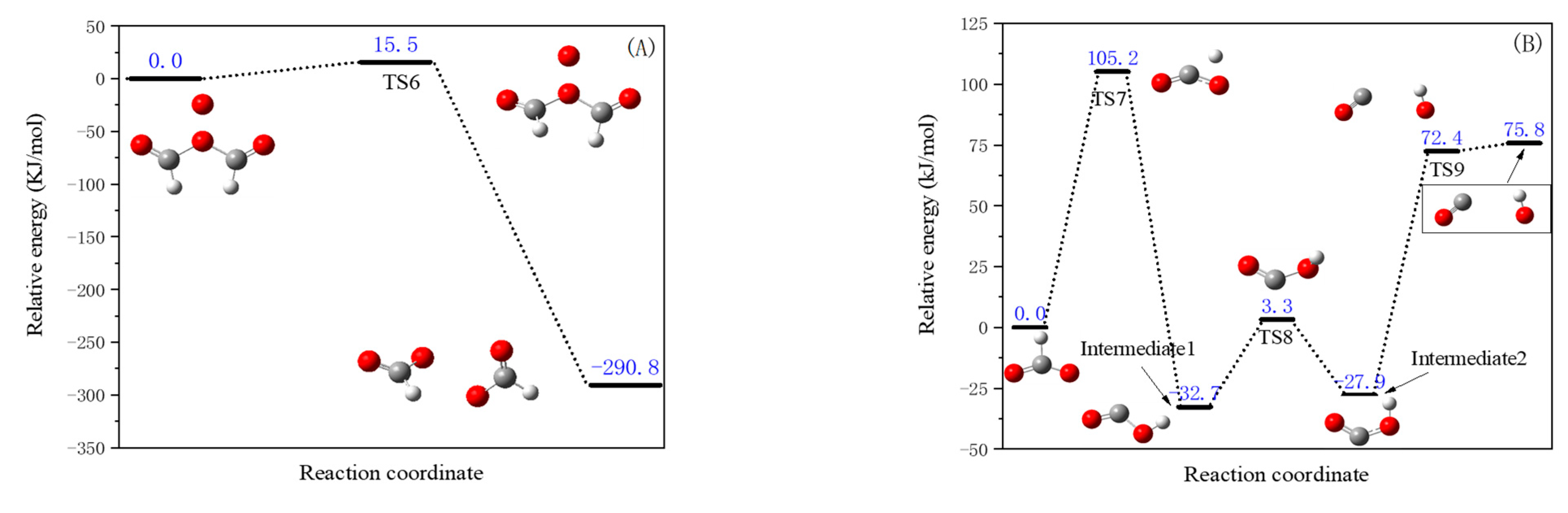
- The terminal carbon of the acetylene radical attracts O2, as shown in Figure 6A. As can be seen from Figure 6A, when the acetylene radical reacts with O2, the C atom attracts O2 to form Intermediate 1. There is no transition state in this reaction process, and the heat released is 233.9 kJ/mol. In Intermediate 1, an O atom transfer reaction occurs. After passing through the transition state TS1, O2 combines with the middle carbon to form Intermediate 2. The potential energy barrier of this reaction is 227.2 kJ/mol, and the heat released is 185.1 kJ/mol. One of the triple bonds in the alkyne of Intermediate 2 is cleaved. After passing through the transition state TS2, the middle carbon forms a C-O bond to generate Intermediate 3. The potential energy barrier of this reaction is 13.8 kJ/mol, and the heat absorbed is 5.0 kJ/mol. The conformation of the C-O bond in Intermediate 3 changes. After passing through the transition state TS3, an oxygen atom migrates to the terminal carbon atom, forming two C-O bonds to generate Intermediate 4. The potential energy barrier of this reaction is 11.2 kJ/mol, and the heat released is 97.6 kJ/mol. The C-O bond on the middle carbon of Intermediate 4 is very unstable and breaks. After passing through TS4, a terminal carbonyl group is formed, and then Product 1 is generated. The potential energy barrier of this reaction is 66.6 kJ/mol, and the heat absorbed is 92.5 kJ/mol. In addition, Intermediate 1 can also form a terminal carbonyl group through the cleavage of the O-O bond in its molecule after passing through the transition state TS11, thus generating Product 1. The potential energy barrier of this reaction is 72.4 kJ/mol.
