1. Introduction
Coffee is an agricultural product with a high global consumption, in which Vietnam, Brazil, and Colombia are the leading exporters [
1]. According to the International Coffee Organization, in September 2022, exports increased to 9.95 million bags, with Colombia being one of the main protagonists worldwide [
2]. Hence, the high production of coffee brings a large amount of waste, usually used for one of the following activities: (i) fertilizer for crops, (ii) disposal as solid waste on farms, or (iii) incineration.
Once the coffee is collected from the plants, a process begins before it can be consumed. This process is divided into the following stages: (i) Pulped consists of removing the coffee’s soft part (cherry), generally accompanied by washing, resulting in high-water consumption; (ii) Once the cherry is removed from the coffee bean, a drying process is conducted to achieve a moisture percentage of around 10%. In Colombia, this process is carried out in the sun in structures called heldas; (iii) Once dry, the coffee is transported to places where it is roasted at temperatures close to 200 °C. During this process, the coffee’s physical and chemical properties are modified, giving it its characteristic flavor at the end; (iv) It is transported and packaged for distribution.
Based on the high amount of waste generated during the coffee harvest, the option of using biomass waste as a raw material for the production of chemicals of commercial interest has been evaluated [
3]. To use biomass waste, which is composed mainly of three fractions: cellulose, hemicellulose, and lignin, as a raw material, the following treatments must be applied: mechanical, physical, and chemical treatments. These treatments allow the biomass to be fractionated and to access monomeric sugars, which are starting molecules for obtaining products of industrial value [
4].
Thus, furfural is one of the products of interest that can be obtained from coffee waste. Furfural is a vital chemical obtained from agricultural or forestry residues and is rich in polymers of five-carbon sugars [
5]. In the same way, furfural is one of the platform chemicals obtained from biomass that can be converted into various useful products used in pharmaceutical, fine chemical, petroleum refining, agrochemical, biofuels, and polymer industries [
6].
Usually, furfural is obtained from biomass using strong mineral acids. Specifically, sulfuric acid hydrolyzes biomass and dehydrates xylose to furfural. Even though this process generates excellent yields, it has several drawbacks: (i) high production of acidic wastewater and corrosion of equipment; (ii) difficulty separating the product of interest; and (iii) high formation of byproducts [
7]. Therefore, the use of solid catalytic materials to obtain furfural from xylose has been proposed. The proposed catalytic process involves two steps: the isomerization of xylose to xylulose mediated by Lewis acid sites; and the dehydration of xylulose to furfural attributed to Brönsted acid sites [
8].
Reports have utilized solid materials as catalysts for this purpose, where furfural yields are around 53% using two
at 160 °C for 4 h, suggesting the importance of the catalysts’ acid sites [
9]. Likewise, using iron catalytic materials supported on activated carbon, gave furfural in around 57% yield [
10].
Thus, this research initially characterized the residual biomass of coffee cultivation in terms of moisture content, ash, and total solids. Furthermore, the percentage of cellulose, hemicellulose, and lignin was determined. Subsequently, hydrothermal treatment was applied to the biomass to evaluate the generation of xylose and furfural. Finally, catalytic iron materials were assessed for the dehydration of xylose commercial and the xylose obtained from the hydrolysates of coffee residues, thus determining the conversion percentages, selectivity, and yield.
In the case of the catalytic materials evaluated, mesoporous iron catalysts supported in silica were prepared and characterized using the sol–gel method. During the synthesis, we evaluated two iron charges, 0.5 and 1.5 wt%, and two calcination temperatures, 450 and 750 °C, and the catalytic conditions were labeled as follows: 0.5Fe-Si-450, 0.5Fe-Si-750, 1.5Fe-Si-450, and 1.5Fe-Si-750. In the proposed catalyst, the iron species act as Lewis acid sites, and in order to identify their role in the catalyzed dehydration process to convert xylose to furfural we evaluated two different loads. Furthermore, the calcination temperature influenced the type of iron species that formed the reaction mixture. When the materials were calcined at 750 °C, iron oxides type hematite with was produced. Therefore, this work successfully obtains a platform molecule, furfural, from agricultural coffee residues using solid acid catalysts.
2. Materials and Methods
2.1. Biomass Material
The residual coffee biomass was collected from a Puente Nacional, Colombia private farm. The percentage of humidity was determined following the NREL/TP-510-42621 standard proposed by the National Renewable Energy Laboratory [
11]. As with the dry biomass, the percentage of ash was determined following the NREL/TP-510-42622 protocol proposed by the National Renewable Energy Laboratory [
12]. Finally, cellulose, hemicellulose, and lignin were determined following the NREL/TP-510-42618 protocol proposed by the National Renewable Energy Laboratory [
13].
2.2. Hydrothermal Treatment of Biomass
The hydrothermal treatments applied to the residual coffee biomass were carried out in a stainless steel reactor, designed and built by our research group, located in Bogotá, Colombia, under a nitrogen atmosphere. In a typical test, 5.00 g of biomass and 100 mL of water were loaded into the reactor. Subsequently, the treatment temperature was programmed to 170 or 190 °C, and the agitation began at 500 rpm; once the programmed temperature was reached, the run time was started for 30 or 60 min. When the treatment was finished, the reactor was cooled for one hour to room temperature and then placed into an ice bath. Finally, the solid was separated, and the hydrolysate was analyzed by high performance liquid chromatography (HPLC) (Agilent Colombia-Bogota) to quantify xylose and furfural.
2.3. Catalysts Preparation
The iron catalysts supported in silicon oxide were prepared according to the procedure described in another article from our research group [
14]. All reagents used were of analytical grade and obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Tetraethyl orthosilicate (TEOS 98%) and iron acetate (99%) were used as precursors for silicon and iron, respectively. Oxalic acid (99%) was used as a structure-directing agent. For the synthesis of the catalysts, a defined mass of oxalic acid was mixed in ethanol and stirred vigorously to obtain a homogeneous solution. A total of 0.5 and 1.5 wt% of the iron precursor material for the purpose of obtaining iron charges was dissolved in ethanol. These two solutions were added to another container containing TEOS. Finally, water was added in a ratio of 1/1/4 TEOS/oxalic acid/water. Once the solution was formed, the gelation process was carried out at 70 °C for 7 h with a stirring rate of 300 rpm. Then, the materials were dried at 105 °C for 12 h and calcined at 450 or 750 °C for 6 h.
2.4. Characterization of Catalysts
X-ray diffraction patterns were obtained on a Rigaku diffractometer (Matsubara-cho, 3-9-12 Akishima-shi, Tokyo, 196-8666 Japan) with a Cu anode and a nominal current tube voltage of 45 kV. Data were collected at room temperature between 10° and 90° with a geometry of 2θ. The infrared spectra (FTIR) were recorded on a Nicolet FTIR iS10 spectrophotometer. The spectra were determined in the 400–4000 cm−1 spectral range. Transmission spectra were obtained from the samples diluted with KBr; the tablet was prepared with around 1 mg of the solid sample and 200 mg of pure KBr. All the spectra were recorded at a resolution of 2 cm−1.
