Activated Carbons from Apricot Kernel Shells for Wastewater Treatment: Adsorption of Pb2+ and Rhodamine B with Equilibrium, Kinetics, Thermodynamics, and DFT Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbons
2.2. Structural Analysis of Activated Carbons
2.3. Adsorption Experiments
2.3.1. Preparation of Stock Solutions of Inorganic and Organic Contaminants
2.3.2. Adsorption Optimizations in the Experimental Procedure
2.4. Adsorption Kinetics and the Equilibrium Models
2.5. Thermodynamic Analysis
2.6. DFT Computations—Methodology
3. Results
3.1. FTIR Analysis of Functional Groups in Prepared Activated Carbons
3.2. Results of the XRD Analysis
3.3. Morphological Comparison of Activated Carbons (SEM Analysis)
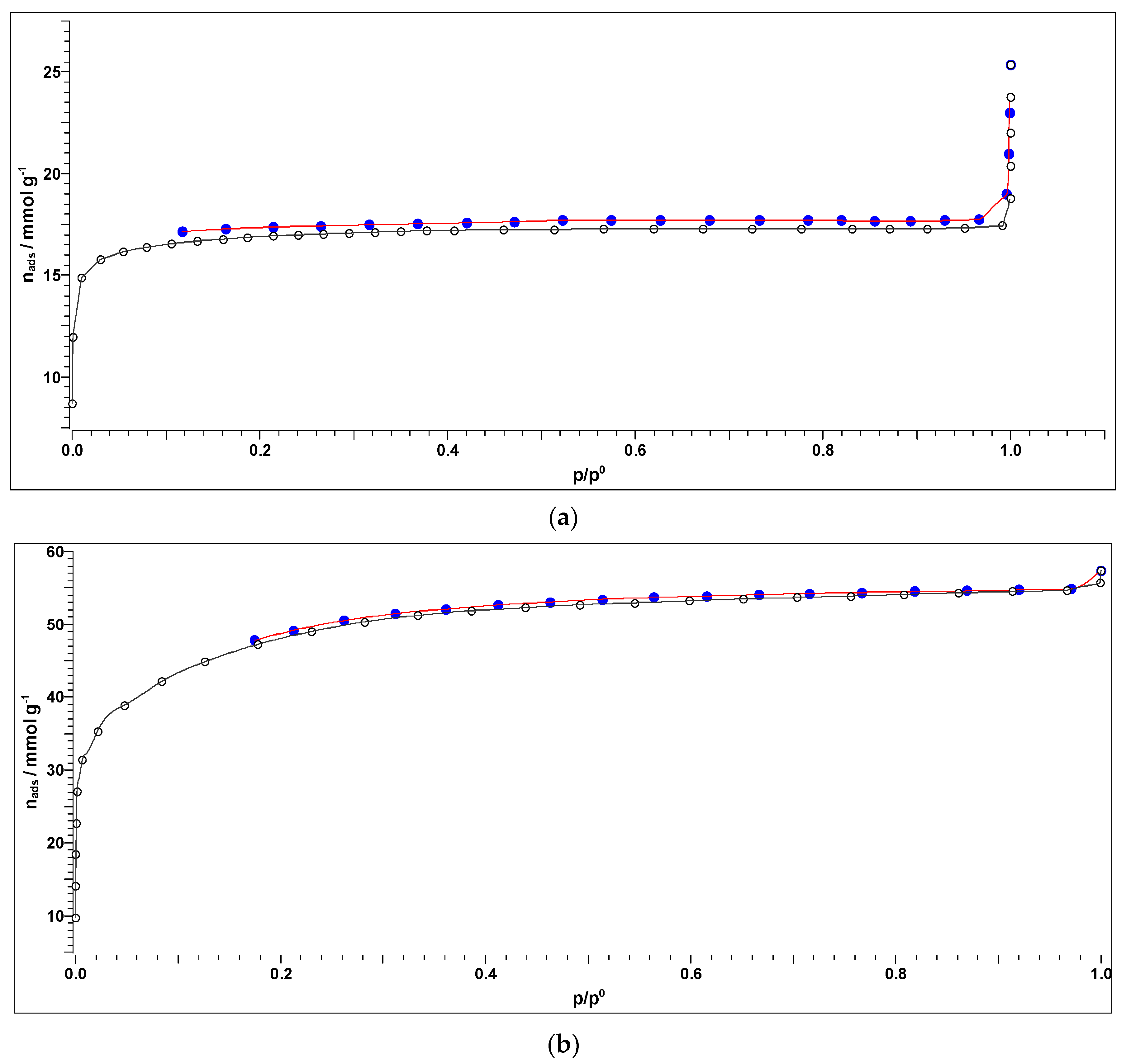
3.4. Textural Properties of Activated Carbons (BET Analysis)
3.5. Removal Efficiency, Equilibrium and Kinetics of Adsorption Process
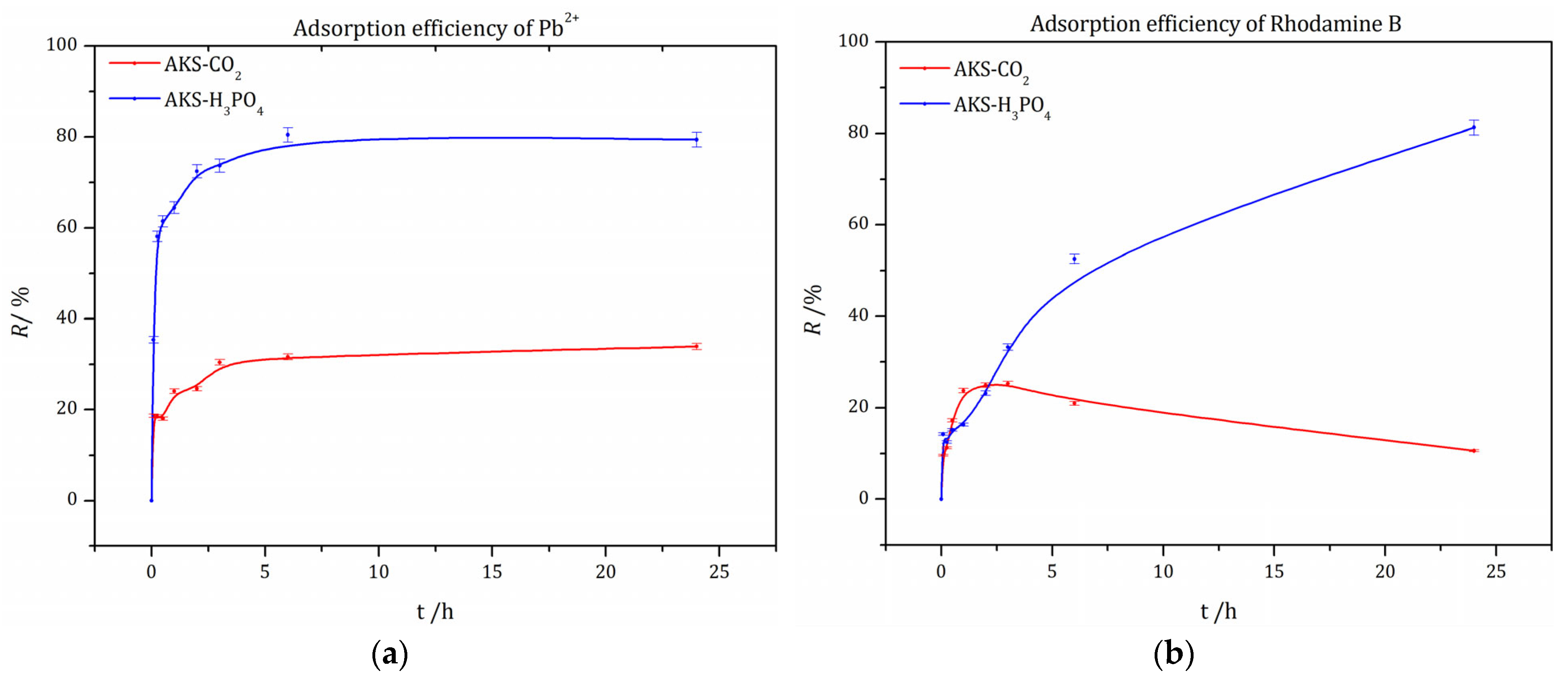
3.5.1. Removal Efficiency of Pb2+ and RhB Using AKS-CO2 and AKS-H3PO4 Adsorbents
3.5.2. Adsorption Isotherms for Pb2+ and RhB onto AKS-H3PO4
3.5.3. Adsorption-Reaction Kinetics of Pb2+ and RhB onto AKS-H3PO4
3.5.4. Adsorption-Diffusion Kinetics of Pb2+ and RhB onto AKS-H3PO4
3.6. Thermodynamic Study of Pb2+ and RhB Adsorption onto AKS-H3PO4
3.6.1. Standard Thermodynamic Parameters
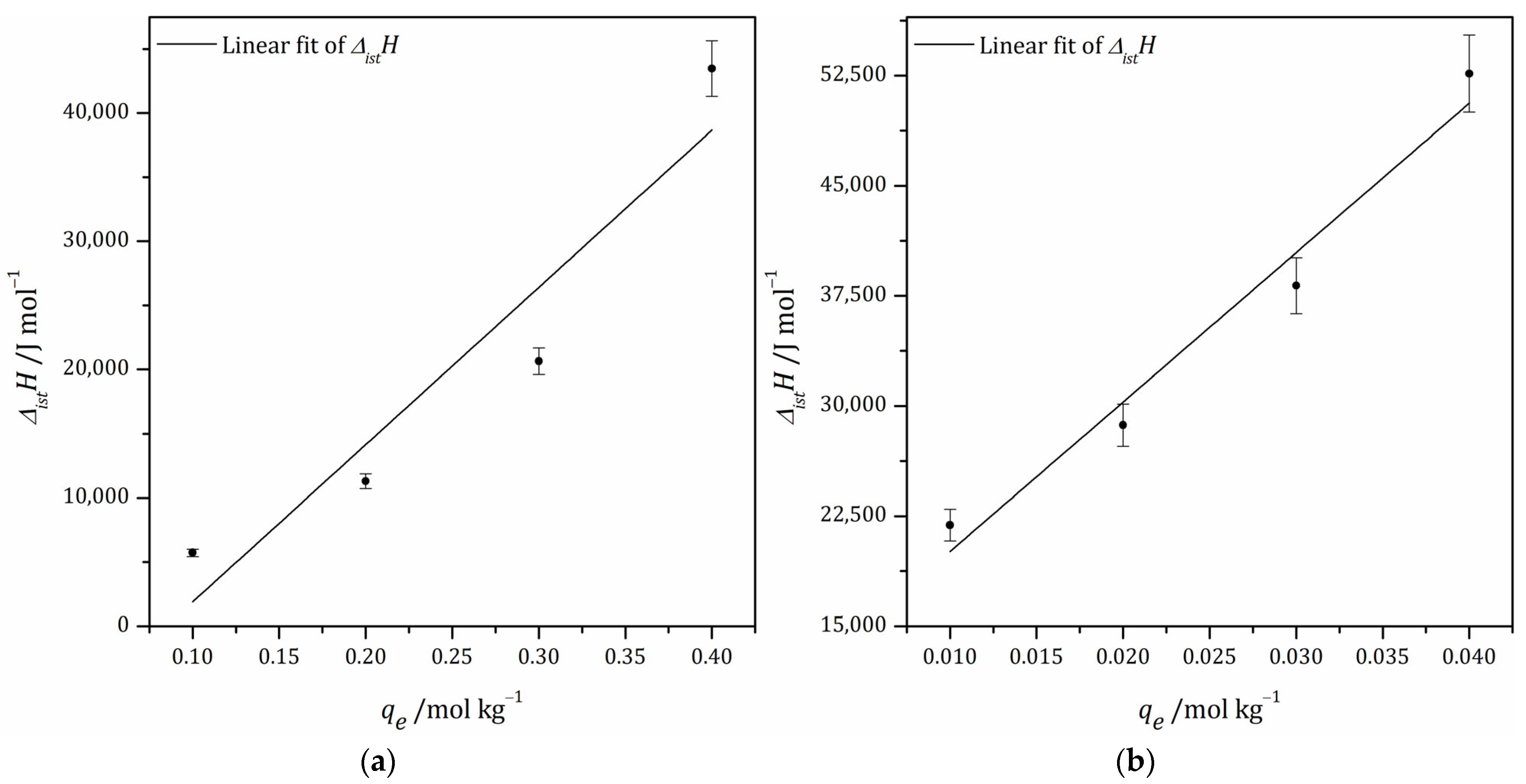
3.6.2. Isosteric Heat of Adsorption
3.7. Summary Regarding Thermodynamics and Mechanisms of Adsorption Processes onto AKS-H3PO4
3.8. Results of DFT Computations
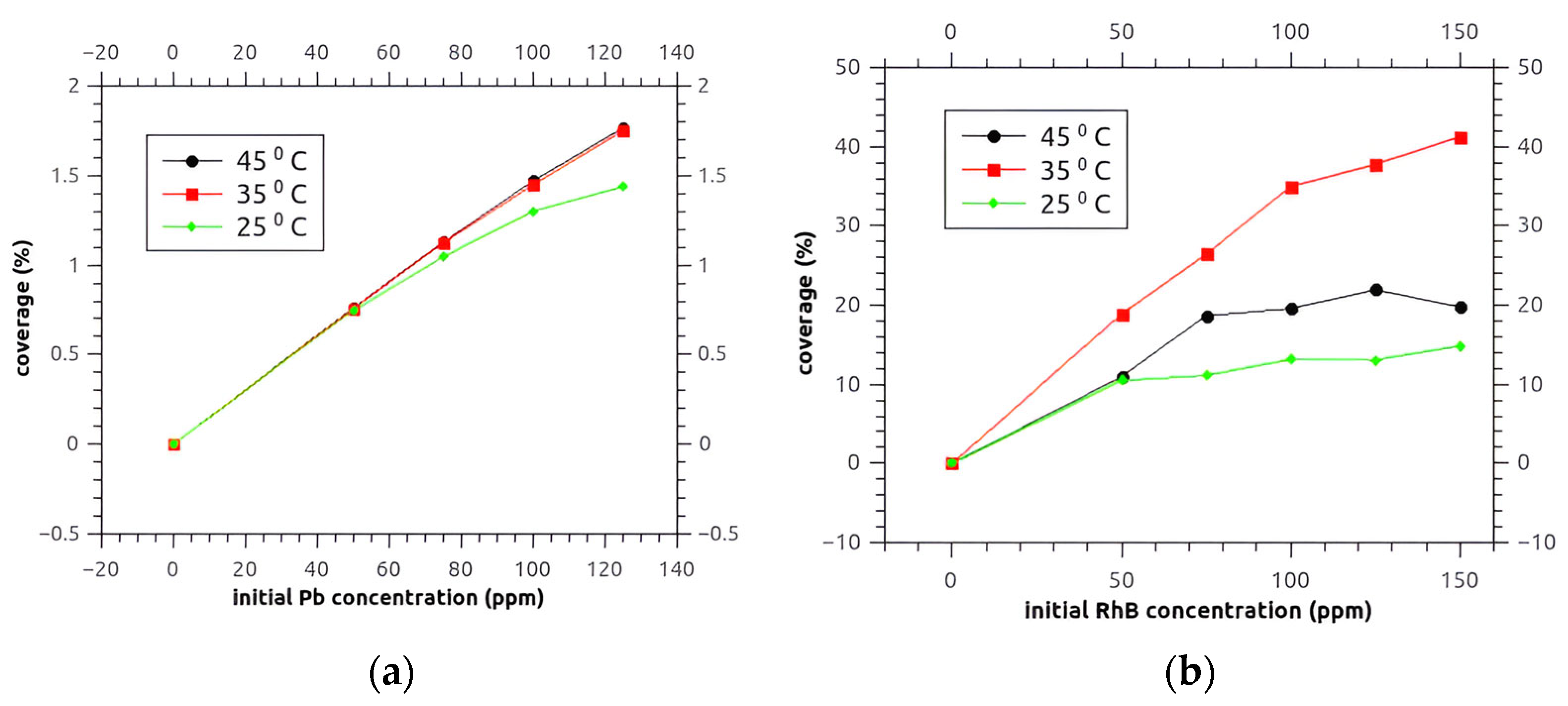
3.8.1. The Estimation of AKS-H3PO4 Surface Coverage
3.8.2. Model Surfaces for Adsorption of Pb2+ and RhB
3.8.3. Adsorption of Pb2+ Ions
3.8.4. Adsorption of RhB Dye
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Letter designations | |
| LC | Lignocellulose |
| AKS | Apricot kernel shells |
| ACs | Activated carbons |
| AKS-CO2 | Physically activated carbon (originated from AKS biomass precursor) |
| AKS-H3PO4 | Chemically activated carbon (originated from AKS biomass precursor) |
| RhB | Rhodamine B |
| PFO | Pseudo-first order |
| PSO | Pseudo-second order |
| IP | Intra-particle |
| DC | Diffusion-chemisorption |
| MV | Mono-vacancy |
| Physical Quantities | |
| CrI | Crystallinity index (%) |
| R2 | Coefficient of determination (dimensionless) |
| RL | The separation factor (dimensionless) |
| E | The average adsorption energy (kJ/mol) |
| C | Boundary layer thickness (mg/g) |
| KDC | Diffusion-chemisorption constant [mg·g−1·h−0.5] |
