Green Synthesis of Cu and Pd Catalysts Using Mexican Oregano (Lippia graveolens) Extract and Their Application in the Conversion of a Biomass-Derived Molecule
Abstract
1. Introduction
2. Materials and Methods
2.1. Oregano Extract
2.2. Synthesis of Cu and Pd Nanoparticles by Conventional and Green Methods
2.3. Synthesis of Cu and Pd Nanoparticles and Catalysts by Conventional Methods
2.4. Synthesis of Cu and Pd Nanoparticles and Catalysts Using a Green Method
2.5. Kinetic Determination of Cu and Pd Nanoparticles Formation
2.6. Characterization Techniques
2.7. Catalytic Oxidation of HMFCA
3. Results and Discussion
3.1. Identification of Phytochemical Agents in Oregano Extract
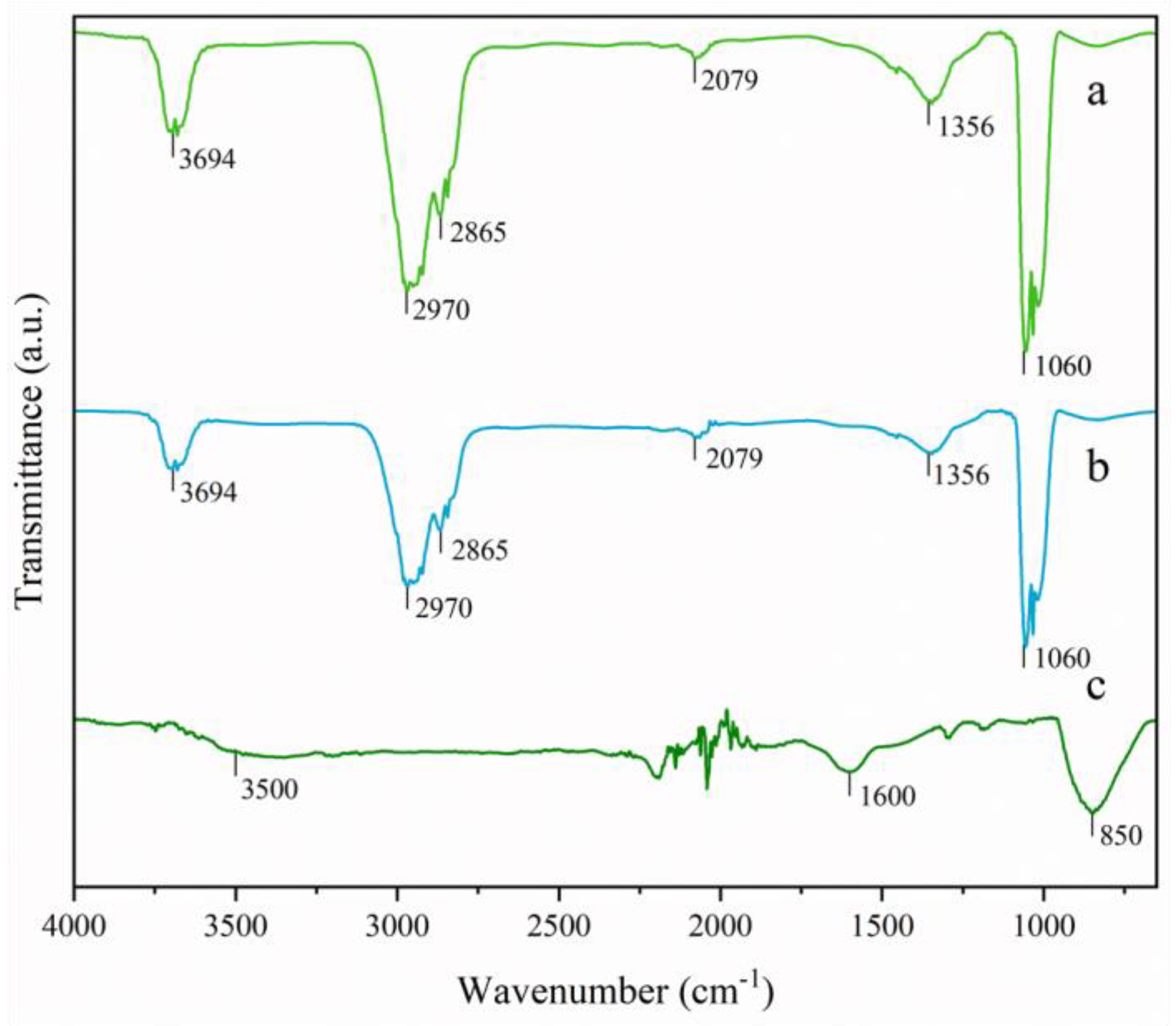
3.2. FT-IR of Catalysts Synthesized by the Green Method
3.3. Microstructural Characterization of Catalysts
3.4. Electronic Microscopy Analysis
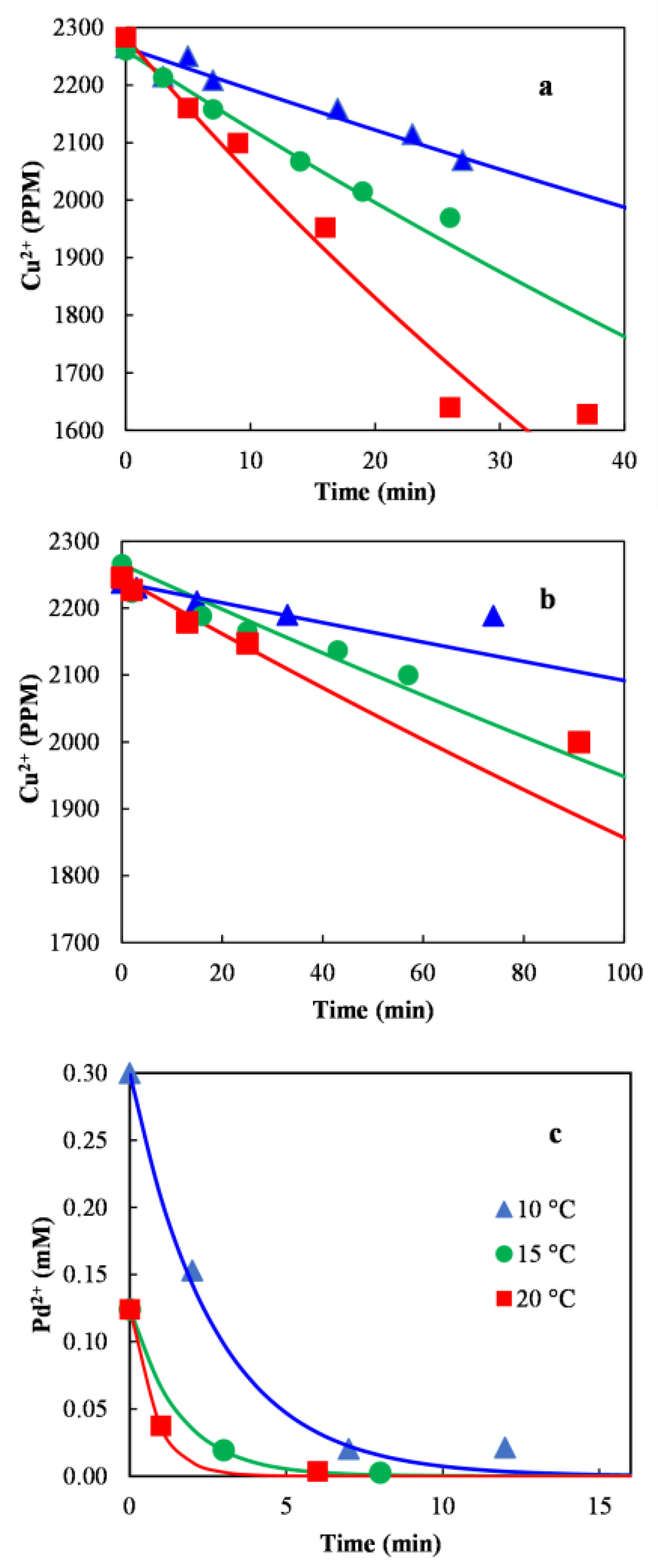
3.5. Kinetics of Cu2+ and Pd2+ Reduction
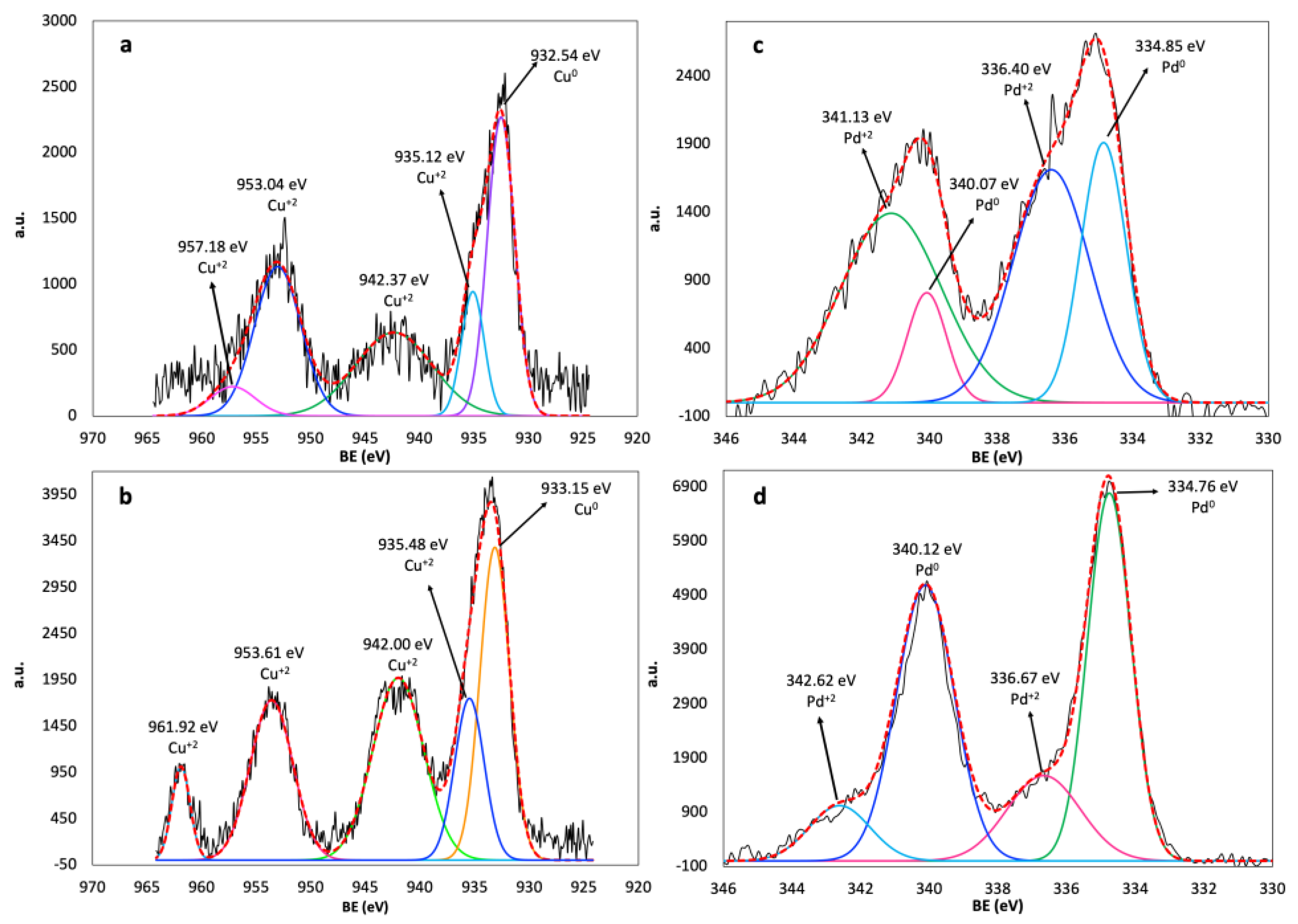
3.6. XPS of Cu and Pd on Catalysts
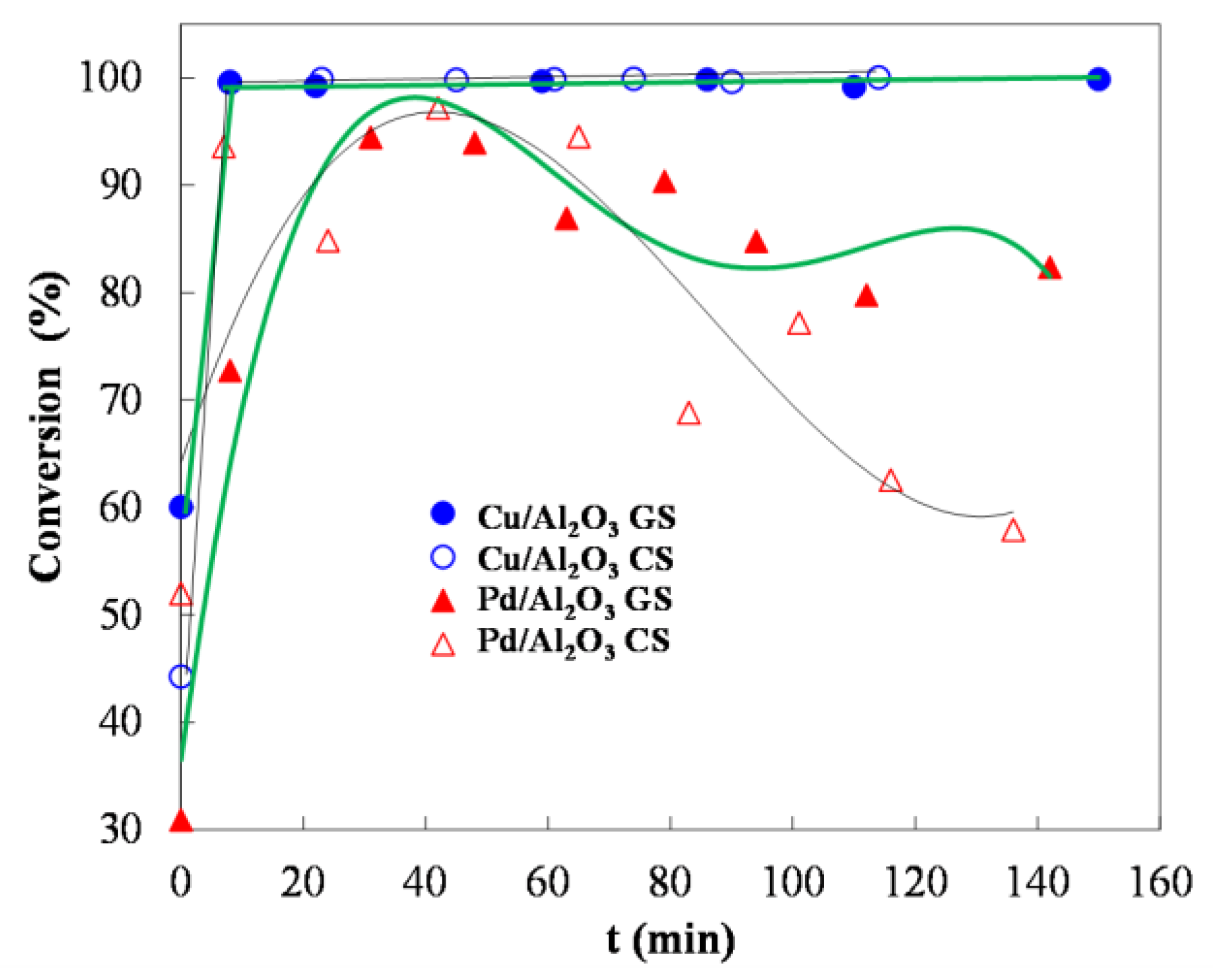
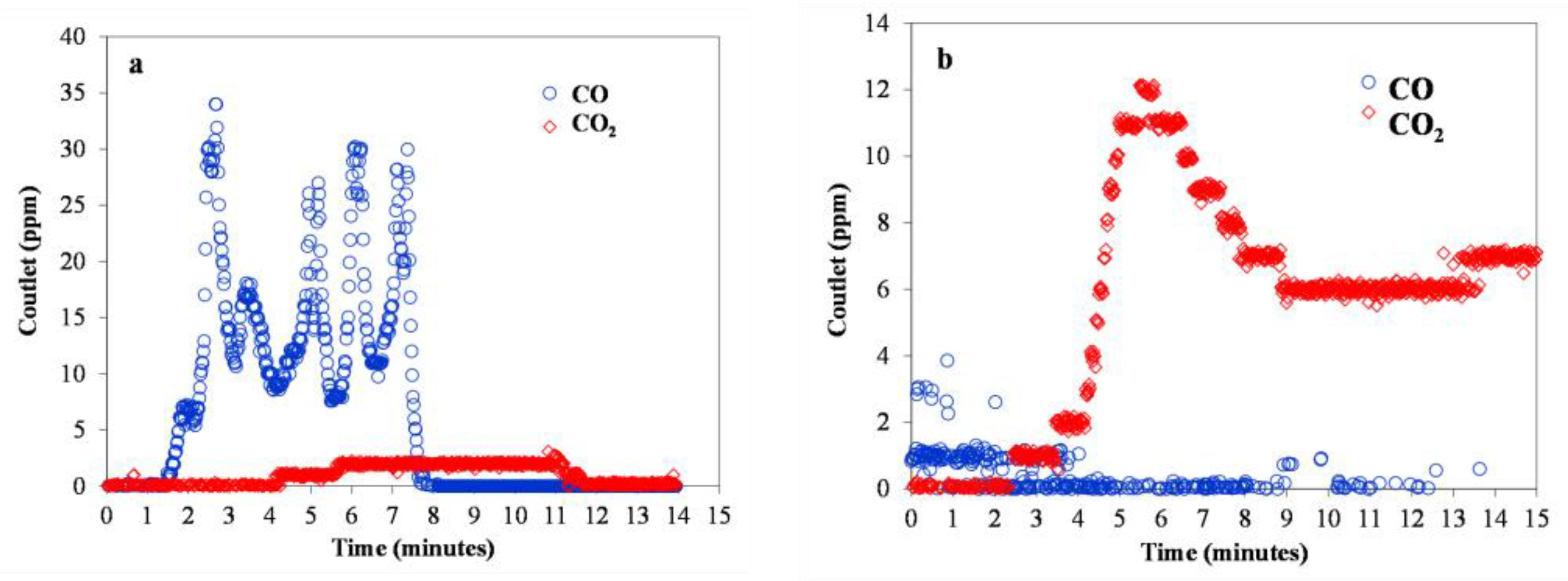
3.7. Catalytic Oxidation of HMFCA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of Green Chemistry and Its Multidimensional Impacts: A Review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, D.; Schramm, K.-W.; Lalah, J.O. Green Chemistry: Some Important Forerunners and Current Issues. Sustain. Chem. Pharm. 2020, 18, 100313. [Google Scholar] [CrossRef]
- Solis Maldonado, C.; Rivera De la Rosa, J.; Lucio-Ortiz, C.J.; Valente, J.S.; Castaldi, M.J. Synthesis and Characterization of Functionalized Alumina Catalysts with Thiol and Sulfonic Groups and Their Performance in Producing 5-Hydroxymethylfurfural from Fructose. Fuel 2017, 198, 134–144. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Luo, J.; Yang, S. Direct Conversion of Biomass Components to the Biofuel Methyl Levulinate Catalyzed by Acid-Base Bifunctional Zirconia-Zeolites. Appl. Catal. B 2017, 200, 182–191. [Google Scholar] [CrossRef]
- Ruiz-Zamora, E.; De la Rosa, J.R.; Maldonado, C.S.; Lucio–Ortiz, C.J.; Del Río, D.A.D.H.; Garza-Navarro, M.A.; Sandoval-Rangel, L.; Morales-Leal, F.J.; Wi, S. Siliceous Self-Pillared Pentasil (SPP) Zeolite with Incorporated Phosphorus Groups in Catalytic Formation of Butadiene by Dehydra-Decyclization of Tetrahydrofuran: Study of Catalyst Stability by NMR and REDOR Analysis. Appl. Catal. A Gen. 2022, 640, 118648. [Google Scholar] [CrossRef]
- Lyu, H.; Lim, J.Y.; Zhang, Q.; Senadheera, S.S.; Zhang, C.; Huang, Q.; Ok, Y.S. Conversion of Organic Solid Waste into Energy and Functional Materials Using Biochar Catalyst: Bibliometric Analysis, Research Progress, and Directions. Appl. Catal. B 2024, 340, 123223. [Google Scholar] [CrossRef]
- Massironi, A.; Biella, S.; de Moura Pereira, P.F.; Scibona, F.; Feni, L.; Sindaco, M.; Emide, D.; Jiménez-Quero, A.; Bianchi, C.L.M.; Verotta, L.; et al. Valorization of Pumpkin Seed Hulls, Cucurbitin Extraction Strategies and Their Comparative Life Cycle Assessment. J. Clean. Prod. 2023, 427, 139267. [Google Scholar] [CrossRef]