Raman spectra were obtained using a Thermo Scientific Raman microscope (Agilent, Bogota, Colombia) with 540 nm excitation from a diode laser through a 10× objective. The scattered radiation from the sample was collected at right angles to the laser beam and was directed to a detector with a photodiode array of thermoelectric cooled to −48 °C. Adsorption–desorption isotherms were carried out in a Quantachrome Autosorb sorptometer (Agilent, Bogota, Colombia), employing N2 as the adsorbent. The samples (200 mg) were degassed for six hours at 200 °C before analysis. The specific surface area was calculated using the standard Brunauer–Emmett–Teller (BET) method based on the adsorption data in a relative pressure range of 0.07 < p/p0 < 0.3. Finally, the physical appearance of the catalysts was examined using scanning electron microscopy (SEM) with Jeol equipment, JSM7100F a 10,000×.
2.5. Catalytic Conversion of Xylose and Coffee Waste to Furfural
The catalytic tests were conducted in a batch reactor of stainless steel and under a nitrogen atmosphere. In a typical procedure, 100 mL of aqueous xylose solution and 100 mg of catalyst were placed inside the reactor. The catalytic system was purged with nitrogen three times, and the reaction mixture was rapidly heated to 170 or 190 °C under 500 rpm stirring. When the programmed temperature was reached, the zero time is taken, and the reaction was continued for 30, 60, or 120 min. Once the reaction was completed, the reactor was cooled for one hour to room temperature and then placed in an ice bath. Finally, the catalyst was separated, and the liquid was analyzed by high performance liquid chromatography (HPLC) to quantify the xylose and furfural content. The same procedure was performed with the hydrolysates from the biomass treatment. Thus, 100 mL of the hydrolysate obtained from the hydrothermal treatment and 100 mg of catalyst was placed inside the reactor. The temperature and agitation were programmed. Once the reaction was finished, the catalyst was separated from the liquid, which was used to quantify the xylose and furfural content by HPLC.
2.6. Xylose and Furfural Quantification
After the reaction was completed, the aqueous phase was filtered, and the concentration of furfural present was quantified using chromatographic equipment SHIMADZU Prominence-i LC2030, equipped with a UV detector and an Optiacua PUR 100 C18 column of 3 μm. The detection and quantification of furfural was developed at 275 nm, using an internal standard furfuryl alcohol that was absorbed at 210 nm. A RID 20 detector and a chromatographic column VDSpher® PUR 100 HILIC-SAC were used to quantify xylose, using inositol as an internal standard.
3. Results and Analysis
3.1. Coffee Waste Biomass
The residual biomass of coffee used is from the pulping process or pulping of the fruit. Thus,
Table 1 presents the results of the percentage of moisture, ash, cellulose, hemicellulose, and lignin compared with the results obtained by other authors.
The results show that the reported values have the same tendency as those obtained in the present work. However, the differences presented were attributed to three main aspects: (i) the separation of each of the residual components of coffee, (ii) the variety of coffee used, and (iii) the particular cultivation conditions. For this specific case, our interest focuses on the amount of hemicellulose since it is a polymer composed of pentoses, the raw material for obtaining furfural [
17].
Variability in biomass composition could be attributed to various factors that influence its structural and chemical properties. Among these, climatic and geographical conditions play a crucial role, since variables such as temperature, humidity, and altitude can affect the accumulation of structural compounds in the biomass. Likewise, the species or variety of coffee studied may present significant differences in its composition due to genetic and metabolic variations specific to each type of plant [
18].
Other factors that could contribute to these differences include the growing conditions and agricultural practices, such as soil type, nutrient availability, and fertilization methods, all of which can influence the relative proportions of biomass components [
19].
Once the biomass was characterized, hydrothermal treatments were applied to evaluate the influence of temperature and time on the amount of xylose and furfural. Thus,
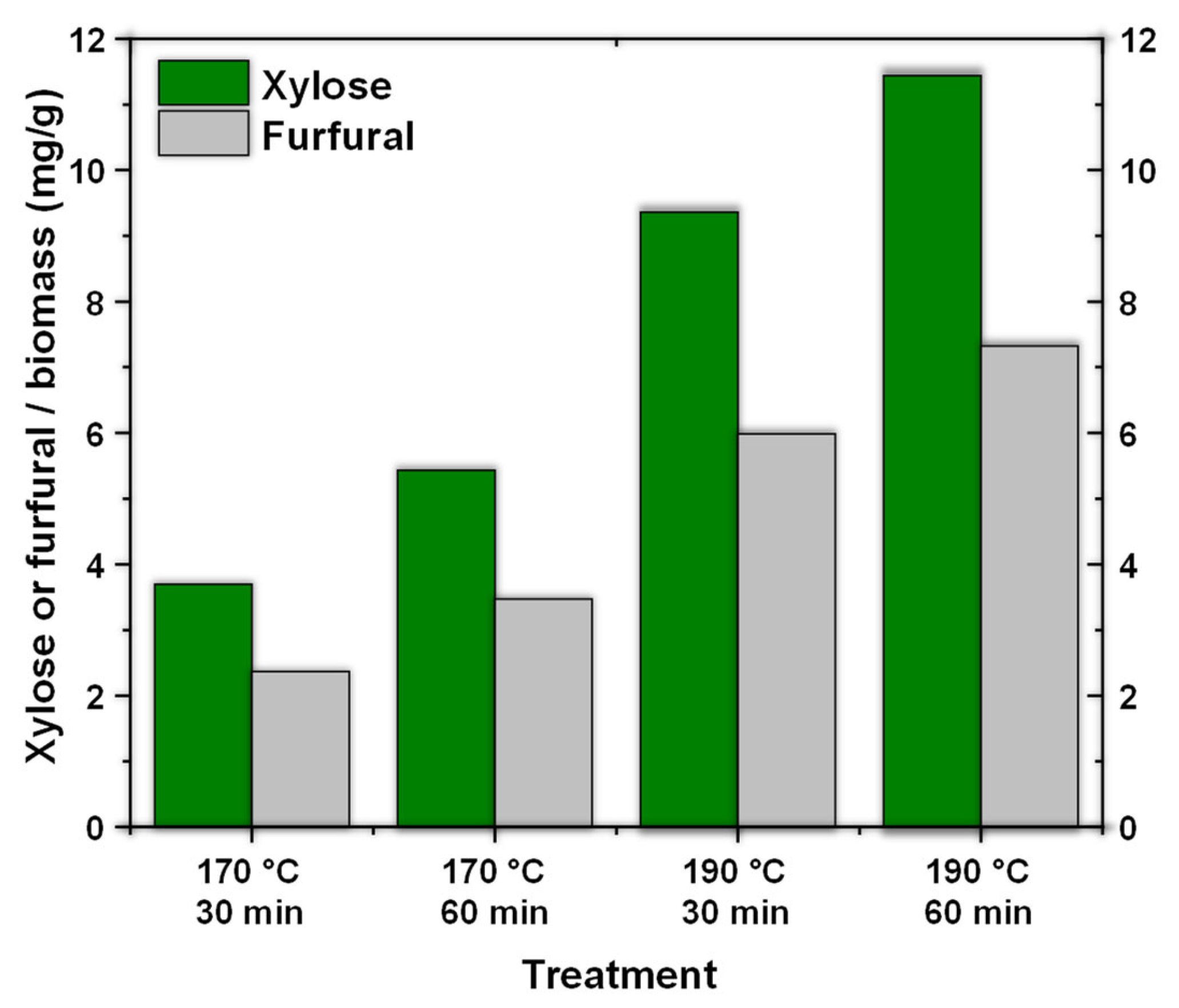
Figure 1 shows the amount of xylose and furfural obtained from the residual coffee biomass.
