Optimization of Immobilization, Characterization, and Environmental Applications of Laccases from Pycnoporus sanguineus UEM-20
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Isolation, Taxonomic Identification, and Molecular Identification of a New Isolate Strain
2.3. Culture Conditions for Laccase Production and Enzyme Extraction
2.4. Standard Laccase Assay
2.5. Partial Purification and Concentration of Laccase
2.6. Electrophoretic Analysis
2.7. Immobilization of Laccase by Cross-Linked Enzyme Aggregates Using Response Surface Methodology
| Run | X1 | X2 | Relative Activity (%) |
|---|---|---|---|
| 1 | −1 | −1 | 18.20 ± 2.13 |
| 2 | 1 | −1 | 42.78 ± 1.61 |
| 3 | −1 | 1 | 53.58 ± 1.02 |
| 4 | 1 | 1 | 92.06 ± 2.31 |
| 5 | −1.41 | 0 | 12.11 ± 0.15 |
| 6 | 1.41 | 0 | 64.42 ± 3.72 |
| 7 | 0 | −1.41 | 31.94 ± 1.89 |
| 8 | 0 | 1.41 | 90.66 ± 2.46 |
| 9 | 0 | 0 | 86.52 ± 1.56 |
| 10 | 0 | 0 | 86.63 ± 0.92 |
| 11 | 0 | 0 | 87.92 ± 2.20 |
2.8. Physico-Chemical Characterization of Free and Immobilized Laccases
2.9. Determination of Kinetic Parameters
2.10. Application of Free and Immobilized Laccases in Bisphenol A (BPA) Degradation
2.11. Application of Free and Immobilized Laccases in the Decolorization of Malachite Green (MG) and Toxicity Analysis
2.12. Statistics
3. Results and Discussion
3.1. Production of Laccase by P. sanguineus UEM-20 in Solid-State Conditions
3.2. Optimization of Laccase Immobilization by Cross-Linked Enzyme Aggregates Using Response Surface Methodology
3.3. Physico-Chemical Characterization of Free and Immobilized P. sanguineus Laccase
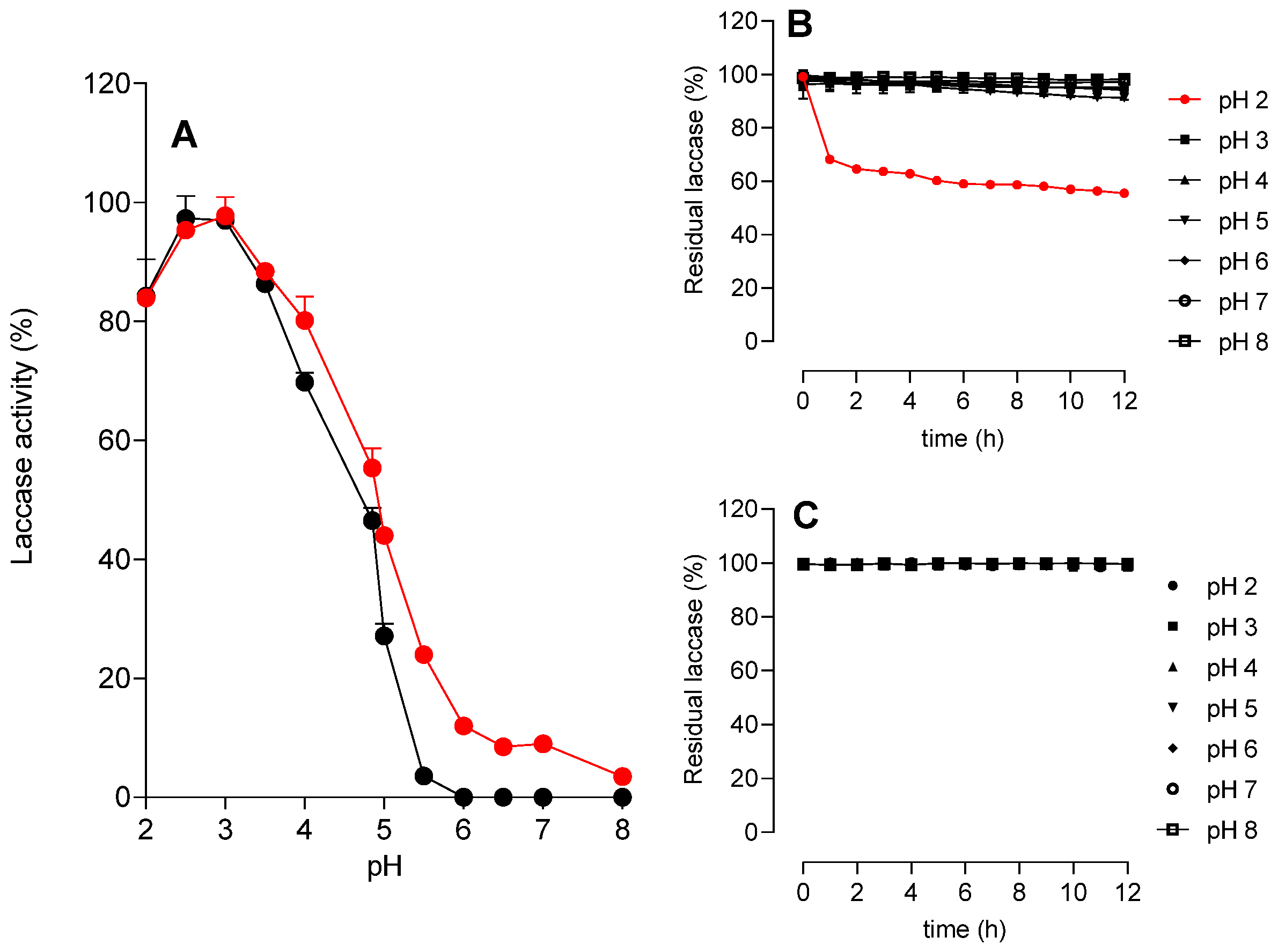
3.3.1. Effects of pH
3.3.2. Effects of Temperature
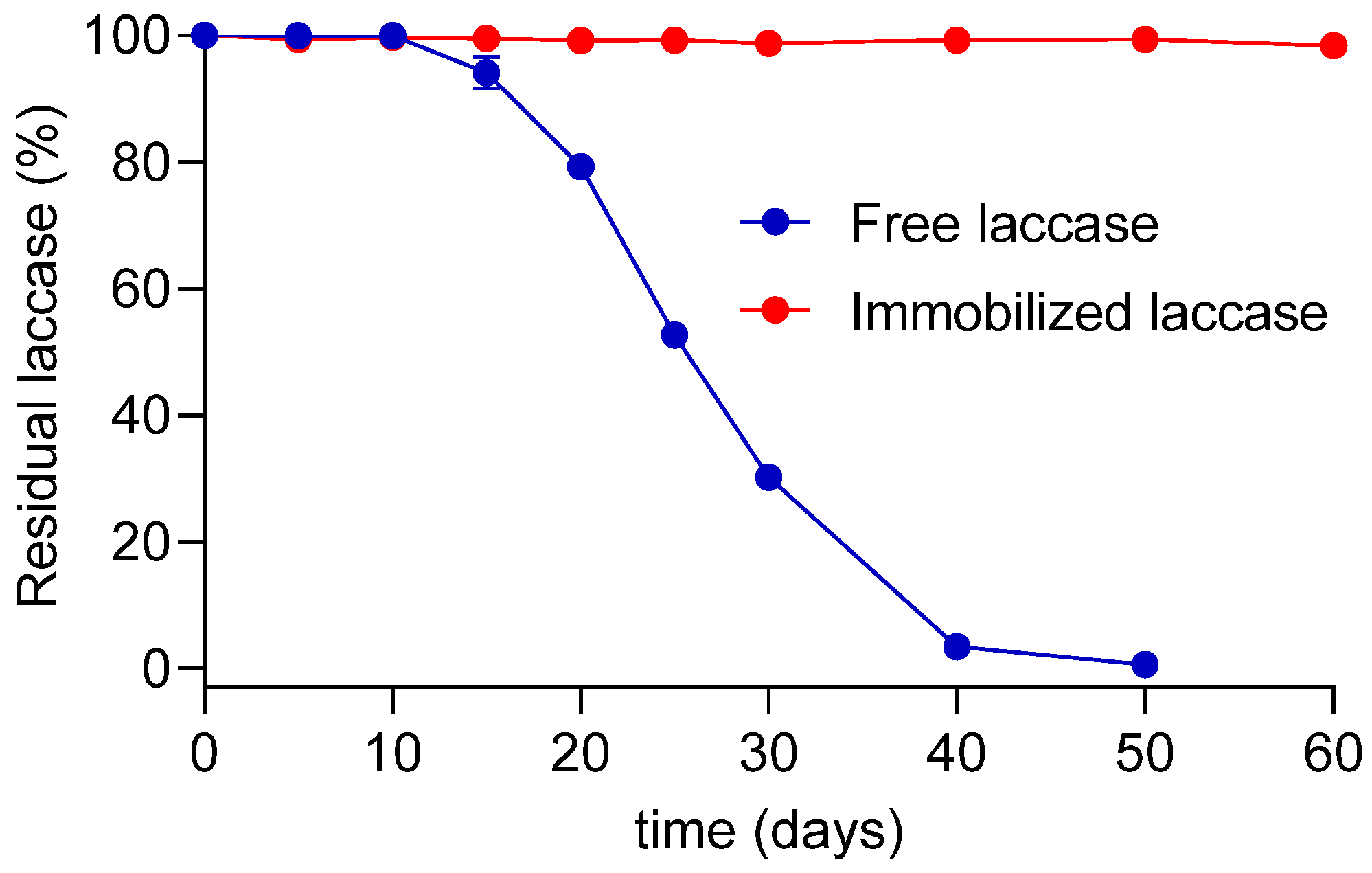
3.3.3. Storage Time
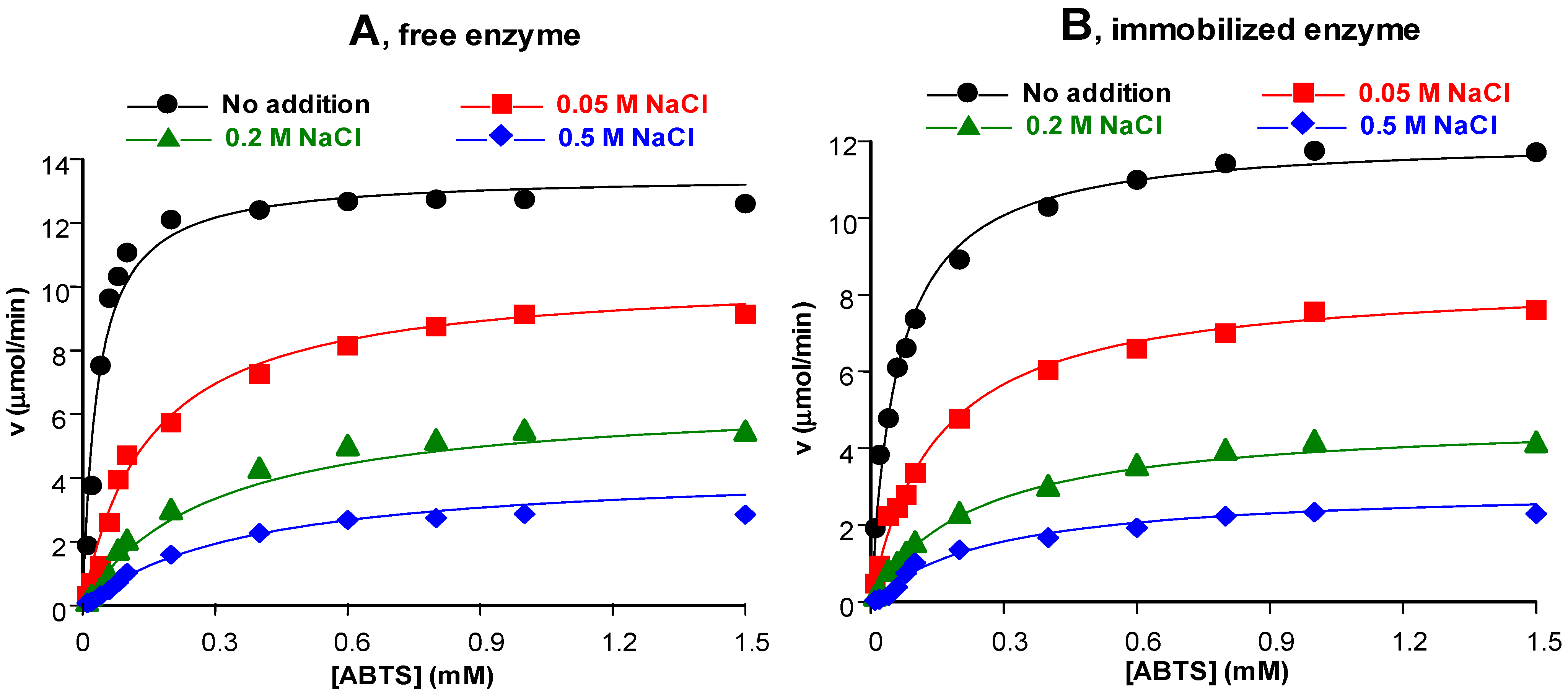
3.3.4. Influence of Salts in the Environment
3.4. Kinetic Properties
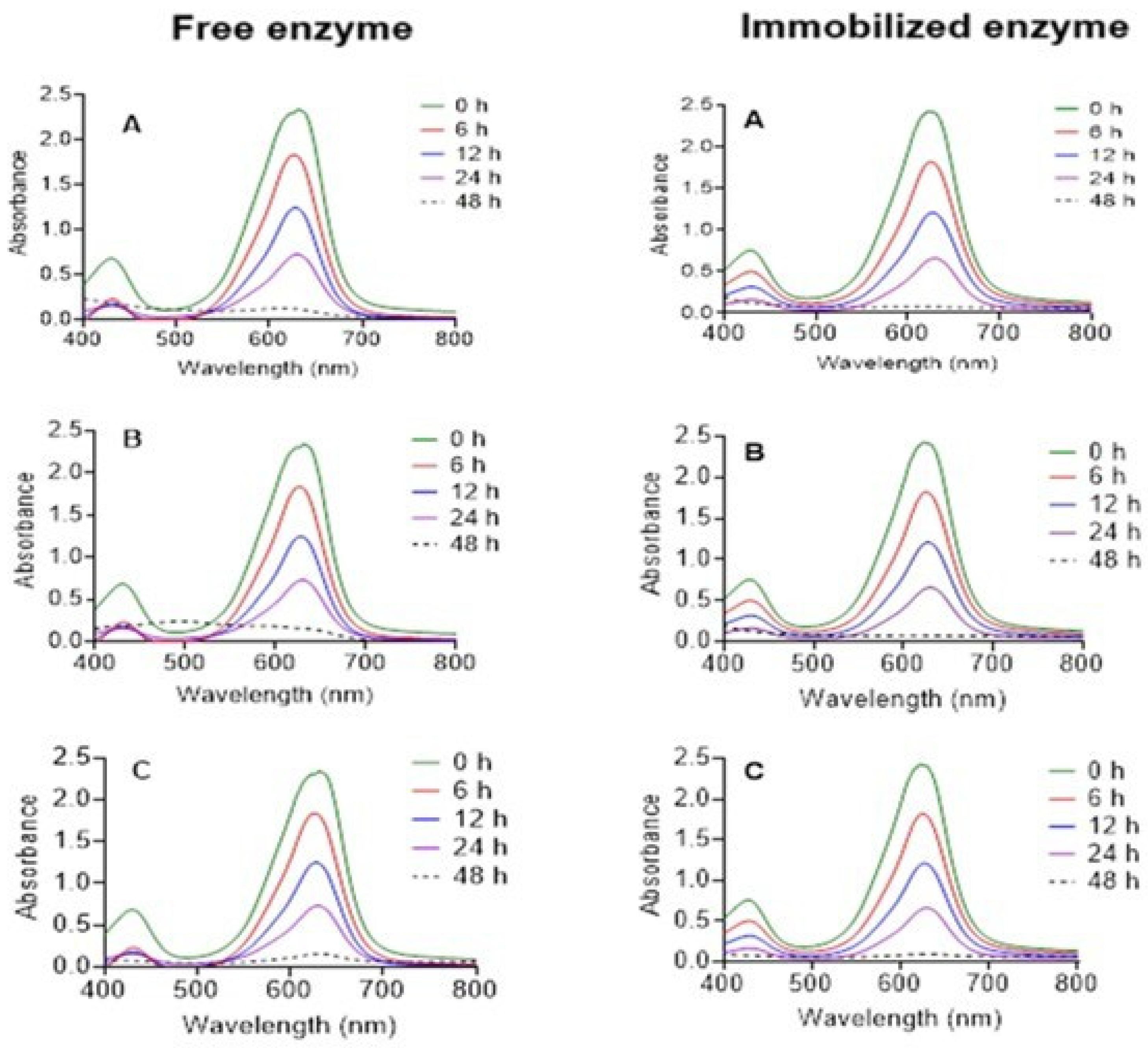
3.5. Degradation of Bisphenol A (BPA) and Malachite Green by Free and Immobilized P. sanguineus Laccases
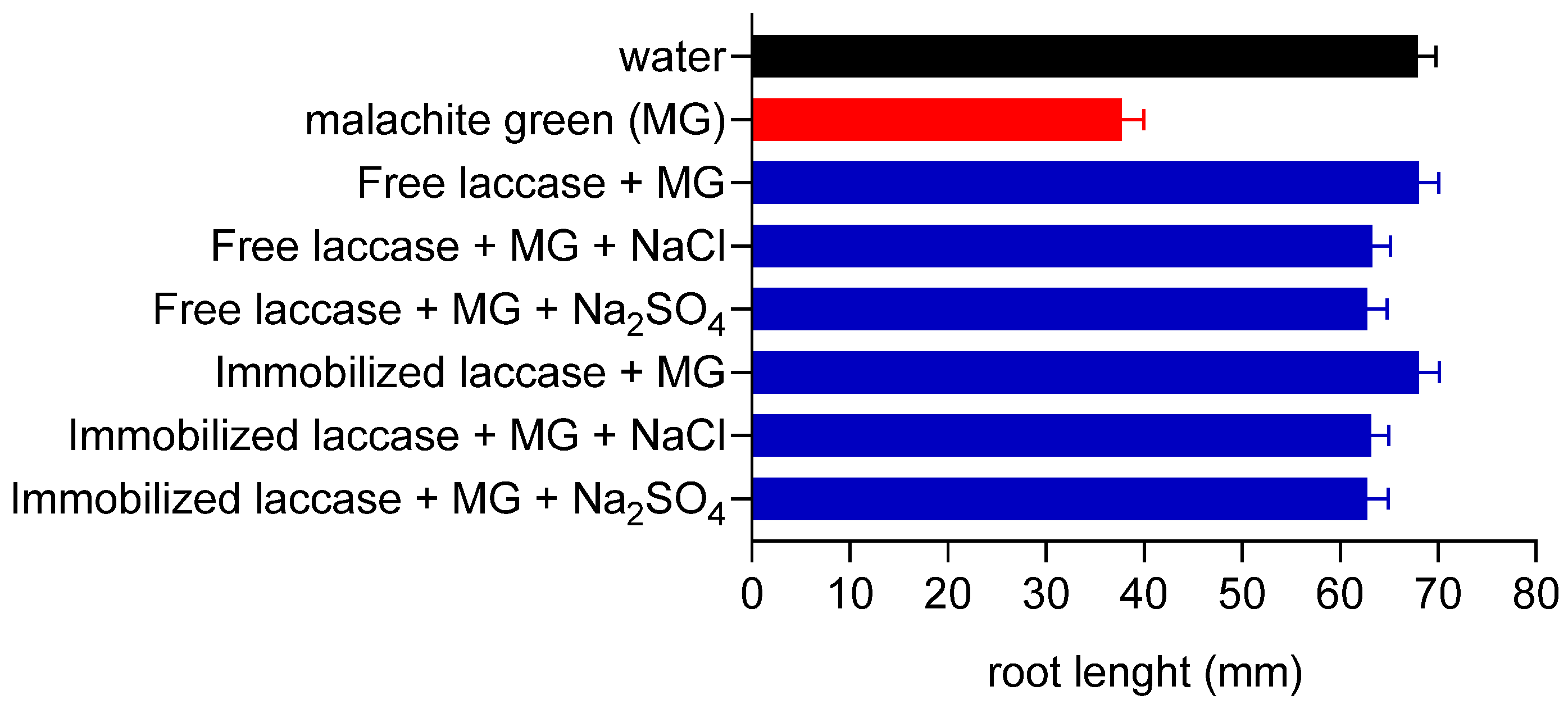
3.6. Actions of Laccase Treatment on Malachite Green Toxicity
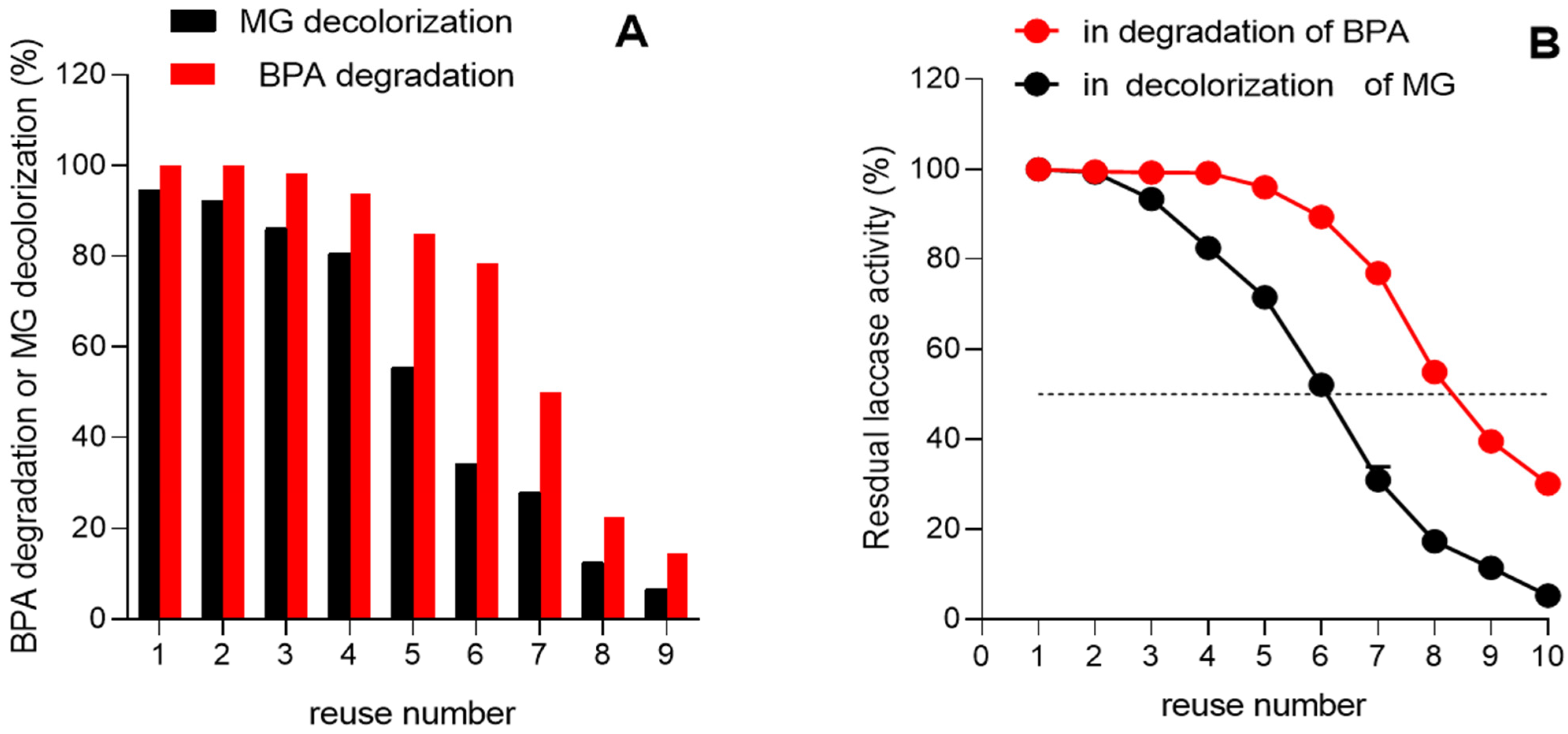
3.7. Reusability of Immobilized Laccase in the Degradation of BPA and Green Malachite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, U.N.; Singh, P.; Pandey, V.P.; Kumar, A. Structure–function relationship among bacterial, fungal and plant laccases. J. Mol. Catal. B Enzym. 2011, 68, 117–128. [Google Scholar] [CrossRef]
- Mahuri, M.; Paul, M.; Thatoi, H. A review of microbial laccase production and activity toward different biotechnological applications. Syst. Microbiol. Biomanuf. 2023, 3, 533–551. [Google Scholar] [CrossRef]