| T | Temperature (K or °C) |
| t | Time (h) |
| θ | Bragg angle (°) |
| qe | The equilibrium adsorption capacity of the adsorbent (mg/g) |
| ce | The equilibrium adsorbate concentration (mg/L) |
| co | The initial adsorbate concentration in the bulk liquid (mg/L) |
| qm | The maximum adsorption capacity (mg/g) |
| K° | The standard equilibrium constant (dimensionless) |
| ΔH° | The standard enthalpy change (kJ/mol) |
| ΔS° | The standard entropy change (J/(mol·K)) |
| ΔG° | The standard Gibbs free energy change (kJ/mol) |
| ΔistH | The isosteric heat of adsorption (kJ/mol) |
| ΔEads | Adsorption energy (eV) |
| Eslab+ads | Total energy of the optimized slab–adsorbate system (eV) |
| Etot,isol | Total energy of the isolated adsorbate (eV) |
| Etot,slab | Total energy of the bare surface slab (eV) |
| nads | The adsorption amount (mmol/g) |
| p/po | The relative pressure (dimensionless) |
| SBET | The BET specific surface area (m2/g) |
| Smeso | The meso-pores surface area (m2/g) |
| Vp(H-K) | The cumulative pore volume determined by Horvath–Kawazoe (cm3/g) |
| rm(H-K) | Median pore radius determined by Horvath–Kawazoe (nm) |
| rmax(H-K) | Maximum pore radius determined by Horvath–Kawazoe (nm) |
| Vmicro | The micro-pore volume (cm3/g) |
| Vp(B.J.H.) | The cumulative pore volume determined by Barrett–Joyner–Halenda (cm3/g) |
| rm(B.J.H.) | Median pore radius determined by Barrett–Joyner–Halenda (nm) |
| rmax(B.J.H.) | Maximum pore radius determined by Barrett–Joyner–Halenda (nm) |
| Vp(C-I) | The cumulative pore volume determined by Cranston–Inkley (cm3/g) |
| rm(C-I) | Median pore radius determined by Cranston–Inkley (nm) |
| rmax(C-I) | Maximum pore radius determined by Cranston–Inkley (nm) |
| Vp(D-H) | The cumulative pore volume determined by Dollimore–Heal (cm3/g) |
| rm(D-H) | Median pore radius determined by Dollimore–Heal (nm) |
| rmax(D-H) | Maximum pore radius determined by Dollimore–Heal (nm) |
References
- Noor, A.; Khan, S.A. Agricultural Wastes as Renewable Biomass to Remediate Water Pollution. Sustainability 2023, 15, 4246. [Google Scholar] [CrossRef]
- Rajput, V.; Saini, I.; Parmar, S.; Pundir, V.; Kumar, V.; Kumar, V.; Naik, B.; Rustagi, S. Biochar Production Methods and Their Transformative Potential for Environmental Remediation. Discov. Appl. Sci. 2024, 6, 408. [Google Scholar] [CrossRef]
- Biochar Standards. Available online: https://biochar-international.org/biochar-standards/ (accessed on 18 February 2025).
