Boosting Agroforestry Waste Valorization: Red Mud Oxygen Carriers with Tailored Oxygen Release for Enhanced Chemical Looping Gasification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
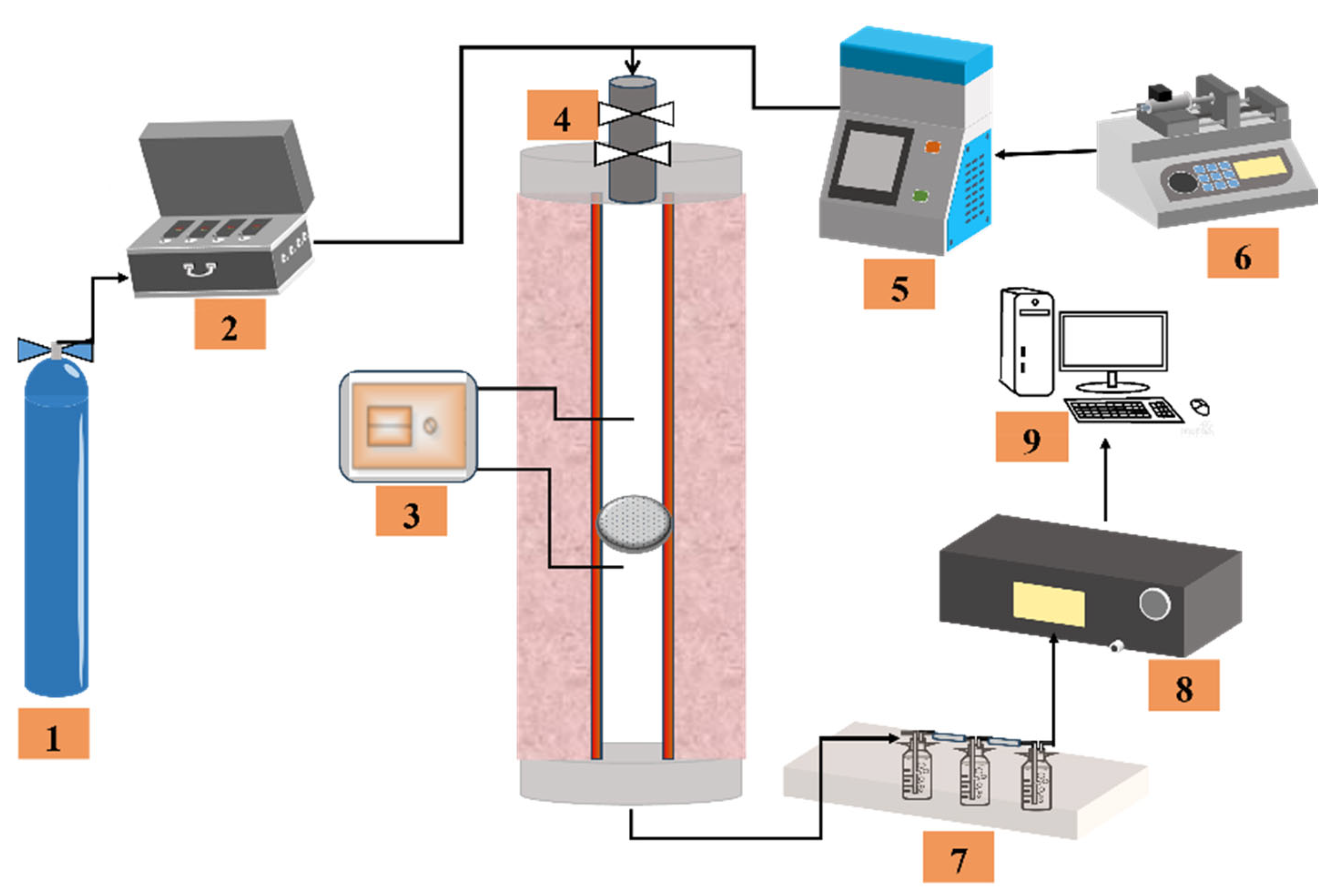
2.2. Experiment Procedures
2.3. Data Processing
3. Results
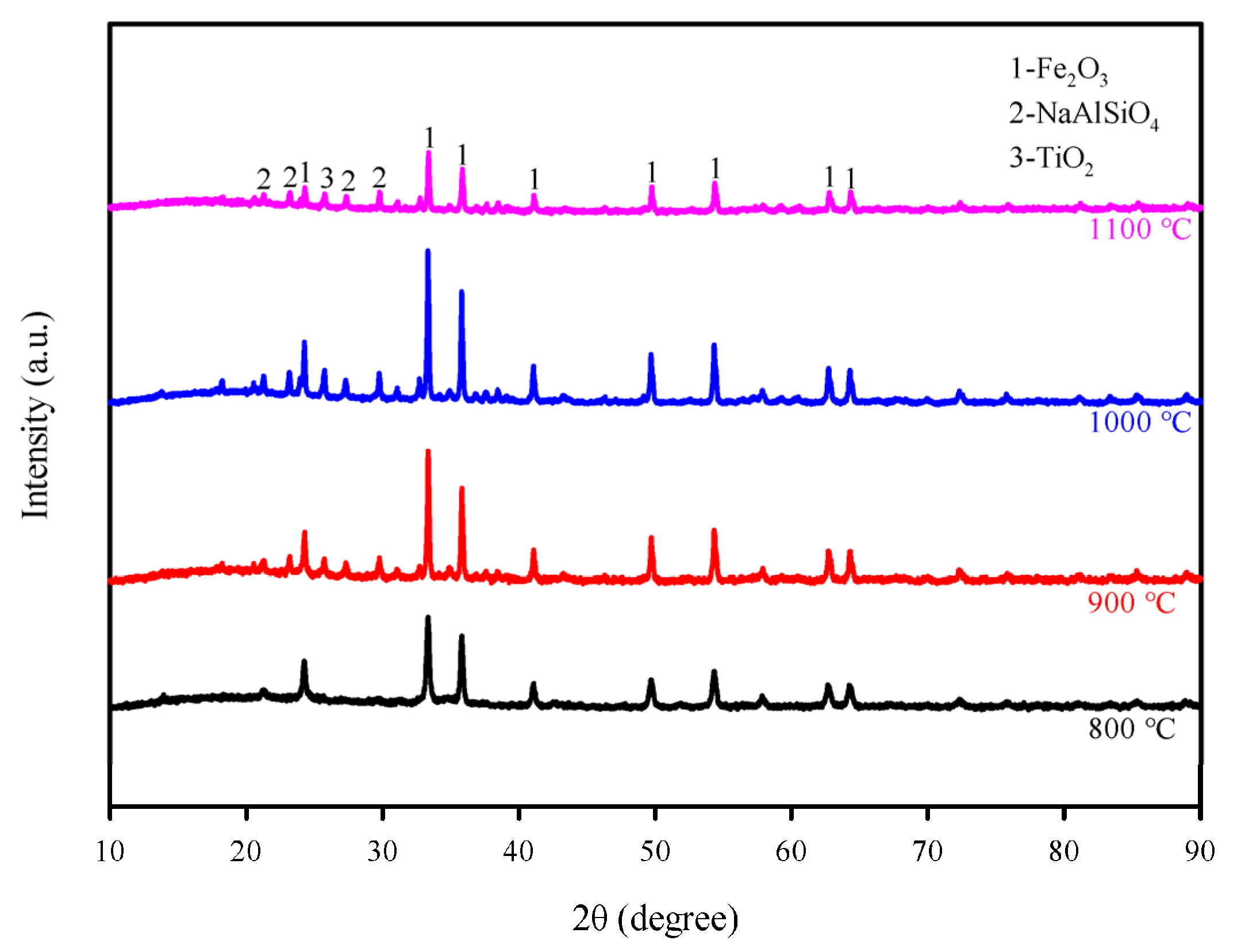
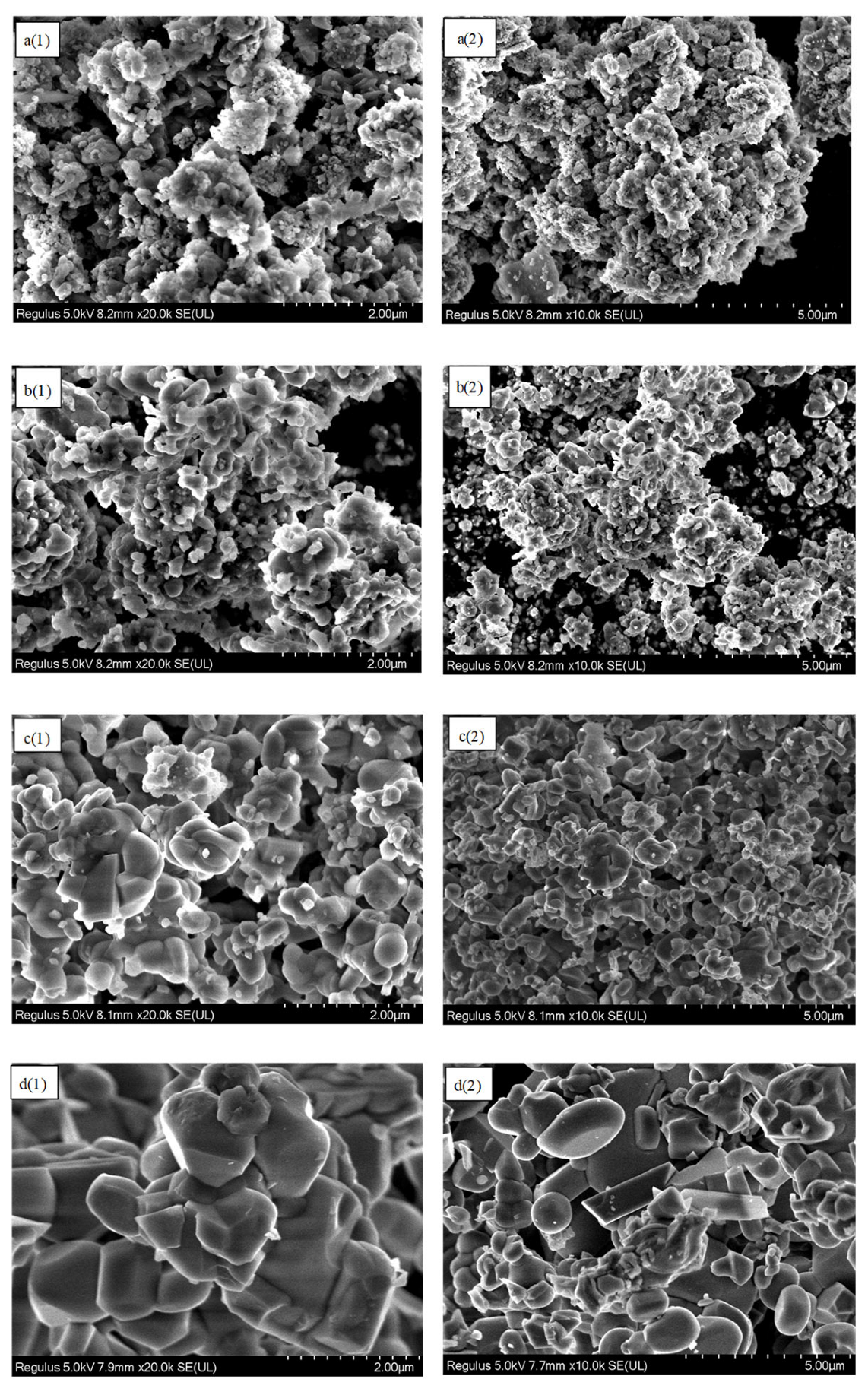
3.1. Characterization of Red Mud Oxygen Carriers
3.2. Effect of Different Preparation Temperatures on the Production and Composition
3.3. Effect of Different Preparation Temperatures on CLG Characteristics
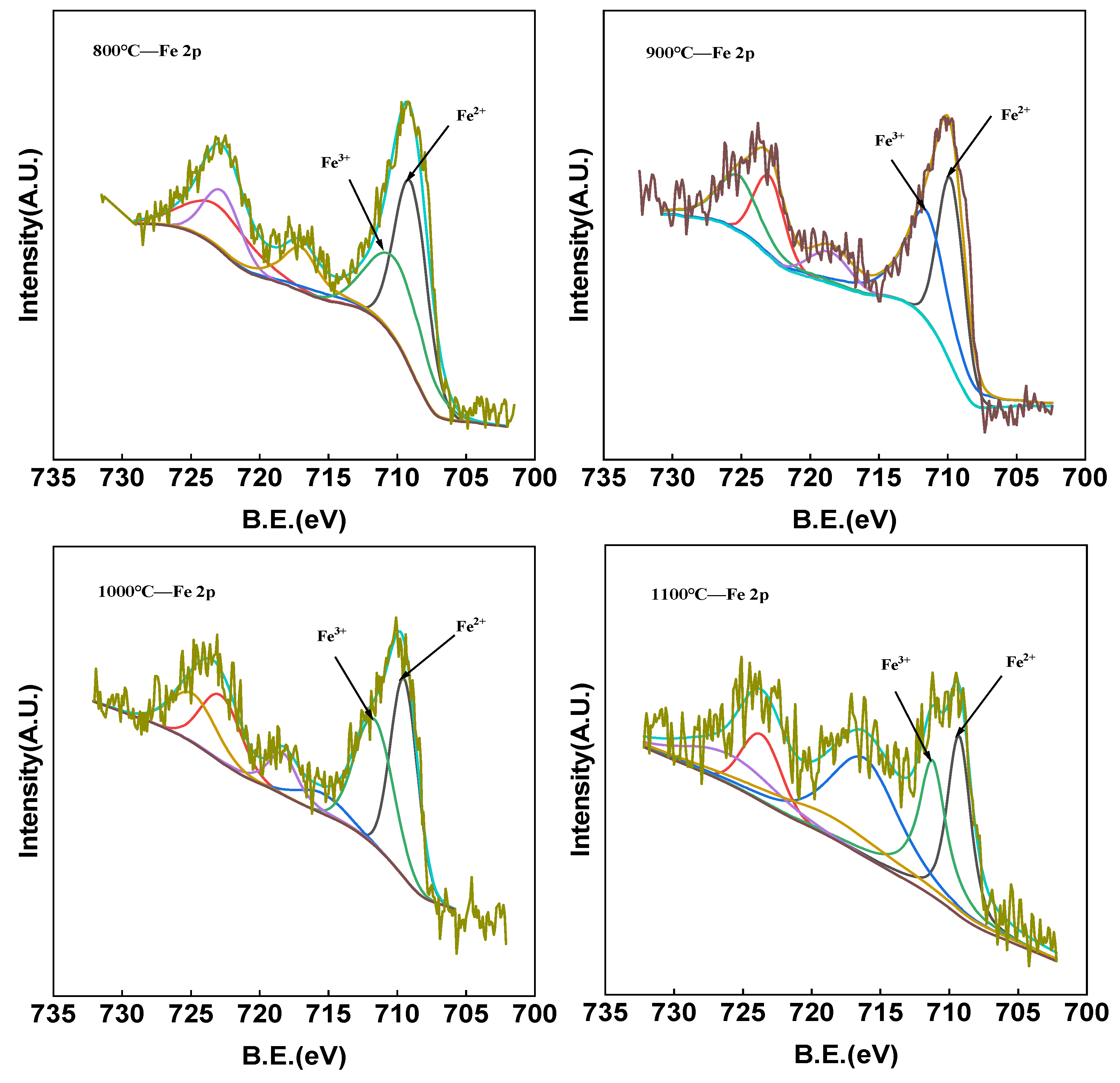
3.4. Analysis of Red Mud Carriers Before and After the Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Riseh, R.S.; Vazvani, M.G.; Hassanisaadi, M.; Thakur, V.K. Agricultural wastes: A practical and potential source for the isolation and preparation of cellulose and application in agriculture and different industries. Ind. Crops Prod. 2024, 208, 117904. [Google Scholar] [CrossRef]
- Shammi, M.; Akter, B. Chapter 3—A practical approach of biochar production and application toward sustainable agricultural waste management and achieving carbon neutrality. In Biochar Ecotechnology for Sustainable Agriculture and Environment; Kumar, A., Prasad, V., Kumari, P., Solanki, M.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 81–105. [Google Scholar]
