Study on the Unblocking Fluid System for Complex Blockages in Weiyuan Shale Gas Wellbores
Abstract
1. Introduction
2. Experimental and Simulation Methods
2.1. Test Samples
2.2. Materials and Instruments
2.3. Analysis of the Physicochemical Properties of Blockages
- (1)
- Formation Water Ion Content Analysis
- (2)
- Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS) Analysis
- (3)
- High-Temperature Combustion Analysis
- (4)
- Inorganic Composition Analysis
- (5)
- Organic Composition Analysis
2.4. Model Construction and Parameter Settings
2.5. Construction and Evaluation Method of the Unblocking Fluid System
- (1)
- Construction of the Unblocking Fluid System
- (2)
- Evaluation of the Unblocking Fluid System
3. Results and Discussion
3.1. Blockage Morphology and Composition Analysis
3.1.1. Formation Water Quality Analysis
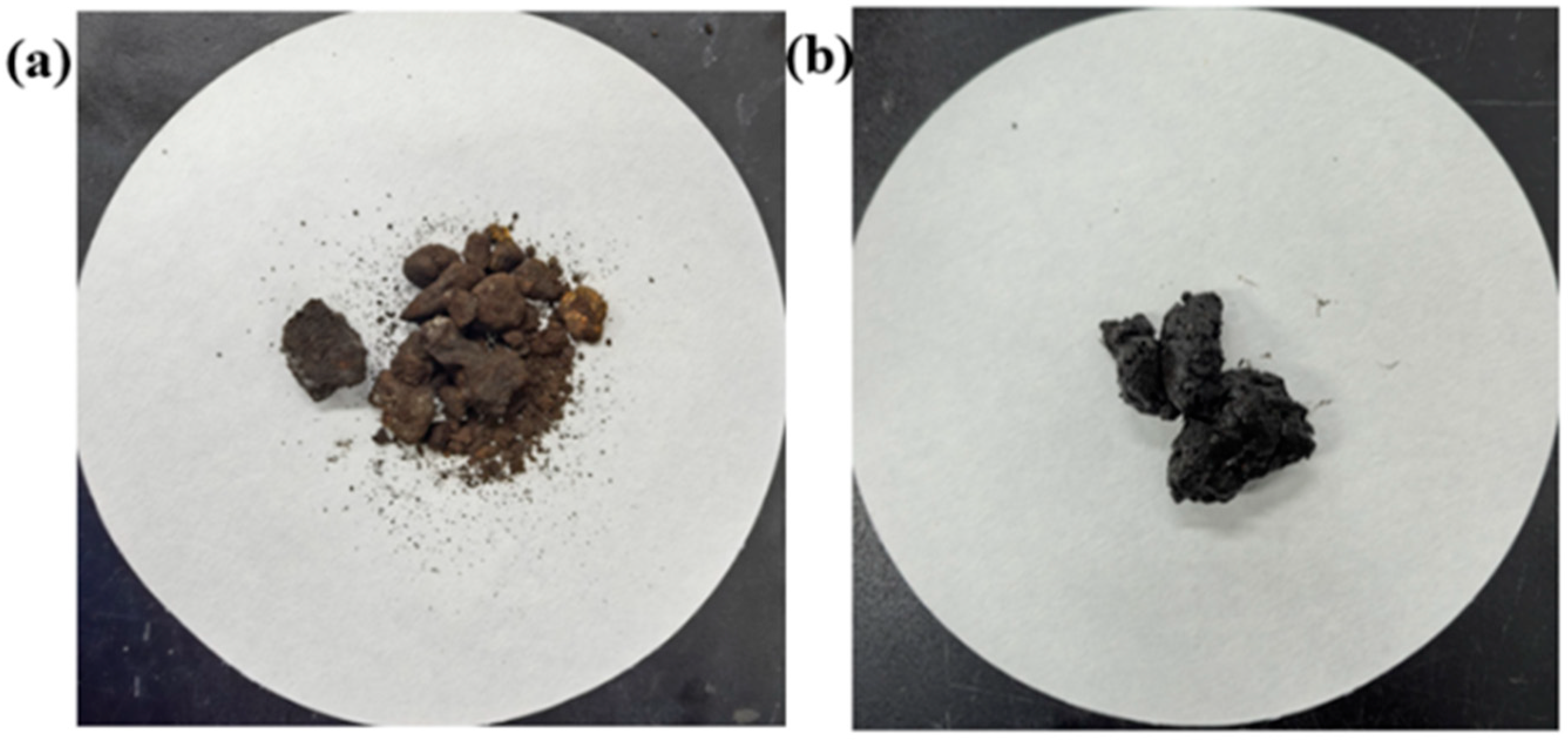
3.1.2. Blockage Morphology
3.1.3. High-Temperature Combustion Experiment Analysis
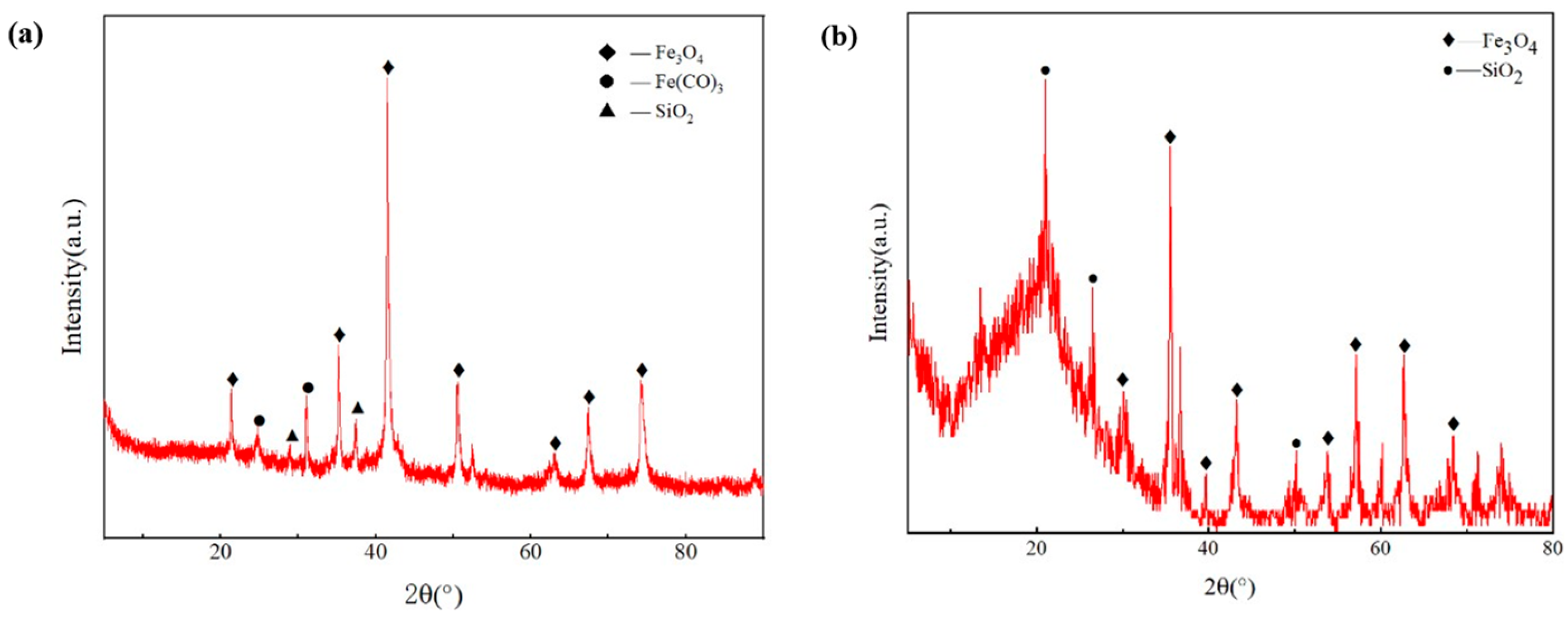
3.1.4. Inorganic Component Analysis of Blockage
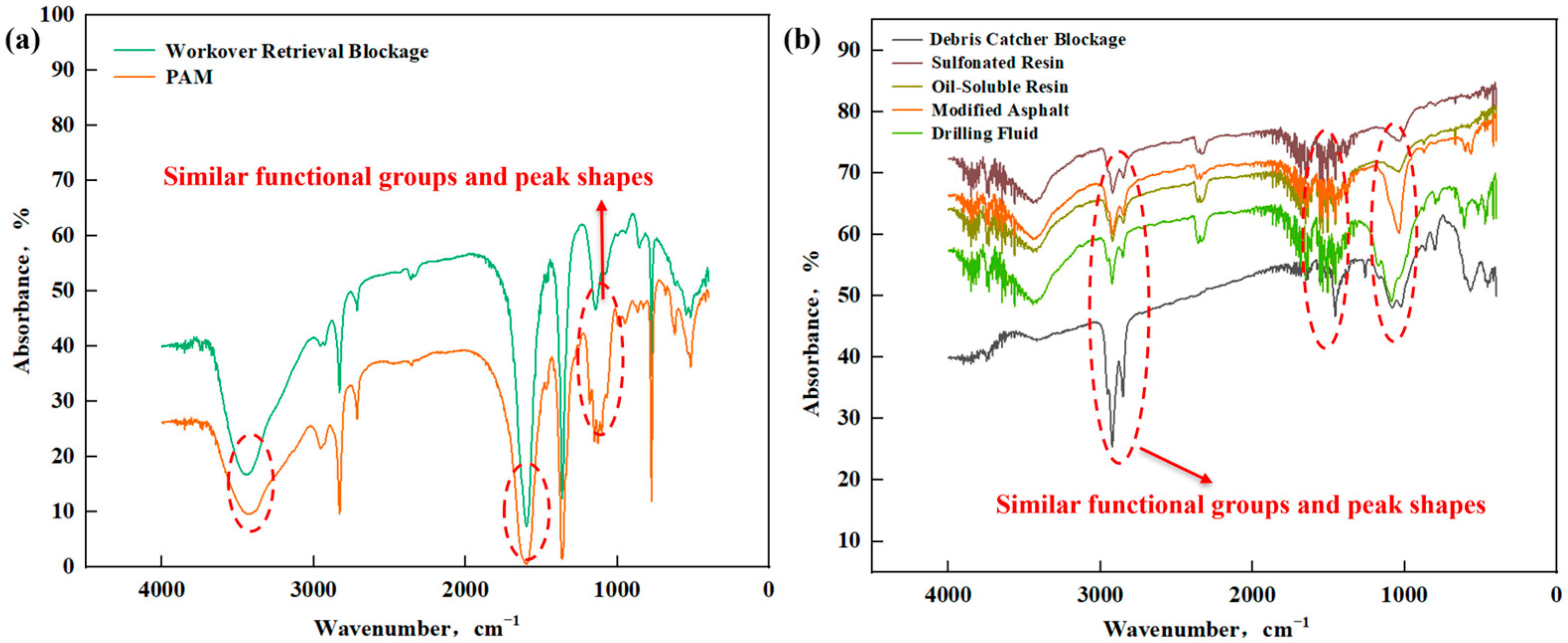
3.1.5. Organic Component Analysis of Blockage
3.2. Construction of Blockage Depolymerization System
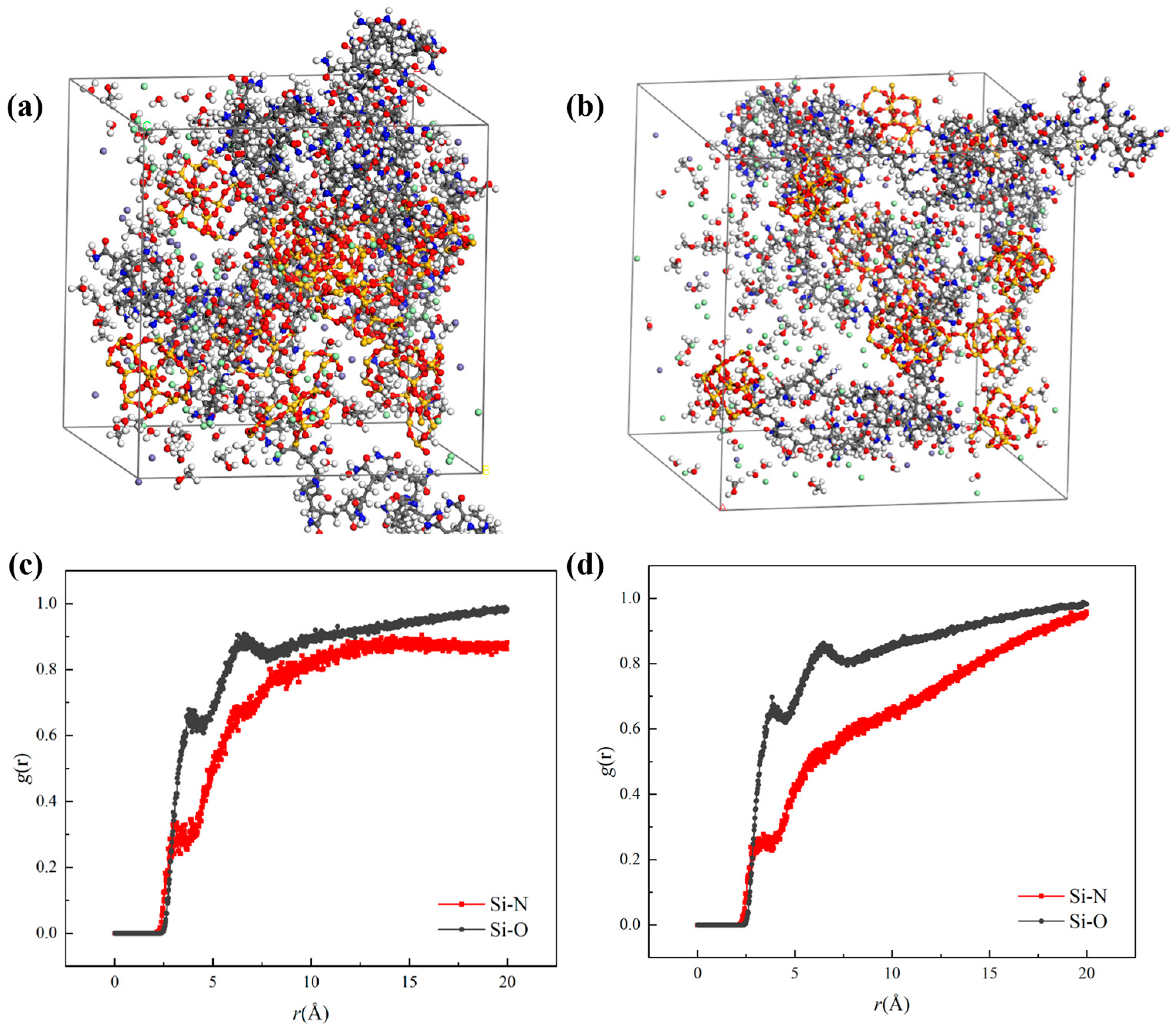
3.2.1. Analysis of Blockage Depolymerization and Dispersion Mechanism
3.2.2. Construction of Unblocking Fluid System
3.3. Performance Evaluation of the Unblocking Fluid System
3.3.1. Compatibility and Static Dissolution Rate Measurement
3.3.2. Corrosion Evaluation
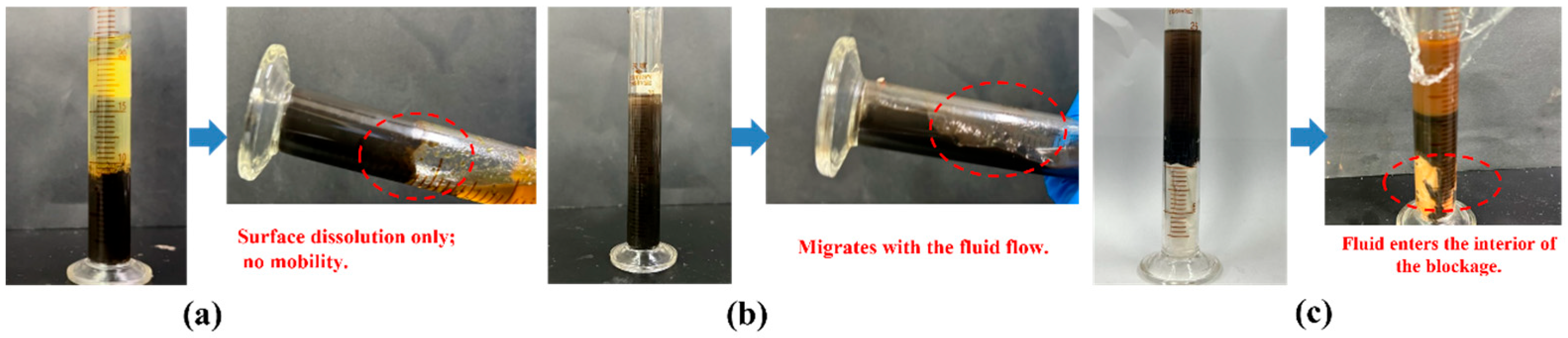
3.3.3. Evaluation of Unblocking Effect Under Simulated Wellbore Conditions
3.4. Wellbore Unblocking Process Integration
3.4.1. Unblocking Process
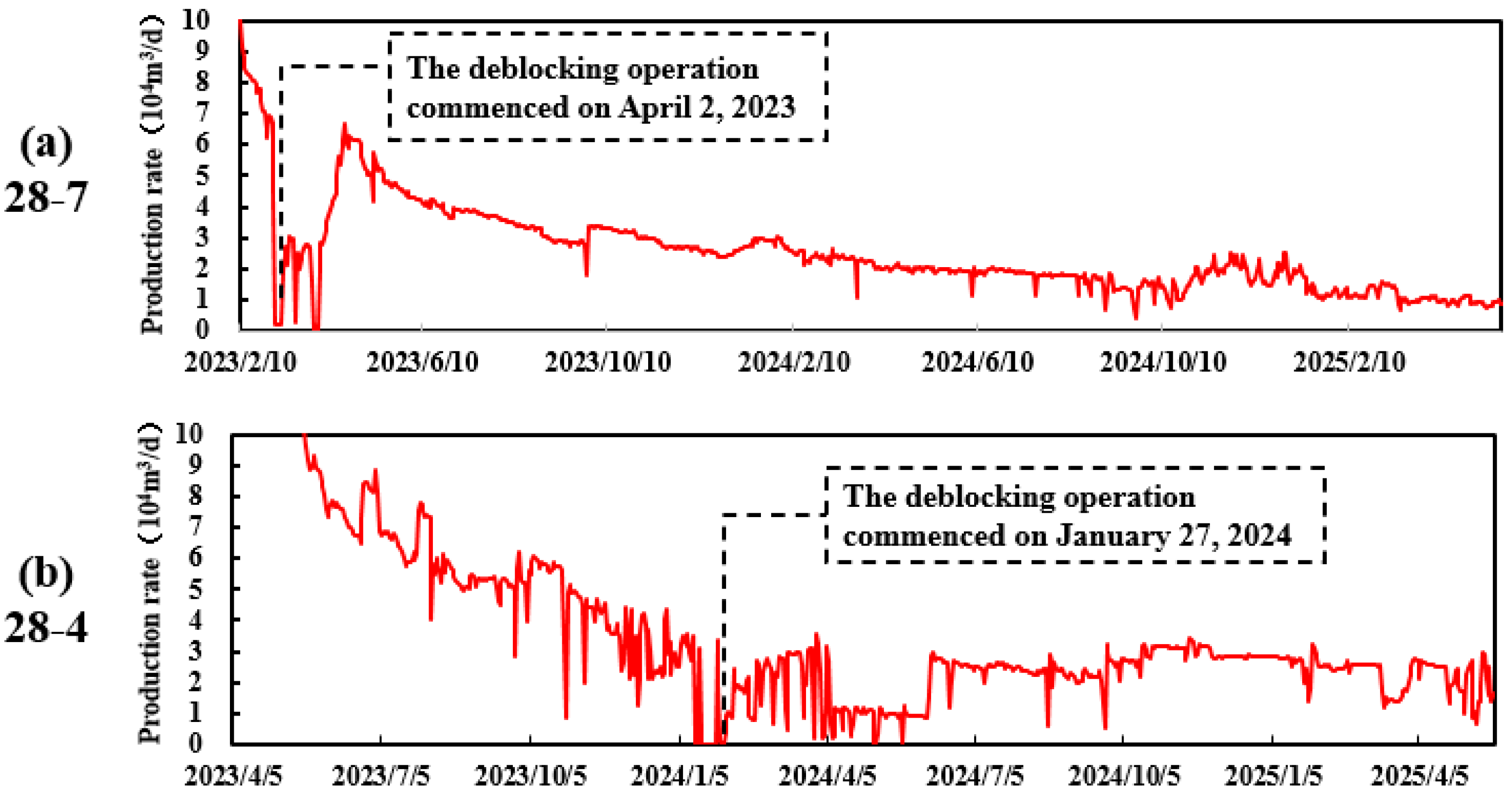
3.4.2. Field Implementation Effect
4. Conclusions
- (1)
- The blockage material in the Weiyuan block primarily exists as an inorganic-dominated aggregate with organic components as a secondary phase, with an inorganic-to-organic ratio of approximately 8:2. The main inorganic components are Fe3O4 and SiO2, while the organic components are mainly associated with polymeric materials from drilling fluids and fracturing fluids;
- (2)