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of Biochar for the Adsorption of Organic Pollutants from Wastewater: Modification Strategies, Mechanisms and Challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Biswal, B.K.; Balasubramanian, R. Use of Biochar as a Low-Cost Adsorbent for Removal of Heavy Metals from Water and Wastewater: A Review. J. Environ. Chem. Eng. 2023, 11, 110986. [Google Scholar] [CrossRef]
- Kobyłecki, R.; Ścisłowska, M.; Bis, Z. Carbonization of Biomass—An Efficient Tool to Decrease the Emission of CO2. Arch. Thermodyn. 2013, 34, 185–195. [Google Scholar] [CrossRef]
- Rampáčková, E.; Mrázová, M.; Čížková, J.; Nečas, T. Pomological Traits and Genome Size of Prunus armeniaca L. Considering to Geographical Origin. Horticulturae 2022, 8, 199. [Google Scholar] [CrossRef]
- Akhone, M.A.; Bains, A.; Tosif, M.M.; Chawla, P.; Fogarasi, M.; Fogarasi, S. Apricot Kernel: Bioactivity, Characterization, Applications, and Health Attributes. Foods 2022, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Cañellas, J.; Femenia, A.; Rosselló, C.; Soler, L. Chemical Composition of the Shell of Apricot Seeds. J. Sci. Food Agric. 1992, 59, 269–271. [Google Scholar] [CrossRef]
- Shaikhiev, I.; Shaykhieva, K.; Sverguzova, S.; Fomina, E.; Vinogradenko, Y.; Fediuk, R.; Amran, M.; Svintsov, A.P.; de Azevedo, A.R.G.; Gunasekaran, M. Removing Pollutants from Sewage Waters with Ground Apricot Kernel Shell Material. Materials 2022, 15, 3428. [Google Scholar] [CrossRef]
- Janković, B.; Manić, N.; Dodevski, V.; Radović, I.; Pijović, M.; Katnić, Đ.; Tasić, G. Physico-Chemical Characterization of Carbonized Apricot Kernel Shell as Precursor for Activated Carbon Preparation in Clean Technology Utilization. J. Clean. Prod. 2019, 236, 117614. [Google Scholar] [CrossRef]
- Okoro, H.K.; Pandey, S.; Ogunkunle, C.O.; Ngila, C.J.; Zvinowanda, C.; Jimoh, I.; Lawal, I.A.; Orosun, M.M.; Adeniyi, A.G. Nanomaterial-Based Biosorbents: Adsorbent for Efficient Removal of Selected Organic Pollutants from Industrial Wastewater. Emerg. Contam. 2022, 8, 46–58. [Google Scholar] [CrossRef]
- Kang, C.; Zhu, L.; Wang, Y.; Wang, Y.; Xiao, K.; Tian, T. Adsorption of Basic Dyes Using Walnut Shell-Based Biochar Produced by Hydrothermal Carbonization. Chem. Res. Chin. Univ. 2018, 34, 622–627. [Google Scholar] [CrossRef]
- World Population Prospects. Available online: https://population.un.org/wpp/ (accessed on 22 May 2025).
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Woerden, F.V. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018; ISBN 978-1-4648-1347-4. [Google Scholar]
- Circular Economy: Definition, Importance and Benefits. Available online: https://www.europarl.europa.eu/topics/en/article/20151201STO05603/circular-economy-definition-importance-and-benefits (accessed on 22 May 2025).
- Sajjadi, B.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Chen, W.Y. Chemical Activation of Biochar for Energy and Environmental Applications: A Comprehensive Review. Rev. Chem. Eng. 2019, 35, 777–815. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A Review of Chemicals to Produce Activated Carbon from Agricultural Waste Biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Derbyshire, F. Activated Carbons from Yellow Poplar and White Oak by H3PO4 Activation. Carbon 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Neme, I.; Gonfa, G.; Masi, C. Preparation and Characterization of Activated Carbon from Castor Seed Hull by Chemical Activation with H3PO4. Results Mater. 2022, 15, 100304. [Google Scholar] [CrossRef]
- El-Hendawy, A.-N.A. An Insight into the KOH Activation Mechanism through the Production of Microporous Activated Carbon for the Removal of Pb2+ Cations. Appl. Surf. Sci. 2009, 255, 3723–3730. [Google Scholar] [CrossRef]
- Satayev, M.I.; Alibekov, R.S.; Satayeva, L.M.; Baiysbay, O.P.; Mutaliyeva, B.Z. Characteristics of Activated Carbons Prepared from Apricot Kernel Shells by Mechanical, Chemical and Thermal Activations. Mod. Appl. Sci. 2015, 9, p104. [Google Scholar] [CrossRef]
- Pet, I.; Sanad, M.N.; Farouz, M.; ElFaham, M.M.; El-Hussein, A.; El-sadek, M.S.A.; Althobiti, R.A.; Ioanid, A. Review: Recent Developments in the Implementation of Activated Carbon as Heavy Metal Removal Management. Water Conserv. Sci. Eng. 2024, 9, 62. [Google Scholar] [CrossRef]
- Natrayan, L.; Kaliappan, S.; Dheeraj Kumar Reddy, C.N.; Karthick, M.; Sivakumar, N.s.; Patil, P.P.; Sekar, S.; Thanappan, S. Development and Characterization of Carbon-Based Adsorbents Derived from Agricultural Wastes and Their Effectiveness in Adsorption of Heavy Metals in Waste Water. Bioinorg. Chem. Appl. 2022, 2022, 1659855. [Google Scholar] [CrossRef] [PubMed]
- Vashchynskyi, V.; Okhay, O.; Boychuk, T. Chemical Activation of Apricot Pit-Derived Carbon Sorbents for the Effective Removal of Dyes in Environmental Remediation. C 2023, 9, 93. [Google Scholar] [CrossRef]
- Erdoğan, S.; Önal, Y.; Akmil-Başar, C.; Bilmez-Erdemoğlu, S.; Sarıcı-Özdemir, Ç.; Köseoğlu, E.; İçduygu, G. Optimization of Nickel Adsorption from Aqueous Solution by Using Activated Carbon Prepared from Waste Apricot by Chemical Activation. Appl. Surf. Sci. 2005, 252, 1324–1331. [Google Scholar] [CrossRef]
- Adhami, S.; Ghorbanpoor, H.; Azak, B.; Kapucu, S.; Nurbas, M.; Avcı, H. A novel approach for water treatment by using activated carbon: Apricot kernel shell. Eskişeh. Osman. Üniversitesi Mühendis. Ve Mimar. Fakültesi Derg. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- CDC Substance Priority List. Available online: https://www.atsdr.cdc.gov/programs/substance-priority-list.html (accessed on 18 February 2025).
- Behera, A.K.; Shadangi, K.P.; Sarangi, P.K. Efficient Removal of Rhodamine B Dye Using Biochar as an Adsorbent: Study the Performance, Kinetics, Thermodynamics, Adsorption Isotherms and Its Reusability. Chemosphere 2024, 354, 141702. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Kokalj, A. XCrySDen—A New Program for Displaying Crystalline Structures and Electron Densities. J. Mol. Graph. Model. 1999, 17, 176–179. [Google Scholar] [CrossRef]
- Kuyanov-Prozument, K.; Choi, M.Y.; Vilesov, A.F. Spectrum and Infrared Intensities of OH-Stretching Bands of Water Dimers. J. Chem. Phys. 2010, 132, 014304. [Google Scholar] [CrossRef]
- Sufian, J.; Babaakbari Sari, M.; Marchelli, F.; Fiori, L.; Avanes, A.; Moradi, S. An Analysis of the Factors Influencing Cadmium Removal in Aquatic Environments by Chlorella Vulgaris-Derived Solids. C 2024, 10, 2. [Google Scholar] [CrossRef]
- Haris, M.R.H.M.; Sathasivam, K. The Removal of Methyl Red from Aqueous Solutions Using Banana Pseudostem Fibers. Am. J. Appl. Sci. 2009, 6, 1690–1700. [Google Scholar] [CrossRef]
- Saka, C. BET, TG–DTG, FT-IR, SEM, Iodine Number Analysis and Preparation of Activated Carbon from Acorn Shell by Chemical Activation with ZnCl2. J. Anal. Appl. Pyrolysis 2012, 95, 21–24. [Google Scholar] [CrossRef]
- Al-rawi, M.; Tomma, J. Synthesis of New 1,2,4-Triazole Derivatives with Expected Biological Activities. Chem. Methodol. 2021, 6, 59–66. [Google Scholar] [CrossRef]
- Ilić, M.; Haegel, F.-H.; Lolić, A.; Nedić, Z.; Tosti, T.; Ignjatović, I.S.; Linden, A.; Jablonowski, N.D.; Hartmann, H. Surface Functional Groups and Degree of Carbonization of Selected Chars from Different Processes and Feedstock. PLoS ONE 2022, 17, e0277365. [Google Scholar] [CrossRef]
- Liu, H.; Kaya, H.; Lin, Y.-T.; Ogrinc, A.; Kim, S.H. Vibrational Spectroscopy Analysis of Silica and Silicate Glass Networks. J. Am. Ceram. Soc. 2022, 105, 2355–2384. [Google Scholar] [CrossRef]
- Aldahasi, R.M.; Shami, A.; Mohammed, A.E. Bimetallic Nanoparticles and Biochar Produced by Adansonia Digitata Shell and Their Effect against Tomato Pathogenic Fungi. PeerJ 2024, 12, e17023. [Google Scholar] [CrossRef]
- Nguyen, L.X.; Do, P.T.M.; Nguyen, C.H.; Kose, R.; Okayama, T.; Pham, T.N.; Nguyen, P.D.; Miyanishi, T. Properties of Biochars Prepared from Local Biomass in the Mekong Delta, Vietnam. BioResources 2018, 13, 7325–7344. [Google Scholar] [CrossRef]