- (2)
- The terminal carbon of the acetylene radical attracts O2, and there may be other reaction pathways, as shown in Figure 6B. The process of the reactants forming Intermediate 1 is the same as that in Figure 6A mentioned above. Intermediate 1 undergoes an O-transfer reaction. After passing through the transition state TS5, all O atoms are added to the terminal carbon to form Intermediate 5. The potential energy barrier of this reaction is 84.3 kJ/mol, and the heat absorbed is 33.6 kJ/mol. Intermediate 5 isomerizes. After passing through the transition state TS6, Intermediate 6 is formed. The potential energy barrier of this reaction is 38.2 kJ/mol, and the heat released is 463.9 kJ/mol. In addition, Intermediate 1 can directly form Intermediate 6 through an O-transfer reaction after passing through the transition state TS5. The potential energy barrier of this reaction is 84.3 kJ/mol, and the heat released is 430.3 kJ/mol. Intermediate 6 undergoes an adjacent migration of the O atom to the CC position, forming a three-membered-ring transition state TS7. The O atom continues to migrate to generate Intermediate 7. The potential energy barrier of this reaction is 255.7 kJ/mol, and the heat absorbed is 87.3 kJ/mol. Intermediate 7 undergoes an adjacent migration of the H atom to the OC position. After passing through the transition state TS8, Intermediate 8 is formed. The potential energy barrier of this reaction is 278.0 kJ/mol, and the heat absorbed is 175.1 kJ/mol. Intermediate 8 undergoes migrations of the H atom and the O atom. After passing through the transition state TS9, Intermediate 9 is generated. The potential energy barrier of this reaction is 33.8 kJ/mol, and the heat released is 0 kJ/mol. The middle C-O bond in Intermediate 9 is cleaved. After passing through the transition state TS10, Product 2, namely the formyl group and the OH radical, is generated, releasing 273.6 kJ/mol of heat. It is worth noting that the energy of the transition state TS10 is lower than that of Intermediate 9, which is inconsistent with the transition-state theory. A possible reason is that there is no transition state in the last step of the reaction, that is, TS10 does not exist, and Intermediate 9 directly cleaves into the formyl group and the OH radical. In addition, Intermediate 7 undergoes a C-C bond cleavage. After passing through the transition state TS72, Product 2 is generated. The potential energy barrier of this reaction is 34.9 kJ/mol, and the heat released is 98.5 kJ/mol.
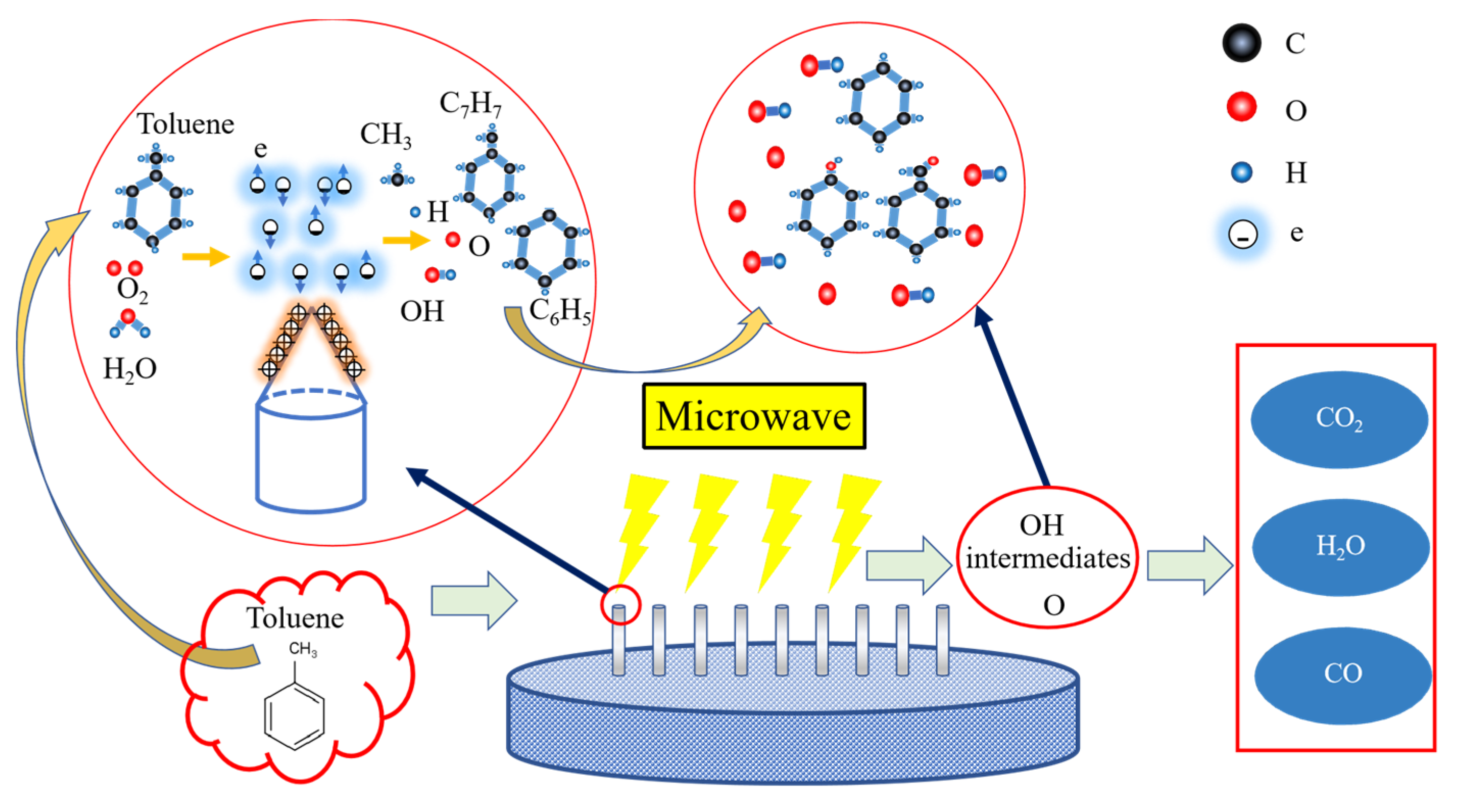
3.3. The Reaction Pathway of Toluene Degradation by Microwave Plasma
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Liu, J.; Shen, C.; Li, J.; Wang, T.; Xue, Y. Effect of Zinc Chloride-Modified Biochar with Varying Pore Structures On Vocs Inhibition and Pavement Performance of Asphalt. Constr. Build. Mater. 2025, 472, 140887. [Google Scholar] [CrossRef]