- Gas wellbore blockage in gas wells is typically induced by the combined effects of multiple factors, with the blockage material often exhibiting a complex multiphase composite structure. To elucidate the mechanism of dispersion and disintegration, this study integrated molecular simulation with laboratory experiments based on the physicochemical properties of the blockage. A synergistic unblocking strategy of “organic dispersion + inorganic dissolution” was proposed from a microscopic perspective. According to the varying proportions of organic and inorganic components in the blockage, three types of unblocking fluid systems—neutral, acidic, and composite—were developed to suit different blockage scenarios. The results demonstrated that, when dealing with dense composite blockages, the composite fluid system exhibited superior penetration and unblocking efficiency in simulated wellbore models, highlighting its strong potential for application in complex downhole environments;
- (3)
- Blockages in shale gas wells typically exhibit pronounced multiphase complexity, posing considerable challenges for effective remediation. This underscores the need for high-efficiency, adaptable unblocking technologies tailored to the organic–inorganic composite nature of such obstructions. In response, this study developed a composite unblocking fluid system with tunable formulation flexibility, wherein the ratio of neutral-to-acidic components can be adjusted according to the specific organic/inorganic composition of the blockage. This design enables the system to accommodate a wide range of reservoir types and complex geological conditions. Field trials were conducted in two shale gas wells (Wei 28-4 and Wei 28-7) to evaluate the system’s performance under real-world conditions. The results demonstrated significant improvements in productivity: the Wei 28-7 well achieved an average post-treatment production increase of 3.2 × 104 m3/d, while the Wei 28-4 well maintained a stable output of 2.2 × 104 m3/d without recurrence of blockage. These outcomes validate the system’s capability to effectively remove complex wellbore blockages and extend well productivity. The technology has now entered the promotion and application phase, offering a robust and adaptable solution for efficient wellbore blockage mitigation in similar shale gas development scenarios;
- (4)