- Ahmad, I.; Pal, S.; Waseem, M.; Jamal, A.; Kamal, M.A.; Ahmad, F.; Haji, E.M.; Siddiqui, S.; Singh, A.K. Catalytic insights into laccase for sustainable remediation of multifaceted pharmaceutically active micropollutants from water matrices: A state-of-art review. J. Water Process. Eng. 2025, 70, 106901. [Google Scholar] [CrossRef]
- Murrill, W.A. The Polyporaceae of North America—VIII. Hapalopilus, Pycnoporus, and New Monotypic Genera. Bull. Torrey Bot. Club 1904, 31, 415–428. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.; Orona-Navar, C.; García-Morales, R.; Hernandez-Luna, C.; Parra, R.; Mahlknecht, J.; Ornelas-Soto, N. Biotransformation Kinetics of Pharmaceutical and Industrial Micropollutants in Groundwaters by a Laccase Cocktail from Pycnoporus sanguineus CS43 Fungi. Int. Biodeterior. Biodegrad. 2016, 108, 34–41. [Google Scholar] [CrossRef]
- Yang, J.; Xu, X.; Yang, X.; Ye, X.; Lin, J. Cross-Linked Enzyme Aggregates of Cerrena Laccase: Preparation, Enhanced NaCl Tolerance and Decolorization of Remazol Brilliant Blue Reactive. J. Taiwan Inst. Chem. Eng. 2016, 65, 1–7. [Google Scholar] [CrossRef]
- Garcia-Morales, R.; Rodríguez-Delgado, M.; Gomez-Mariscal, K.; Orona-Navar, C.; Hernandez-Luna, C.; Torres, E.; Ornelas-Soto, N. Biotransformation of Endocrine-Disrupting Compounds in Groundwater: Bisphenol A, Nonylphenol, Ethynylestradiol and Triclosan by a Laccase Cocktail from Pycnoporus sanguineus CS43. Water Air Soil Pollut. 2015, 226, 1–14. [Google Scholar] [CrossRef]
- Cheute, V.M.S.; Uber, T.M.; dos Santos, L.F.O.; Backes, E.; Dantas, M.P.; Contato, A.G.; Castoldi, R.; de Souza, C.G.M.; Corrêa, R.C.G.; Bracht, A.; et al. Biotransformation of Pollutants by Pycnoporus spp. in Submerged and Solid-State Fermentation: Mechanisms, Achievements, and Perspectives. Biomass 2024, 4, 313–328. [Google Scholar] [CrossRef]
- Ramírez-Cavazos, L.I.; Junghanns, C.; Ornelas-Soto, N.; Cárdenas-Chávez, D.L.; Hernández-Luna, C.; Demarche, P.; Parra, R. Purification and Characterization of Two Thermostable Laccases from Pycnoporus sanguineus and Potential Role in Degradation of Endocrine Disrupting Chemicals. J. Mol. Catal. B Enzym. 2014, 108, 32–42. [Google Scholar] [CrossRef]
- Brugnari, T.; Pereira, M.G.; Bubna, G.A.; de Freitas, E.N.; Contato, A.G.; Corrêa, R.C.G.; Castoldi, R.; de Souza, C.G.M.; Polizeli, M.L.T.M.; Bracht, A.; et al. A highly reusable MANAE-agarose-immobilized Pleurotus ostreatus laccase for degradation of bisphenol A. Sci. Total. Environ. 2018, 634, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Veena, R.; Samuel, M.S.; Selvarajan, E. Immobilization of Laccases and Applications for the Detection and Remediation of Pollutants: A Review. Environ. Chem. Lett. 2021, 19, 521–538. [Google Scholar] [CrossRef]
- Ortolan, G.G.; Contato, A.G.; Aranha, G.M.; Salgado, J.C.S.; Alnoch, R.C.; Polizeli, M.L.T.M. Enhancing Laccase Production by Trametes hirsuta GMA-01 Using Response Surface Methodology and Orange Waste: A Novel Breakthrough in Sugarcane Bagasse Saccharification and Synthetic Dye Decolorization. Reactions 2024, 5, 635–650. [Google Scholar] [CrossRef]