- Elemike, E.E.; Ibe, K.A.; Mbonu, J.I.; Onwudiwe, D.C. Chapter 5—Phytonanotechnology and Synthesis of Silver Nanoparticles. In Phytonanotechnology; Thajuddin, N., Mathew, S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–96. ISBN 978-0-12-822348-2. [Google Scholar]
- González-Casamachin, D.A.; Rivera De la Rosa, J.; Lucio-Ortiz, C.J.; Elizondo Villareal, N.; Verastegui Domínguez, L.H.; Bustos Martínez, D.; Sandoval Rangel, L. Green Synthesis of Pd–Fe Catalyst Supported on ɣ-Al2O3 Using Gobernadora Extract (Larrea Tridentata) for the Hydrogenation of 2-Methylfuran to Biorefinery Products. Top. Catal. 2022, 65, 1244–1250. [Google Scholar] [CrossRef]
- Patra, S.; Mishra, S.; Parhi, B.; Mishra, H.; Swain, S.K. Role of Transition Metal Nanocomposites in Organic Reactions: A State of Art as an Alternative to Conventional Catalysts. Results Chem. 2023, 6, 101172. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface Area and Pore Texture of Catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Cheirmadurai, K.; Biswas, S.; Murali, R.; Thanikaivelan, P. Green Synthesis of Copper Nanoparticles and Conducting Nanobiocomposites Using Plant and Animal Sources. RSC Adv. 2014, 4, 19507–19511. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Green Synthesis and Characterizations of Silver and Gold Nanoparticles Using Leaf Extract of Rosa Rugosa. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 34–41. [Google Scholar] [CrossRef]
- Alshammari, S.O.; Mahmoud, S.Y.; Farrag, E.S. Synthesis of Green Copper Nanoparticles Using Medicinal Plant Krameria Sp. Root Extract and Its Applications. Molecules 2023, 28, 4629. [Google Scholar] [CrossRef]
- Quintana-Solórzano, R.; Neri-Gómez, A.A.; Armendáriz-Herrera, H.; Valente, J.S. Manufacture Process Scale-Up and Industrial Testing of Novel Catalysts for SO x -Emissions Control in FCC Units. Catal. Lett. 2019, 149, 272–282. [Google Scholar] [CrossRef]
- Mahmoudi, B.; Soleimani, F.; Keshtkar, H.; Ali Nasseri, M.; Kazemnejadi, M. Green Synthesis of Trimetallic Oxide Nanoparticles and Their Use as an Efficient Catalyst for the Green Synthesis of Quinoline and Spirooxindoles: Antibacterial, Cytotoxicity and Anti-Colon Cancer Effects. Inorg. Chem. Commun. 2021, 133, 108923. [Google Scholar] [CrossRef]
- Rafi Shaik, M.; Ali, Z.J.Q.; Khan, M.; Kuniyil, M.; Assal, M.E.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, M.; Adil, S.F. Green Synthesis and Characterization of Palladium Nanoparticles Using Origanum Vulgare L. Extract and Their Catalytic Activity. Molecules 2017, 22, 165. [Google Scholar] [CrossRef]
- Rasool, A.; Kiran, S.; Abrar, S.; Iqbal, S.; Farooq, T.; Jahan, N.; Munir, B.; Yusuf, M.; Mukhtar, N. Synthesis and Characterization of Bio-Fabricated Silver Nanoparticles as Green Catalysts for Mitigation of Synthetic Dyes: A Sustainable Environmental Remedial Approach. J. Mol. Liq. 2024, 396, 124061. [Google Scholar] [CrossRef]