- Wellbore scaling and blockages are long-term issues that require repeated unblocking operations. Further research is needed to better understand the formation mechanisms of such complex blockages, allowing for the development of more targeted and long-lasting unblocking fluid systems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, J.; Zhao, S. Hydrocarbon accumulation and key exploration & development technologies of Changning-Weiyuan marine shale gas field, southern Sichuan. Pet. Res. 2021, 6, 1–15. [Google Scholar] [CrossRef]
- Tan, X. Composite technology of plug removal, cleanup and descaling for shale gas well and its application: A case study of Weiyuan and Rongxian operation areas, Sichuan Basin. Nat. Gas Explor. Dev. 2022, 45, 116–122. [Google Scholar] [CrossRef]
- Sheng, G.; Su, Y.; Wang, W. A new fractal approach for describing induced-fracture porosity/permeability/ compressibility in stimulated unconventional reservoirs. J. Pet. Sci. Eng. 2019, 179, 12. [Google Scholar] [CrossRef]
- Lv, M.; Guo, T.; Jia, X.; Wen, D.; Chen, M.; Wang, Y.; Qu, Z.; Ma, D. Study on the pump schedule impact in hydraulic fracturing of unconventional reservoirs on proppant transport law. Energy 2024, 286, 129569. [Google Scholar] [CrossRef]
- Lei, Q.; Yun, X.U.; Cai, B.; Guan, B.; Wang, X.; Bi, G.; Li, H.; Li, S.; Ding, B.; Fu, H.; et al. Progress and prospects of horizontal well fracturing technology for shale oil and gas reservoirs. Pet. Explor. Dev. 2022, 49, 166–172. [Google Scholar] [CrossRef]
- Xu, C.; Kang, Y.; You, Z.; Chen, M. Review on formation damage mechanisms and processes in shale gas reservoir: Known and to be known. J. Nat. Gas Sci. Eng. 2016, 36, 1208–1219. [Google Scholar] [CrossRef]
- Yan, X.; You, L.; Kang, Y.; Deng, S.; Xu, C. Formation Damage Induced by Oil-Based Drilling Fluid in a Longmaxi Shale Gas Reservoir: A Comprehensive View of the Drilling, Stimulation, and Production Processes. Energy Fuels 2023, 37, 945–954. [Google Scholar] [CrossRef]
- Sun, J.; XU, C.; Kang, Y.; Zhang, J. Research Progress and Development Recommendations Covering Damage Mechanisms and Protection Technologies for Tight/Shale Oil and Gas Reservoirs. Pet. Drill. Tech. 2020, 48, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, D.; Hill, A.D. Water-Induced Fracture Conductivity Damage in Shale Formations. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 3–5 February 2015. [Google Scholar] [CrossRef]