Figure 1 shows the direct influence of temperature and hydrothermal treatment time in obtaining xylose and furfural. For xylose, we observed that at 170 °C increasing the time increases the generation of sugar, which is mainly attributed to the influence of the reaction time on the hydrolysis of hemicellulose. The same trend was observed for furfural, only in a smaller proportion. By increasing the temperature to 190 °C and a time of 30 min, the observed increase was slightly more than double the production of xylose from coffee waste. Under these same conditions, the production of furfural triples. This is attributed to the influence of temperature, which generates the breaking of glycosidic bonds, thus obtaining monomers of sugars (xylose) which are subsequently dehydrated for the formation of furfural [
20].
It is important to note that, although an increase in time allows the increased generation of both xylose and furfural at the two temperatures evaluated, its influence is not as extensive as that of the temperature. Thus, it can be stated that at higher temperatures the reaction conditions are more ideal, positively affecting the hydrolysis process of hemicellulose. In the same way, hydrothermal treatment is mainly a hybrid treatment between a physical process and a chemical process; for instance, when water is in a subcritical state, it acts as a Brönsted acid and promotes the hydrolysis of biomass [
21].
3.2. Characterization of Catalysts
The catalysts were characterized by XRD, FTIR, Raman, SEM, and optometry to identify the influence of the calcination temperature on their synthesis. The XRD patterns are shown in
Figure 2.
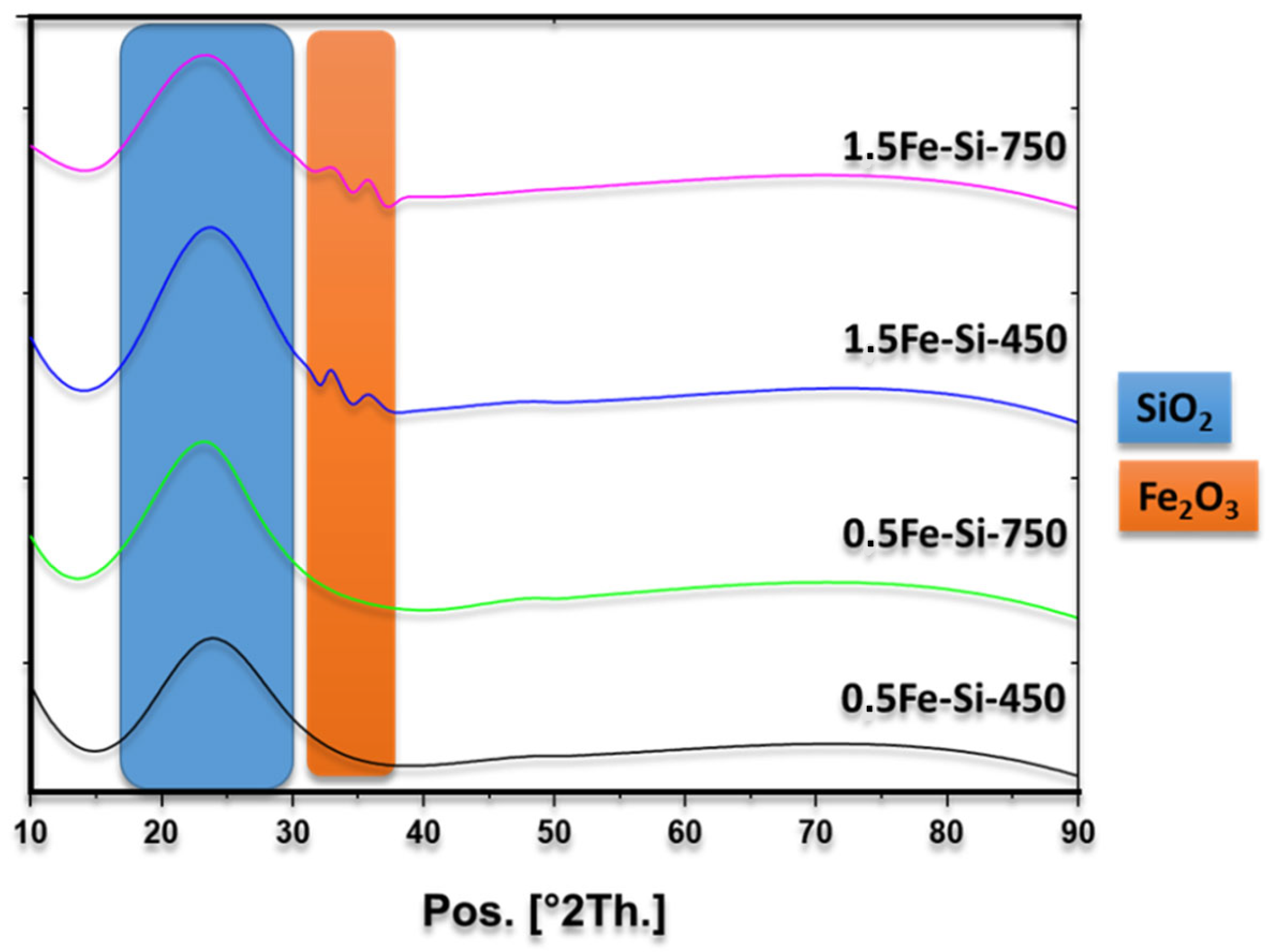
Figure 2 shows a broad peak at 2θ = 22°, which is assigned to amorphous silica [
22,
23,
24]. No peaks attributed to iron species are observed for materials with an iron charge of 0.5 wt% (0.5Fe-450 and 0.5Fe-750). This is due to the high degree of dispersion of active species associated with the process of sol–gel synthesis. For materials with an iron charge of 1.5 wt% (1.5Fe-450, 1.5Fe-750), two broad peaks related to silica are observed at 2θ = 33 and 35° which can be assigned to species of iron oxide type hematite JCPDS: 33-0664 [
25,
26,
27]. It is essential to highlight the presence of hematite-type iron in which the metal has an oxidation state of Fe
3+ [
28]. For the reaction of xylose dehydration to furfural, Fe
3+ acts as a Lewis acid, promoting the isomerization of xylose to xylulose.