- Rehman, S.; Zheng, X.; Aujla, M.I.; Mehmood, T. Recent Advances in Adsorptive Removal of Hazardous Vocs by Metal-Organic-Framework-Based Materials. Chem. Eng. J. 2025, 505, 159257. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, H.; Wang, G. Revealing the Nonlinear Responses of Pm2.5 and O3 to Voc and Nox Emissions From Various Sources in Shandong, China. J. Hazard. Mater. 2025, 489, 137655. [Google Scholar] [CrossRef]
- Yadav, M.; Soni, K.; Soni, B.K.; Singh, N.K.; Bamniya, B.R. Source Apportionment of Particulate Matter, Gaseous Pollutants, and Volatile Organic Compounds in a Future Smart City of India. Urban Clim. 2019, 28, 100470. [Google Scholar] [CrossRef]
- Liang, W.; Wang, J.; Zhang, C.; Liu, J. Synergetic Emission Reduction of Vocs and Co2 in the Automotive Coating Industry Using Life Cycle Assessment (Lca) Technology. J. Environ. Chem. Eng. 2025, 13, 115534. [Google Scholar] [CrossRef]
- Drdova, S.; Zhao, S.; Giannakou, M.; Sivaraman, D.; Alburquerque, N.G.; Bonnin, A.; Pauer, R.; Pan, Z.; Billeter, E.; Siqueira, G.; et al. Biomimetic Light-Driven Aerogel Passive Pump for Volatile Organic Pollutant Removal. Adv. Sci. 2022, 9, 2105819. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, R.; Ge, J.; Xu, P. Vocs Absorption Using Ionic Liquids. Sep. Purif. Technol. 2025, 363, 132265. [Google Scholar] [CrossRef]
- Rostami, R.; Moussavi, G.; Jafari, A.J.; Darbari, S. A Modeling Concept On Removal of Vocs in Wire-Tube Non-Thermal Plasma, Considering Electrical and Structural Factors. Environ. Monit. Assess. 2020, 192, 280. [Google Scholar] [CrossRef]
- Panda, P.; Mahanta, R.K.; Mohanty, S.; Paikaray, R.; Das, S.P. Abatement of Gas-Phase Vocs Via Dielectric Barrier Discharge Plasmas. Environ. Sci. Pollut. Res. 2021, 28, 28666–28679. [Google Scholar] [CrossRef]
- Veerapandian, S.; Leys, C.; De Geyter, N.; Morent, R. Abatement of Vocs Using Packed Bed Non-Thermal Plasma Reactors: A Review. Catalysts 2017, 7, 113. [Google Scholar] [CrossRef]
- Nyuyki, D.; Museba, H.N.; Balue, Y.K.; Lee, B. Abatement of Volatile Organic Compounds (Vocs) and Fluorine Gases by a Microwave Plasma Torch (Mpt). Process Saf. Environ. Prot. 2025, 193, 43–53. [Google Scholar] [CrossRef]
- Perroud, H.; Miraux, J.; Lions, M.; Caillot, T.; Ferronato, C.; Kaddouri, A.; Meunier, F.C. Combustion of Volatile Organic Compounds in a Domestic Microwave Oven Using Regenerable Sorbent-Catalyst Combinations. Sep. Purif. Technol. 2024, 330, 125387. [Google Scholar] [CrossRef]
- Yamamoto, T.; Imamura, Y.; Kishida, M. Development and Combustion Characteristics of Microwave Plasma-Assisted Fluidized Bed Combustor. Adv. Powder Technol. 2023, 34, 104203. [Google Scholar] [CrossRef]
- Sanito, R.C.; You, S.-J.; Chang, G.-M.; Wang, Y.-F. Effect of Shell Powder On Removal of Metals and Volatile Organic Compounds (Vocs) From Resin in an Atmospheric-Pressure Microwave Plasma Reactor. J. Hazard. Mater. 2020, 394, 122558. [Google Scholar] [CrossRef]