- Reinicke, A.; Rybacki, E.; Stanchits, S.; Huenges, E.; Dresen, G. Hydraulic fracturing stimulation techniques and formation damage mechanisms—Implications from laboratory testing of tight sandstone–proppant systems. Chem. Erde—Geochem.—Interdiscip. J. Chem. Probl. Geosci. Geoecology 2010, 70 (Suppl. S3), 107–117. [Google Scholar] [CrossRef]
- Huang, W.; Lei, M.; Qiu, Z.; Leong, Y.-K.; Zhong, H.; Zhang, S. Damage mechanism and protection measures of a coalbed methane reservoir in the Zhengzhuang block. J. Nat. Gas Sci. Eng. 2015, 26, 683–694. [Google Scholar] [CrossRef]
- Dai, C. Formation Damage During Chemical Flooding. In Formation Damage During Improved Oil Recovery; Yuan, B., Wood, D.A., Eds.; Gulf Professional Publishing: Houston, TX, USA, 2018; pp. 275–304. [Google Scholar] [CrossRef]
- Bai, J.; Kang, Y.; Chen, Z.; You, L.; Chen, M.; Li, X. Changes in retained fracturing fluid properties and their effect on shale mechanical properties. J. Nat. Gas Sci. Eng. 2020, 75, 103163. [Google Scholar] [CrossRef]
- King, G.E.; Durham, D. Chemicals in drilling, stimulation, and production. Advances in chemical pollution, environmental management and protection. Adv. Chem. Pollut. Environ. Manag. Prot. 2017, 1, 47–61. [Google Scholar] [CrossRef]
- Sun, J.; Xu, C.; Kang, Y.; Jing, H.; Zhang, J.; Yang, B.; You, L.; Zhang, H.; Long, Y. Formation damage mechanism and control strategy of the compound function of drilling fluid and fracturing fluid in shale reservoirs. Pet. Explor. Dev. 2024, 51, 430–439. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Wang, S. Adsorption damage and control measures of slick-water fracturing fluid in shale reservoirs. Pet. Explor. Dev. 2018, 45, 336–342. [Google Scholar] [CrossRef]
- Ekanem, E.M.; Rücker, M.; Yesufu-Rufai, S.; Spurin, C.; Ooi, N.; Georgiadis, A.; Berg, S.; Luckham, P.F. Novel adsorption mechanisms identified for polymer retention in carbonate rocks. JCIS Open 2021, 4, 100026. [Google Scholar] [CrossRef]
- Liu, S.Q.; Zhang, J.C.; Xu, J.C. Investigation on Polymer Adsorption and Retention in a Polymer Flooded Reservoir. Adv. Mater. Res. 2013, 734–737, 1200–1203. [Google Scholar] [CrossRef]
- Al-Hajri, S.; Mahmood, S.M.; Akbari, S.; Abdulelah, H.; Yekeen, N.; Saraih, N. Experimental investigation and development of correlation for static and dynamic polymer adsorption in porous media. J. Pet. Sci. Eng. 2020, 189, 106864. [Google Scholar] [CrossRef]