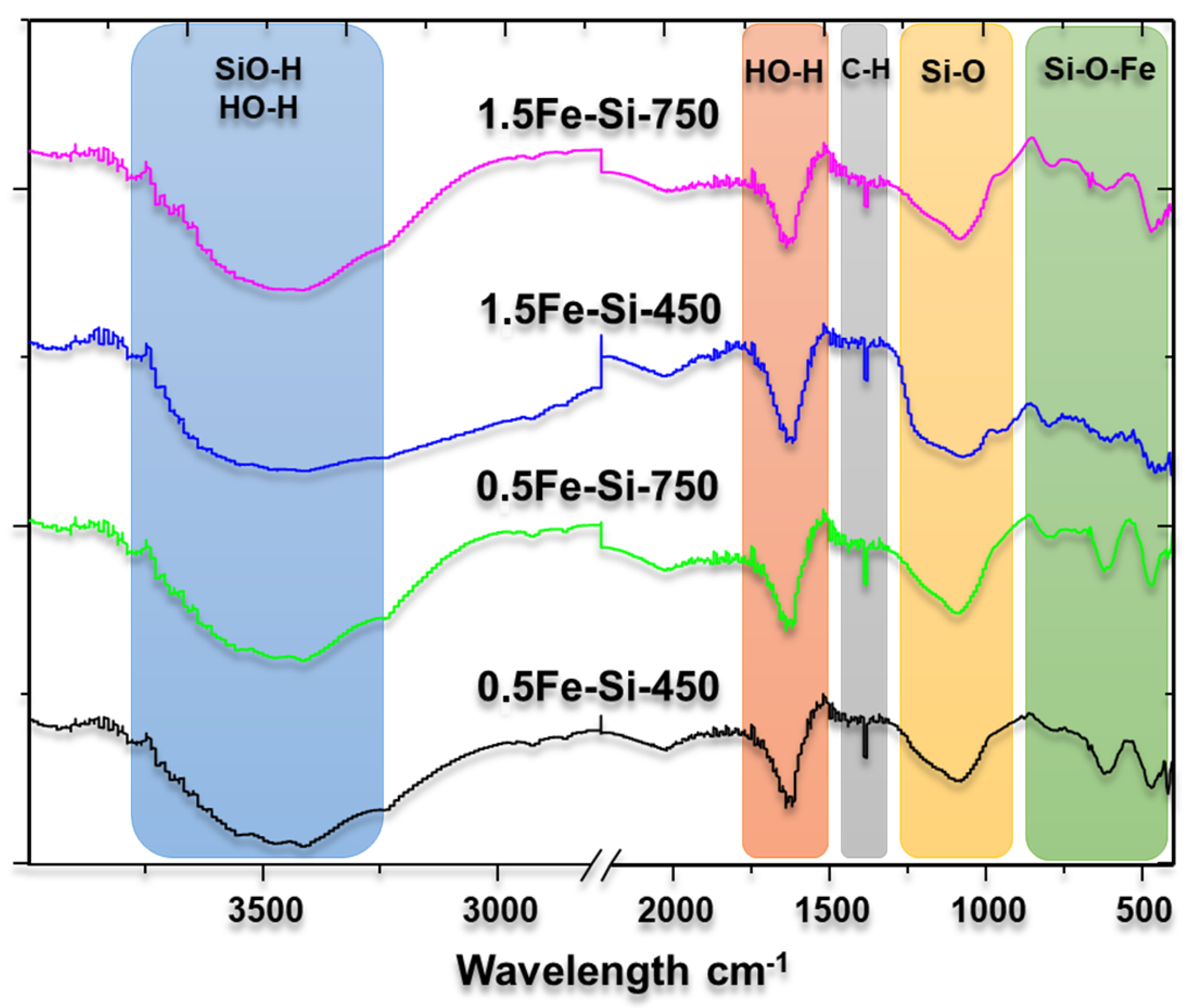
Figure 3 shows the FTIR spectra of the synthesized catalysts in the range of 400–4000 cm
−1. The peak around 3500 cm
−1 corresponds to the modes of extension of the OH groups [
22,
29]. These OH groups can bond to a hydrogen atom forming HO-H, or a silicon atom forming Si-OH. Si-OH has a vital role in a catalytic process. Although many authors suggest that the OH group is inert to the supports in the catalysts, it can act as a Brönsted acid, favoring the dehydration of xylulose to furfural.
The broadband around 3500 cm
−1 is related to the superposition of SiO-H extension [
30].
Table 2 details the wavelengths and their assignments.
The band highlighted at 1082 cm
−1 is attributed to Si-O-Si extension vibrations [
31,
32]. A shoulder is observed around 1200 cm
−1 due to the vibration of the extension of the Si-O bond, and another small band is observed at 981 cm
−1 due to the Si-O bond in the Si-OH silanol groups [
33]. Likewise, 461 and 798 cm
−1 bands are assigned to the Si-O-Si bond ring structure [
34].