- Chen, S.C.-i.; Dang, X.; Xu, Q.-q.; Own, C.-M. Transforming Waste into Value: Sustainable Recycling of Agricultural Resources Under the ‘Carbon Peak and Carbon Neutrality’ Vision. Sustainability 2025, 17, 55. [Google Scholar] [CrossRef]
- Condori, O.; García-Labiano, F.; de Diego, L.F.; Izquierdo, M.T.; Abad, A.; Adánez, J. Biomass chemical looping gasification for syngas production using ilmenite as oxygen carrier in a 1.5 kWth unit. Chem. Eng. J. 2021, 405, 126679. [Google Scholar] [CrossRef]
- Samprón, I.; de Diego, L.F.; García-Labiano, F.; Izquierdo, M.T.; Abad, A.; Adánez, J. Biomass Chemical Looping Gasification of pine wood using a synthetic Fe2O3/Al2O3 oxygen carrier in a continuous unit. Bioresour. Technol. 2020, 316, 123908. [Google Scholar] [CrossRef]
- Wei, G.; He, F.; Zhao, Z.; Huang, Z.; Zheng, A.; Zhao, K.; Li, H. Performance of Fe–Ni bimetallic oxygen carriers for chemical looping gasification of biomass in a 10 kWth interconnected circulating fluidized bed reactor. Int. J. Hydrogen Energy 2015, 40, 16021–16032. [Google Scholar] [CrossRef]