- Shi, B.; Wang, Z.; Zhang, Z.; Xu, Y.; Ling, K. A State of the Art Review on the Wellbore Blockage of Condensate Gas Wells: Towards Understanding the Blockage Type, Mechanism, and Treatment. Lithosphere 2022, 2022, 8076631. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.; Mahmoud, M.; Sultan, A.S.; Saad, M.A. Oilfield scale formation and chemical removal: A review. J. Pet. Sci. Eng. 2018, 171, 127–139. [Google Scholar] [CrossRef]
- Feng, B.; Ke, Z.; Liu, Y.; Shangguan, S.; Li, X.; Cui, Z. Plugging mechanism and plugging removal technologies for enhanced geothermal system reservoirs. Nat. Gas Ind. 2022, 42, 165. [Google Scholar] [CrossRef]
- Yuan, Y.; Du, J.; Liu, P.; Zou, Q.; Chen, X.; Liu, J. Research on Wellbore Blockage Mechanism and Blockage Removal Technology of Shale Gas Wells. J. Phys. Conf. Ser. 2024, 2834, 012167. [Google Scholar] [CrossRef]
- Lv, Y.; Ou, J.; Fu, H.; Li, J.; Luo, Z.; Yang, X.; Zhao, J.; Yang, T. Unblocking Process of Complex Sulfur–Iron Scale Blockage in Sulfur-Bearing Gas Wells and Its Mechanism. Nat. Gas Ind. 2022, 42, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sheng, L.; Shi, J.; Fei, H.; Guo, D.; Bai, H.; Yao, E. Mechanism of Salt Precipitation Blockage in Low-Producing Gas Wells and the Method of Acidification Blockage Removal. ACS Omega 2024, 9, 10886–10896. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Roostaei, M.; Fattahpour, V.; Uzcatequi, A.; Cyre, J.; Sutton, C.; Fermaniuk, B. Standalone sand control failure: The role of wellbore and near wellbore hydro-thermo-chemical phenomenon on the plugging and the flow performance impairments of the standalone sand screen. In Proceedings of the SPE Thermal Well Integrity and Design Symposium, Banff, AB, Canada, 27–29 November 2018. [Google Scholar] [CrossRef]
- Mubarak, G.; Verma, C.; Barsoum, I.; Alfantazi, A.; Rhee, K.Y. Internal corrosion in oil and gas wells during casings and tubing: Challenges and opportunities of corrosion inhibitors. J. Taiwan Inst. Chem. Eng. 2023, 150, 105027. [Google Scholar] [CrossRef]
- Nikoo, A.H.; Ghaedi, M.; Malayeri, M.R.; Riazi, M. Analysis of wellbore clogging by asphaltene deposition using interaction energies. Fuel 2023, 352, 129111. [Google Scholar] [CrossRef]
- SY/T 5405-2019; Performance Test Methods and Evaluation Indicators for Corrosion Inhibitors for Acidizing. China’s Ministry of Petroleum and Chemical Industry: Beijing, China, 2019.
