Abstract
In this work, a gradient leaching strategy for stepwise extraction of tungsten and cerium from a spent W-Ce/TiO2 catalyst has been developed. Results of a thermodynamic analysis indicated that high-temperature alkaline leaching and low-temperature acid leaching were conducive to the extraction of W and Ce, respectively. The effects of leaching agent type, concentration, temperature, and liquid-to-solid ratio on the leaching rates of W and Ce were systematically investigated. Experimental results revealed that the leaching ratio of W reached 90.92% under optimized conditions of 3 mol/L NaOH, 100 °C, 1 h, and a liquid-to-solid ratio of 20:1 for the alkaline leaching in the first stage. The leaching ratio for Ce reached 91.96% under optimized conditions of 1 mol/L H2SO4, 50 °C, 2 h, and a liquid-to-solid ratio of 12:1 for acidic leach in the second stage. The leaching ratios of titanium and aluminum were limited to 1.76% and 4.42%, respectively, indicating that >90% of these elements were virtually undissolved during the two-stage leaching process. The final leaching residue after the two-stage leaching contained >91.88 wt% TiO2. Consequently, this study not only demonstrated effective separation of W, Ce, and Ti, but also provided an innovative solution for the environmentally friendly treatment and resource utilization for spent W-Ce/TiO2 catalysts.
1. Introduction
Nitrogen oxides (NOx) are one of the most characteristic atmospheric pollutants emitted from direct combustion of coal in coal-fired power plants [1,2,3]. A substantial emission of NOx can lead to complex environmental pollution issues such as acid rain, photochemical smog, and greenhouse effects. Additionally, these pollutants exert a strong irritating effect on human organs, including eyes and the respiratory tract, posing a significant threat to both environmental safety and public health [4,5]. Selective catalytic reduction (SCR) has become the predominant flue gas denitrification technology due to its favorable reaction temperature, high denitrification efficiency (>90%), excellent reaction selectivity, and well-developed operation of the equipment [6,7]. SCR catalyst is the most crucial component of the selective catalytic reduction denitrification system, in which the active constituents induce the reductant to selectively react with and convert NOx to environmentally benign N2 in the flue gas at a certain temperature [8,9,10]. However, SCR catalyst inevitably undergoes attrition and corrosion caused by alkali metals and arsenic present in the flue dust during prolonged operation, leading to a decrease in the catalytic activity, a reduction in lifespan, and even a decommissioning of the SCR catalyst [11,12,13]. Statistics indicate that approximately 800,000 tons of solid catalysts are consumed globally each year, resulting in about 500,000–700,000 tons of spent catalysts [14]. Spent SCR catalysts have been classified as hazardous waste since 2014. They typically contain 75–90% TiO2, 3–8% WO3/MoO3, and other valuable metals such as vanadium, cerium, or other rare earth metals [15]. If these spent catalysts are not properly disposed of or recycled, they can lead to severe environmental pollution and wasting of resources. Therefore, the recovery of valuable metals from spent SCR catalysts is of significant importance.
Recovery techniques for spent SCR catalysts are broadly categorized into pyrometallurgical and hydrometallurgical methods. The pyrometallurgical method involves a pre-treatment using alkali chemicals such as CaO [16], NaOH [17,18,19], or Na2CO3 [20,21] to roast the spent catalysts, followed by leaching vanadium and tungsten with hot water or acid, and separating tungsten and/or vanadium from titanium and other impurities. Compared to single-component roasting, using a mix of strong bases often yields better results. For example, Wang et al. [19] used the NaOH-NaCl roasting methods, where the decomposition of NaCl produced chlorine gas, acting as an oxidizing agent and catalyst, enhancing the oxidation of lower-valence vanadium and improving its leachability, while the lower melting point of NaOH facilitates the decrease in roasting temperatures and an enhancement of mass transfer. Under optimal roasting conditions, the leaching efficiencies of V and W can reach up to 93.25% and 99.17%, respectively. Chen et al. [22] used a NaOH-Na2CO3 mixed roasting system, followed by hydrochloric acid leaching, achieving recovery ratios of 99.2% for Ti and 99.6% for V. These pyrometallurgical methods extract all valuable metals simultaneously; therefore, they require a subsequent wet process for separation of metals.
The hydrometallurgical method includes acid and alkaline leaching to treat spent SCR catalysts. Nie et al. [23] noted that tungsten compounds in spent SCR catalysts transformed into tungstic acid, depositing on the surface of solid particles during leaching, hindering acid transport within the catalyst particles and reducing leaching ratios. Consequently, direct acid leaching has not yet proven to be a suitable method for recycling spent SCR catalysts. Alkaline leaching has shown promising results. Choi et al. [24] achieved extraction ratios of 91.5% V and 87% W using NaOH under optimal leaching conditions at a temperature of 250 °C, a concentration of 3 mol/L, and a particle size distribution under 150 μm, and a solid-to-liquid ratio (S/L) of 40% wt/vol. Temperature, pressure, and concentration of alkaline leaching agent significantly affect the leaching ratio of tungsten [25,26,27]. High-pressure leaching conducted in an autoclave could significantly enhanced the recovery of tungsten from 64.8% to 96.0% with increasing experimental temperatures from 200 °C to 300 °C, respectively [28]. However, high-temperature and -pressure leaching imposed high demands on equipment and resulted in a higher energy load, and it also required subsequent steps to separate metals such as W and V.
The method of recycling tungsten from spent SCR catalyst is summarized in Table 1. It can be seen in the table that there is a lack of research data on spent W-Ce/TiO2 SCR catalysts, as they are emerging catalysts with enhanced thermal stability and environmental performance. Therefore, this paper proposes a method on gradient recovery of tungsten, cerium, and titanium from the spent W-Ce/TiO2 catalysts with two-steps leaching (first alkaline leaching for W recovery, then acid leaching for Ce extraction, and the remaining TiO2 in the final leaching residue). Thermodynamic analyses of W-H2O and Ce-H2O systems were first carried out by employing HSC Chemistry 10 software [29]. Then, the effects of reaction temperature, reaction time, leaching agent concentration, and solid/liquid ratio on alkaline leaching of W and acidic leaching of Ce were investigated in detail. The optimal leaching conditions for alkaline and acidic leaching stages were determined. Furthermore, XRD, XPS, SEM, and EDS analyses were employed to analyze the solid samples before and after leaching for better understanding of the leaching steps. The results obtained in this study facilitate a comprehensive re-utilization of resources from the spent W-Ce/TiO2 SCR catalysts in a cleaner way.

Table 1.
A summary of recycling tungsten from spent W-V/TiO2 SCR catalyst.