- Giannenas, I.; Bonos, E.; Christaki, E.; Florou-Paneri, P. Oregano: A Feed Additive With Functional Properties. In Therapeutic Foods; Academic Press: Cambridge, MA, USA, 2018; pp. 179–208. [Google Scholar] [CrossRef]
- Calamaco, Z.G.; Ramos, G.; Montfort, C.; Marszalek, J.E.; Vargas González, G. Revisión Sobre El Orégano Mexicano Lippia Graveolens HBK. (Sinonimia Lippia berlandieri Schauer) y Su Aceite Esencial; Universidad Autónoma de Nuevo León (UANL): San Nicolás de los Garza, NL, Mexico, 2023; Volume 8. [Google Scholar] [CrossRef]
- Cid-Pérez, T.S.; Nevárez-Moorillón, G.V.; Torres-Muñoz, J.V.; Palou, E.; López-Malo, A. Chapter 63—Mexican Oregano (Lippia berlandieri and Poliomintha longiflora) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 551–560. ISBN 978-0-12-416641-7. [Google Scholar]
- Jurado-Guerra, P.; Velázquez-Martínez, M.; Sánchez-Gutiérrez, R.A.; Álvarez-Holguín, A.; Domínguez-Martínez, P.A.; Gutiérrez-Luna, R.; Garza-Cedillo, R.D.; Luna-Luna, M.; Chávez-Ruiz, M.G. The Grasslands and Scrublands of Arid and Semi-Arid Zones of Mexico: Current Status, Challenges and Perspectives. Rev. Mex. Cienc. Pecu. 2021, 12, 261–285. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Gardini, F.; Lanciotti, R. Effects of Sub-Lethal Concentrations of Thyme and Oregano Essential Oils, Carvacrol, Thymol, Citral and Trans-2-Hexenal on Membrane Fatty Acid Composition and Volatile Molecule Profile of Listeria Monocytogenes, Escherichia Coli and Salmonella Enteritidis. Food Chem. 2015, 182, 185–192. [Google Scholar] [CrossRef]
- Shang, X.; Wang, Y.; Zhou, X.; Guo, X.; Dong, S.; Wang, D.; Zhang, J.; Pan, H.; Zhang, Y.; Miao, X. Acaricidal Activity of Oregano Oil and Its Major Component, Carvacrol, Thymol and p-Cymene against Psoroptes cuniculi in Vitro and in Vivo. Vet. Parasitol. 2016, 226, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Castillo, B.; Morones-Ramírez, J.R.; Rivera-De la Rosa, J.; Alcalá-Rodríguez, M.M.; Cerdán Pasarán, A.Q.; Díaz-Barriga Castro, E.; Escárcega-González, C.E. Organic Waste as Reducing and Capping Agents for Synthesis of Silver Nanoparticles with Various Applications. ChemistrySelect 2022, 7, e202201023. [Google Scholar] [CrossRef]
- Sitthisa, S.; Pham, T.; Prasomsri, T.; Sooknoi, T.; Mallinson, R.G.; Resasco, D.E. Conversion of Furfural and 2-Methylpentanal on Pd/SiO2 and Pd–Cu/SiO2 Catalysts. J. Catal. 2011, 280, 17–27. [Google Scholar] [CrossRef]
- Baruah, D.; Hussain, F.L.; Suri, M.; Saikia, U.P.; Sengupta, P.; Dutta, D.K.; Konwar, D. Bi (NO3)3·5H2O and Cellulose Mediated Cu-NPs—A Highly Efficient and Novel Catalytic System for Aerobic Oxidation of Alcohols to Carbonyls and Synthesis of DFF from HMF. Catal. Commun. 2016, 77, 9–12. [Google Scholar] [CrossRef]
- Zhen, Z.; Li, A.; Yang, Y.; Hu, Y.; Lv, J.; Huang, S.; Wang, Y.; Ma, X. Engineering Stable Active Cu0-Cuσ+ Sites via in Situ Surface Reconstruction over Cu/CeO2 Catalyst for the Hydrogenation of Carbon-Oxygen Bonds. Catal. Today 2024, 437, 114766. [Google Scholar] [CrossRef]