- SY/T 0600-2009; Prediction Methods for Scaling Tendency in Oil-Field Water. China’s Ministry of Petroleum and Chemical Industry: Beijing, China, 2009.
- Davudov, D.; Moghanloo, R.G.; Akita, E.; Karami, H. A diagnostic approach to predict asphaltene deposition in reservoir and wellbore. In Proceedings of the SPE Western Regional Meeting, Garden Grove, CA, USA, 22–26 April 2018. [Google Scholar] [CrossRef]
- Xu, H.; Wang, S.; Cheng, C.; Li, Q.; Liu, Y.; Qi, J.; Wu, J. Organic Solid Blocking Mechanism and Unblocking Technology for Ultra-Deep and High Pressure Oil Wells from Tarim Basin. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 1–3 March 2023. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, S.; Liu, D.; Li, Y.; Luo, W.; Li, J. Wellbore cleaning technologies for shale-gas horizontal wells: Difficulties and countermeasures. Nat. Gas Ind. B 2020, 7, 190–195. [Google Scholar] [CrossRef]
- Hoa, L.Q.; Bäßler, R.; Bettge, D.; Buggisch, E.; Schiller, B.N.; Beck, M. Corrosion Study on Wellbore Materials for the CO₂ Injection Process. Processes 2021, 9, 115. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; He, Y.; Yang, Z.; Liang, X.; Wang, X.; Mao, Q. Study on the Mechanism of Wellbore Blockage and Scaling Trend Prediction of Keshen Block. Processes 2024, 12, 782. [Google Scholar] [CrossRef]
- Xiao, L.; Hou, J.; Li, J. Performance evaluation and application of organic plug removal agent and non-acid plug removal system. Oilfield Chem. 2020, 37, 658–664. [Google Scholar]
- Yang, J.; Feng, Y.; Zhang, B.; Tang, Y.; Jiang, Z. A blockage removal technology for natural gas hydrates in the wellbore of an ultra-high pressure sour gas well. Nat. Gas Ind. B 2021, 8, 188–194. [Google Scholar] [CrossRef]