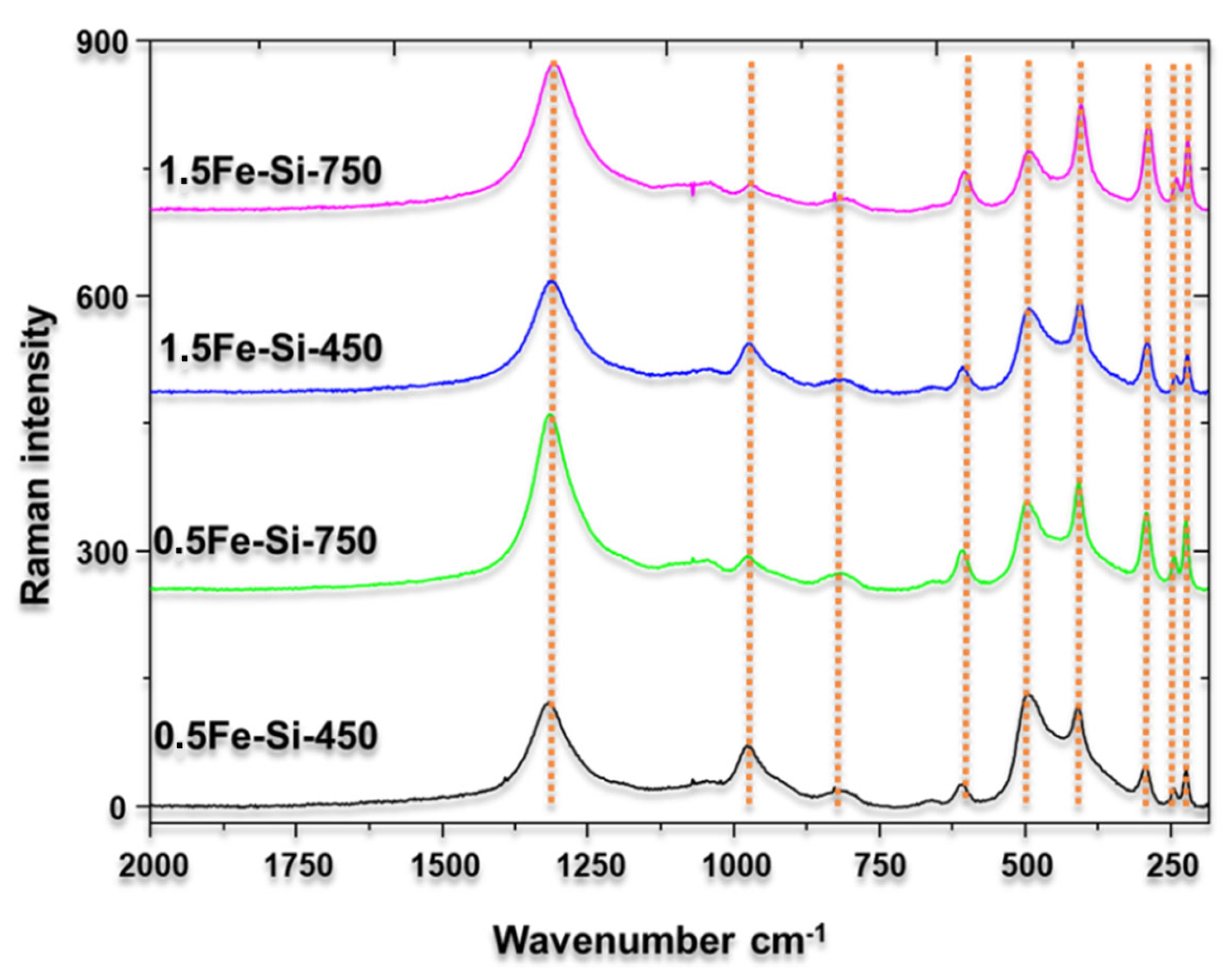
Figure 4 shows the Raman peaks of the synthesized iron materials. An intense peak at around 1300 cm
−1 is associated with hematite-type iron species [
35]. In this regard, a greater intensity is observed in materials synthesized at 750 °C than those calcined at 450 °C. This is attributed to the more significant number of species formed at 750 °C, the temperature at which iron mostly has an oxidation state of Fe
3+. At 450 °C, a mixture of iron Fe
2+ and Fe
3+ is present. Additionally, peaks at 225, 245, 292, 390, 500, 615, and 960 are associated with hematite-type iron [
36,
37]. Based on the above information, the presence of iron is complemented and confirmed by its higher oxidation state, which acts as a Lewis acid during the dehydration reaction of xylose to furfural.
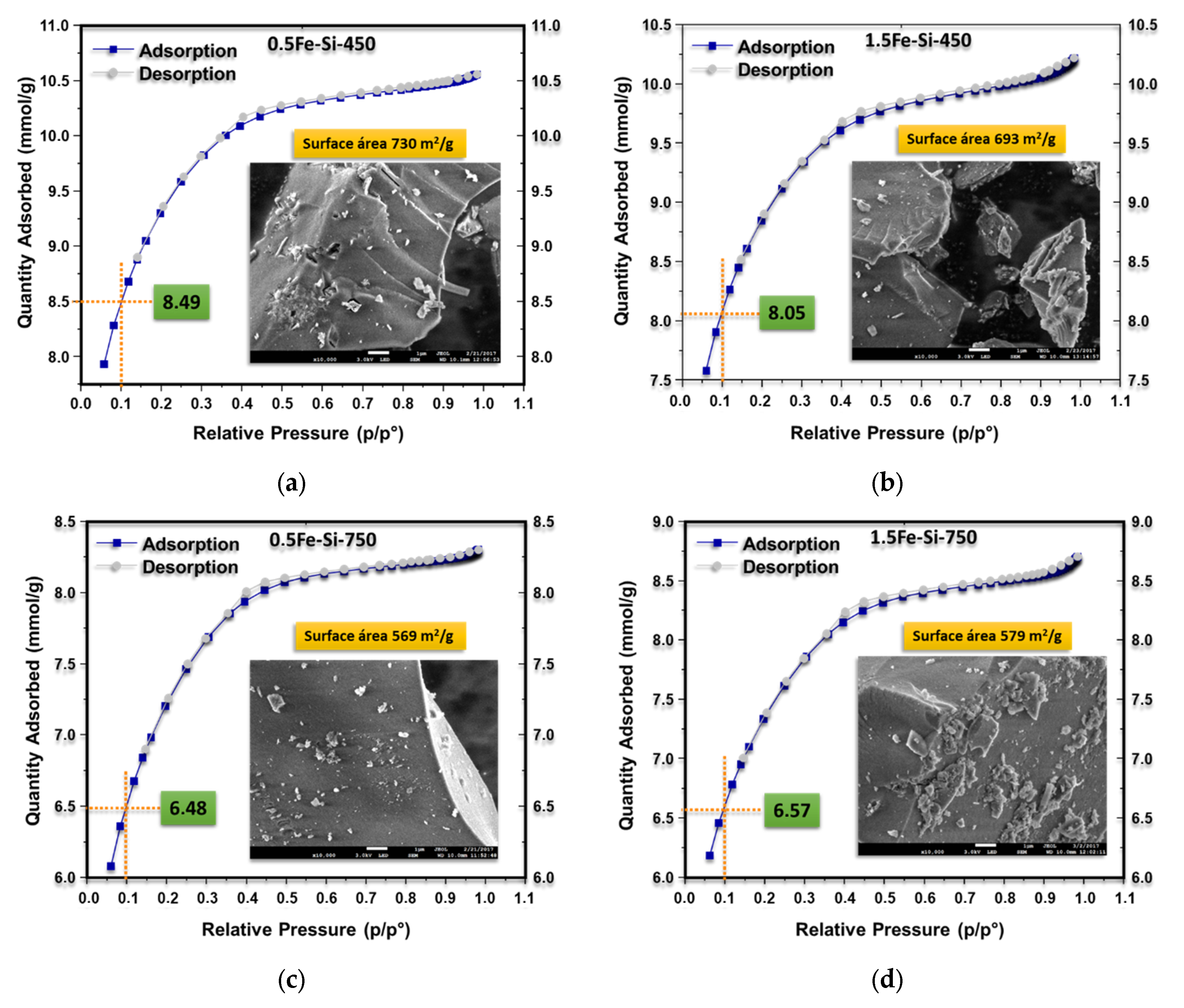
The adsorption and desorption isotherms of iron materials supported on silicon oxide are presented in
Figure 5. Importantly, during nitrogen adsorption at pressures lower than 0.1 (
p/
p0 < 0.1), a filling of the surface by a monolayer of N
2 occurs [
38,
39]; hence, a limit was determined at this relative pressure to identify the adsorbed volume of N
2, which was related to the synthesis conditions of each material. Type II isotherms are observed when comparing the samples calcined at 450 °C with a load of 0.5 and 1.5% in mol of iron (
Figure 5a,b) [
39]. In this case, the surface is 730 and 693 m
2/g, respectively. Based on the surface area values, the sample with the lowest iron load and calcined at 450 °C (0.5Fe-450) required a more significant amount of nitrogen to fill the monolayer (8.49 mmol/g). The material with the highest load and calcined at the same temperature (1.5Fe-450) required 8.05 mmol/g of N
2. This allows us to observe the influence of iron loading on the structural formation of the silicon lattice. The higher iron loading produces structures with a lower surface area due to the higher formation of Si-O-Fe bonds.