2. Materials and Methods
The spent W-Ce/TiO2 SCR catalysts were supplied by a smelter in Ganzhou, Jiangxi, China. The main compositions of spent SCR catalysts are listed in Table 2. The morphology of solid samples was examined using SEM. As shown in Figure 1a, the surface of spent SCR catalyst raw materials was relatively smooth, with the presence of small pores, together with some rod-like grains. According to the EDS analyses in Figure 1b, the main component elements of the catalyst are Ti, W, and Ce, uniformly distributed in the catalyst. The embedded rod-like grains were glass fibers, mainly composed of SiO2 and Al2O3. The phase composition was determined using XRD, and the results are shown in Figure 1c. The main component of the catalyst was TiO2. There was a small peak between 15° and 20°, which could not be attributed to a certain phase. The substances such as SiO2, WO3 or CeO2 might exist in an amorphous or microcrystalline form.

Table 2.
The main chemical composition of spent SCR catalyst.
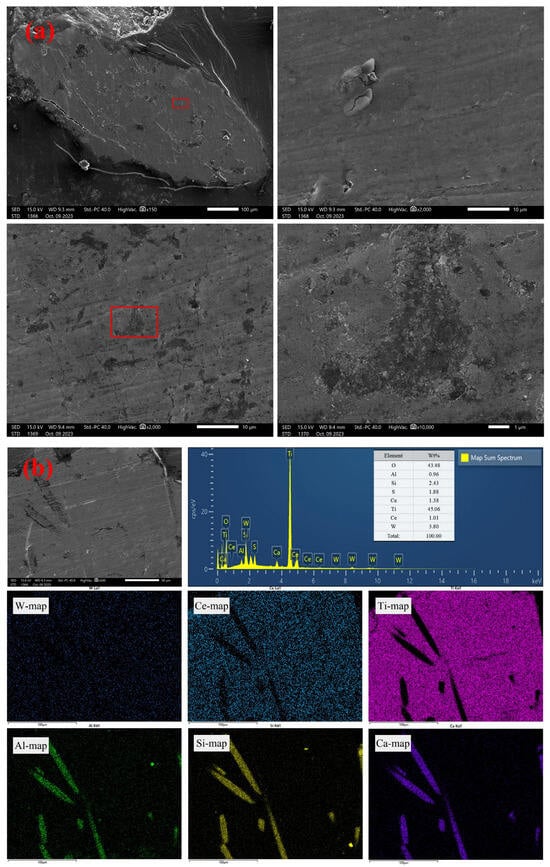
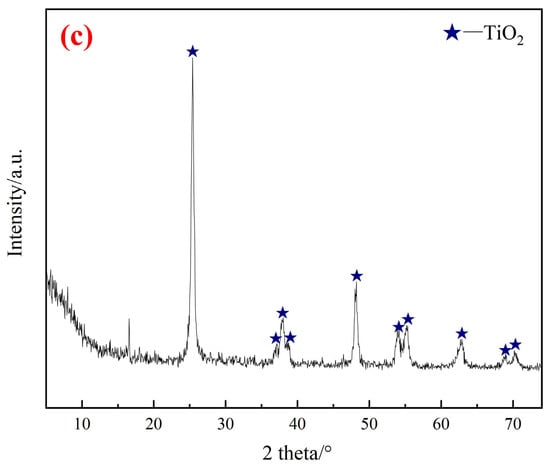
Figure 1.
Micrographs and composition of the solid sample (spent SCR catalyst); (a) SEM images, (b) EDS analyses, and (c) XRD patterns.
After drying the spent SCR catalyst for 24 h, a light-yellow powder was obtained and used in the experiment after grinding with a vibromill for 3 min with a particle size D50 of 2.294 μm and D90 of 22.47 μm. All reagents used in the experiments were analytically pure, and deionized water was used.
The leaching process was carried out in a constant temperature water bath for atmospheric pressure reactions (T ≤ 100 °C) and in a pressure autoclave (DY-8, machinery plant affiliated with Central South University, Changsha, China) for pressure conditions (T > 100 °C). The volume of the water bath and the autoclave pressure are 250 mL and 60 mL, respectively. The ground powder of raw materials was combined with the leaching agent in a specific liquid-to-solid ratio and added to the leaching reactor. The stirring rates for low- and high-temperature reactions were controlled by magnetic stirring and steel balls of different diameters, respectively. The mixture was stirred under a set temperature for a designated duration. After leaching, the mixture was separated by vacuum filtration to obtain the residue and filtrate. Then, the filtrate was used to determine the concentration of W and Ce. And the residue was washed and dried for 24 h in an oven at 45 °C for further analyses.
Information such as elemental composition, content, and chemical state were examined using X-ray Photoelectron Spectroscopy (PHI VersaProbe 4, ULVAC, Choshi City, Japan). The phase of the solid samples was determined by X-ray Diffraction (Empyrean 2, PANalytical B.V., Malvern, UK) with Cu Kα radiation (λ = 1.5406 Å). The particle size distribution (PSD) was recorded by a laser particle analyzer (MS 2000, Malvern Instruments, Malvern, UK). The elemental composition and distribution on the material surface were characterized using an energy-dispersive X-ray spectrometer (ULTIM MAX 40, Oxford Instruments, Oxford, UK). The morphology of the solid samples was tested using a field-emission scanning electron microscope (JSM-6360LV, JEOL, Shima City, Tokyo Prefecture, Japan). The WO3 content in the filtrate was determined with the spectrophotometric thiocyanate method (SP-752, Shanghai Spectral Instrument, CHN, Shanghai, China). The concentrations of Ce, Ti, and Al in the filtrate were analyzed using an Inductively Coupled Plasma (ICP) Emission Spectroscopy (ICAP 7400 radial, Thermo, Waltham, MA, USA), allowing for the calculation of the leaching ratios using the formula
where the suffix X is the type of metal, cX is the mass concentration of the metal X in the leaching solution (g/L), VX is the volume of the leaching solution (L), wX is the mass fraction of metal X in the raw material, and m0 is the raw material mass (g).
3. Results and Discussion
3.1. Thermodynamic Calculation
By referencing pertinent thermodynamic data [30], this study employed HSC Chemistry 10 software [29] to calculate and plot the E-pH diagrams for the W-H2O and Ce-H2O systems at 25 °C, 50 °C, 100 °C, and 140 °C, with the results presented in Figure 2 and Figure 3, respectively. Under acidic conditions, tungsten predominantly exists in the form of solid tungstic acid, H2WO4, which can lead to the formation of H2WO4 during acidic leaching, causing sedimentation and blockage of the catalyst, thereby hindering the reaction between catalyst and leaching agent. Therefore, the use of an alkaline leaching method is more effective for the extraction of WO3. By increasing the temperature, the boundary of the WO42− stability region shifts towards a lower pH, providing a greater thermodynamic driving force for the leaching of WO3. Figure 3 indicates that the favorable region for cerium oxides is in the upper right portion of the diagram. By controlling the reaction conditions to the left and lower part of the diagram, that is, under acidic and reductive conditions, Ce can be readily converted to Ce3+. With the increment in temperature, the favorable region for Ce3+ shifts to the left, indicating that the acidity required to obtain Ce3+ also increases, making the reaction conditions more stringent. Thus, lower temperatures are more conducive to the leaching of Ce.