- Adeniyi, A.; Ighalo, J.O.; Onifade, D.; Popoola, A. Production of Hybrid Biochar by Retort-Heating of Elephant Grass (Pennisetum purpureum) and Low Density Polyethylene (LDPE) for Waste Management and Product Development. J. Mater. Environ. Sci. 2020, 11, 1940–1952. [Google Scholar]
- Samsonowicz, M.; Hrynaszkiewicz, T.; Świsłocka, R.; Regulska, E.; Lewandowski, W. Experimental and Theoretical IR, Raman, NMR Spectra of 2-, 3- and 4-Aminobenzoic Acids. J. Mol. Struct. 2005, 744–747, 345–352. [Google Scholar] [CrossRef]
- Guo, Y.; Bustin, R.M. FTIR Spectroscopy and Reflectance of Modern Charcoals and Fungal Decayed Woods: Implications for Studies of Inertinite in Coals. Int. J. Coal Geol. 1998, 37, 29–53. [Google Scholar] [CrossRef]
- Ramola, S.; Mishra, T.; Rana, G.; Srivastava, R.K. Characterization and Pollutant Removal Efficiency of Biochar Derived from Baggase, Bamboo and Tyre. Environ. Monit. Assess. 2014, 186, 9023–9039. [Google Scholar] [CrossRef]
- Maguana, Y.E.; Elhadiri, N.; Bouchdoug, M.; Benchanaa, M. Optimization of Preparation Conditions of Activated Carbon from Nut Oil Cake Using Response Surface Methodology. Moroc. J. Chem. 2018, 6, 92–105. [Google Scholar] [CrossRef]
- Ahmad, A.; Jini, D.; Aravind, M.; Parvathiraja, C.; Ali, R.; Kiyani, M.Z.; Alothman, A. A Novel Study on Synthesis of Egg Shell Based Activated Carbon for Degradation of Methylene Blue via Photocatalysis. Arab. J. Chem. 2020, 13, 8717–8722. [Google Scholar] [CrossRef]
- Gupta, V.; Sharma, N.; Singh, U.; Arif, M.; Singh, A. Higher Oxidation Level in Graphene Oxide. Optik 2017, 143, 115–124. [Google Scholar] [CrossRef]
- Mopoung, S.; Amornsakchai, P. Production of KMnO4 Modified Activated Carbon Fiber Filter from Pineapple Leaf Carbon Fiber for Fe3+ and Ca2+ Ions Adsorption. Indian J. Sci. Technol. 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Dutournié, P.; Bruneau, M.; Brendlé, J.; Limousy, L.; Pluchon, S. Mass transfer modelling in clay-based material: Estimation of apparent diffusivity of a molecule of interest. Comptes Rendus Chim. 2019, 22, 250–257. [Google Scholar] [CrossRef]
- Jiao, W.; Liu, Z.; Huang, T.; Tan, L.; Hao, Y.; Wang, Y.; Lin, T.; Yang, H.; Sai, H. Fabrication of Hydrophilic Cellulose Graft Copolymer/SiO2 Coated Mesh for Efficient Oil-Water Separation. Colloids Surf. Physicochem. Eng. Asp. 2024, 696, 134333. [Google Scholar] [CrossRef]
- Nelson, E.S.; Iyuke, S.; Daramola, M.O.; Okewale, A. Extraction and Characterization of Silica from Empty Palm Fruit Bunch (EPFB) Ash. Processes 2023, 11, 1684. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Kodiweera, N.K.A.C.; Stallworth, P.E.; Greenbaum, S.; Bandosz, T.J. Effect of Surface Phosphorus Functionalities of Activated Carbons Containing Oxygen and Nitrogen on Electrochemical Capacitance. Carbon 2009, 47, 1576–1584. [Google Scholar] [CrossRef]
- Ruiz-Rosas, R.; García-Mateos, F.J.; Gutiérrez, M.d.C.; Rodríguez-Mirasol, J.; Cordero, T. About the Role of Porosity and Surface Chemistry of Phosphorus-Containing Activated Carbons in the Removal of Micropollutants. Front. Mater. 2019, 6, e00134. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Activated Carbons from KOH and H3PO4-Activation of Olive Residues and Its Application as Supercapacitor Electrodes. Electrochim. Acta 2017, 229, 219–228. [Google Scholar] [CrossRef]
- Horikawa, T.; Do, D.D.; Nicholson, D. Capillary Condensation of Adsorbates in Porous Materials. Adv. Colloid Interface Sci. 2011, 169, 40–58. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Zorpas, A.A. Heat of Adsorption, Adsorption Energy and Activation Energy in Adsorption and Ion Exchange Systems. Desalination Water Treat. 2012, 39, 149–157. [Google Scholar] [CrossRef]
- Suter, E.; Rutto, H.; Kohitlhetse, I. Adsorptive Removal of Pb2+ from Aqueous Solution by Magnetic Biodegradable and Polymeric Cellulose/Nylon 6 @Fe3O4 Nanocomposite Encapsulated with Chitosan. Desalination Water Treat. 2024, 320, 100782. [Google Scholar] [CrossRef]
- Kamal, M.H.M.A.; Azira, W.M.K.W.K.; Kasmawati, M.; Haslizaidi, Z.; Saime, W.N.W. Sequestration of Toxic Pb(II) Ions by Chemically Treated Rubber (Hevea brasiliensis) Leaf Powder. J. Environ. Sci. 2010, 22, 248–256. [Google Scholar] [CrossRef]
- El Bardiji, N.; Ziat, K.; Naji, A.; Saidi, M. Fractal-Like Kinetics of Adsorption Applied to the Solid/Solution Interface. ACS Omega 2020, 5, 5105–5115. [Google Scholar] [CrossRef]
- Youssif, M.M.; El-Attar, H.G.; Hessel, V.; Wojnicki, M. Recent Developments in the Adsorption of Heavy Metal Ions from Aqueous Solutions Using Various Nanomaterials. Materials 2024, 17, 5141. [Google Scholar] [CrossRef]
- Módenes, A.N.; Hinterholz, C.L.; Neves, C.V.; Sanderson, K.; Trigueros, D.E.G.; Espinoza-Quiñones, F.R.; Borba, C.E.; Steffen, V.; Scheufele, F.B.; Kroumov, A.D. A New Alternative to Use Soybean Hulls on the Adsorptive Removal of Aqueous Dyestuff. Bioresour. Technol. Rep. 2019, 6, 175–182. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: Cambridge, MA, USA, 2013; ISBN 978-0-08-097036-3. [Google Scholar]
- Salvestrini, S.; Leone, V.; Iovino, P.; Canzano, S.; Capasso, S. Considerations about the Correct Evaluation of Sorption Thermodynamic Parameters from Equilibrium Isotherms. J. Chem. Thermodyn. 2014, 68, 310–316. [Google Scholar] [CrossRef]
- Saha, P.; Chowdhury, S. Insight Into Adsorption Thermodynamics. In Thermodynamics; Tadashi, M., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-544-0. [Google Scholar]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaedi, M.; Haghdoust, S. Chapter 1—Fundamentals of Adsorption Technology. In Interface Science and Technology; Ghaedi, M., Ed.; Adsorption: Fundamental Processes and Applications; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 1–70. [Google Scholar]
- Gökırmak Söğüt, E.; Gülcan, M. Chapter 1—Adsorption: Basics, Properties, and Classification. In Adsorption Through Advanced Nanoscale Materials; Verma, C., Aslam, J., Khan, M.E., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–21. ISBN 978-0-443-18456-7. [Google Scholar]
- Hoenke, S.; Serbian, I.; Deigner, P.; Csuk, R. Mitocanic Di- and Triterpenoid Rhodamine B Conjugates. Molecules 2020, 25, 5443. [Google Scholar] [CrossRef]
- Jinendra, U.; Bilehal, D.; Nagabhushana, B.M.; Kumar, A.P. Adsorptive Removal of Rhodamine B Dye from Aqueous Solution by Using Graphene–Based Nickel Nanocomposite. Heliyon 2021, 7, e06851. [Google Scholar] [CrossRef]
- Adeola, A.O.; Cui, M.; Naccache, R. Rhodamine B Sequestration Using Acid-Precipitated and Microwave-Treated Softwood Lignin: Comparative Isotherm, Kinetics and Thermodynamic Studies. Environ. Technol. Innov. 2023, 32, 103419. [Google Scholar] [CrossRef]
- Shoaib, M.; Al-Swaidan, H.M. Effect of CO2 Flow Rate on the Synthesis of Sliced Activated Carbon from Date Palm Tree Fronds (Agro Waste) by Physical Activation. Asian J. Chem. 2014, 26, 7025–7028. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; Elchaghaby, G.A. Influence of Operating Conditions on the Removal of Cu, Zn, Cd and Pb Ions from Wastewater by Adsorption. Int. J. Environ. Sci. Technol. 2007, 4, 451–456. [Google Scholar] [CrossRef]
- Amen, R.; Yaseen, M.; Mukhtar, A.; Klemeš, J.J.; Saqib, S.; Ullah, S.; Al-Sehemi, A.G.; Rafiq, S.; Babar, M.; Fatt, C.L.; et al. Lead and Cadmium Removal from Wastewater Using Eco-Friendly Biochar Adsorbent Derived from Rice Husk, Wheat Straw, and Corncob. Clean. Eng. Technol. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Arabahmadi, V.; Ghorbani, M. Pb (II) Removal from Water Using Surface-Modified Polythiophene-Coated Rice Husk Ash Nanocomposite. Inorg. Nano-Met. Chem. 2017, 47, 1614–1624. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Biosorptive Removal of Lead by Lentil Husk. J. Environ. Chem. Eng. 2015, 3, 1088–1095. [Google Scholar] [CrossRef]
- Çelebi, H.; Gök, O. Evaluation of Lead Adsorption Kinetics and Isotherms from Aqueous Solution Using Natural Walnut Shell. Int. J. Environ. Res. 2017, 11, 83–90. [Google Scholar] [CrossRef]
- Chaduka, M.; Guyo, U.; Zinyama, N.P.; Tshuma, P.; Matsinha, L.C. Modeling and Optimization of Lead (II) Adsorption by a Novel Peanut Hull-g-Methyl Methacrylate Biopolymer Using Response Surface Methodology (RSM). Anal. Lett. 2020, 53, 1294–1311. [Google Scholar] [CrossRef]