- Uber, T.M.; Pateis, V.O.; Cheute, V.M.S.; dos Santos, L.F.O.; Trindade, A.R.F.; Contato, A.G.; dos Santos Filho, J.R.; Corrêa, R.C.G.; Castoldi, R.; de Souza, C.G.M.; et al. Immobilization of Trametes versicolor Laccase by Interlinked Enzyme Aggregates with Improved pH Stability and Its Application in the Degradation of Bisphenol A. Reactions 2025, 6, 9. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Mateos-Díaz, J.C.; Favela-Torres, E. Cross-Linked Enzyme Aggregates (CLEA) in Enzyme Improvement—A Review. Biocatalysis 2016, 1, 166–177. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Noreen, S.; Shah, S.Z.H.; Bharagava, R.N.; Iqbal, H.M.N. Modifying Bio-Catalytic Properties of Enzymes for Efficient Biocatalysis: A Review from Immobilization Strategies Viewpoint. Biocatal. Biotransform. 2019, 37, 159–182. [Google Scholar] [CrossRef]
- Mota, T.R.; Kato, C.G.; Peralta, R.A.; Bracht, A.; de Morais, G.R.; Baesso, M.L.; Peralta, R.M. Decolourization of Congo Red by Ganoderma lucidum Laccase: Evaluation of Degradation Products and Toxicity. Water Air Soil Pollut. 2015, 226, 1–11. [Google Scholar] [CrossRef]
- Uber, T.M.; Buzzo, A.J.D.R.; Scaratti, G.; Amorim, S.M.; Helm, C.V.; Maciel, G.M.; Peralta, R.M. Comparative Detoxification of Remazol Brilliant Blue R by Free and Immobilized Laccase of Oudemansiella canarii. Biocatal. Biotransform. 2020, 40, 17–28. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Patel, H.; Gupte, S.; Gahlout, M.; Gupte, A. Purification and Characterization of an Extracellular Laccase from Solid-State Culture of Pleurotus ostreatus HP-1. 3 Biotech 2013, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Brugnari, T.; Contato, A.G.; Pereira, M.G.; Freitas, E.N.D.; Bubna, G.A.; Aranha, G.M.; Peralta, R.M. Characterisation of Free and Immobilised Laccases from Ganoderma lucidum: Application on Bisphenol A Degradation. Biocatal. Biotransform. 2021, 39, 71–80. [Google Scholar] [CrossRef]
- Coelho-Moreira, J.S.; Brugnari, T.; Sá-Nakanishi, A.B.; Castoldi, R.; Souza, C.G.M.; Bracht, A.; Peralta, R.M. Evaluation of Diuron Tolerance and Biotransformation by the White-Rot Fungus Ganoderma lucidum. Fungal Biol. 2018, 122, 471–488. [Google Scholar] [CrossRef]
- Durand, A. Bioreactor Designs for Solid State Fermentation. Biochem. Eng. J. 2003, 13, 113–125. [Google Scholar] [CrossRef]
- Abu Yazid, N.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef]