- Yao, M.; Ji, Y.; Wang, H.; Ao, Z.; Li, G.; An, T. Adsorption Mechanisms of Typical Carbonyl-Containing Volatile Organic Compounds On Anatase Tio2 (001) Surface: A Dft Investigation. J. Phys. Chem. C 2017, 121, 13717–13722. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Liu, D.; Du, K. Modified Mn/Zsm-5 for Non-Thermal Plasma Mineralization of Vocs and Dft Simulation Using a Novel Y-Type Zsm-5 Model. Catalysts 2022, 12, 906. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Z.; Yi, L.; Li, Y.; Xiao, H.; Chen, D. Theoretical Study of the Interaction of Sf6 Molecule On Ag(1 1 1) Surfaces: A Dft Study. Appl. Surf. Sci. 2018, 457, 745–751. [Google Scholar] [CrossRef]
- Dai, Z.; Li, D.; Ao, Z.; Wang, S.; An, T. Theoretical Exploration of Vocs Removal Mechanism by Carbon Nanotubes through Persulfate-Based Advanced Oxidation Processes: Adsorption and Catalytic Oxidation. J. Hazard. Mater. 2021, 405, 124684. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, Z.; Jin, X.; Tian, H.; Song, Z.; Zhang, Q.; Xu, D.; Hong, R. Theoretical Investigation of Aryl/Alkyl Halide Reduction with Hydrated Electrons From Energy and Aimd Aspects. J. Mol. Model. 2023, 29, 142. [Google Scholar] [CrossRef]
- Jiang, B.; Wen, Y.; Li, Z.; Xia, D.; Liu, X. Theoretical Analysis On the Removal of Cyclic Volatile Organic Compounds by Non-Thermal Plasma. Water Air Soil Pollut. 2018, 229, 35. [Google Scholar] [CrossRef]
- Mai, J.-L.; Yang, W.-W.; Zeng, Y.; Guan, Y.-F.; Chen, S.-J. Volatile Organic Compounds (Vocs) in Residential Indoor Air During Interior Finish Period: Sources, Variations, and Health Risks. Hyg. Environ. Health Adv. 2024, 9, 100087. [Google Scholar] [CrossRef]
- Gu, S.; Guenther, A.; Faiola, C. Effects of Anthropogenic and Biogenic Volatile Organic Compounds On Los Angeles Air Quality. Environ. Sci. Technol. 2021, 55, 12191–12201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, X.; Wang, C.; Zhou, H. Environmental and Human Health Impacts of Volatile Organic Compounds: A Perspective Review. Chemosphere 2023, 313, 137489. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Malekmohammadi, B.; Tajalli, S. Interaction of Benzene, Toluene, Ethylbenzene, and Xylene with Human’S Body: Insights Into Characteristics, Sources and Health Risks. J. Hazard. Mater. Adv. 2024, 16, 100459. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, W.; Wang, Y.; Sun, J.; Zhang, C.; Shahzad, Q.; Mao, Y.; Zhao, X.; Song, Z. Experimental Study of Destruction of Acetone in Exhaust Gas Using Microwave-Induced Metal Discharge. Sci. Total Environ. 2018, 645, 788–795. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, W.; Wang, Y.; Sun, J.; Zhang, C.; Mao, Y.; Zhao, X.; Song, Z. Experimental Study on In-Situ Decomposition of Vocs Using Microwave-Induced Metal Discharge. Waste Biomass Valorization 2019, 10, 3921–3929. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Leys, C.; Van Langenhove, H. Combining Non-Thermal Plasma with Heterogeneous Catalysis in Waste Gas Treatment: A Review. Appl. Catal. B Environ. 2008, 78, 324–333. [Google Scholar] [CrossRef]
- Oda, T.; Takahashi, T.; Yamaji, K. Tce Decomposition by the Non-Thermal Plasma Process Concerning Ozone Effect. IEEE Trans. Ind. Appl. 2004, 40, 1249–1256. [Google Scholar] [CrossRef]