- Chamarthy, S.; Seo, C.W.; Marshall, W.E. Adsorption of Selected Toxic Metals by Modified Peanut Shells. J. Chem. Technol. Biotechnol. 2001, 76, 593–597. [Google Scholar] [CrossRef]
- Fooladgar, S.; Teimouri, A.; Ghanavati Nasab, S. Highly Efficient Removal of Lead Ions from Aqueous Solutions Using Chitosan/Rice Husk Ash/Nano Alumina with a Focus on Optimization by Response Surface Methodology: Isotherm, Kinetic, and Thermodynamic Studies. J. Polym. Environ. 2019, 27, 1025–1042. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, D.; Gaur, J.P. Kinetic and Isotherm Modeling of Lead(II) Sorption onto Some Waste Plant Materials. Chem. Eng. J. 2009, 148, 226–233. [Google Scholar] [CrossRef]
- Imamoglu, M.; Tekir, O. Removal of Copper (II) and Lead (II) Ions from Aqueous Solutions by Adsorption on Activated Carbon from a New Precursor Hazelnut Husks. Desalination 2008, 228, 108–113. [Google Scholar] [CrossRef]
- Iyagba, E.T.; Opete, O.S.E. Removal of Chromium and Lead from Drill Cuttings Using Activated Palm Kernel Shell and Husk. Afr. J. Environ. Sci. Technol. 2009, 3, 171–179. [Google Scholar]
- Janyasuthiwong, S.; Phiri, S.M.; Kijjanapanich, P.; Rene, E.R.; Esposito, G.; Lens, P.N.L. Copper, Lead and Zinc Removal from Metal-Contaminated Wastewater by Adsorption onto Agricultural Wastes. Environ. Technol. 2015, 36, 3071–3083. [Google Scholar] [CrossRef] [PubMed]
- Kamari, S.; Ghorbani, F.; Sanati, A.M. Adsorptive Removal of Lead from Aqueous Solutions by Amine–Functionalized magMCM-41 as a Low–Cost Nanocomposite Prepared from Rice Husk: Modeling and Optimization by Response Surface Methodology. Sustain. Chem. Pharm. 2019, 13, 100153. [Google Scholar] [CrossRef]
- Kazemipour, M.; Ansari, M.; Tajrobehkar, S.; Majdzadeh, M.; Kermani, H.R. Removal of Lead, Cadmium, Zinc, and Copper from Industrial Wastewater by Carbon Developed from Walnut, Hazelnut, Almond, Pistachio Shell, and Apricot Stone. J. Hazard. Mater. 2008, 150, 322–327. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Fekry, N.A.; Abdelfattah, A.M. A Novel Nanobiosorbent of Functionalized Graphene Quantum Dots from Rice Husk with Barium Hydroxide for Microwave Enhanced Removal of Lead (II) and Lanthanum (III). Bioresour. Technol. 2020, 298, 122514. [Google Scholar] [CrossRef]
- Masoumi, A.; Hemmati, K.; Ghaemy, M. Low-Cost Nanoparticles Sorbent from Modified Rice Husk and a Copolymer for Efficient Removal of Pb(II) and Crystal Violet from Water. Chemosphere 2016, 146, 253–262. [Google Scholar] [CrossRef]
- Meena, A.K.; Kadirvelu, K.; Mishraa, G.K.; Rajagopal, C.; Nagar, P.N. Adsorption of Pb(II) and Cd(II) Metal Ions from Aqueous Solutions by Mustard Husk. J. Hazard. Mater. 2008, 150, 619–625. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, P.; Liu, X.; Han, L. Facile Fabrication of Magnetic Bio-Derived Chars by Co-Mixing with Fe3O4 Nanoparticles for Effective Pb2+ Adsorption: Properties and Mechanism. J. Clean. Prod. 2020, 262, 121350. [Google Scholar] [CrossRef]
- Meunier, N.; Laroulandie, J.; Blais, J.F.; Tyagi, R.D. Cocoa Shells for Heavy Metal Removal from Acidic Solutions. Bioresour. Technol. 2003, 90, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.N. Fruit Stones from Industrial Waste for the Removal of Lead Ions from Polluted Water. Environ. Monit. Assess. 2006, 119, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.; Sakthi, V.; Rengaraj, S. Kinetics and Equilibrium Adsorption Study of Lead(II) onto Activated Carbon Prepared from Coconut Shell. J. Colloid Interface Sci. 2004, 279, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Pai, R.S. Removal of Pb(II) from Solution Using Cellulose-Containing Materials. J. Chem. Technol. Biotechnol. 2005, 80, 176–183. [Google Scholar] [CrossRef]
- Sun, C.; Chen, T.; Huang, Q.; Wang, J.; Lu, S.; Yan, J. Enhanced Adsorption for Pb(II) and Cd(II) of Magnetic Rice Husk Biochar by KMnO4 Modification. Environ. Sci. Pollut. Res. 2019, 26, 8902–8913. [Google Scholar] [CrossRef]
- Zulkali, M.M.D.; Ahmad, A.L.; Norulakmal, N.H.; Sharifah, N.S. Comparative Studies of Oryza sativa L. Husk and Chitosan as Lead Adsorbent. J. Chem. Technol. Biotechnol. 2006, 81, 1324–1327. [Google Scholar] [CrossRef]
- Yan, J.; Lan, G.; Qiu, H.; Chen, C.; Liu, Y.; Du, G.; Zhang, J. Adsorption of Heavy Metals and Methylene Blue from Aqueous Solution with Citric Acid Modified Peach Stone. Sep. Sci. Technol. 2018, 53, 1678–1688. [Google Scholar] [CrossRef]
- Bello, O.S.; Adegoke, K.A.; Fagbenro, S.O.; Lameed, O.S. Functionalized Coconut Husks for Rhodamine-B Dye Sequestration. Appl. Water Sci. 2019, 9, 189. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hassani, A.J.; Mohamed, A.R.; Najafpour, G.D. Removal of Rhodamine B from Aqueous Solution Using Palm Shell-Based Activated Carbon: Adsorption and Kinetic Studies. J. Chem. Eng. Data 2010, 55, 5777–5785. [Google Scholar] [CrossRef]
- Sureshkumar, M.V.; Namasivayam, C. Adsorption Behavior of Direct Red 12B and Rhodamine B from Water onto Surfactant-Modified Coconut Coir Pith. Colloids Surf. Physicochem. Eng. Asp. 2008, 317, 277–283. [Google Scholar] [CrossRef]
- Atalay, E.H.; Kadıoğlu, E.N.; Akbal, F. High-Efficiency Removal of Rhodamine B Using Modified Biochar from Agricultural Waste Pine Nut Shell: Investigation of Kinetics, Isotherms, and Artificial Neural Network Modeling. Biomass Convers. Biorefinery 2024, 15, 12137–12150. [Google Scholar] [CrossRef]
- Tsamo, C.; Kidwang, G.D.; Dahaina, D.C. Removal of Rhodamine B from Aqueous Solution Using Silica Extracted from Rice Husk. SN Appl. Sci. 2020, 2, 256. [Google Scholar] [CrossRef]
- da Silva Lacerda, V.; López-Sotelo, J.B.; Correa-Guimarães, A.; Hernández-Navarro, S.; Sánchez-Báscones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; Martín-Gil, J. Rhodamine B Removal with Activated Carbons Obtained from Lignocellulosic Waste. J. Environ. Manag. 2015, 155, 67–76. [Google Scholar] [CrossRef]
- Bello, O.S.; Adegoke, K.A.; Sarumi, O.O.; Lameed, O.S. Functionalized Locust Bean Pod (Parkia biglobosa) Activated Carbon for Rhodamine B Dye Removal. Heliyon 2019, 5, e02323. [Google Scholar] [CrossRef]
- Periasamy, K. Efficient Usage of Rice Husk Carbon and Chitosan Composite for Adsorption of Rhodamine B from Waste Water. J. Pharm. Chem. Biol. Sci. 2018, 6, 107–121. [Google Scholar]
- Chebbi, M.; Youcef, S.; Youcef, L.; Soudani, A.; Dridi, C.; Sahli, A.; Houchet, A.; Deroues, C. Single and Combined Treatment Processes for Rhodamine B Removal by Coagulation–Flocculation and Adsorption. RSC Adv. 2024, 14, 37833–37845. [Google Scholar] [CrossRef]
- Namasivayam, C.; Radhika, R.; Suba, S. Uptake of Dyes by a Promising Locally Available Agricultural Solid Waste: Coir Pith. Waste Manag. 2001, 21, 381–387. [Google Scholar] [CrossRef]
- Ding, L.; Zou, B.; Gao, W.; Liu, Q.; Wang, Z.; Guo, Y.; Wang, X.; Liu, Y. Adsorption of Rhodamine-B from Aqueous Solution Using Treated Rice Husk-Based Activated Carbon. Colloids Surf. Physicochem. Eng. Asp. 2014, 446, 1–7. [Google Scholar] [CrossRef]
- Gad, H.M.H.; El-Sayed, A.A. Activated Carbon from Agricultural By-Products for the Removal of Rhodamine-B from Aqueous Solution. J. Hazard. Mater. 2009, 168, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, K.S.; Ramesh, S.T. Removal of Dyes Using Agricultural Waste as Low-Cost Adsorbents: A Review. Appl. Water Sci. 2013, 3, 773–790. [Google Scholar] [CrossRef]
- Doğan, M.; Abak, H.; Alkan, M. Adsorption of Methylene Blue onto Hazelnut Shell: Kinetics, Mechanism and Activation Parameters. J. Hazard. Mater. 2009, 164, 172–181. [Google Scholar] [CrossRef]
- Porselvi, E.; Krishnamoorthy, P. Removal of Acid Yellow by Agricultural Waste. J. Mater. Environ. Sci. 2014, 5, 408–415. [Google Scholar]
- Walhout, P.K.; He, Z.; Dutagaci, B.; Nawrocki, G.; Feig, M. Molecular Dynamics Simulations of Rhodamine B Zwitterion Diffusion in Polyelectrolyte Solutions. J. Phys. Chem. B 2022, 126, 10256–10272. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Babamale, H.F. A Short Review on the Removal of Rhodamine B Dye Using Agricultural Waste-Based Adsorbents. Asian J. Chem. Sci. 2020, 25–37. [Google Scholar] [CrossRef]