- Huijun, G.; Laihong, S.; Fei, F.; Shouxi, J. Experiments on biomass gasification using chemical looping with nickel-based oxygen carrier in a 25 kWth reactor. Appl. Therm. Eng. 2015, 85, 52–60. [Google Scholar] [CrossRef]
- Goel, A.; Moghaddam, E.M.; Liu, W.; He, C.; Konttinen, J. Biomass chemical looping gasification for high-quality syngas: A critical review and technological outlooks. Energy Convers. Manag. 2022, 268, 116020. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Feng, Y.; Zhao, K.; Zheng, A.; Chang, S.; Li, H. Synthesis gas production through biomass direct chemical looping conversion with natural hematite as an oxygen carrier. Bioresour. Technol. 2013, 140, 138–145. [Google Scholar] [CrossRef]
- Taneez, M.; Hurel, C. A review on the potential uses of red mud as amendment for pollution control in environmental media. Environ. Sci. Pollut. Res. 2019, 26, 22106–22125. [Google Scholar] [CrossRef]
- Chen, L.; Liu, F.; Heather, S.N.; Fan, Z.; Liu, K. Coal char-fueled chemical looping combustion use different iron-based oxygen carriers. Energy Procedia 2014, 63, 73–79. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; De Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Mendiara, T.; Gayán, P.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Performance of a bauxite waste as oxygen-carrier for chemical-looping combustion using coal as fuel. Fuel Process. Technol. 2013, 109, 57–69. [Google Scholar] [CrossRef]