- Eugenio, M.E.; Carbajo, J.M.; Martín, J.A.; González, A.E.; Villar, J.C. Laccase production by Pycnoporus sanguineus under different culture conditions. J. Basic Microbiol. 2009, 49, 433–440. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, M.; Zhang, B.B.; Yu, S.Y.; Bian, X.J.; Wang, W.; Wang, Y. Purification and Characterization of Laccase from Pycnoporus sanguineus and Decolorization of an Anthraquinone Dye by the Enzyme. Appl. Microbiol. Biotechnol. 2007, 74, 1232–1239. [Google Scholar] [CrossRef]
- Niladevi, K.N.; Sukumaran, R.K.; Prema, P. Utilization of Rice Straw for Laccase Production by Streptomyces psammoticus in Solid-State Fermentation. J. Ind. Microbiol. Biotechnol. 2007, 34, 665–674. [Google Scholar] [CrossRef]
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; de Souza Vandenberghe, L.P. Recent Developments and Innovations in Solid State Fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Zimbardi, A.L.; Camargo, P.F.; Carli, S.; Aquino Neto, S.; Meleiro, L.P.; Rosa, J.C.; Furriel, R.P. A High Redox Potential Laccase from Pycnoporus sanguineus RP15: Potential Application for Dye Decolorization. Int. J. Mol. Sci. 2016, 17, 672. [Google Scholar] [CrossRef] [PubMed]
- Iracheta-Cárdenas, M.M.; Rocha-Peña, M.A.; Galán-Wong, L.J.; Arévalo-Niño, K.; Tovar-Herrera, O.E. A Pycnoporus sanguineus Laccase for Denim Bleaching and Its Comparison with an Enzymatic Commercial Formulation. J. Environ. Manag. 2016, 177, 93–100. [Google Scholar] [CrossRef]
- Scarpa, J.D.C.P.; Marques, N.P.; Monteiro, D.A.; Martins, G.M.; de Paula, A.V.; Boscolo, M.; Bocchini, D.A. Saccharification of Pretreated Sugarcane Bagasse Using Enzymes Solution from Pycnoporus sanguineus MCA 16 and Cellulosic Ethanol Production. Ind. Crop. Prod. 2019, 141, 111795. [Google Scholar] [CrossRef]
- Fathali, Z.; Rezaei, S.; Faramarzi, M.A.; Habibi-Rezaei, M. Catalytic Phenol Removal Using Entrapped Cross-Linked Laccase Aggregates. Int. J. Biol. Macromol. 2019, 122, 359–366. [Google Scholar] [CrossRef]
- Cabana, H.; Jones, J.P.; Agathos, S.N. Preparation and Characterization of Cross-Linked Laccase Aggregates and Their Application to the Elimination of Endocrine Disrupting Chemicals. J. Biotechnol. 2007, 132, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; Kumar, M.P.; Thiruvenkadaravi, K.V.; Baskaralingam, P.; Kumar, P.S.; Sivanesan, S. Preparation and Characterization of Porous Cross Linked Laccase Aggregates for the Decolorization of Triphenyl Methane and Reactive Dyes. Bioresour. Technol. 2012, 119, 28–34. [Google Scholar] [CrossRef]
- Daâssi, D.; Rodríguez-Couto, S.; Nasri, M.; Mechichi, T. Biodegradation of Textile Dyes by Immobilized Laccase from Coriolopsis gallica into Ca-Alginate Beads. Int. Biocatal. Biotransform. 2014, 90, 71–78. [Google Scholar] [CrossRef]
- Zheng, F.; Cui, B.K.; Wu, X.J.; Meng, G.; Liu, H.X.; Si, J. Immobilization of Laccase onto Chitosan Beads to Enhance Its Capability to Degrade Synthetic Dyes. Int. Biocatal. Biotransform. 2016, 110, 69–78. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a Strategy for Improving Enzyme Properties—Application to Oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef]