- Li, S.; Du, J.; He, J.; Sun, D.; Qiao, L.; Wang, S. Research progress of natural gas well wellbore declogging working fluid. Appl. Chem. Ind. 2024, 53, 238. [Google Scholar] [CrossRef]



| No. | Well | Abnormal Type | Unblocking Type | Unblocking Result |
|---|---|---|---|---|
| 1 | W202H22-8 | Suspected horizontal section blockage | Minor Workover and Flushing | Decline |
| 2 | W204H48-4 | Tubing installation obstruction after pressure channeling | Microbubble Well Cleaning | Not Increased |
| 3 | W204H48-5 | Tubing installation obstruction after pressure channeling | Microbubble Well Cleaning | Not Increased |
| 4 | W202H16-8 | Wellbore contamination, tubing blockage | Nitrogen Foam Well Cleaning | Not Increased |
| 5 | W204H51-5 | Sand blockage in tubing | Nitrogen Foam Well Cleaning | Not Increased |
| 6 | W204H33-1 | Flow restriction in the annulus | Surfactant Well Cleaning | Not Increased |
| 7 | W204H10-2 | Wellbore contamination, tubing blockage | Surfactant Well Cleaning | Not Increased |
| Well | pH | Fe3+/Fe2+ | Na+ | Mg2+ | K+ | Ba2+ | Cl− | CO32− | SO42− | NO3− | Total Salinity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| W204H47 | 7.39 | 8.26 | 11,789.64 | 96.63 | 150.51 | 190.43 | 19,590.12 | 9.39 | 1.19 | 96.82 | 31,924.73 |
| W204H49 | 7.23 | 141.81 | 6417.51 | 31.14 | 311.19 | 539.11 | 10,769.28 | 318.28 | 2560.28 | 55.14 | 21,001.93 |
| W204H41 | 7.19 | 48.84 | 4812.57 | 35.44 | 60.11 | 10.15 | 6031.28 | 28.15 | 329.95 | 28.58 | 11,336.23 |
| W204H51 | 7.79 | 73.51 | 4669.97 | 27.25 | 42.47 | 7.64 | 6793.51 | 21.37 | 230.12 | 21.34 | 11,813.67 |
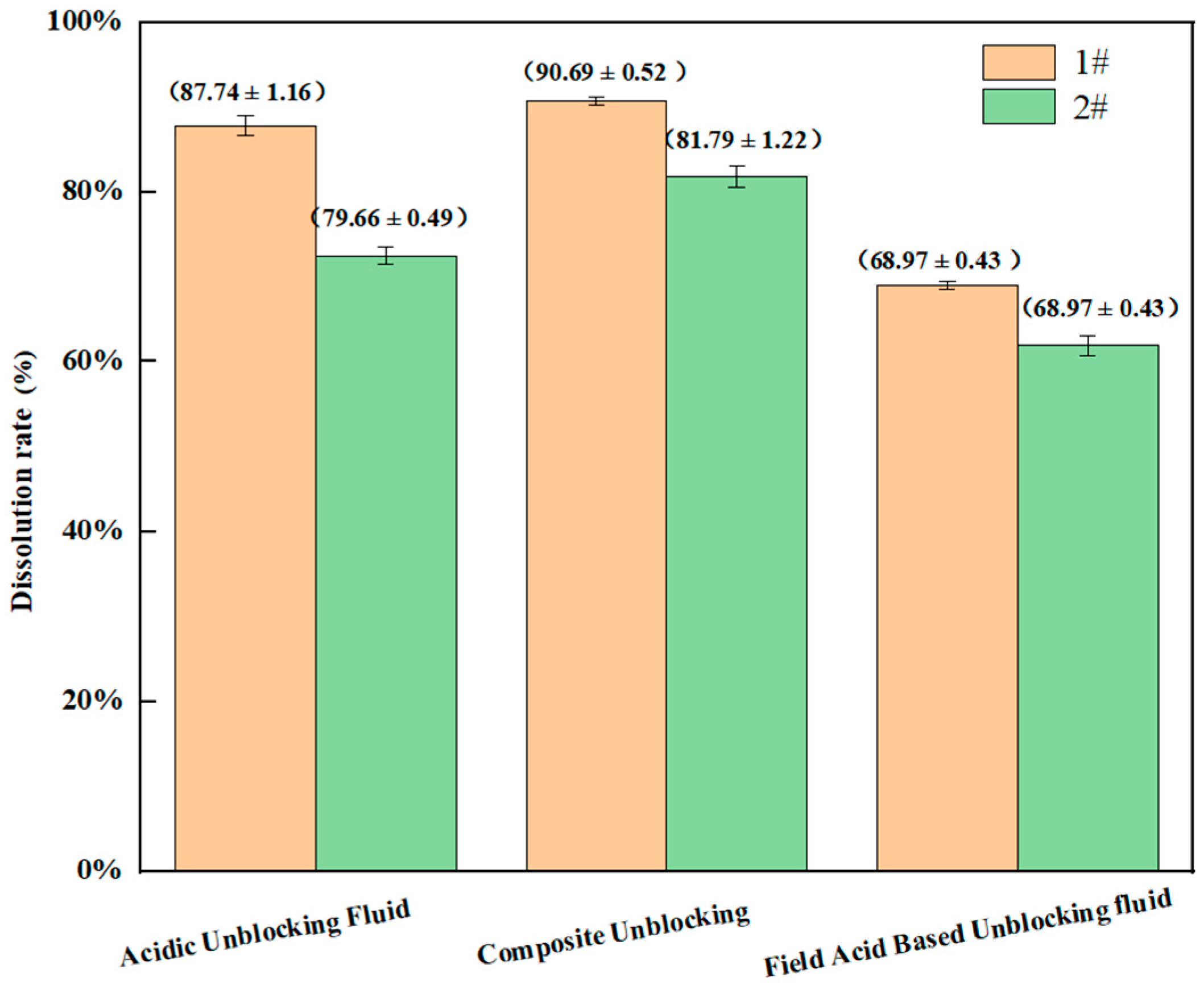
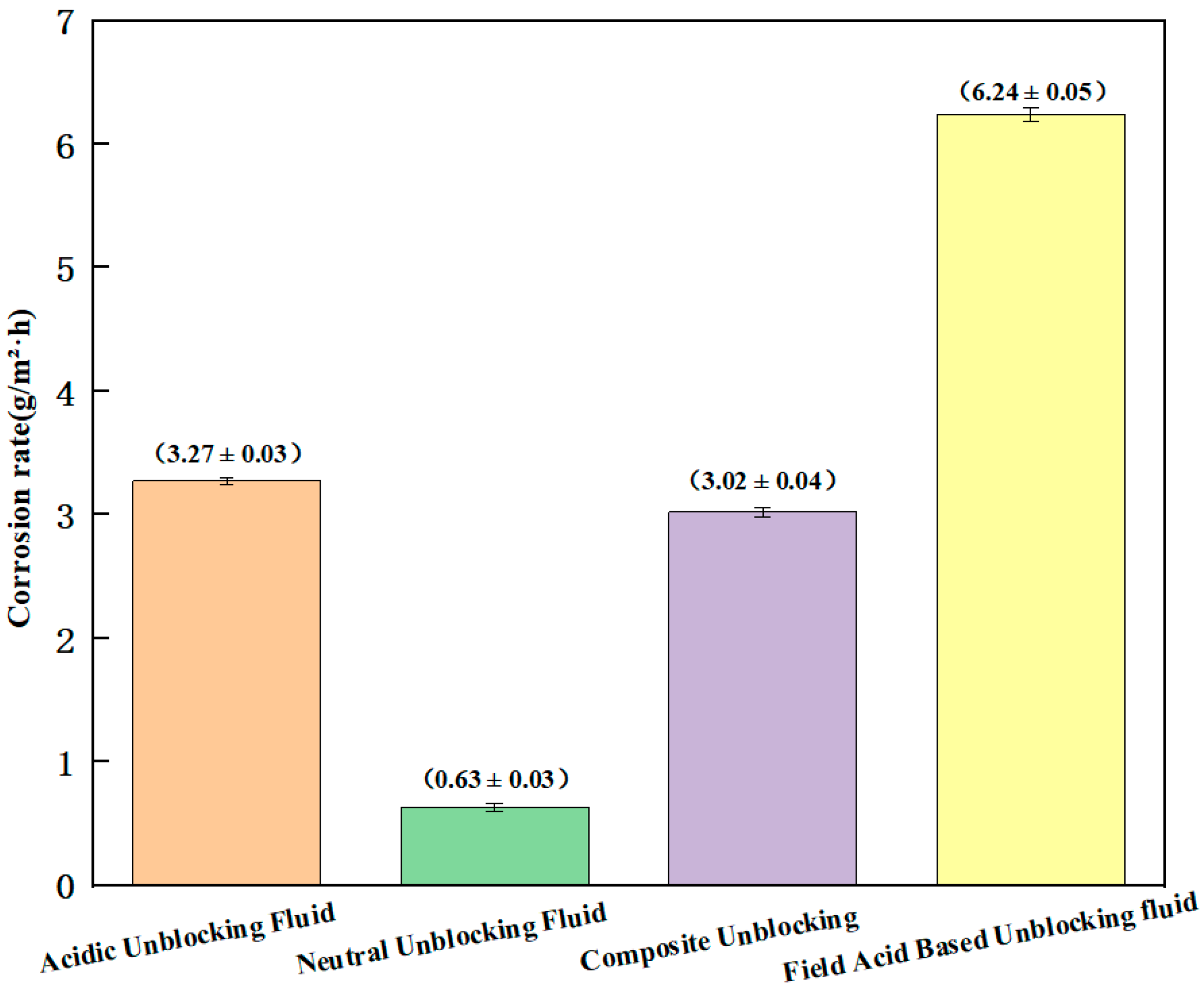