- Hasan, I.H.; Mhaibes, R.M.; Kadhum, A.A.H.; Al-Bahrani, H.A.; Imeer, A.T.A.; Al-Rashedi, N.A.M.; Shu, G. Recent Advances on Pd Schiff Base Catalysts in Suzuki-Miyaura Cross-Coupling Reaction: A Review. J. Organomet. Chem. 2025, 1024, 123444. [Google Scholar] [CrossRef]
- Dai, J. Synthesis of 2,5-Diformylfuran from Renewable Carbohydrates and Its Applications: A Review. Green Energy Environ. 2021, 6, 22–32. [Google Scholar] [CrossRef]
- Liu, P.; Huai, L.; Zhu, B.; Zhong, Y.; Zhang, J.; Chen, C. Interaction between Copper and Nickel Species for Electrooxidation of 2,5-Bis(Hydroxymethyl)Furan. Green Chem. 2024, 26, 5377–5385. [Google Scholar] [CrossRef]
- Mittal, N.; Kaur, M.; Singh, V. Mild and Selective Catalytic Oxidation of 5-HMF to 2,5-FDCA over Metal Loaded Biomass Derived Graphene Oxide Using Hydrogen Peroxide in Aqueous Media. Mol. Catal. 2023, 546, 113223. [Google Scholar] [CrossRef]
- Li, Z.; Du, E.; Hao, P.; Huai, L.; Zhong, Y.; El-Hout, S.I.; Chen, C.; Zhang, J. Diluted Oxygen Realizes High Productivity of 2,5-Furandicarboxylic Acid under Ambient Temperature. Catal. Today 2023, 423, 114277. [Google Scholar] [CrossRef]
- Li, Z.; Huai, L.; Hao, P.; Zhao, X.; Wang, Y.; Zhang, B.; Chen, C.; Zhang, J. Oxidation of 2,5-Bis(Hydroxymethyl)Furan to 2,5-Furandicarboxylic Acid Catalyzed by Carbon Nanotube-Supported Pd Catalysts. Chin. J. Catal. 2022, 43, 793–801. [Google Scholar] [CrossRef]
- Elizondo, N.; Segovia, P.; Coello, V.; Arriaga, J.; Belmares, S.; Alcorta, A.; Hernández, F.; Obregón, R.; Torres, E.; Paraguay, F. Green Synthesis and Characterizations of Silver and Gold Nanoparticles. In Green Chemistry; Kidwai, M., Mishra, N.K., Eds.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Mehrabadi, B.A.T.; Eskandari, S.; Khan, U.; White, R.D.; Regalbuto, J.R. A Review of Preparation Methods for Supported Metal Catalysts. Adv. Catal. 2017, 61, 1–35. [Google Scholar] [CrossRef]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Borohydride Reduction of Nickel and Copper Ions in Aqueous and Nonaqueous Media. Controllable Chemistry Leading to Nanoscale Metal and Metal Boride Particles. Langmuir 1994, 10, 4726–4730. [Google Scholar] [CrossRef]
- Wojnicki, M.; Fitzner, K.; Luty-Błocho, M. Kinetic Studies of Nucleation and Growth of Palladium Nanoparticles. J. Colloid Interface Sci. 2016, 465, 190–199. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Issaabadi, Z.; Sajadi, S.M. Green Synthesis of Cu/Al2O3 Nanoparticles as Efficient and Recyclable Catalyst for Reduction of 2,4-Dinitrophenylhydrazine, Methylene Blue and Congo Red. Compos. B Eng. 2019, 166, 112–119. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef]
- Sankarganesh, P.; Ganesh Kumar, A.; Parthasarathy, V.; Joseph, B.; Priyadharsini, G.; Anbarasan, R. Synthesis of Murraya Koenigii Mediated Silver Nanoparticles and Their In Vitro and In Vivo Biological Potential. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2971–2979. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Javanshir, Z.; Mehr, R.; Fekri, M.; Hossein, M. Experimental and Computational Studies on the Electrochemical Behavior of Carvacrol and Menthol. Iran. J. Chem. Chem. Eng. 2021, 40, 487–499. [Google Scholar]