- Cao, L. Immobilised Enzymes: Science or Art? Curr. Opin. Chem. Biol. 2005, 9, 217–226. [Google Scholar] [CrossRef]
- Backes, E.; Kato, C.G.; da Silva, T.B.V.; Uber, T.M.; Pasquarelli, D.L.; Bracht, A.; Peralta, R.M. Production of fungal laccase on pineapple waste and application in detoxification of malachite green. J. Environ. Sci. Health 2022, 57, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Isanapong, J.; Suwannoi, K.; Lertlattanapong, S.; Panchal, S. Purification, characterization of laccase from Pleurotus ostreatus HK35, and optimization for congo red biodecolorization using Box–Behnken design. 3 Biotech 2024, 14, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Songulashvili, G.; Flahaut, S.; Demarez, M.; Tricot, C.; Bauvois, C.; Debaste, F.; Penninckx, M.J. High yield production in seven days of Coriolopsis gallica 1184 laccase at 50 L scale; enzyme purification and molecular characterization. Fungal Biol. 2016, 120, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, A.K.; Mikkelsen, J.D.; Meyer, A.S. Structure, functionality and tuning up of laccases for lignocellulose and other industrial applications. Crit. Rev. Biotechnol. 2016, 36, 70–86. [Google Scholar] [CrossRef]
- Kuddus, M.; Joseph, B.; Ramteke, P.W. Production of laccase from newly isolated Pseudomonas putida and its application in bioremediation of synthetic dyes and industrial effluents. Biocatal. Agric. Biotechnol. 2013, 2, 333–338. [Google Scholar] [CrossRef]
- Xu, K.; Huo, Y.; Tang, S.; Han, S.; Lin, Y.; Zheng, S. A novel laccase for alkaline medium temperature environments in the textile industry. Biotechnol. J. 2024, 19, e2400383. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcaide, M. Laccase: A multi-purpose biocatalyst at the forefront of biotechnology. Microb. Biotechnol. 2017, 10, 1457–1467. [Google Scholar] [CrossRef]
- Gonzalez-Coronel, L.A.; Cobas, M.; Rostro-Alanis, M.J.; Parra-Saldívar, R.; Hernandez-Luna, C.; Pazos, M.; Ángeles Sanromán, M. Immobilization of laccase of Pycnoporus sanguineus CS43. New Biotechnol. 2017, 39, 141–149. [Google Scholar] [CrossRef]
- Asgher, M.; Kamal, S.; Iqbal, H.M. Improvement of Catalytic Efficiency, Thermo-stability and Dye Decolorization Capability of Pleurotus ostreatus IBL-02 laccase by Hydrophobic Sol Gel Entrapment. Chem. Cent. J. 2012, 6, 110. [Google Scholar] [CrossRef]
- Lettera, V.; Pezzella, C.; Cicatiello, P.; Piscitelli, A.; Giacobelli, V.G.; Galano, E.; Amoresano, A.; Sannia, G. Efficient immobilization of a fungal laccase and its exploitation in fruit juice clarification. Food Chem. 2016, 196, 1272–1278. [Google Scholar] [CrossRef]