- Kainth, S.; Sharma, P.; Pandey, O.P. Green Sorbents from Agricultural Wastes: A Review of Sustainable Adsorption Materials. Appl. Surf. Sci. Adv. 2024, 19, 100562. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, G.; Zhang, Y.; Su, Y.; Zhang, H. The Deactivation Mechanisms, Regeneration Methods and Devices of Activated Carbon in Applications. J. Clean. Prod. 2024, 476, 143751. [Google Scholar] [CrossRef]
- Wambu, E.W. The Graphene Surface Chemistry and Adsorption Science. In Chemistry of Graphene—Synthesis, Reactivity, Applications and Toxicities; IntechOpen: London, UK, 2024; ISBN 978-1-83769-283-5. [Google Scholar]
- Tsubouchi, L.M.S.; de Almeida, E.A.; Santo, D.E.; Bona, E.; Pereira, G.L.D.; Jegatheesan, V.; Cardozo-Filho, L.; Peron, A.P.; Junior, O.V. Production and Characterization of Graphene Oxide for Adsorption Analysis of the Emerging Pollutant Butylparaben. Water 2024, 16, 3703. [Google Scholar] [CrossRef]
- Lombardo, C.; Varrica, G.; Forte, G. Computational Study on Thiolated and Functionalized Graphene Oxide for Heavy Metal Recovery. Comput. Theor. Chem. 2025, 1248, 115211. [Google Scholar] [CrossRef]
- Raza, S.A.; Naqvi, S.Q.; Usman, A.; Jennings, J.R.; Soon, Y.W. Spectroscopic Study of the Interaction between Rhodamine B and Graphene. J. Photochem. Photobiol. Chem. 2021, 418, 113417. [Google Scholar] [CrossRef]
- Pijović, M.; Manić, N.; Anićijević, D.V.; Krstić, A.; Mitrić, M.; Matić, T.; Janković, B. Simple and Effective One-Step Production of High-Quality Mesoporous Pyrolytic Char from Waste Tires: Rhodamine B Adsorption Kinetics and Density Functional Theory (DFT) Study. Diam. Relat. Mater. 2022, 121, 108768. [Google Scholar] [CrossRef]
- Guo, S.; Zou, Z.; Chen, Y.; Long, X.; Liu, M.; Li, X.; Tan, J.; Chen, R. Synergistic Effect of Hydrogen Bonding and π-π Interaction for Enhanced Adsorption of Rhodamine B from Water Using Corn Straw Biochar. Environ. Pollut. 2023, 320, 121060. [Google Scholar] [CrossRef]
- Şenol, Z.M.; Messaoudi, N.E.; Fernine, Y.; Keskin, Z.S. Bioremoval of Rhodamine B Dye from Aqueous Solution by Using Agricultural Solid Waste (Almond Shell): Experimental and DFT Modeling Studies. Biomass Convers. Biorefinery 2024, 14, 17927–17940. [Google Scholar] [CrossRef]
- Aygün, A.; Yenisoy-Karakaş, S.; Duman, I. Production of Granular Activated Carbon from Fruit Stones and Nutshells and Evaluation of Their Physical, Chemical and Adsorption Properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Turk Sekulić, M.; Pap, S.; Stojanović, Z.; Bošković, N.; Radonić, J.; Šolević Knudsen, T. Efficient Removal of Priority, Hazardous Priority and Emerging Pollutants with Prunus armeniaca Functionalized Biochar from Aqueous Wastes: Experimental Optimization and Modeling. Sci. Total Environ. 2018, 613–614, 736–750. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, C.; Yang, J.; Yan, B.; Liu, J.; Wang, S.; Li, Q.; Zhou, M. Preparation and Characterization of Apricot Kernel Shell Biochar and Its Adsorption Mechanism for Atrazine. Sustainability 2022, 14, 4082. [Google Scholar] [CrossRef]
- Pap, S.; Paunovic, O.; Prosen, H.; Kraševec, I.; Trebše, P.; Niemi, L.; Taggart, M.A.; Turk Sekulic, M. Removal of Benzotriazole Derivatives by Biochar: Potential Environmental Applications. Environ. Pollut. 2023, 334, 122205. [Google Scholar] [CrossRef]
- Sklivaniotis, L.N.; Economou, P.; Karapanagioti, H.K.; Manariotis, I.D. Chlorine Removal from Water by Biochar Derived from Various Food Waste Natural Materials. Environ. Process. 2022, 10, 4. [Google Scholar] [CrossRef]
- Abdel-Raouf, M.E. Chapter 5—Fruit Stones as Green Materials for Wastewater Remediation. In Emerging Techniques for Treatment of Toxic Metals from Wastewater; Ahmad, A., Kumar, R., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 83–101. ISBN 978-0-12-822880-7. [Google Scholar]
- Sharififard, H.; Sharififard, I. Removal of Reactive Red Dye 198 by Modified Apricot Kernel Shell Derived-Activated Carbon Bio-Composite: Kinetics, Equilibrium, and Thermodynamics. J. Text. Sci. Technol. 2024, 12, 1–17. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal Crystallinity Index Revisited by the Simulation of X-Ray Diffraction Patterns of Cotton Cellulose Iβ and Cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Liu, Y.; He, Z.; Hinchliffe, D.J.; Fang, D. Assessment of Segal Method for Identifying Crystallinity Evolution in Developing Cotton Fibers. Agric. Environ. Lett. 2024, 9, e20138. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Cranston, R.W.; Inkley, F.A. 17 The Determination of Pore Structures from Nitrogen Adsorption Isotherms. In Advances in Catalysis; Farkas, A., Ed.; Proceedings of the International Congress on Catalysis; Academic Press: Cambridge, MA, USA, 1957; Volume 9, pp. 143–154. [Google Scholar] [CrossRef]
- Dollimore, D.; Heal, G.R. Pore-Size Distribution in Typical Adsorbent Systems. J. Colloid Interface Sci. 1970, 33, 508–519. [Google Scholar] [CrossRef]
- Galarneau, A.; Villemot, F.; Rodriguez, J.; Fajula, F.; Coasne, B. Validity of the T-Plot Method to Assess Microporosity in Hierarchical Micro/Mesoporous Materials. Langmuir 2014, 30, 13266–13274. [Google Scholar] [CrossRef]
- Horváth, G.; Kawazoe, K. Method for the Calculation of Effective Pore Size Distribution in Molecular Sieve Carbon. J. Chem. Eng. Jpn. 1983, 16, 470–475. [Google Scholar] [CrossRef]
- Zamri, N.I.I.; Zulmajdi, S.L.N.; Daud, N.Z.A.; Mahadi, A.H.; Kusrini, E.; Usman, A. Insight into the Adsorption Kinetics, Mechanism, and Thermodynamics of Methylene Blue from Aqueous Solution onto Pectin-Alginate-Titania Composite Microparticles. SN Appl. Sci. 2021, 3, 222. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Xu, M.; Liu, S.; Yao, J. Fast Adsorption of Methyl Blue on Zeolitic Imidazolate Framework-8 and Its Adsorption Mechanism. RSC Adv. 2016, 6, 109608–109612. [Google Scholar] [CrossRef]
- Ediati, R.; Putra Hidayat, A.R.; Syukrie, T.D.; Zulfa, L.L.; Jannah, M.; Harmami, H.; Fansuri, H.; Ibnu Ali, B.T. Investigation the Adsorption Kinetic and Isotherm Studies of Remazol Red 5B Dye on Benzoic Acid Modified Al2O3/UiO-66 Composite. Arab. J. Chem. 2025, 18, 106078. [Google Scholar] [CrossRef]
- Maccarino, L.; Miglio, V.; Paul, G.; Golemme, G.; Bisio, C.; Marchese, L. Swellable Hybrid Silicas for the Removal of Rhodamine B Dye from Aqueous Phase. Microporous Mesoporous Mater. 2024, 375, 113178. [Google Scholar] [CrossRef]
- Abdel Salam, O.E.; Reiad, N.A.; ElShafei, M.M. A Study of the Removal Characteristics of Heavy Metals from Wastewater by Low-Cost Adsorbents. J. Adv. Res. 2011, 2, 297–303. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Negm, N.A.; Hefni, H.H.H.; Wahab, M.M.A. Metal Adsorption by Agricultural Biosorbents: Adsorption Isotherm, Kinetic and Biosorbents Chemical Structures. Int. J. Biol. Macromol. 2015, 81, 400–409. [Google Scholar] [CrossRef]
- Humelnicu, I.; Băiceanu, A.; Ignat, M.-E.; Dulman, V. The Removal of Basic Blue 41 Textile Dye from Aqueous Solution by Adsorption onto Natural Zeolitic Tuff: Kinetics and Thermodynamics. Process Saf. Environ. Prot. 2017, 105, 274–287. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of Nickel(II) Ions onto Sargassum Wightii: Application of Two-Parameter and Three-Parameter Isotherm Models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef]
- Ghasemi, M.; Javadian, H.; Ghasemi, N.; Agarwal, S.; Gupta, V.K. Microporous Nanocrystalline NaA Zeolite Prepared by Microwave Assisted Hydrothermal Method and Determination of Kinetic, Isotherm and Thermodynamic Parameters of the Batch Sorption of Ni (II). J. Mol. Liq. 2016, 215, 161–169. [Google Scholar] [CrossRef]
- Arshadi, M.; Amiri, M.J.; Mousavi, S. Kinetic, Equilibrium and Thermodynamic Investigations of Ni(II), Cd(II), Cu(II) and Co(II) Adsorption on Barley Straw Ash. Water Resour. Ind. 2014, 6, 1–17. [Google Scholar] [CrossRef]
- Angin, D. Utilization of Activated Carbon Produced from Fruit Juice Industry Solid Waste for the Adsorption of Yellow 18 from Aqueous Solutions. Bioresour. Technol. 2014, 168, 259–266. [Google Scholar] [CrossRef]
- Demiral, H.; Güngör, C. Adsorption of Copper(II) from Aqueous Solutions on Activated Carbon Prepared from Grape Bagasse. J. Clean. Prod. 2016, 124, 103–113. [Google Scholar] [CrossRef]