- Tonello, N.V.; D’Eramo, F.; Marioli, J.M.; Crevillen, A.G.; Escarpa, A. Extraction-Free Colorimetric Determination of Thymol and Carvacrol Isomers in Essential Oils by PH-Dependent Formation of Gold Nanoparticles. Microchim. Acta 2018, 185, 352. [Google Scholar] [CrossRef]
- Noguchi, T. FTIR Detection of Water Reactions in the Oxygen-Evolving Centre of Photosystem II. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wang, X.; Ding, Q.; Jin, S.; Feng, Z.; Li, C. Effect of Pt Cocatalyst in Pt/TiO2 Studied by in Situ FTIR of CO Adsorption. Chin. J. Catal. 2014, 35, 1900–1906. [Google Scholar] [CrossRef]
- Maldonado, C.S.; De la Rosa, J.R.; Lucio-Ortiz, C.J.; Hernández-Ramírez, A.; Castillón Barraza, F.F.; Valente, J.S. Low Concentration Fe-Doped Alumina Catalysts Using Sol-Gel and Impregnation Methods: The Synthesis, Characterization and Catalytic Performance during the Combustion of Trichloroethylene. Materials 2014, 7, 2062–2086. [Google Scholar] [CrossRef]
- Dealba-Montero, I.; Guajardo-Pacheco, J.; Morales-Sánchez, E.; Araujo-Martínez, R.; Loredo-Becerra, G.M.; Martínez-Castañón, G.A.; Ruiz, F.; Jasso, M.E.C. Antimicrobial Properties of Copper Nanoparticles and Amino Acid Chelated Copper Nanoparticles Produced by Using a Soya Extract. Bioinorg. Chem. Appl. 2017, 2017, 1064918. [Google Scholar] [CrossRef]
- Morales–Leal, F.J.; Rivera De la Rosa, J.; Lucio–Ortiz, C.J.; De Haro Del Río, D.A.; Garza–Navarro, M.A.; Tian, W.; Herrera, J.E. Monometallic Platinum and Palladium–Based Catalysts in the Competitive Oxidation of Methanol over the Liquid–Phase Methanol–Ethanol Mixtures. Chem. Eng. J. 2021, 426, 131623. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Urzhuntsev, G.A.; Stadnichenko, A.I.; Svintsitskiy, D.A.; Ishchenko, A.V.; Boronin, A.I.; Ismagilov, Z.R. Effect of Pd- Precursor and Support Acid Properties on the Pd Electronic State and the Hydrodesulfurization Activity of Pd-Zeolite Catalysts. Catal. Today 2019, 323, 257–270. [Google Scholar] [CrossRef]
- Sánchez-De La Torre, F.; Rivera De La Rosa, J.; Kharisov, B.I.; Lucio-Ortiz, C.J. Preparation and Characterization of Cu and Ni on Alumina Supports and Their Use in the Synthesis of Low-Temperature Metal-Phthalocyanine Using a Parallel-Plate Reactor. Materials 2013, 6, 4324. [Google Scholar] [CrossRef]
- Morales-Leal, F.J.; de la Rosa, J.R.; Lucio-Ortiz, C.J.; Martínez, D.B.; De Haro Del Rio, D.A.; Garza-Navarro, M.A.; Martínez-Vargas, D.X.; Garcia, C.D. Comparison between the Catalytic and Photocatalytic Activities of Cu/Al2O3 and TiO2 in the Liquid Phase Oxidation of Methanol/Ethanol Mixtures. Development of a Kinetic Model for the Preparation of Catalyst. Appl. Catal. A Gen. 2018, 562, 184–197. [Google Scholar] [CrossRef]