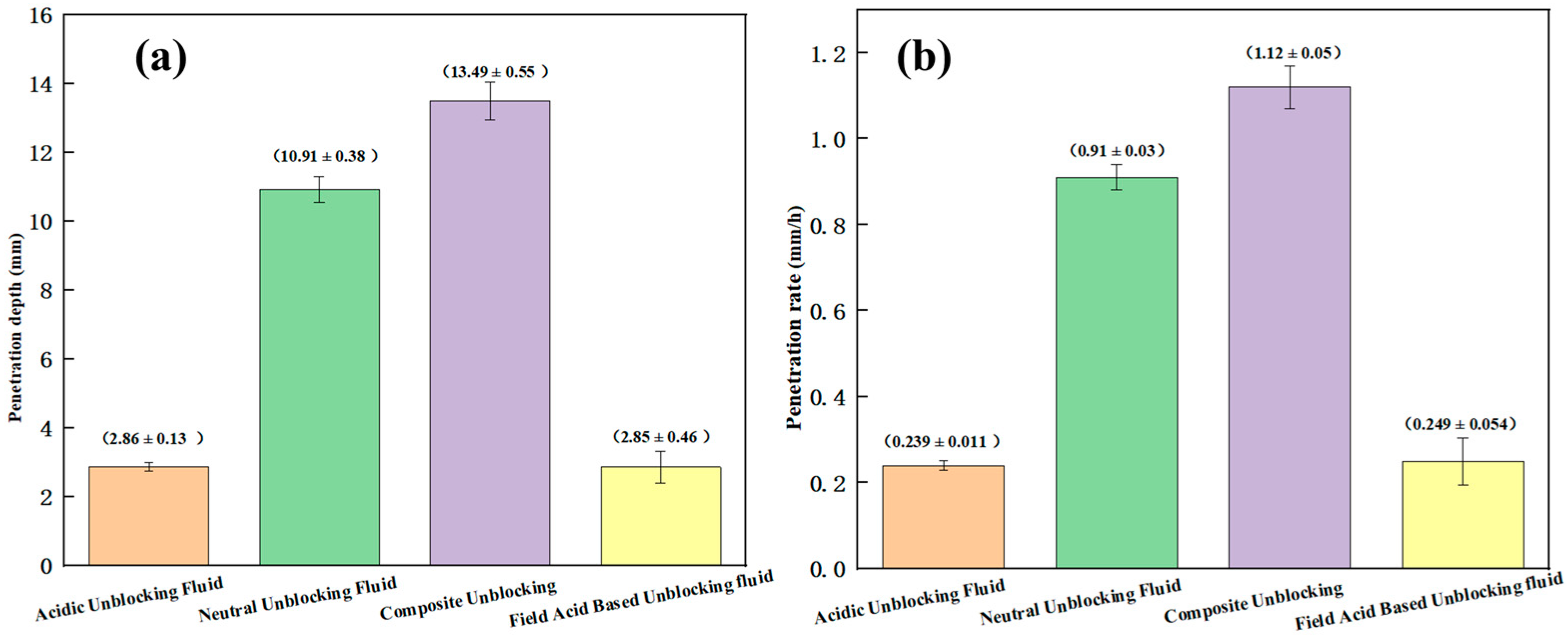
| Unblocking Fluid System | Dissolution Rate of Blockage Sample #1 (%) | Dissolution Rate of Blockage Sample #2 (%) | Corrosion Rate (g/m2·h) | Penetration Depth (mm) | Penetration Rate (mm/h) |
|---|---|---|---|---|---|
| Acidic Unblocking Fluid | 87.74 (±1.16) | 79.66 (±0.49) | 3.27 (±0.03) | 2.86 (±0.13) | 0.239 (±0.011) |
| Neutral Unblocking Fluid | / | / | 0.63 (±0.03) | 10.91 (±0.38) | 0.9 (±0.03) |
| Composite Unblocking | 90.69 (±0.52) | 81.79 (±1.22) | 3.02 (±0.04) | 13.49 (±0.55) | 1.12 (±0.05) |
| Field Acid-Based Unblocking Fluid | 68.97 (±0.43) | 61.90 (±1.18) | 6.24 (±0.05) | 2.85 (±0.46) | 0.249 (±0.054) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, Y.; Zou, L.; Xiao, J.; He, Q.; Zhang, T.; Qiu, B.; Zhu, J. Study on the Unblocking Fluid System for Complex Blockages in Weiyuan Shale Gas Wellbores. Processes 2025, 13, 1684. https://doi.org/10.3390/pr13061684
Yang Y, Wang Y, Zou L, Xiao J, He Q, Zhang T, Qiu B, Zhu J. Study on the Unblocking Fluid System for Complex Blockages in Weiyuan Shale Gas Wellbores. Processes. 2025; 13(6):1684. https://doi.org/10.3390/pr13061684
Chicago/Turabian StyleYang, Yadong, Yixuan Wang, Longqing Zou, Jianfeng Xiao, Qiyue He, Teng Zhang, Bangkun Qiu, and Jingyi Zhu. 2025. "Study on the Unblocking Fluid System for Complex Blockages in Weiyuan Shale Gas Wellbores" Processes 13, no. 6: 1684. https://doi.org/10.3390/pr13061684
APA StyleYang, Y., Wang, Y., Zou, L., Xiao, J., He, Q., Zhang, T., Qiu, B., & Zhu, J. (2025). Study on the Unblocking Fluid System for Complex Blockages in Weiyuan Shale Gas Wellbores. Processes, 13(6), 1684. https://doi.org/10.3390/pr13061684