- Ben-Ali, S.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and Adsorption Capacity of Raw Pomegranate Peel Biosorbent for Copper Removal. J. Clean. Prod. 2017, 142, 3809–3821. [Google Scholar] [CrossRef]
- Yang, C. Statistical Mechanical Aspects of Adsorption Systems Obeying the Temkin Isotherm. J. Phys. Chem. 1993, 97, 7097–7101. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic Parameters of Cadmium Adsorption onto Orange Peel Calculated from Various Methods: A Comparison Study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Januszewicz, K.; Kazimierski, P.; Cymann-Sachajdak, A.; Hercel, P.; Barczak, B.; Wilamowska-Zawłocka, M.; Kardaś, D.; Łuczak, J. Conversion of Waste Biomass to Designed and Tailored Activated Chars with Valuable Properties for Adsorption and Electrochemical Applications. Environ. Sci. Pollut. Res. 2023, 30, 96977–96992. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Wu, F.-C.; Juang, R.-S. Characteristics and Applications of the Lagergren’s First-Order Equation for Adsorption Kinetics. J. Taiwan Inst. Chem. Eng. 2010, 41, 661–669. [Google Scholar] [CrossRef]
- Naushad, M.; ALOthman, Z.A.; Inamuddin; Javadian, H. Removal of Pb(II) from Aqueous Solution Using Ethylene Diamine Tetra Acetic Acid-Zr(IV) Iodate Composite Cation Exchanger: Kinetics, Isotherms and Thermodynamic Studies. J. Ind. Eng. Chem. 2015, 25, 35–41. [Google Scholar] [CrossRef]
- Chinoune, K.; Bentaleb, K.; Bouberka, Z.; Nadim, A.; Maschke, U. Adsorption of Reactive Dyes from Aqueous Solution by Dirty Bentonite. Appl. Clay Sci. 2016, 123, 64–75. [Google Scholar] [CrossRef]
- McKay, G.; Ho, Y.S.; Ng, J.C.Y. Biosorption of Copper from Waste Waters: A Review. Sep. Purif. Methods 1999, 28, 87–125. [Google Scholar] [CrossRef]
- El-Khaiary, M.I.; Malash, G.F. Common Data Analysis Errors in Batch Adsorption Studies. Hydrometallurgy 2011, 105, 314–320. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the Adsorption Kinetics Models for the Removal of Contaminants from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic Models of Sorption: A Theoretical Analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Al-Odayni, A.-B.; Alsubaie, F.S.; Saeed, W.S. Nitrogen-Rich Polyaniline-Based Activated Carbon for Water Treatment: Adsorption Kinetics of Anionic Dye Methyl Orange. Polymers 2023, 15, 806. [Google Scholar] [CrossRef]
- Lopes, E.C.N.; dos Anjos, F.S.C.; Vieira, E.F.S.; Cestari, A.R. An Alternative Avrami Equation to Evaluate Kinetic Parameters of the Interaction of Hg(II) with Thin Chitosan Membranes. J. Colloid Interface Sci. 2003, 263, 542–547. [Google Scholar] [CrossRef]
- Cestari, A.R.; Vieira, E.F.S.; Lopes, E.C.N.; da Silva, R.G. Kinetics and Equilibrium Parameters of Hg(II) Adsorption on Silica–Dithizone. J. Colloid Interface Sci. 2004, 272, 271–276. [Google Scholar] [CrossRef]
- Heydari-Gorji, A.; Sayari, A. CO2 Capture on Polyethylenimine-Impregnated Hydrophobic Mesoporous Silica: Experimental and Kinetic Modeling. Chem. Eng. J. 2011, 173, 72–79. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Hameed, B.H.; El-Khaiary, M.I. Equilibrium, Kinetics and Mechanism of Malachite Green Adsorption on Activated Carbon Prepared from Bamboo by K2CO3 Activation and Subsequent Gasification with CO2. J. Hazard. Mater. 2008, 157, 344–351. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S., Jr. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics1. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Kajjumba, G.W.; Aydın, S.; Güneysu, S. Adsorption Isotherms and Kinetics of Vanadium by Shale and Coal Waste. Adsorpt. Sci. Technol. 2018, 36, 936–952. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. Pesticides Removal from Waste Water by Activated Carbon Prepared from Waste Rubber Tire. Water Res. 2011, 45, 4047–4055. [Google Scholar] [CrossRef]
- Little, J.; Moler, C. MATLAB; MathWorks: Natick, MA, USA, 2023. [Google Scholar]
- Kaushal, A.; Singh, S.K. Critical Analysis of Adsorption Data Statistically. Appl. Water Sci. 2017, 7, 3191–3196. [Google Scholar] [CrossRef]
- Peng, M.; Nguyen, A.V.; Wang, J.; Miller, R. A Critical Review of the Model Fitting Quality and Parameter Stability of Equilibrium Adsorption Models. Adv. Colloid Interface Sci. 2018, 262, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Rajahmundry, G.K.; Garlapati, C.; Kumar, P.S.; Alwi, R.S.; Vo, D.-V.N. Statistical Analysis of Adsorption Isotherm Models and Its Appropriate Selection. Chemosphere 2021, 276, 130176. [Google Scholar] [CrossRef]
- Andreu-Vilarroig, C.; Cortés, J.-C.; Navarro-Quiles, A.; Sferle, S.-M. Statistical Analysis of a General Adsorption Kinetic Model with Randomness in Its Formulation. An Application to Real Data. Match-Commun. Math. Comput. Chem. 2023, 90, 19–51. [Google Scholar] [CrossRef]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Hai, T.N. Comments on “Effect of Temperature on the Adsorption of Methylene Blue Dye onto Sulfuric Acid–Treated Orange Peel". Chem. Eng. Commun. 2017, 204, 134–139. [Google Scholar] [CrossRef]
- Zhou, X. Comment on the Thermodynamic Calculation Using the Non-Standard Equilibrium Constant Re Jemutai-Kimosop et al. (2020) and Conde-Cid et al. (2019). Environ. Res. 2020, 187, 109610. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry, Revised and Expanded, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-315-27428-7. [Google Scholar]
- Atkins, P.W.; Paula, J.D.; Keeler, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2018; ISBN 978-0-19-876986-6. [Google Scholar]
- Biggar, J.W.; Cheung, M.W. Adsorption of Picloram (4-Amino-3,5,6-Trichloropicolinic Acid) on Panoche, Ephrata, and Palouse Soils: A Thermodynamic Approach to the Adsorption Mechanism. Soil Sci. Soc. Am. J. 1973, 37, 863–868. [Google Scholar] [CrossRef]
- Khan, A.A.; Singh, R.P. Adsorption Thermodynamics of Carbofuran on Sn (IV) Arsenosilicate in H+, Na+ and Ca2+ Forms. Colloids Surf. 1987, 24, 33–42. [Google Scholar] [CrossRef]
- Tran, H.N.; Lima, E.C.; Juang, R.-S.; Bollinger, J.-C.; Chao, H.-P. Thermodynamic Parameters of Liquid–Phase Adsorption Process Calculated from Different Equilibrium Constants Related to Adsorption Isotherms: A Comparison Study. J. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Rawajfih, Z.; Mohammad, H.A.; Nsour, N.; Ibrahim, K. Study of Equilibrium and Thermodynamic Adsorption of α-Picoline, β-Picoline, and γ-Picoline by Jordanian Zeolites: Phillipsite and Faujasite. Microporous Mesoporous Mater. 2010, 132, 401–408. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Martyna, G.J.; Tuckerman, M.E. A Reciprocal Space Based Method for Treating Long Range Interactions in Ab Initio and Force-Field-Based Calculations in Clusters. J. Chem. Phys. 1999, 110, 2810–2821. [Google Scholar] [CrossRef]



| AKS-CO2 | AKS-H3PO4 | ||||
|---|---|---|---|---|---|
| Frequency (cm−1) | Allocation of Bands | Components | Frequency (cm−1) | Allocation of Bands | Components |
| 3551 | The stretching vibration of O−H bonds in hydroxyl groups | Alcohols | 3551 | The stretching vibration of O–H bonds in hydroxyl groups | Alcohols |
| 3480 | Dimeric OH stretch | The water dimer | 3478 | Dimeric OH stretch | The water dimer |
| 3415 | O–H tensile vibration | The presence of phenols, alcohols, or carboxylic acid | 3413 | O–H tensile vibration | The presence of phenols, alcohols, or carboxylic acid |
| 3234 | Vibrational (stretching) of O–H bond with hydrogen bonding | Present in physically activated biochar | 3234 | Vibrational (stretching) of O–H bond with hydrogen bonding | Present in chemically activated biochar |
| 3120 | Aromatic C–H stretching band in polynuclear system | Aromatics present in physically activated biochar | 2348 | P–H stretching of ester compound | P-molecule-specific functional group in chemically activated biochar |
| 2027 | C–H (alkane) stretching vibrations in aromatic compound | Aromatics present in physically activated biochar | 1797 | Carbonyl (C = O) vibration of ester | Esters present in chemically activated biochar |
| 1636 | C = O group stretching vibration | Presence of aldehyde, esters, carboxylic acid, and ketone, in physically activated biochar | 1636 | C = O group stretching vibration | Presence of aldehyde, esters, carboxylic acid, and ketone, in physically activated biochar |