- Rostami-Tapeh-Esmaeil, E.; Golshan, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Synthesis of Copper and Copper Oxide Nanoparticles with Different Morphologies Using Aniline as Reducing Agent. Solid. State Commun. 2021, 334–335, 114364. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Qing, W.; Chen, K.; Wang, Y.; Liu, X.; Lu, M. Green Synthesis of Silver Nanoparticles by Waste Tea Extract and Degradation of Organic Dye in the Absence and Presence of H2O2. Appl. Surf. Sci. 2017, 423, 1019–1024. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass Transfer Operations, 3rd ed.; Brown, J.V., Eichberg, M., Eds.; McGraw-Hill chemical engineering series; McGraw Hill: New York, NY, USA, 1981; ISBN 0-07-066615-6. [Google Scholar]
- Zhao, J.; Zhang, G.; Liu, H.; Shu, Q.; Zhang, Q. Improved Charge Transfer and Morphology on Ti-Modified Cu/γ-Al2O3/Al Catalyst Enhance the Activity for Methanol Steam Reforming. Int. J. Hydrogen Energy 2022, 47, 18294–18304. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Yang, W.B.; Song, H.; Ma, Q.X.; Gao, X.H.; Li, P.; Zhao, T.S. Formic Acid Assisted Synthesis of Cu-ZnO-Al2O3 Catalyst and Its Performance in CO2 Hydrogenation to Methanol. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2023, 51, 120–128. [Google Scholar] [CrossRef]
- Prabu, P.; Losetty, V. Green Synthesis of Copper Oxide Nanoparticles Using Macroptilium lathyroides (L) Leaf Extract and Their Spectroscopic Characterization, Biological Activity and Photocatalytic Dye Degradation Study. J. Mol. Struct. 2024, 1301, 137404. [Google Scholar] [CrossRef]
- Zhu, C.; Fang, Y.; Luo, Z.; Zhang, C.; Zhang, X.; Li, J.; Chen, J.; Tan, L. Direct Dimethyl Ether Synthesis over Mesoporous Cu–Al2O3 Catalyst via CO Hydrogenation. Res. Chem. Intermed. 2019, 45, 5863–5876. [Google Scholar] [CrossRef]
- Al Soubaihi, R.M.; Saoud, K.M.; Awadallah-F, A.; Elkhatat, A.M.; Al-Muhtaseb, S.A.; Dutta, J. Investigation of Palladium Catalysts in Mesoporous Silica Support for CO Oxidation and CO2 Adsorption. Heliyon 2023, 9, e18354. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Yuan, H.; Feng, K.; Sun, X. Silica-Supported Schiff-Based Palladium Nanocatalyzed Cross-Coupling Reactions and Its Applications in the Synthesis of Antiplatelet and Fungicidal. Arab. J. Chem. 2023, 16, 105203. [Google Scholar] [CrossRef]
- Boucly, A.; Artiglia, L.; Roger, M.; Zabilskiy, M.; Beck, A.; Ferri, D.; van Bokhoven, J.A. Water Inhibition and Role of Palladium Adatoms on Pd/Al2O3 Catalysts during Methane Oxidation. Appl. Surf. Sci. 2022, 606, 154927. [Google Scholar] [CrossRef]
- Lv, Q.; Meng, Q.; Liu, W.; Sun, N.; Jiang, K.; Ma, L.; Peng, Z.; Cai, W.; Liu, C.; Ge, J.; et al. Pd–PdO Interface as Active Site for HCOOH Selective Dehydrogenation at Ambient Condition. J. Phys. Chem. C 2018, 122, 2081–2088. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, P.; Ding, D.; Yang, Z.; Wang, W.; Xin, H.; Xu, J.; Han, Y.F. Revealing the Active Species of Cu-Based Catalysts for Heterogeneous Fenton Reaction. Appl. Catal. B 2019, 258, 117985. [Google Scholar] [CrossRef]