- Mendiara, T.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Adánez, J. Behaviour of a bauxite waste material as oxygen carrier in a 500Wth CLC unit with coal. Int. J. Greenh. Gas Control. 2013, 17, 170–182. [Google Scholar] [CrossRef]
- Bao, J.; Chen, L.; Liu, F.; Fan, Z.; Nikolic, H.S.; Liu, K. Evaluating the Effect of Inert Supports and Alkali Sodium on the Performance of Red Mud Oxygen Carrier in Chemical Looping Combustion. Ind. Eng. Chem. Res. 2016, 55, 8046–8057. [Google Scholar] [CrossRef]
- Chen, L.; Yang, L.; Liu, F.; Nikolic, H.S.; Fan, Z.; Liu, K. Evaluation of multi-functional iron-based carrier from bauxite residual for H2-rich syngas production via chemical-looping gasification. Fuel Process. Technol. 2017, 156, 185–194. [Google Scholar] [CrossRef]
- Gai, D.; Shao, D.; An, F.; Zhong, Z.; Wang, X.; Chen, L. Targeted migration mechanisms of nitrogen-containing pollutants during chemical looping co-gasification of coal and microalgae. J. Hazard. Mater. 2025, 487, 137237. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, G.; Lu, Y.; Zhang, H. Redox performance of Fe2O3/Al2O3 oxygen carrier calcined at different temperature in chemical looping process. Fuel 2022, 310, 122381. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Yu, J.; Wang, J.; Wang, B.; Li, P.; Wan, Y. Catalytic effects of red mud on coal char gasification and the transformation of active lattice oxygen in iron oxides. Fuel 2025, 380, 133241. [Google Scholar] [CrossRef]
- Cho, P.; Mattisson, T.; Lyngfelt, A. Comparison of iron-, nickel-, copper- and manganese-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1215–1225. [Google Scholar] [CrossRef]





| Oxygen Carrier | Oxygen Carrier Synthesis Methods | Fuel | Reaction Conditions | Gasification Effect | Reference |
|---|---|---|---|---|---|
| Bauxite waste | Calcined at 1200 °C for 18 h | Bituminous coal | N2/H2O/CO2, 900 °C | Combustion efficiencies > 0.95 at 900 and 980 °C | [13] |
| Red mud | Calcined at 1150 °C for 6 h in air | Char from Kentucky Coal (USA) | 50 vol.% H2O/N2 | / | [11] |
| Alumina | Calcined at 1200 °C for 18 h | Colombian coal | TFR = 870–930 °C, 100 vol.% H2O | ηcomb,FR = 93.1% | [14] |
| Red mud | Inert supports (Al2O3,SiO2,TiO2 and CaO) and alkali sodium modification | Coal char | 50 vol.% H2O/N2, 950 °C | 30th cycle activity | [15] |
| Red mud | Calcined at 800 °C, 900 °C, 1000 °C, 1100 °C for 8 h | Water hyacinth | 0.2 mL/min steam, 950 °C | Gas yield = 1.02 Nm3/kg, LHV = 12.06 MJ/Nm3, CGE = 91.49%, ηc = 82.65%. | This work |