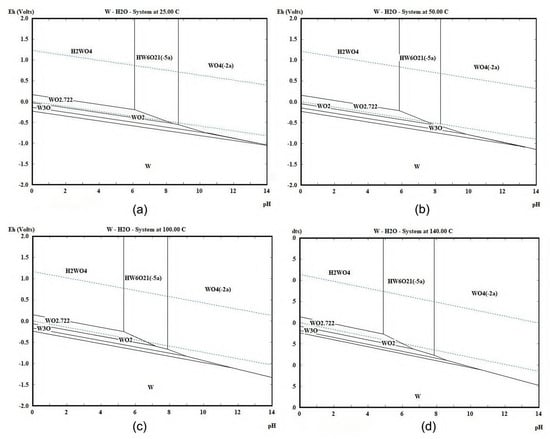
Figure 2.
E-pH diagram of the W-H2O system calculated with HSC chemistry software at (a) 25 °C, (b) 50 °C, (c) 100 °C, and (d) 140 °C.
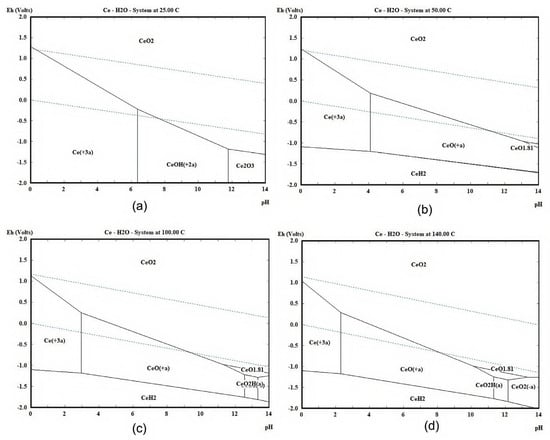
Figure 3.
E-pH diagram of the Ce-H2O system calculated with HSC chemistry software at (a) 25 °C, (b) 50 °C, (c) 100 °C, and (d) 140 °C.
3.2. Alkaline Leaching of Tungsten
The effects of leach agents on leaching efficiency of tungsten are shown in Figure 4a under the following experimental conditions: a temperature of 140 °C, a liquid/solid ratio of 5:1, and a leaching time of 2 h. From Figure 4a, strong bases such as NaOH and Na2CO3 had a good leaching effect on W, in which the leaching ratio of NaOH could reach 74.94%. Tungsten could hardly be leached due to both leaching efficiencies in weak bases and weak acids of less than 50%. Therefore, optimization conditions were performed with NaOH as the leaching agent in this study.
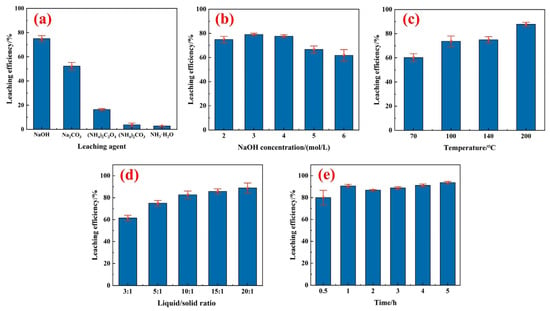
Figure 4.
Tungsten leaching efficiency under different reaction conditions: (a) effect of leaching agent (140 °C, 5:1, 2 h), (b) effect of NaOH concentration (140 °C, 5:1, 2 h), (c) effect of temperature (2 M NaOH, 5:1, 2 h), (d) effect of liquid/solid ratio (140 °C, 2 M NaOH, 2 h), and (e) effect of reaction time (140 °C, 3 M NaOH, 20:1).
Figure 4b illustrates the effect of NaOH concentration on the W leaching ratio. With increasing NaOH concentration, the leaching of W increased gradually at first but decreased slightly after the concentration reached 3 mol/L. The leaching ratio reached 79.03% with 3 mol/L NaOH, but the leaching ratio was only 61.79% with 6 mol/L. This can be interpreted as increased NaOH concentration strengthening the mass transfer, which is conducive to the diffusion and dissolution of W [31]. As the concentration of NaOH increases, the leaching reaction of the valuable metal was interrupted, as other elements having very low reactivity, such as SiO2, tend to react [28,32]. In the leaching experiment, Ti and Ce can hardly be leached in the NaOH solution, and NaOH concentration had almost no effect on the leaching ratios of Ti and Ce. Additionally, the leaching rate of Al was less than 3%. In this case, NaOH preferentially reacted with W. Consequently, the alkalinity of the solution post-reaction was insufficient for effective aluminum leaching. Therefore, the alkali leaching stage could only leach W into the solution and leave Ti and Ce in the residue.
The effects of temperature on the leaching of W were examined at 70, 100, 140, and 200 °C, with the results shown in Figure 4c. The leaching ratio at 70 °C was only 60.14%, while if the temperature is raised to 100 °C, the leaching ratio could increase nearly 20%. At higher temperatures, the increasing rate of leaching slows down, which can be explained by NaOH tending to react with impurities in the SCR rather than with WO3 [33]. At the same time, the higher the temperature, the higher the reaction equipment requirements and the larger the load is. Therefore, 100 °C was selected as the optimal reaction temperature.
The effects of the liquid/solid ratio on the leaching efficiency of W were examined, as shown in Figure 4d. The leaching temperature was 140 °C, the concentration of the NaOH was 2 mol/L, and the leaching time was 2 h. The leaching efficiency of W increased rapidly from 61.47% to 88.82%, as the liquid/solid ratio increased from 3 to 20. The increasing of the liquid/solid ratio expands the contact area of the liquid phase and the solid phase and reduces the leach liquid concentration, which makes the mass transfer resistance smaller, accelerating the leaching process [34].
Figure 4e shows the effect of leaching time on the W leaching ratio under the conditions of a leaching temperature of 140 °C, NaOH concentration of 3 mol/L, and liquid/solid ratio of 20:1. As the leaching time extended, the leaching ratio of tungsten gradually increased. Specifically, the leaching ratio of tungsten reached 90.65% with a leaching time of 1 h. Prolonging the leaching time further resulted in a negligible increment and slight fluctuation in the leaching ratio of tungsten. A kinetic analysis was attempted in order to reveal the tungsten leaching step by different kinetic models with the present data but failed due to the close fitting R2 value with present data. Therefore, 1 h was selected as the optimal leaching time.
In conclusion, the optimized leaching conditions in the alkali leaching stage were as follows: NaOH concentration of 3 mol/L, liquid/solid ratio of 20:1, reaction temperature of 100 °C, and reaction time of 1 h, where the leaching ratio of tungsten was 90.92%.