- Abdulaziz, F.; Jamal, A.; Faizi, M.S.H.; Peedikakkal, A.M.P.; Hussain, A.; Al-Busaidi, I.J.; Dege, N.; Alenezi, K.M.; Haque, A. Experimental and Theoretical Studies of Pyrazole-4-Carbaldehyde Derivatives by X-Ray Crystallography, Dft, Molecular Docking, and Molecular Dynamics Simulation. J. Mol. Struct. 2025, 1321, 139796. [Google Scholar] [CrossRef]
- Pandit, S.; Dash, M.R. In Pursuit of Novel Pyriporphyrin—A Porphyrin Ring Expansion Congener Containing a Built-in Pyridine Moiety by Ch Radical: A Dft Investigation. Struct. Chem. 2023, 34, 1457–1468. [Google Scholar] [CrossRef]
- Luo, Y. Comprehensive Handbook of Chemical Bond Energies; China Science Publishing & Media Ltd.: Beijing, China, 2005. [Google Scholar]
- Sun, J.; Wang, Q.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y.; Ma, C. Novel Treatment of a Biomass Tar Model Compound Via Microwave-Metal Discharges. Fuel 2017, 207, 121–125. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, D.-G.; Chen, F.; Zhan, X. The Decomposition of Aromatic Hydrocarbons During Coal Pyrolysis in Hydrogen Plasma: A Density Functional Theory Study. Int. J. Hydrogen Energy 2012, 37, 18040–18049. [Google Scholar] [CrossRef]
- Kashiwa, K.; Kitahara, T.; Arai, M.; Kobayashi, Y. Benzene Pyrolysis and Pm Formation Study Using a Flow Reactor. Fuel 2018, 230, 185–193. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, W.; Sun, J.; Fu, L.; Song, Z.; Zhao, X.; Mao, Y. Microwave-Induced Electrical Discharge of Metal Strips for the Degradation of Biomass Tar. Energy 2017, 126, 42–52. [Google Scholar] [CrossRef]
- Li, L.; Tan, Y.; Sun, J.; Zhang, Y.; Zhang, L.; Deng, Y.; Cai, D.; Song, Z.; Zou, G.; Bai, Y. Characteristics and Kinetic Analysis of Pyrolysis of Forestry Waste Promoted by Microwave-Metal Interaction. Energy 2021, 232, 121095. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Q.; Wang, W.; Wang, K. Plasma Catalytic Steam Reforming of a Model Tar Compound by Microwave-Metal Discharges. Fuel 2018, 234, 1278–1284. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E.; Crehuet, R.; Anglada, J.; Gräfenstein, J. The Ozone–Acetylene Reaction: Concerted Or Non-Concerted Reaction Mechanism? A Quantum Chemical Investigation. Chem. Phys. Lett. 2001, 347, 268–276. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Du, P.; Ma, Y.; Zhuang, Z.; Ma, X. Study on the Analysis of Toluene Degradation via Microwave Plasma Based on Density Functional Theory Calculations. Processes 2025, 13, 1824. https://doi.org/10.3390/pr13061824
Feng Y, Du P, Ma Y, Zhuang Z, Ma X. Study on the Analysis of Toluene Degradation via Microwave Plasma Based on Density Functional Theory Calculations. Processes. 2025; 13(6):1824. https://doi.org/10.3390/pr13061824
Chicago/Turabian StyleFeng, Yukun, Pengzhou Du, Yang Ma, Zhaoyi Zhuang, and Xiaoxu Ma. 2025. "Study on the Analysis of Toluene Degradation via Microwave Plasma Based on Density Functional Theory Calculations" Processes 13, no. 6: 1824. https://doi.org/10.3390/pr13061824
APA StyleFeng, Y., Du, P., Ma, Y., Zhuang, Z., & Ma, X. (2025). Study on the Analysis of Toluene Degradation via Microwave Plasma Based on Density Functional Theory Calculations. Processes, 13(6), 1824. https://doi.org/10.3390/pr13061824