The catalysts calcined at 750 °C (0.5Fe-750 and 1.5Fe-750) possess lower surface area values and, therefore, lower N
2 expenditure for monolayer filling. Thus, areas of 569 and 579 m
2/g and nitrogen values of 6.48 and 7.57 mmol/g were observed, respectively. The lower values of area and N
2 expenditure are attributed to the calcination temperature since it influences both the decrease in the surface area and the pore diameter, causing loss of the chemically bonded water, (i) reduction in silanol groups (Si-OH), which generates a contraction of the material structure, and (ii) modification of the texture by sintering [
40]. In this aspect, small crystals or particles become larger structures due to the decrease in surface energy caused by the reduction in surface area and the elimination of solid interfaces. Thus, the final structure resulting from the sintering process generates grain growth and decreases pore size.
In general, the obtained SEM images show materials with angular shapes that are characteristic of silica [
31]. In the same way, an amorphous structure with agglomerates is observed in all cases, which is also characteristic of silica materials prepared by the sol–gel method [
24]. Likewise, irregular shapes of different sizes are observed, in which a defined microstructure is absent.
3.3. Production of Furfural from Xylose
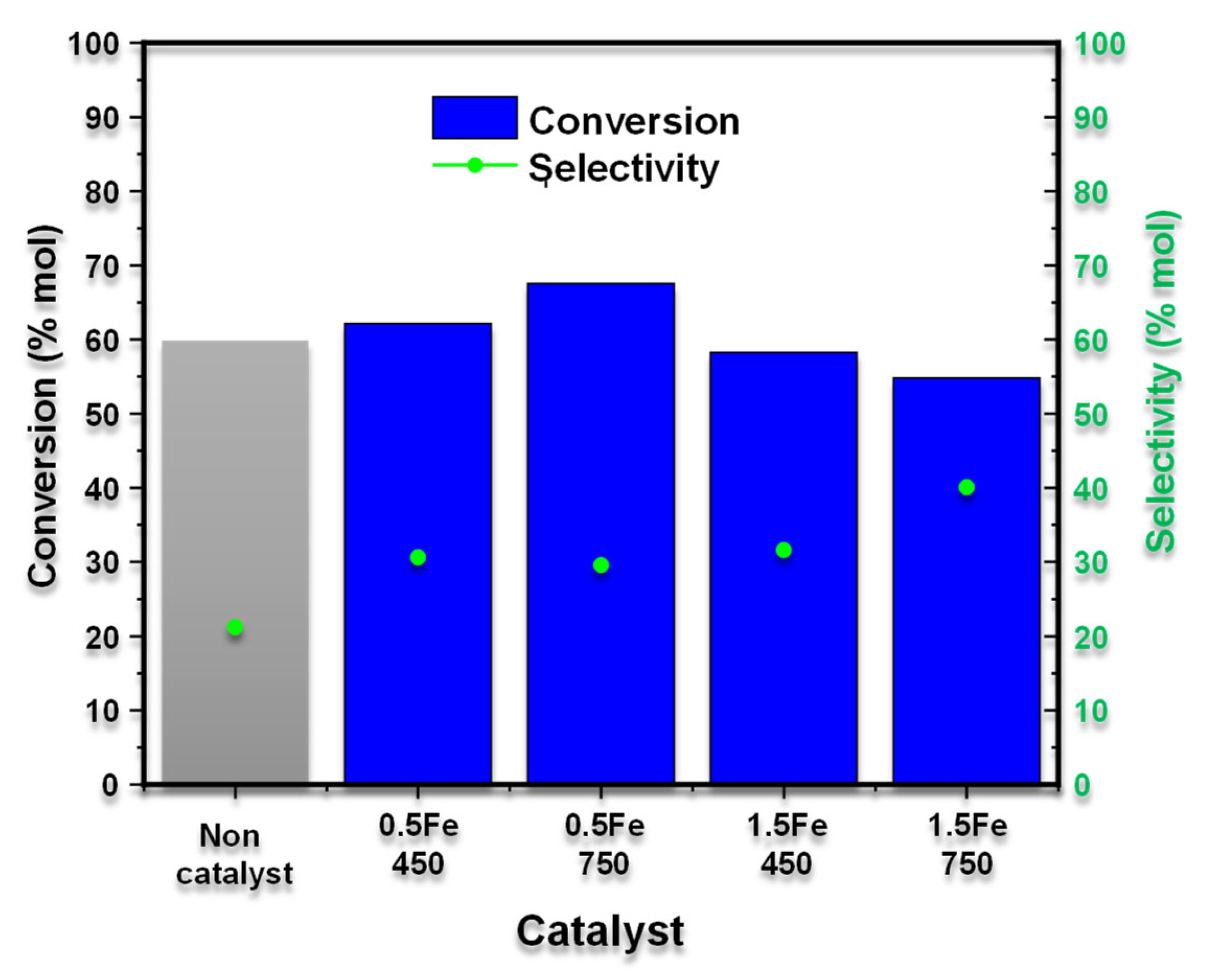
The effect of iron loading and calcination temperature of the materials on xylose to furfural dehydration at 170 °C for 120 min is shown in
Figure 6. In the background reaction, in the absence of a catalyst, the xylose conversion was 59.82% in mol, and a selectivity of 21.17% in mol was observed. In this context, “conversion” refers to the proportion of the precursor (in this case, xylose) that undergoes a transformation into any product, whether it is the desired molecule, furfural, or any other byproducts formed during the process. Additionally, “selectivity” indicates the fraction of the converted precursor that specifically results in furfural formation. In other words, conversion measures the degree to which xylose reacts, while selectivity reflects the efficiency with which the process directs that reaction toward furfural production [
41].
In this aspect, it is essential to emphasize that at 170 °C in a pressurized system, the water acts as an acid, promoting the dehydration of xylose [
42,
43]. The presence of the iron materials enhances the reaction’s selectivity to furfural as well as the process’s performance. In this regard, iron catalysts, supported by silicon oxide, fulfill a double function. The hydrogen of the silane groups of the support (Si-OH) acts as a Brönsted acid, promoting the isomerization of xylose to xylulose [
44]. However, the active centers of iron (Fe
3+) act as a Lewis acid, generating the dehydration of xylulose and the formation of furfural [
45].