3.3. Acidic Leaching of Cerium
Under identical acidity conditions, six leaching agents—sulfuric acid, nitric acid, hydrochloric acid, acetic acid, and oxalic acid—were used to investigate the leaching efficiency of element Ce. The experimental results are shown in Figure 5a. The three strong acids, sulfuric acid, hydrochloric acid, and nitric acid, demonstrated a certain leaching capacity for Ce, in good agreement with thermodynamic predictions. In contrast, organic acids such as acetic acid and oxalic acid were less effective, possibly due to the poor kinetic characteristics of weak acids. Therefore, sulfuric acid was selected as the leaching agent for Ce.
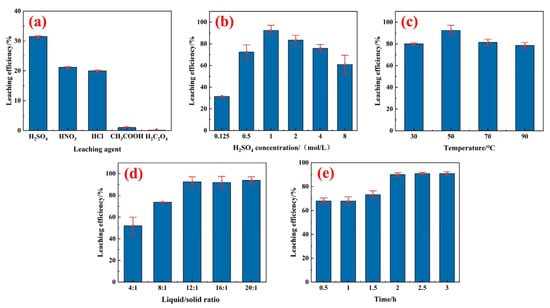
Figure 5.
Cerium leaching efficiency under different reaction conditions: (a) effect of leaching agent (50 °C, 6:1, 0.125 M H2SO4, 2 h), (b) effect of H2SO4 concentration (50 °C, 12:1, 2 h), (c) effect of temperature (1 M H2SO4, 12:1, 2 h), (d) effect of liquid/solid ratio (50 °C, 1 M H2SO4, 2 h), and (e) effect of reaction time (50 °C, 1 M H2SO4, 12:1).
The effects of H2SO4 concentration on the leaching efficiency of Ce were examined under the conditions of T = 50 °C, liquid/solid ratio of 12:1, and leaching time of 2 h, with results shown in Figure 5b. With the increment of sulfuric acid concentration, there is an escalation initially, followed by a subsequent decline of Ce leaching efficiency. The leaching efficiency of Ce reached a maximum of 92.45% with a sulfuric acid concentration of 1 mol/L. When the sulfuric acid concentration was low, fewer hydrogen ions were provided, leading to an incomplete reaction. The increment in sulfuric acid concentration enhanced the concentration gradient at the surface of the spent catalyst and promoted the diffusion of H+ and SO42− into its interior, thereby facilitating the leaching of Ce. When the concentration of sulfuric acid was excessively high, residual WO3 might react with the acid to form solid tungstic acid [35], which covered the catalyst particles and inhibited the leaching of Ce.
The effects of leaching temperature on the leaching efficiency of Ce were examined and are shown in Figure 5c under the experimental conditions of a sulfuric acid concentration of 1 mol/L, liquid/solid ratio of 12:1, and leaching time of 2 h. By increasing the temperature, the leaching ratio of Ce first increased and then decreased, reaching a maximum of 92.45% at 50 °C. A low temperature may lead to a slower diffusion rate and a lower leaching ratio of Ce, while a high temperature causes boundary movement of the equilibrium region of cerium ions, thermodynamically reinforcing the limitations of the leaching reaction. Therefore, the optimal leaching temperature was selected as a moderate 50 °C.
The effect of the liquid/solid ratio on the leaching efficiency of Ce was investigated under the experimental conditions of a temperature of 50 °C, reaction time of 2 h, and H2SO4 concentration of 1 mol/L. The results are shown in Figure 5d. Overall, the leaching ratio increased with an increment in the liquid-to-solid ratio. When the liquid-to-solid ratio increased from 5:1 to 12:1, the leaching rate of cerium significantly improved and reached 92.45%. When the liquid-to-solid ratio exceeded 12:1, the leaching ratio of cerium fluctuated around 90%, with a fluctuation range of <3%. Therefore, a liquid-to-solid ratio of 12:1 was considered as more appropriate to reduce leaching costs and minimize the discharge of acidic wastewater.
Figure 5e shows the effect of leaching time on the Ce leaching ratio under the conditions of a leaching temperature of 50 °C, H2SO4 concentration of 2 mol/L, and liquid-to-solid ratio of 12:1. The leaching ratio of Ce continued to increase by increasing the leaching time from 0.5 h to 3 h. Nevertheless, the increment in leaching efficiency was not significant after 2 h. A similar kinetic analysis was attempted to reveal Ce leaching with an unsuccessful result. Therefore, 2 h was considered as the optimal leaching time.
In summary, the optimal experimental conditions for the acid leaching of Ce were as follows: leaching agent of sulfuric acid of 1 mol/L, liquid-to-solid ratio of 12:1, leaching time of 2 h, and temperature of 50 °C. Under these conditions, the leaching ratio of the Ce element was 91.96%.
3.4. Characterization of Leaching Residue
XRD patterns of the spent SCR catalyst and leaching residue are shown in Figure 6. They only exhibited diffraction peaks of TiO2 and did not reveal the presence of other materials such as WO3 or CeO2, indicating that the content of W and Ce was very low or in an amorphous state. On the other hand, the characteristic diffraction peak intensity of TiO2 enhanced after leaching, suggesting an increment in the content of TiO2 post-leaching.
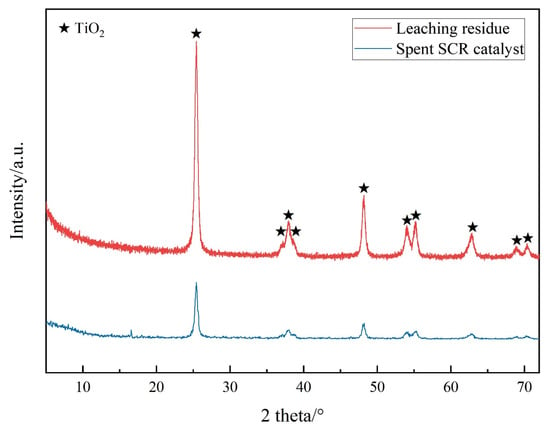
Figure 6.
XRD patterns of the spent SCR catalyst and leaching residue.
According to the SEM images of the final leaching residue (Figure 7), the leaching residue particles were irregular and ellipsoidal; no flaky particles were present in the form of sodium titanate [36]. Therefore, it was assumed that TiO2 did not react with NaOH and H2SO4 under the optimized conditions. From the EDS maps, little tungsten was evenly and dispersedly distributed in the leaching residue, and this residual W might be the inner lattice embedded in the TiO2 [31]. Thus, a further leaching ratio of W was required that breaks the TiO2 skeleton. Relative to the W element, there was slightly lower Ce in the leaching residue, indicating that sulfuric acid is a better leaching agent for Ce. Furthermore, the uniform distribution of W and Ce in the raw material and leaching residue possibly explained the failed kinetic analyses and revealed the leaching steps with a diffusion control in the leaching end stage.