- Wlizło, K.; Polak, J.; Jarosz-Wilkołazka, A.; Pogni, R.; Petricci, E. Novel textile dye obtained through transformation of 2-amino-3-methoxybenzoic acid by free and immobilised laccase from a Pleurotus ostreatus strain. Enzym. Microb. Technol. 2020, 132, 109398. [Google Scholar] [CrossRef] [PubMed]
- Almulaiky, Y.Q.; Alkabli, J.; El-Shishtawy, R.M. Improving enzyme immobilization: A new carrier-based magnetic polymer for enhanced covalent binding of laccase enzyme. Int. J. Biol. Macromol. 2024, 282, 137362. [Google Scholar] [CrossRef] [PubMed]
- Backes, E.; Alnoch, R.C.; Contato, A.G.; Castoldi, R.; Souza, C.G.M.; Kato, C.G.; Peralta, R.A.; Moreira, R.F.P.M.; Polizeli, M.L.T.M.; Bracht, A.; et al. Properties and Kinetic Behavior of Free and Immobilized Laccase from Oudemansiella canarii: Emphasis on the Effects of NaCl and Na2SO4 on Catalytic Activities. Int. J. Biol. Macromol. 2024, 281, 136565. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, J.L.; Vallejos, S.; Trigo-López, M.; García, J.M.; Busto, M.D. Optimization and stability of a reusable laccase-polymer hybrid film for the removal of bisphenol A in water. Environ. Technol. Innov. 2025, 38, 104093. [Google Scholar] [CrossRef]
- Zilly, A.; da Silva Coelho-Moreira, J.; Bracht, A.; De Souza, C.G.M.; Carvajal, A.E.; Koehnlein, E.A.; Peralta, R.M. Influence of NaCl and Na2SO4 on the Kinetics and Dye Decolorization Ability of Crude Laccase from Ganoderma lucidum. Int. Biodeterior. Biodegrad. 2011, 65, 340–344. [Google Scholar] [CrossRef]
- Botts, J.; Morales, M. Analytical Description of the Effects of Modifiers and of Enzyme Multivalency upon the Steady State Catalyzed Reaction Rate. Trans. Faraday Soc. 1953, 49, 696–707. [Google Scholar] [CrossRef]
- Baici, A. Kinetics of Enzyme-Modifier Interactions. In The General Modifier Mechanism; Springer: Berlin, Germany, 2015; pp. 65–102. [Google Scholar] [CrossRef]
- Pan, Z.; Song, C.; Li, L.; Wang, H.; Pan, Y.; Wang, C.; Feng, X. Membrane Technology Coupled with Electrochemical Advanced Oxidation Processes for Organic Wastewater Treatment: Recent Advances and Future Prospects. Chem. Eng. J. 2019, 376, 120909. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of Antibiotics by Advanced Oxidation Processes: An Overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Ghobadi Nejad, Z.; Borghei, S.M.; Yaghmaei, S. Kinetic Studies of Bisphenol A in Aqueous Solutions by Enzymatic Treatment. Int. J. Environ. Sci. Technol. 2019, 16, 821–832. [Google Scholar] [CrossRef]
- Lassouane, F.; Aït-Amar, H.; Rodriguez-Couto, S. High BPA removal by immobilized crude laccase in a batch fluidized bed bioreactor. Biochem. Eng. J. 2022, 184, 108489. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, P.; Saharan, V.; Kapoor, R.K. Simultaneous Laccase Production and Transformation of Bisphenol-A and Triclosan Using Trametes versicolor. 3 Biotech 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Himanshu; Behera, B.; Kumari, N.; Maruthi, M.; Singh, R.K.; Saini, J.K. Appraisal of malachite green biodegradation and detoxification potential of laccase from Trametes cubensis. Bioresour. Technol. 2025, 417, 131869. [Google Scholar] [CrossRef] [PubMed]