Figure 6 shows that regardless of the calcination temperature, the highest iron load (1.5Fe-450 and 1.5Fe-750) generates the lowest values of xylose conversion, 58.27 and 54.76% in mol. However, the higher iron loading increased the selectivity to furfural, 31.62 and 40.09% in mol. In this way, the higher iron loading decreases the number of silanol groups (Si-OH) and, therefore, the activation and initial conversion of xylose. However, this higher iron load generates more active iron centers, which favor the formation of furfural. Likewise, comparing the calcination temperature between materials with a higher iron load (1.5Fe-450 and 1.5Fe-750), we observed that the catalyst calcined at 750 °C was more selective towards furfural, 40.09% in mol. Therefore, the higher calcination temperature promotes the formation of hematite-type iron species (Fe
3+), which act as a Lewis acid and favor the formation of furfural [
46,
47].
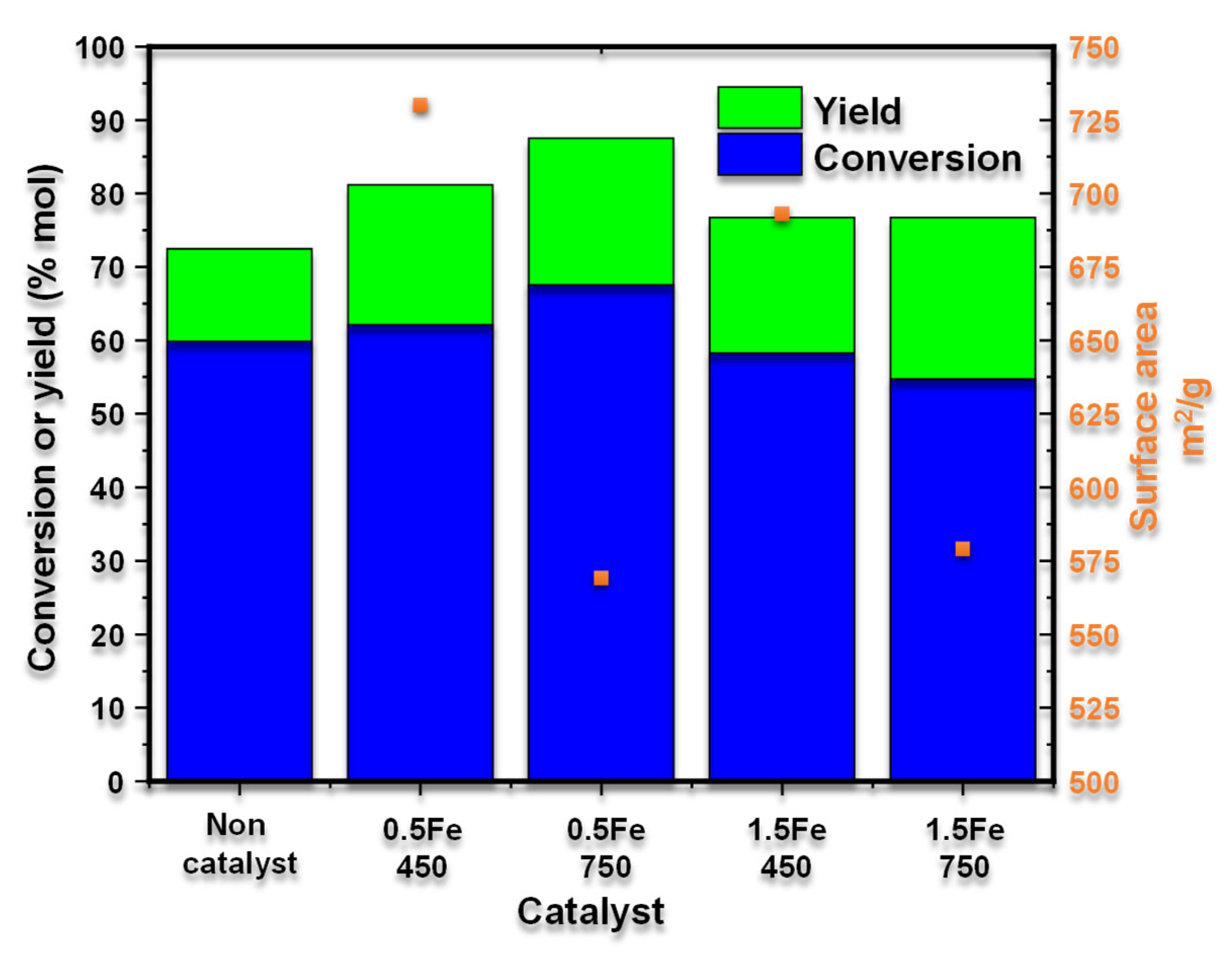
The effect of the surface area on xylose conversion and yield to furfural is presented in
Figure 7. It highlights that the material with the most significant area (730 m
2/g) is obtained at the lowest calcination temperature (450 °C), has a low iron load (0.5Fe-450), and generates a conversion of xylose and selectivity to furfural of 62.14 and 30.61% in mol, respectively. Hence, a larger surface area maximizes the access of the reagents, promoting collisions between them, and influencing the selectivity of the process.
We observed that the material with the lowest specific surface area (579 m2/g) was obtained at a higher calcination temperature (750 °C), presenting a high iron content (1.5Fe-750) and resulting in a xylose conversion of 54.76% and a furfural selectivity of 40.09% in moles. This is because high calcination temperatures can induce sintering processes, which explains the decrease in the specific surface area. However, high temperatures also promote the formation of Fe3+ species, which act as Lewis acid sites that facilitate the isomerization of xylose to xylulose, increasing the selectivity toward furfural. In contrast, calcination processes at lower temperatures produce materials with a higher specific surface area and a greater proportion of Fe2+ species or a mixture of Fe2+ and Fe3+, which reduces furfural selectivity. In conclusion, a higher calcination temperature decreases the material’s specific surface area but enhances the furfural production process.