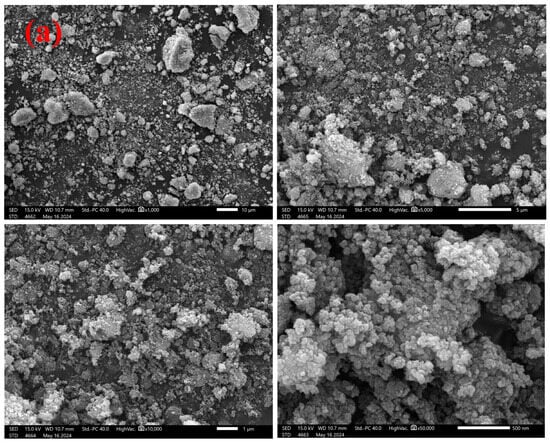
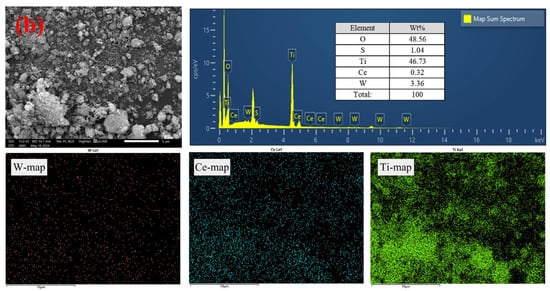
Figure 7.
SEM-EDS analyses of the final leaching residue. (a) SEM images and (b) EDS analysis.
Furthermore, rod-shaped solids were observed during the SEM scanning process, which were presumed to be the glass fibers observed in the raw material. The SEM images and EDS analysis results are shown in Figure 8. From the elemental distribution, it was evident that the main components of the rod-shaped fibers were Si, Al, and O, which are consistent with the analysis of the raw material. Moreover, their shape and surface condition has shown no significant changes, indicating that the two-stage leaching processes did not disrupt the structure of these glass fibers.
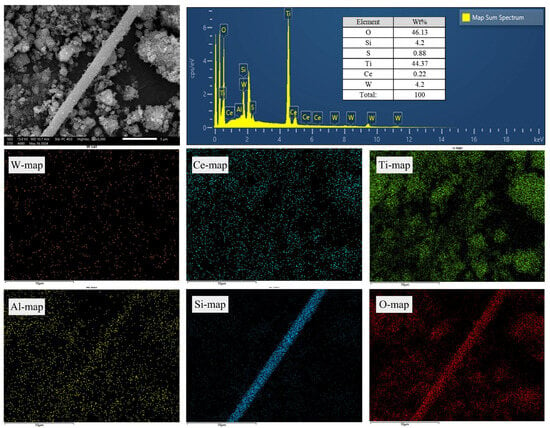
Figure 8.
SEM-EDS analyses of rod-shaped fibers in the final leaching residue.
XPS was used to further confirm the elemental composition and valence states. Figure 9a presents the full XPS survey spectrum of the spent catalyst and final leach residue, mainly showing W, Ce, Ti, and O elements. Figure 9b shows the W 4f electronic spectrum of the spent SCR catalyst and final leach residue. The peaks attributed to W 4f5/2 (37.38 eV and 40.71 eV) and W 4f7/2 (36.01 eV) orbitals were observed in the spent SCR catalyst, which corresponded to the oxidation state +6 of W [37,38,39]. Hence, W primarily existed in the form of WO3 in the spent SCR catalysts. However, there was only one peak in the W 4f5/2 electronic spectrum of final leach residue recorded at 37.35 eV, which was shifted towards lower binding energies compared to spent SCR catalyst. But the binding energy of this peak still fell within the range of W6+ oxidation state [39].
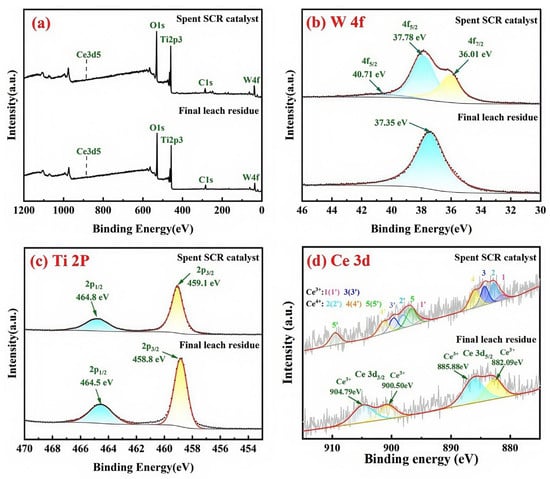
Figure 9.
XPS spectra of the spent catalyst and final leach residue. (a) full spectrum, (b) W 4f, (c) Ti 2p, (d) Ce 3d.
Figure 9c shows ESCA (Electron Spectroscopy for Chemical Analysis) data of the Ti 2p region obtained from the spent SCR catalyst and final leach residue. In the spent SCR catalyst, two characteristic peaks were observed at binding energies (BEs) of 464.8 eV and 459.1 eV, evoked by the Ti 2p3/2 and Ti 2p1/2 states, respectively. In the final leach residue, the two characteristic peaks were observed at positions around 464.5 eV and 458.8 eV, respectively. According to the literature data, the peak location and the splitting of the doublet (ΔBE = 5.7 eV) indicate an oxidation state of 4+ for titanium [18,40,41]. In detail, the binding energy value of 458.8 eV was the characteristic peak of TiO2 [37,42], and the binding energy value of 459.1 eV was the characteristic peak of highly sulfated TiO2 with sulfuric acid [43,44]. Compared to the spent SCR catalyst, the positions of characteristic peaks for Ti 2p1/2 and Ti 2p3/2 in the final leach residue were shifted towards lower binding energies by approximately 0.3 eV. This change was more likely due to the alkaline leaching removal of W, resulting in an increment in the charge density around the oxygen atoms shifting the electron density of the Ti-O bond to the Ti atoms [45].
The Ce 3d spectra of the spent catalyst and final leach residue are shown in Figure 9d. The Ce 3d peak could be separated into ten oxidation peaks. The characteristic peaks of Ce3+ were denoted as 1 (881.45 eV), 3 (885.43 eV), 1′ (900.10 eV), and 3′ (906.93 eV); the six characteristic peaks of Ce4+ were labeled as 2 (883.29 eV), 4 (887.86 eV), 5 (902.75 eV), 2′ (904.70 eV), 4′ (909.34 eV), and 5′ (920.75 eV) [46,47]. Consequently, cerium in the spent SCR catalyst existed in multiple valence states. By resolving the Ce 3d3/2 and Ce 3d5/2 peaks, the proportions of Ce3+ and Ce4+ were calculated, with Ce4+ representing 81.71%. After leaching, the XPS of leached residue showed a Ce with only the +3 valence state. This indicated that during the leaching process, the higher valence oxides of cerium reacted with sulfuric acid to form the corresponding sulfates.