- Thoa, L.T.K.; Thao, T.T.P.; Hung, N.B.; Khoo, K.S.; Quang, H.T.; Lan, T.T.; Hoang, V.D.; Park, S.-M.; Ooi, C.W.; Show, P.L.; et al. Biodegradation and Detoxification of Malachite Green Dye by Extracellular Laccase Expressed from Fusarium oxysporum. Waste Biomass-Valorization 2022, 13, 2511–2518. [Google Scholar] [CrossRef]
- Enaud, E.; Trovaslet, M.; Naveau, F.; Decristoforo, A.; Bizet, S.; Vanhulle, S.; Jolivalt, C. Laccase chloride inhibition reduction by an anthraquinonic substrate. Enzym. Microb. Technol. 2011, 49, 517–525. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef]
- Culp, S.J.; Beland, F.A. Malachite green: A toxicological review. J. Am. Coll. Toxicol. 1996, 15, 219–238. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Malachite green “a cationic dye” and its removal from aqueous solution by adsorption. Appl. Water Sci. 2017, 7, 3407–3445. [Google Scholar] [CrossRef]
- He, J.; Mo, P.; Luo, Y.-S.; Yang, P.-H. Strategies for solving the issue of malachite green residues in aquatic products: A review. Aquac. Res. 2023, 2023, 578570. [Google Scholar] [CrossRef]
- Raeder, U.; Broda, P. Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1985, 1, 17–20. [Google Scholar] [CrossRef]
- Pamphile, J.A.; Azevedo, J.L. Molecular characterization of endophytic strains of Fusarium verticillioides. World J. Microbiol. Biotechnol. 2002, 18, 391–396. [Google Scholar] [CrossRef]
- Van Den Ende, A.H.G.; De Hoog, G.S. Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana. Stud. Mycol. 1999, 43, 151–162. [Google Scholar]
- Justo, A.; Miettinen, O.; Floudas, D.; Ortiz-Santana, B.; Sjökvist, E.; Lindner, D.; Nakasone, K.; Niemelã, T.; Larsson, K.-H.; Ryvarden, L.; et al. A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol. 2017, 121, 798–824. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]






| Coded Values | Actual Values | |
|---|---|---|
| X1 (%, wt/v) | X2 (mM) | |
| 1.41 | 78.2 | 170.5 |
| 1 | 70.0 | 150.0 |
| 0 | 50.0 | 100.0 |
| −1 | 30.0 | 50.0 |
| −1.41 | 21.8 | 29.5 |
| Factor | Effect | Standard Error | T (5) | p-Value |
|---|---|---|---|---|
| Mean | 87.0119 | 1.306560 | 66.5962 | <0.000001 |
| Linear | ||||
| X1 | 34.3050 | 1.602607 | 21.4057 | 0.000004 |
| X2 | −47.9839 | 1.912327 | −25.0919 | 0.000002 |
| Quadratic | ||||
| X1 | 41.9872 | 1.602607 | 26.1993 | 0.000002 |
| X2 | −24.8078 | 1.912327 | −12.9725 | 0.000049 |
| Interactive | ||||
| X1 X2 | 6.9527 | 2.263055 | 3.0722 | 0.027713 |