| Proximate Analysis (wt.%) | Elemental Analysis (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| Moisture | Volatiles | Ash | Fixed Carbon | C | H | N | S |
| 11.31 | 60.43 | 10.95 | 17.31 | 34.71 | 5.03 | 1.00 | 0.32 |
| Composition | Fe2O3 | Al2O3 | SiO2 | Na2O | TiO2 | CaO | MgO | SO3 | CuO | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| Mass fraction (wt.%) | 50.06 | 21.41 | 11.19 | 6.77 | 5.43 | 1.07 | 0.13 | 0.44 | 0.02 | 3.47 |
| Temperature (°C) | H2 (%) | CO (%) | CO2 (%) | CH4 (%) | Yg (Nm3/kg) |
|---|---|---|---|---|---|
| 800 | 44.44 | 26.94 | 19.27 | 9.36 | 0.87 |
| 900 | 46.37 | 26.65 | 16.02 | 10.96 | 1.01 |
| 1000 | 47.40 | 27.18 | 15.62 | 9.80 | 1.02 |
| 1100 | 45.90 | 27.03 | 17.09 | 9.98 | 0.97 |
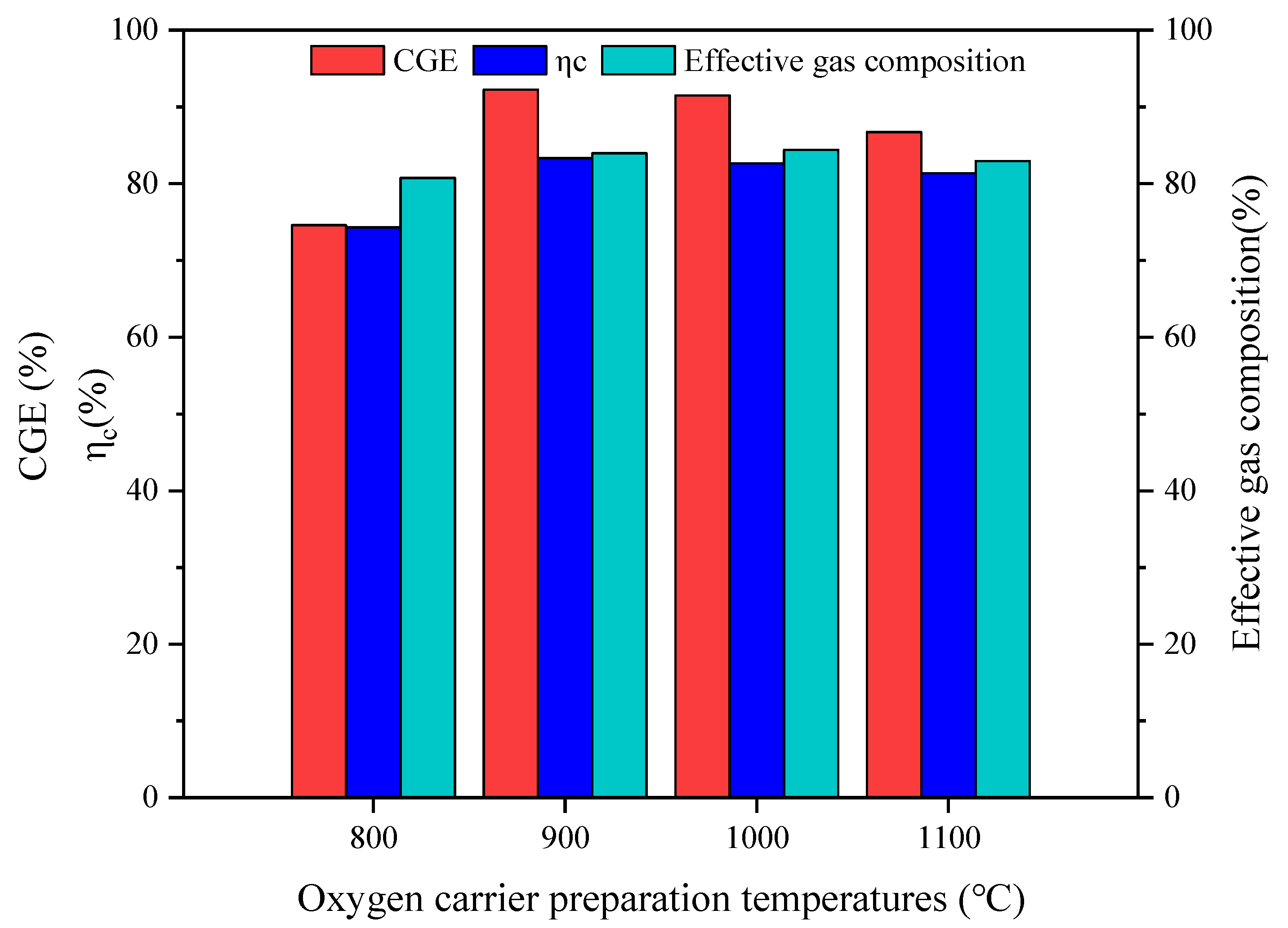
| Temperature (°C) | LHV (MJ/Nm3) | CGE (%) | ηc (%) | Effective Gas Composition (%) | H2/CO |
|---|---|---|---|---|---|
| 800 | 11.55 | 74.57 | 74.29 | 80.73 | 1.65 |
| 900 | 12.30 | 92.24 | 83.30 | 83.98 | 1.74 |
| 1000 | 12.06 | 91.49 | 82.65 | 84.38 | 1.74 |
| 1100 | 11.95 | 86.72 | 81.34 | 82.91 | 1.70 |
| Oxygen Carrier | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| Before reaction | 2.451 | 0.006 | 10.529 |