- Dimas-Rivera, G.L.; de la Rosa, J.R.; Lucio-Ortiz, C.J.; de los Reyes Heredia, J.A.; González, V.G.; Hernández, T. Desorption of Furfural from Bimetallic Pt-Fe Oxides/Alumina Catalysts. Materials 2014, 7, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Miranda, B.; Díaz, E.; Ordóñez, S.; Vega, A.; Díez, F. V Oxidation of Trichloroethene over Metal Oxide Catalysts: Kinetic Studies and Correlation with Adsorption Properties. Chemosphere 2007, 66, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Lucio-Ortiz, C.J.; De la Rosa, J.R.; Hernández-Ramírez, A.; López-Cuellar, E.M.; Beltrán-Pérez, G.; del Carmen Miranda Guardiola, R.; Pedroza-Solís, C.D. La-, Mn- and Fe-Doped Zirconia Catalysts by Sol-Gel Synthesis: TEM Characterization, Mass-Transfer Evaluation and Kinetic Determination in the Catalytic Combustion of Trichloroethylene. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 352. [Google Scholar] [CrossRef]
- Dimas-Rivera, G.L.; Rivera De la Rosa, J.; Lucio-Ortiz, C.J.; Martínez-Vargas, D.X.; Sandoval-Rangel, L.; García Gutiérrez, D.I.; Solis Maldonado, C. Bimetallic Pd-Fe Supported on Gamma-Al2O3 Catalyst Used in the Ring Opening of 2-Methylfuran to Selective Formation of Alcohols. Appl. Catal. A Gen. 2017, 543, 133–140. [Google Scholar] [CrossRef]
- Xulú Martínez-Vargas, D.; Rivera, J.; La Rosa, D.; Sandoval-Rangel, L.; Guzmán-Mar, J.L.; Garza-Navarro, M.A.; Lucio-Ortiz, C.J.; De, D.A.; Río, H.-D. 5-Hydroxymethylfurfural Catalytic Oxidation under Mild Conditions by Co (II), Fe (III) and Cu (II) Salen Complexes Supported on SBA-15: Synthesis, Characterization and Activity. Appl. Catal. A Gen. 2017, 547, 132–145. [Google Scholar] [CrossRef]
- Gonzalez-Casamachin, D.A.; Rivera De la Rosa, J.; Lucio–Ortiz, C.J.; Sandoval-Rangel, L.; García, C.D. Partial Oxidation of 5-hydroxymethylfurfural to 2,5-Furandicarboxylic Acid Using O2 and a Photocatalyst of a Composite of ZnO/PPy under Visible-Light: Electrochemical Characterization and Kinetic Analysis. Chem. Eng. J. 2020, 393, 124699. [Google Scholar] [CrossRef]
- Davis, S.E.; Benavidez, A.D.; Gosselink, R.W.; Bitter, J.H.; de Jong, K.P.; Datye, A.K.; Davis, R.J. Kinetics and Mechanism of 5-Hydroxymethylfurfural Oxidation and Their Implications for Catalyst Development. J. Mol. Catal. A Chem. 2014, 388–389, 123–132. [Google Scholar] [CrossRef]
- Cang, R.; Shen, L.Q.; Yang, G.; Zhang, Z.D.; Huang, H.; Zhang, Z.G. Highly Selective Oxidation of 5-Hydroxymethylfurfural to 5-Hydroxymethyl-2-Furancarboxylic Acid by a Robust Whole-Cell Biocatalyst. Catalysts 2019, 9, 526. [Google Scholar] [CrossRef]
- Ardagh, M.A.; Birol, T.; Zhang, Q.; Abdelrahman, O.A.; Dauenhauer, P.J. Catalytic Resonance Theory: SuperVolcanoes, Catalytic Molecular Pumps, and Oscillatory Steady State. Catal. Sci. Technol. 2019, 9, 5058–5076. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Y.; Gao, Y.; Ma, J.; Zhao, H.; Gu, Q.; Su, Y.; Xu, X.; Wang, A.; Yang, B.; et al. Redox-Driven Surface Generation of Highly Active Pd/PdO Interface Boosting Low-Temperature Methane Combustion. Chin. J. Catal. 2024, 60, 242–252. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering; John Wiley & Sons: Hoboken, NJ, USA, 1999; ISBN 047125424X. [Google Scholar]