3.4. Production of Furfural from Coffee Residual Biomass Hydrolysates
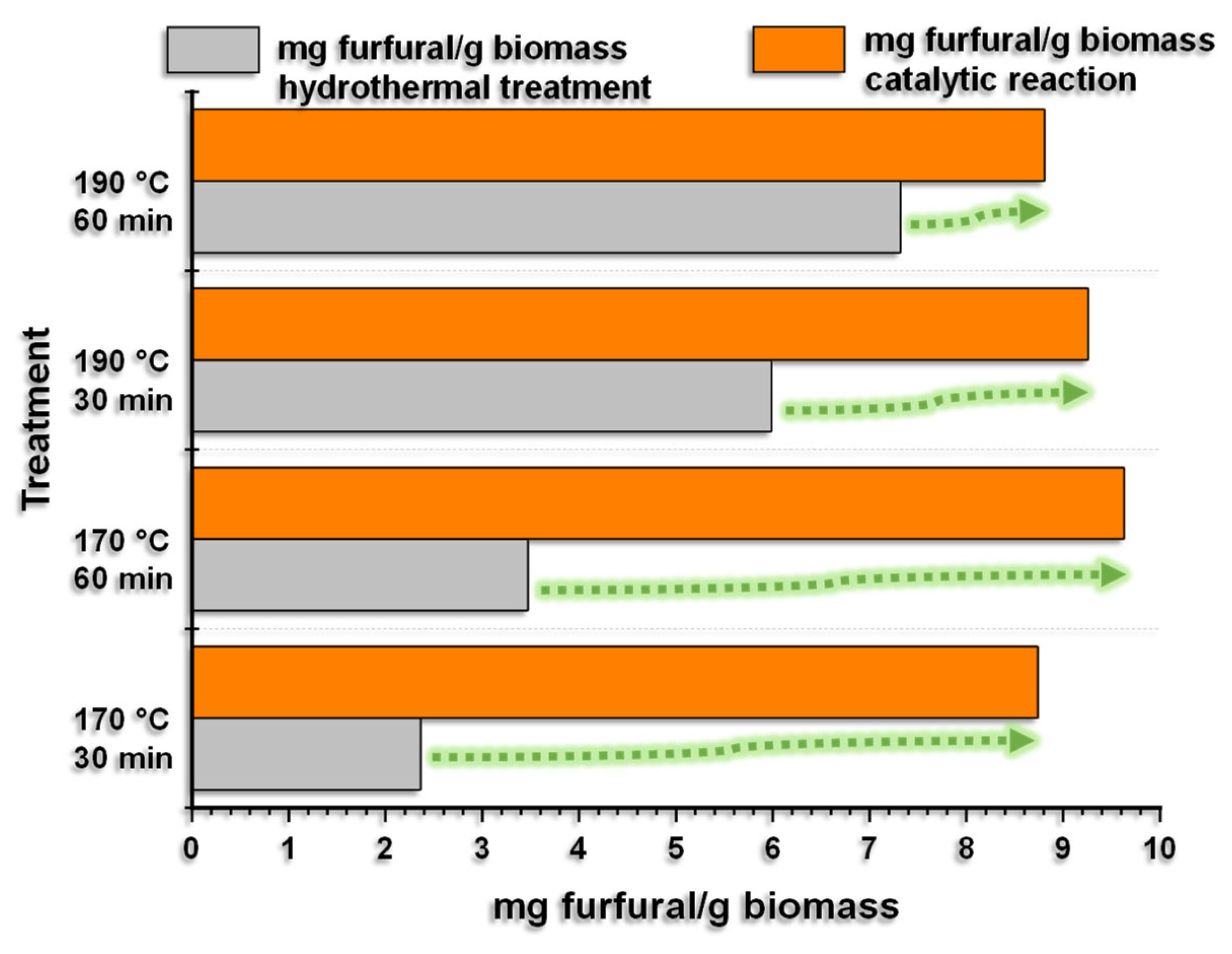
Once the residual coffee biomass had been hydrothermally treated, xylose was used as a raw material to obtain furfural (1.5Fe-750) based on the results of the best catalyst. The catalytic reactions were carried out at 170 °C for 30 and 60 min, each hydrolysate containing 100 mg of catalyst. The results are presented in
Figure 8.
Figure 8 shows that when carrying out the catalytic reaction at 170 °C for 30 and 60 min with each of the hydrolyzates and using the catalyst with the highest iron load of 1.5 wt% and calcined at 750 °C (1.5Fe-750), the amount of furfural obtained increases in all tests. In this sense, as previously mentioned, the biomass was subjected to hydrothermal treatments at 170 and 190 °C for 30 or 60 min, which, in addition to producing xylose, allowed furfural to be obtained. However, when using the hydrolysates obtained at 170 °C (30 and 60 min) and the iron catalyst, an increase in furfural production was observed, from 2.36 to 8.74 mg of furfural/g of biomass and from 3.47 to 9.63 mg of furfural/g of biomass, respectively. This is attributed to the catalytic role of iron species (Lewis acid) and silanol groups (Brönsted acids).
Regarding the hydrolysates obtained at 190 °C (30 and 60 min), we observed that the hydrothermal process carried out for 30 min and then catalytically for 30 min shows increased furfural production from 5.99 to 9.26 mg of furfural/g of biomass, while for 60 min, the hydrothermal and catalytic process increases from 7.32 to 8.81 mg of furfural/g of biomass. Therefore, in all cases, furfural production increases using an iron catalyst supported by silicon oxide. The most significant increases were observed in the hydrolysates obtained at a lower temperature (170 °C) than those obtained at 190 °C.
Finally, our study determined that the catalyst with a 1.5% Fe loading, calcined at 750 °C, showed a selectivity toward furfural of 40.09% from the structural sugars present in coffee crop residues. This adds value by utilizing residual biomass. These results are comparable to those reported by other researchers who used heterogeneous catalysts, such as aluminum- and hafnium-pillared clays, starting from xylose and achieving selectivity ranging from 40% to 65% [
48].
It is important to note that the literature studies reached high selectivity after 4 h of processing, whereas in the present study, the reactions were carried out in 30 and 60 min. Additionally, other studies using iron-impregnated activated carbon derived from lignin reported a maximum selectivity of 65% from xylose, using reaction times of 3 h at 170 °C in a biphasic water/methyl isobutyl ketone system.
These findings demonstrate that our results fall within a comparable range to those reported in the literature for heterogeneous catalytic processes. This supports the feasibility of continuing to evaluate Fe3+, in the form of hematite, as a Lewis acid site that promotes the isomerization of xylose to xylulose for subsequent dehydration, in combination with the hydroxyl groups from the xylanol groups present in the catalyst support.
4. Conclusions
According to our results, when carrying out the catalytic reaction at 170 °C for 2 h with each of the hydrolyzates and using the catalyst with the highest iron loading of 1.5 wt% and calcined at 750 °C (1.5Fe-750), the amount of furfural obtained increases in all tests. Additionally, the biomass was subjected to hydrothermal treatments at 170 and 190 °C for 30 or 60 min, which, in addition to producing xylose, allowed furfural to be obtained. However, when using the hydrolysates obtained at 170 °C (30 and 60 min) and in the presence of the iron catalyst, an increase in furfural production was observed, going from 2.36 to 8.74 mg of furfural/g of biomass and from 3.47 to 9.63 mg of furfural/g of biomass, respectively. This is attributed to the catalytic role of iron species (Lewis acid) and silanol groups (Brönsted acids).
Regarding the hydrolysates obtained at 190 °C (30 and 60 min), we observed that the hydrothermal process carried out for 30 min and then catalytically for 2 h, generates an increase in furfural production from 5.99 to 9.26 mg of furfural/g of biomass and from 7.32 to 8.81 mg. In all cases, furfural production increased using an iron catalyst supported by silicon oxide. The most significant increases were observed in the hydrolysates obtained at a lower temperature (170 °C) than those obtained at 190 °C.