| After reaction | 2.668 | 0.007 | 12.485 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, F.; Chen, J.; Zhuang, K.; Gai, D.; Yu, Y.; Shen, F.; Wang, X.; Wang, S. Boosting Agroforestry Waste Valorization: Red Mud Oxygen Carriers with Tailored Oxygen Release for Enhanced Chemical Looping Gasification. Processes 2025, 13, 1716. https://doi.org/10.3390/pr13061716
An F, Chen J, Zhuang K, Gai D, Yu Y, Shen F, Wang X, Wang S. Boosting Agroforestry Waste Valorization: Red Mud Oxygen Carriers with Tailored Oxygen Release for Enhanced Chemical Looping Gasification. Processes. 2025; 13(6):1716. https://doi.org/10.3390/pr13061716
Chicago/Turabian StyleAn, Fengxia, Jiajun Chen, Ke Zhuang, Didi Gai, Ying Yu, Fanhui Shen, Xiaojia Wang, and Sheng Wang. 2025. "Boosting Agroforestry Waste Valorization: Red Mud Oxygen Carriers with Tailored Oxygen Release for Enhanced Chemical Looping Gasification" Processes 13, no. 6: 1716. https://doi.org/10.3390/pr13061716
APA StyleAn, F., Chen, J., Zhuang, K., Gai, D., Yu, Y., Shen, F., Wang, X., & Wang, S. (2025). Boosting Agroforestry Waste Valorization: Red Mud Oxygen Carriers with Tailored Oxygen Release for Enhanced Chemical Looping Gasification. Processes, 13(6), 1716. https://doi.org/10.3390/pr13061716