| Source | SS | df | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Regression Residual | 9314.1982 25.6071 | 5 5 | 1862.840 5.121 | 363.735 | 0.000002 |
| Lack of fit Pure error | 24.395 1.212 | 3 2 | 8.132 0.606 | 13.419 | 0.070145 |
| Total | 9339.805 | 10 |
| Factor | Optimal Condition | Predicted (%) | Experimental (%) | Relative Error (%) |
|---|---|---|---|---|
| X1 | 0.423 (58.46%, wt/v) | 100.14 | 98.79 ± 0.33 | 1.22 |
| X2 | 0.905 (145.27 mM) |
| Parameter | Free Enzyme | Immobilized Enzyme |
|---|---|---|
| V1 (=Vmax) (µmol/min) | 13.49 ± 0.25 * | 12.09 ± 0.12 * |
| V2 (µmol/min) | 1.81 ± 0.53 * | 1.21 ± 0.19 * |
| KM (mM) | 0.0328 ± 0.0029 * | 0.0602 ± 0.0026 * |
| Ki1 (M) | 0.00968 ± 0.00133 * | 0.0187 ± 0.00728 * |
| Ki2 (M) | 0.138 ± 0.024 * | 0.0987 ± 0.00728 * |
| Correlation | 0.993 | 0.998 |
| MSC | 4.165 | 5.438 |
| Parameter | Free Enzyme | Immobilized Enzyme |
|---|---|---|
| V1 (=Vmax) (µmol/min) | 13.57 ± 0.25 * | 12.21 ± 0.21 * |
| V2 (µmol/min) | 5.45 ± 0.87 | 6.77 |
| KM (mM) | 0.0321 ± 0.0029 * | 0.0602 ± 0.0043 * |
| Ki1 (M) | 0.167 ± 0.049 | 0.746 |
| Ki2 (M) | 0.246 ± 0.065 | 1.63 |
| Correlation | 0.988 | 0.995 |
| MSC | 3.479 | 4.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheute, V.M.S.; Backes, E.; Pateis, V.d.O.; de Oliveira Junior, V.A.; Uber, T.M.; dos Santos Filho, J.R.; dos Santos, L.F.O.; Castoldi, R.; de Souza, C.G.M.; Polonio, J.C.; et al. Optimization of Immobilization, Characterization, and Environmental Applications of Laccases from Pycnoporus sanguineus UEM-20. Processes 2025, 13, 1800. https://doi.org/10.3390/pr13061800
Cheute VMS, Backes E, Pateis VdO, de Oliveira Junior VA, Uber TM, dos Santos Filho JR, dos Santos LFO, Castoldi R, de Souza CGM, Polonio JC, et al. Optimization of Immobilization, Characterization, and Environmental Applications of Laccases from Pycnoporus sanguineus UEM-20. Processes. 2025; 13(6):1800. https://doi.org/10.3390/pr13061800
Chicago/Turabian StyleCheute, Vinícius Mateus Salvatori, Emanueli Backes, Vanesa de Oliveira Pateis, Verci Alves de Oliveira Junior, Thaís Marques Uber, José Rivaldo dos Santos Filho, Luís Felipe Oliva dos Santos, Rafael Castoldi, Cristina Giatti Marques de Souza, Julio Cesar Polonio, and et al. 2025. "Optimization of Immobilization, Characterization, and Environmental Applications of Laccases from Pycnoporus sanguineus UEM-20" Processes 13, no. 6: 1800. https://doi.org/10.3390/pr13061800
APA StyleCheute, V. M. S., Backes, E., Pateis, V. d. O., de Oliveira Junior, V. A., Uber, T. M., dos Santos Filho, J. R., dos Santos, L. F. O., Castoldi, R., de Souza, C. G. M., Polonio, J. C., Contato, A. G., Bracht, A., & Peralta, R. M. (2025). Optimization of Immobilization, Characterization, and Environmental Applications of Laccases from Pycnoporus sanguineus UEM-20. Processes, 13(6), 1800. https://doi.org/10.3390/pr13061800