| 1618 | Aromatic skeletal vibration, C = C stretching | Aromatic structures present in physically activated biochar | 1618 | Aromatic skeletal vibration, C = C stretching | Aromatic structures present in chemically activated biochar |
| 1370 | Phenolic OH in-plain deformation | From lignin | 1383 | C = O symmetric stretching vibration of COO− groups | Oxygen-containing groups in chemically activated biochar |
| 618 | C–H bond in aromatic and hetero-atomic compounds | Aromatics present in physically activated biochar | 754 | Aromatic hydrocarbons (δCH); aromatic C–H stretching vibration | Aromatics structures present in chemically activated biochar |
| 480 | Bending motions of the Si–O–Si bonds | Silica (SiO2) | 618 | C–H bond in aromatic and hetero-atomic compounds | Aromatics present in chemically activated biochar |
| 480 | Bending motions of the Si–O–Si bonds | Silica (SiO2) | |||
| Sample | SBET (m2/g) | Smeso (m2/g) | Vp(H-K) (cm3/g) | rm(H-K) (nm) | rmax(H-K) (nm) | Vmicro (cm3/g) | Vp(B.J.H.) (cm3/g) | rm(B.J.H.) (nm) | rmax(B.J.H.) (nm) | Vp(C-I) (cm3/g) | rm(C-I) (nm) | rmax(C-I) (nm) | Vp(D-H) (cm3/g) | rm(D-H) (nm) | rmax(D-H) (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AKS-CO2 | 378.08 | 59.089 | 0.2148 | 0.2635 | 1.4330 | 0.1839 | 0.0051 | 1.7776 | 1.3414 | 0.0051 | 1.7793 | 1.7796 | 0.0050 | 1.7795 | 1.7792 |
| AKS-H3PO4 | 1159.4 | 547.55 | 0.5902 | 0.3512 | 0.2887 | 0.2966 | 0.0694 | 1.7883 | 1.3283 | 0.0694 | 1.7884 | 1.3283 | 0.0688 | 1.7905 | 1.3283 |
| Pb2+ | RhB | |||||
|---|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C | |
| R2 | 0.999 | 0.997 | 0.995 | 0.977 | 0.992 | 0.916 |
| RSS | 2.60 × 10−4 | 1.12 × 10−4 | 4.53 × 10−5 | 0.08113 | 8.7453 × 10−4 | 0.11949 |
| F-value | 2552.7 | 969.7 | 623.1 | 129.11968 | 420.90161 | 32.9 |
| RL | 0.027–0.077 | 0.033–0.092 | 0.046–0.127 | 0.12–0.28 | 0.06–0.17 | 0.11–0.27 |
| E, kJ/mol | 15.9 | 13.6 | 12.8 | 15.9 | 13.4 | 13.4 |
| R, % | 80.5 | 82.7 | 85.1 | 30.1 | 80.3 | 44.9 |
| Pb2+ | |||||||
|---|---|---|---|---|---|---|---|
| Adsorbent | qm (mg/g) | R (%) | pH | D (g/L) | C (mg/L) | T (°C) | Reference |
| Rice husks | − | 99.0 | 6.5 | 5.0–40.0 | 25.0 | 25.0 | [76] |
| Rice husk biochar | − | 96.4 | 5.5 | 1.0–4.0 | 1950.0 | 25.0 | [77] |
| Polythiophene-coated rice husk ash nanocomposite | 34.5 | 98.1 | 4.0 | 5.0–20.0 | 50.0–400.0 | 25.0–65.0 | [78] |
| Lentil husk | 81.4 | 98.0 | 5.0 | 2.0 | 20.0–250.0 | 20.0–35.0 | [79] |
| Walnut shell | 9.9 | 92.3 | 4.0 | 1.0–50.0 | 100.0 | 25.0 | [80] |
| Peanut hull-g-methyl methacrylate | 370.4 | 99.3 | 5.7 | 2.0–12.0 | 5.0–100.0 | 20.0–50.0 | [81] |
| Modified peanut shells | 130.5 | − | 4.6–5.0 | − | 4144.0 | 25.0 | [82] |
| Chitosan/rice husk ash/nano-γ alumina | 181.8 | 91.0 | 5.0 | − | 250.0–550.0 | 10.0–40.0 | [83] |
| Peanut hulls | 69.8 | − | 5.0 | 0.1–10.0 | 15.0–200.0 | 25.0 | [84] |
| Hazelnut husks | 13.1 | 97.2 | 5.7 | 2.0–20.0 | 5.0–200.0 | 18.0 | [85] |
| Palm kernel husk | − | 88.0 | 5.0 | 20.0–100.0 | 5.0–15.0 | 25.0 | [86] |
| Groundnut shell | − | 98.0 | 3.0 | 2.4–8.8 | 5.0–105.0 | 25.0 | [87] |
| Rice husk nanocomposite | 1665.0 | 96.8 | 5.2 | 0.1–2.0 | 15.0–150.0 | 25.0 | [88] |
| Apricot stone | − | 89.6 | 6.0–10.0 | − | 30.0 | 25.0 | [89] |
| Functionalized graphene from rice husk | 748.5 | 99.8 | 7.0 | 10.0–50.0 | 20.7 | 18.0–80.0 | [90] |
| Treated rice husk | 93.5 | 95.0 | 7.0 | 5.0 | 100.0–800.0 | 20.0–50.0 | [91] |
| Mustard husk | 30.5 | 100.0 | 6.0 | 6.0–12.0 | 1.0–5.0 | 20.0–60.0 | [92] |
| Magnetic coconut husk-activated carbon | 43.4 | − | 2.0–6.0 | − | 50.0–500.0 | 25.0 | [93] |
| Cocoa shells | 33.4 | 95.0 | 2.0 | 15.0 | 100.0 | 22.0 | [94] |
| Apricot stone | 1.3 | 95.3 | 7.0 | 10.0–40.0 | 5.0–500.0 | 25.0 | [95] |
| Peach stone | 2.3 | 97.6 | 7.0 | 10.0–40.0 | 5.0–500.0 | 25.0 | [95] |
| Coconut shell-activated carbon | 26.5 | 92.5 | 4.5 | 0.2–2.0 | 10.0–50.0 | 35.0–45.0 | [96] |
| Shells of groundnut | 22.0 | 82.8 | 4.9 | 20.0 | 116.0–651.4 | 30.0 | [97] |
| Magnetic rice husk biochar | 148.0 | 95.0 | 2.5–5.8 | 2.5 | 10.0–500.0 | 25.0 | [98] |
| Rice husk | 5.7 | − | 5.0 | 2.0–20.0 | 10.0–200.0 | 30.0–60.0 | [99] |
| Peach stone modified by the citric acid | 118.8 | 93.4 | 2.0–7.0 | − | 6.0–120.0 | 30.0 | [100] |
| AKS-H3PO4 | 127.6 | 85.1 | 5.0 a | 1.0 | 50.0–150.0 | 45.0 | This study |
| RhB | |||||||
| Adsorbent | qm (mg/g) | R (%) | pH | D (g/L) | C (mg/L) | T (°C) | Reference |
| Sulfuric acid-activated coconut husk | 16.7 | 99.2 | 7.0 | − | 200.0–1000.0 | 30.0–50.0 | [101] |
| Palm shell-based activated carbon | − | 95.0 | 3.0 | − | 41.8–208.8 b | 50.0 | [102] |
| Surfactant-modified coconut coir pith | 14.9 | 90.0 | 10.0 | 20.0 | 10.0 | − | [103] |
| Modified pine nut shell biochar | 110.7 | ≈ 100.0 | 3.0 | 0.5 | 10.0 | 25.0 | [104] |
| Rice husk ash (RHA) | 6.0 | 84.0 | 7.0 | 0.05–0.5 | 10.0 | 27.0 | [105] |
| Acid-functionalized coconut husk | 1666.7 | − | 3.0–11.0 | 0.1 | 200.0–1000.0 | 30.0–50.0 | [101] |
| Pine nut shell impregnated with CaCl2 | 34.0 | 84.0 | 2.0–10.0 | 2.0–8.0 | 65.0–140.0 | 25.0–45.0 | [106] |
| Pine nut shell impregnated with H3PO4 | 33.1 | 82.5 | 2.0–10.0 | 2.0–8.0 | 65.0–140.0 | 25.0–45.0 | [106] |
| Acid modified locust bean pod (H3PO4) | 1111.1 | 95.0 | 3.0–11.0 | 0.1 | 200.0–1000.0 | 30.0–50.0 | [107] |
| Rich husk with chitosan composited activated with H3PO4 | 1.2 | 94.0 | 2.0–8.0 | 0.1–1.2 | 4.0–20.0 | 30.0–70.0 | [108] |
| Olive stones biochar | 2.5 | 97.8 | 2.0−12.0 | 4.0 | 1.0–100.0 | 20.0–50.0 | [109] |
| Coir pith biochar | 2.6 | 50.0 | 2.1–11.1 | 7.0 | 10.0–40.0 | 32.0 | [110] |
| Treated rice husk-activated carbon (KOH) | 290.0 | 95.3 | 1.3–10.2 | 10.0 | 300.0 | 19.8 | [111] |
| Bagasse pith activated carbon (H3PO4) | 198.6 | − | 5.7 | 1.0 | 100.0–600.0 | 20.0 | [112] |
| AKS-H3PO4 | 94.7 | 81.3 | 5.0 a | 1.0 | 50.0–150.0 | 35.0 | This study |
| Surface Model | Optimized Geometry | ΔEads |
|---|---|---|
| Bare graphene | 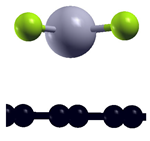 | −1.42 eV |
| H-saturated MV | 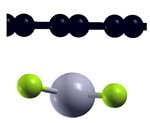 | n.s. |
| H-saturated MV with OH group | 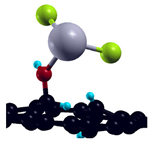 | −1.81 eV |
| H-saturated MV with COOH group |  | −0.25 eV |
| Simple MV with PH2 group | 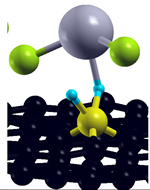 | −2.67 eV |
| Surface Model | Optimized Geometry | ΔEads |
|---|---|---|
| Bare graphene | 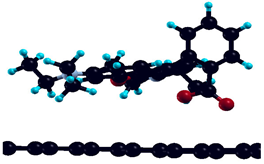 | −0.63 eV |
| H-saturated MV | 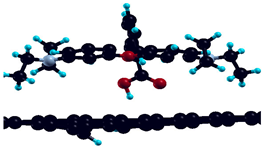 | −0.771 eV |
| Simple MV with PH2 group |  | −3.92 eV |
| Simple MV with OH group |  | −3.41 eV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pijović Radovanović, M.; Ječmenica Dučić, M.; Vasić Anićijević, D.; Dodevski, V.; Živković, S.; Pavićević, V.; Janković, B. Activated Carbons from Apricot Kernel Shells for Wastewater Treatment: Adsorption of Pb2+ and Rhodamine B with Equilibrium, Kinetics, Thermodynamics, and DFT Analysis. Processes 2025, 13, 1715. https://doi.org/10.3390/pr13061715
Pijović Radovanović M, Ječmenica Dučić M, Vasić Anićijević D, Dodevski V, Živković S, Pavićević V, Janković B. Activated Carbons from Apricot Kernel Shells for Wastewater Treatment: Adsorption of Pb2+ and Rhodamine B with Equilibrium, Kinetics, Thermodynamics, and DFT Analysis. Processes. 2025; 13(6):1715. https://doi.org/10.3390/pr13061715
Chicago/Turabian StylePijović Radovanović, Milena, Marija Ječmenica Dučić, Dragana Vasić Anićijević, Vladimir Dodevski, Sanja Živković, Vladimir Pavićević, and Bojan Janković. 2025. "Activated Carbons from Apricot Kernel Shells for Wastewater Treatment: Adsorption of Pb2+ and Rhodamine B with Equilibrium, Kinetics, Thermodynamics, and DFT Analysis" Processes 13, no. 6: 1715. https://doi.org/10.3390/pr13061715
APA StylePijović Radovanović, M., Ječmenica Dučić, M., Vasić Anićijević, D., Dodevski, V., Živković, S., Pavićević, V., & Janković, B. (2025). Activated Carbons from Apricot Kernel Shells for Wastewater Treatment: Adsorption of Pb2+ and Rhodamine B with Equilibrium, Kinetics, Thermodynamics, and DFT Analysis. Processes, 13(6), 1715. https://doi.org/10.3390/pr13061715