- Mortensen, R.L.; Noack, H.D.; Pedersen, K.; Dunstan, M.A.; Wilhelm, F.; Rogalev, A.; Pedersen, K.S.; Mielby, J.; Mossin, S. Understanding the Reversible and Irreversible Deactivation of Methane Oxidation Catalysts. Appl. Catal. B 2024, 344, 123646. [Google Scholar] [CrossRef]




| Sample | Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| Al2O3 | 206 | 0.43 | 8.35 |
| Cu/Al2O3 GS-A | 184 | 0.34 | 7.42 |
| Cu/Al2O3 GS-N | 182 | 0.34 | 7.42 |
| Cu/Al2O3 CS-A | 190 | 0.39 | 6.18 |
| Pd/Al2O3 GS-A | 161 | 0.30 | 3.71 |
| Pd/Al2O3 CS-A | 210 | 0.41 | 5.82 |
| Kinetic Data | Activation Energy (J/mol) | Pre-Exponential Constant (min−1) | r2 |
|---|---|---|---|
| Cu2+ over Al2O3 CS | 84,019 | 1.0397 × 1013 | 0.9996 |
| Cu2+ over Al2O3 GS | 71, 646 | 1.2192 × 1010 | 0.9130 |
| Pd2+ over Al2O3 CS | 83,493 | 8.9367 × 1014 | 0.9932 |
| Cu/Al2O3-GS | Cu/Al2O3-CS | Pd/Al2O3-GS | Pd/Al2O3-CS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Phase | B.E. (eV) | % Area | B.E. (eV) | % Area | Phase | B.E. (eV) | % Area | B.E. (eV) | % Area |
| Cu0 | 933.15 | 29.02 | 932.54 | 31.75 | Pd0 | 334.85 | 22.3 | 334.76 | 38.3 |
| Cu+2 | 935.48 | 15.01 | 935.13 | 11.01 | Pd0 | 340.07 | 8.1 | 340.12 | 38.5 |
| Cu+2 | 942.00 | 29.25 | 942.37 | 25.04 | Pd+2 | 336.40 | 33.7 | 336.67 | 15.0 |
| Cu+2 | 953.61 | 21.38 | 953.04 | 27.05 | Pd+2 | 341.13 | 35.9 | 342.62 | 8.1 |
| Cu+2 | 961.92 | 5.34 | 957.18 | 5.15 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lino Galarza, B.J.; Rivera De la Rosa, J.; Lucio-Ortiz, C.J.; Garza-Navarro, M.A.; Solis Maldonado, C.; Sandoval Rángel, L.; Busto Martínez, D.; Escarcega-González, C.E. Green Synthesis of Cu and Pd Catalysts Using Mexican Oregano (Lippia graveolens) Extract and Their Application in the Conversion of a Biomass-Derived Molecule. Processes 2025, 13, 1681. https://doi.org/10.3390/pr13061681
Lino Galarza BJ, Rivera De la Rosa J, Lucio-Ortiz CJ, Garza-Navarro MA, Solis Maldonado C, Sandoval Rángel L, Busto Martínez D, Escarcega-González CE. Green Synthesis of Cu and Pd Catalysts Using Mexican Oregano (Lippia graveolens) Extract and Their Application in the Conversion of a Biomass-Derived Molecule. Processes. 2025; 13(6):1681. https://doi.org/10.3390/pr13061681
Chicago/Turabian StyleLino Galarza, Bárbara Jazmín, Javier Rivera De la Rosa, Carlos J. Lucio-Ortiz, Marco Antonio Garza-Navarro, Carolina Solis Maldonado, Ladislao Sandoval Rángel, Diana Busto Martínez, and Carlos Enrique Escarcega-González. 2025. "Green Synthesis of Cu and Pd Catalysts Using Mexican Oregano (Lippia graveolens) Extract and Their Application in the Conversion of a Biomass-Derived Molecule" Processes 13, no. 6: 1681. https://doi.org/10.3390/pr13061681
APA StyleLino Galarza, B. J., Rivera De la Rosa, J., Lucio-Ortiz, C. J., Garza-Navarro, M. A., Solis Maldonado, C., Sandoval Rángel, L., Busto Martínez, D., & Escarcega-González, C. E. (2025). Green Synthesis of Cu and Pd Catalysts Using Mexican Oregano (Lippia graveolens) Extract and Their Application in the Conversion of a Biomass-Derived Molecule. Processes, 13(6), 1681. https://doi.org/10.3390/pr13061681