3.5. Process Recommendation
According to the above results presented in Section 3.4, a novel alkaline leaching–acidic leaching gradient recovery process is proposed for the recycling of W and Ce elements from spent SCR catalysts (Figure 10). In the first stage, the raw material was leached with 3 mol/L NaOH using a liquid-to-solid ratio of 20:1 at 100 °C for 1 h. In the second stage, the residue obtained from the first leaching was leached with 1 mol/L H2SO4 using a liquid-to-solid ratio of 12:1 at 50 °C for 2 h. Under the optimized conditions, the leaching efficiencies for W and Ce were 90.92% and 91.96%, respectively. Meanwhile, the leaching ratios of other impurities such as titanium and aluminum were only 1.76% and 4.42%, respectively. The final leaching residue after alkaline leaching–acidic leaching contained >91.88 wt% TiO2, as shown in Table 3, which can be used as a raw material in titanium dioxide production. These facilitated the efficient gradient separation and recovery process for tungsten, cerium, and titanium.
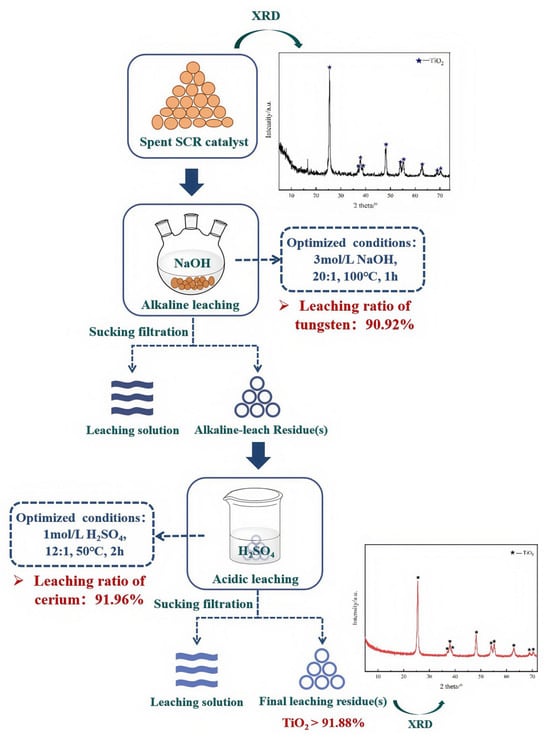
Figure 10.
Alkaline leaching–acidic leaching gradient recovery process for spent W-Ce/TiO2 catalysts.

Table 3.
The main chemical composition of the final leaching residue.
Compared with methods reported in the literature for treating spent W-V/TiO2 catalysts, shown in Table 1, comprehensive analyses were carried out, as shown in Table 4. The roasting–leaching methods obtained a high tungsten recovery, but came with a higher product cost and environmental impact. Hydrometallurgical treatment can avoid high energy consumption and environmental impacts. The high-pressure leaching could also generate high W and V recovery, while the recovery with no-pressure leaching reported by Su et al. [26] got a low recovery yield. This work achieved a 90.92% W and 91.96% Ce leaching yield at a temperature of ≤100 °C with no need for additional pressure. The gradient leaching of W and Ce also makes it easy to utilize these metals in subsequent steps. All this indicates the process has a low environmental impact and is economically viable.

Table 4.
Comprehensive analyses on this work and previous studies.
4. Conclusions
Based on the thermodynamic calculations and experimental examination in this work, the following conclusions were drawn:
- (1)
- Thermodynamic analysis results show that alkaline leaching and increased temperature are more effective for the extraction of WO3, while acid leaching at lower temperatures is more conducive to the leaching of Ce. These observations provide a thermodynamic basis for the alkali–acid gradient treatment of spent SCR catalysts.
- (2)
- The optimized conditions for the alkali leaching process were as follows: temperature of 100 °C, reaction time of 1 h, NaOH concentration of 3 mol/L, and a liquid/solid ratio of 20:1. The values for the acid leaching process were as follows: temperature of 50 °C, reaction time of 2 h, H2SO4 concentration of 1 mol/L, and liquid/solid ratio of 12:1. During the two-stage leaching process, the leaching ratios of W and Ce were 90.92% and 91.96%, respectively.
- (3)
- During the alkaline leaching–acidic leaching gradient recovery process, the leaching ratios of other impurities such as titanium and aluminum were only 1.76% and 4.42%, respectively. The final leaching residue after two-stage leaching was TiO2 with a content > 91.88 wt%. This condition allowed the preliminary separation of W, Ce, and Ti. In the final process, W and Ce were readily enriched and recovered from the leaching solutions, while titanium dioxide remained in final leaching residue.
From the gradient recovery process for spent W-Ce/TiO2 catalysts, a Na2WO4 solution is obtained during the alkaline leaching of spent W-Ce/TiO2 catalyst, which is an intermediate material during the present industrial production of ammonium paratungstate. An acidic cerous sulfate solution is generated during the following acidic leaching, which can be used to produce cerous sulfate. The final leaching residue with >91.88 wt% TiO2 will be a raw material for titanium dioxide production. Furthermore, strengthening measures such as ultrasound-assisted leaching [48] and the high-value utilization [49,50] of spent catalysts could be considered to increase metal recovery and economic benefits.
Author Contributions
H.W.: Investigation, Methodology, Software, Data curation, Writing—original draft. Z.P.: Conceptualization, Supervision, Writing—review and editing. J.H.: Investigation, Software, Data curation. F.T.: Writing—review and editing. L.S.: Conceptualization, Methodology, Validation, Writing—review and editing, Funding acquisition, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Hunan Natural Science Foundation for Youth Science, Fund Category B (former Outstanding Youth Science Fund, No. 2025JJ40046), National Natural Science Foundation of China (No. 52304374), Ganzhou Science and Technology Planning Project (No. 2022-KJH1259), and Starting-up Funding in Central South University (Faculty No. 220208).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| SCR | Selective Catalytic Reduction |
References
- Zhao, J.; Zhang, X.; Yang, F.; Ai, Y.; Chen, Y.; Pan, D. Strategy and Technical Progress of Recycling of Spent Vanadium–Titanium-Based Selective Catalytic Reduction Catalysts. ACS Omega 2024, 9, 6036–6058. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Fang, X.; Zhao, J.; Hu, J. Spatiotemporal Characteristics of Consumption Based CO2 Emissions from China’s Power Sector. Resour. Conserv. Recycl. 2017, 121, 156–163. [Google Scholar] [CrossRef]
- Wang, S.; Hao, J. Air Quality Management in China: Issues, Challenges, and Options. J. Environ. Sci. 2012, 24, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.; Van Heyst, B. Nitrogen Oxide (NOx) Emissions as an Indicator for Sustainability. Environ. Sustain. Indic. 2022, 15, 100188. [Google Scholar] [CrossRef]
- Alves, L.; Holz, L.I.V.; Fernandes, C.; Ribeirinha, P.; Mendes, D.; Fagg, D.P.; Mendes, A. A Comprehensive Review of NOx and N2O Mitigation from Industrial Streams. Renew. Sustain. Energy Rev. 2022, 155, 111916. [Google Scholar] [CrossRef]
- Tripathi, G.; Dhar, A.; Sadiki, A. Recent Advancements in After-Treatment Technology for Internal Combustion Engines—An Overview. In Advances in Internal Combustion Engine Research; Srivastava, D.K., Agarwal, A.K., Datta, A., Maurya, R.K., Eds.; Energy, Environment, and Sustainability; Springer: Singapore, 2018; pp. 159–179. [Google Scholar] [CrossRef]
- Liu, Z.; Han, J.; Zhao, L.; Wu, Y.; Wang, H.; Pei, X.; Xu, M.; Lu, Q.; Yang, Y. Effects of Se and SeO2 on the Denitrification Performance of V2O5-WO3/TiO2 SCR Catalyst. Appl. Catal. Gen. 2019, 587, 117263. [Google Scholar] [CrossRef]
- Shelef, M.; Gandhi, H.S. Ammonia Formation in Catalytic Reduction of Nitric Oxide by Molecular Hydrogen. I. Base Metal Oxide Catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1972, 11, 2–11. [Google Scholar] [CrossRef]
- Rasmussen, S.B.; Abrams, B.L. Fundamental Chemistry of V-SCR Catalysts at Elevated Temperatures. Catal. Today 2017, 297, 60–63. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Kang, M. Recent Advances in Heighten Sulfur Resistance of SCR Catalysts: A Review. Environ. Eng. Res. 2021, 27, 200642-0. [Google Scholar] [CrossRef]
- Li, M.; Liu, B.; Wang, X.; Yu, X.; Zheng, S.; Du, H.; Dreisinger, D.; Zhang, Y. A Promising Approach to Recover a Spent SCR Catalyst: Deactivation by Arsenic and Alkaline Metals and Catalyst Regeneration. Chem. Eng. J. 2018, 342, 1–8. [Google Scholar] [CrossRef]
- Qi, L.; Li, J.; Yao, Y.; Zhang, Y. Heavy Metal Poisoned and Regeneration of Selective Catalytic Reduction Catalysts. J. Hazard. Mater. 2019, 366, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, J.; Ge, M. The Poisoning Effect of Alkali Metals Doping over Nano V2O5–WO3/TiO2 Catalysts on Selective Catalytic Reduction of NOx by NH3. Chem. Eng. J. 2011, 170, 531–537. [Google Scholar] [CrossRef]
- Marberger, A.; Ferri, D.; Elsener, M.; Kröcher, O. The Significance of Lewis Acid Sites for the Selective Catalytic Reduction of Nitric Oxide on Vanadium-based Catalysts. Angew. Chem. Int. Ed. 2016, 55, 11989–11994. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Wang, X.; Li, H.; Zhao, C. Iron Removal and Titanium Dioxide Support Recovery from Spent V2O5-WO3/TiO2 Catalyst. Sep. Purif. Technol. 2022, 301, 121934. [Google Scholar] [CrossRef]
- Choi, I.; Moon, G.; Lee, J.-Y.; Jyothi, R.K. Hydrometallurgical Processing of Spent Selective Catalytic Reduction (SCR) Catalyst for Recovery of Tungsten. Hydrometallurgy 2018, 178, 137–145. [Google Scholar] [CrossRef]
- Jeon, J.H.; Cueva Sola, A.B.; Lee, J.-Y.; Jyothi, R.K. Hydrometallurgical Process Development to Recycle Valuable Metals from Spent SCR deNOX Catalyst. Sci. Rep. 2021, 11, 22131. [Google Scholar] [CrossRef]
- Bange, K.; Ottermann, C.R.; Anderson, O.; Jeschkowski, U.; Laube, M.; Feile, R. Investigations of TiO2 Films Deposited by Different Techniques. Thin Solid Films 1991, 197, 279–285. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q. Optimization of Roasting Parameters for Recovery of Vanadium and Tungsten from Spent SCR Catalyst with Composite Roasting. Processes 2021, 9, 1923. [Google Scholar] [CrossRef]
- Choi, I.-H.; Kim, H.-R.; Moon, G.; Jyothi, R.K.; Lee, J.-Y. Spent V2O5-WO3/TiO2 Catalyst Processing for Valuable Metals by Soda Roasting-Water Leaching. Hydrometallurgy 2018, 175, 292–299. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, J.; Wang, W.; Liu, C.; Zhou, D.; Yang, L. Extraction and Separation of Tungsten and Vanadium from Spent V2O5–WO3/TiO2 SCR Catalysts and Recovery of TiO2 and Sodium Titanate Nanorods as Adsorbent for Heavy Metal Ions. Colloids Surf. Physicochem. Eng. Asp. 2020, 601, 124963. [Google Scholar] [CrossRef]
- Chen, H.-J.; Wang, R.; Yang, Y.-L.; Shi, X.-L.; Lu, S.; Chen, Z.-G. Environmentally-Friendly Harvesting TiO2 Nanospheres and V2O5 Microrods from Spent Selective Catalytic Reduction Catalysts. Prog. Nat. Sci. Mater. Int. 2021, 31, 858–864. [Google Scholar] [CrossRef]
- Nie, Z.; Ma, L.; Xi, X.; Guo, F.; Nie, Z. Studying the leaching mechanism of spent SCR catalyst with different leaching agents (NaOH, H2SO4, HCl and HNO3) using DFT calculations. Appl. Surf. Sci. 2022, 584, 152577. [Google Scholar] [CrossRef]
- Choi, I.-H.; Moon, G.; Lee, J.-Y.; Jyothi, R.K. Extraction of Tungsten and Vanadium from Spent Selective Catalytic Reduction Catalyst for Stationary Application by Pressure Leaching Process. J. Clean. Prod. 2018, 197, 163–169. [Google Scholar] [CrossRef]
- Chen, J.P.; Ma, L.W.; Cao, M.X.; Xi, X.L. Extraction of Tungsten and Vanadium from the Spent SCR Catalyst by High Pressure Alkaline Leaching Method. Mater. Sci. Forum 2018, 913, 954–960. [Google Scholar] [CrossRef]
- Su, Q.; Miao, J.; Li, H.; Chen, Y.; Chen, J.; Wang, J. Optimizing Vanadium and Tungsten Leaching with Lowered Silicon from Spent SCR Catalyst by Pre-Mixing Treatment. Hydrometallurgy 2018, 181, 230–239. [Google Scholar] [CrossRef]
- Choi, I.-H.; Moon, G.; Lee, J.-Y.; Jyothi, R.K. Alkali Fusion Using Sodium Carbonate for Extraction of Vanadium and Tungsten for the Preparation of Synthetic Sodium Titanate from Spent SCR Catalyst. Sci. Rep. 2019, 9, 12316. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, W.G.; Hwang, I.S.; Lee, J.Y.; Han, C. Recovery of Tungsten from Spent Selective Catalytic Reduction Catalysts by Pressure Leaching. J. Ind. Eng. Chem. 2015, 28, 73–77. [Google Scholar] [CrossRef]
- HSC Chemistry. Metso. Available online: https://www.metso.com/portfolio/hsc-chemistry/ (accessed on 26 November 2024).
- Han, B.; Khoroshilov, A.V.; Tyurin, A.V.; Baranchikov, A.E.; Razumov, M.I.; Ivanova, O.S.; Gavrichev, K.S.; Ivanov, V.K. WO3 Thermodynamic Properties at 80–1256 K Revisited. J. Therm. Anal. Calorim. 2020, 142, 1533–1543. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Hou, X.; Li, H.; Wang, X.; Hu, W.; Ge, T.; Zhang, J.; Zhu, G.; Xie, H. Extraction of W, V, and As from Spent SCR Catalyst by Alkali Pressure Leaching and the Pressure Leaching Mechanism. J. Environ. Manag. 2023, 347, 119107. [Google Scholar] [CrossRef]
- Wiewiorowski, E.I. Selective Extraction of Molybdenum and Vanadium from Spent Catalysts by Oxidative Leaching with Sodium Aluminate and Caustic. US4666685A, 19 May 1987. [Google Scholar]
- Kim, H.S. Inter-Particle Distance and Toughening Mechanisms in Particulate Thermosetting Composites. In Synthetic Polymer-Polymer Composites; Bhattacharyya, D., Fakirov, S., Eds.; Carl Hanser Verlag GmbH & Co. KG: München, Germany, 2012; pp. 65–115. [Google Scholar] [CrossRef]
- Cao, Y.; Yuan, J.; Du, H.; Dreisinger, D.; Li, M. A Clean and Efficient Approach for Recovery of Vanadium and Tungsten from Spent SCR Catalyst. Miner. Eng. 2021, 165, 106857. [Google Scholar] [CrossRef]
- Li, X.; Shen, L.; Zhou, Q.; Peng, Z.; Liu, G.; Qi, T. Scheelite Conversion in Sulfuric Acid Together with Tungsten Extraction by Ammonium Carbonate Solution. Hydrometallurgy 2017, 171, 106–115. [Google Scholar] [CrossRef]
- Nakahira, A.; Kubo, T.; Numako, C. Formation Mechanism of TiO2-Derived Titanate Nanotubes Prepared by the Hydrothermal Process. Inorg. Chem. 2010, 49, 5845–5852. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Q.; Sun, J.; Liu, Q.; Zhao, D.; Gao, W.; Liu, L. Effects of Mercury Oxidation on V2O5–WO3/TiO2 Catalyst Properties in NH3-SCR Process. Catal. Commun. 2015, 59, 78–82. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Zhang, T.; Wang, H.; Ma, Y.; Wang, J.; Ning, P. Influence of Preparation Methods on Iron-Tungsten Composite Catalyst for NH3-SCR of NO: The Active Sites and Reaction Mechanism. Appl. Surf. Sci. 2020, 503, 144190. [Google Scholar] [CrossRef]
- Valigi, M.; Gazzoli, D.; Pettiti, I.; Mattei, G.; Colonna, S.; De Rossi, S.; Ferraris, G. WOx/ZrO2 Catalysts Part 1. Preparation, Bulk and Surface Characterization. Appl. Catal. Gen. 2002, 231, 159–172. [Google Scholar] [CrossRef]
- Ong, J.L.; Lucas, L.C.; Raikar, G.N.; Gregory, J.C. Electrochemical Corrosion Analyses and Characterization of Surface-Modified Titanium. Appl. Surf. Sci. 1993, 72, 7–13. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Hong, S.-H.; Hong, S.-J.; Hong, S.-C.; Park, T.-S.; Lee, J.-Y.; Cho, S.-P. Vanadium/Titania-Based Catalyst for Removing Introgen Oxide at Low Temperature Window, and Process of Removing Nitrogen Oxide Using the Same. US20050069477A1, 31 March 2005. [Google Scholar]
- Jung, S.M.; Grange, P. Evidence of Correlation between Electronic Density and Surface Acidity of Sulfated TiO2. Catal. Lett. 2001, 76, 27–30. [Google Scholar] [CrossRef]
- Guo, X.; Bartholomew, C.; Hecker, W.; Baxter, L.L. Effects of Sulfate Species on V2O5/TiO2 SCR Catalysts in Coal and Biomass-Fired Systems. Appl. Catal. B Environ. 2009, 92, 30–40. [Google Scholar] [CrossRef]
- Feng, E.; Gao, D.; Yu, F.; Chen, J.; Xu, Z.; Zhang, W.; Wang, C.; Gao, Y.; Wen, J.; Huang, G.; et al. TiO2 Recovered from Spent Selective Catalytic Reduction Catalysts as Anode Material for Lithium-Ion Batteries. J. Clean. Prod. 2024, 444, 141120. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.H.; Hong, S.C. Influence of Calcination Temperature on Ce/TiO2 Catalysis of Selective Catalytic Oxidation of NH3 to N2. Appl. Catal. Gen. 2014, 470, 189–198. [Google Scholar] [CrossRef]
- Kim, G.J.; Lee, S.H.; Nam, K.B.; Hong, S.C. A Study on the Structure of Tungsten by the Addition of Ceria: Effect of Monomeric Structure over W/Ce/TiO2 Catalyst on the SCR Reaction. Appl. Surf. Sci. 2020, 507, 145064. [Google Scholar] [CrossRef]
- Bu, X.N.; Danstan, J.K.; Hassanzadeh, A.; Vakylabad, A.V.; Chelgani, S.C. Metal extraction from ores and waste materials by ultrasound-assisted leaching—An overview. Miner. Process. Extr. Metall. Rev. 2024, 45, 28–45. [Google Scholar] [CrossRef]
- Darezereshki, E.; Vakylabad, A.B.; Hassanzadeh, A.; Niedoba, T.; Surowiak, A.; Koohestani, B. Hydrometallurgical Synthesis of Nickel Nano-Sulfides from Spent Lithium-Ion Batteries. Minerals 2021, 11, 419. [Google Scholar] [CrossRef]
- Behrad Vakylabad, A.; Darezereshki, E.; Hassanzadeh, A. Selective Recovery of Cobalt and Fabrication of Nano-Co3S4 from Pregnant Leach Solution of Spent Lithium-Ion Batteries. J. Sustain. Metall. 2021, 7, 1027–1044. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).