Preparation of Strontium Hydroxystannate by a Hydrothermal Method and Its Photocatalytic Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Strontium Hydroxystannate by Hydrothermal Method
2.3. Characterization Methods
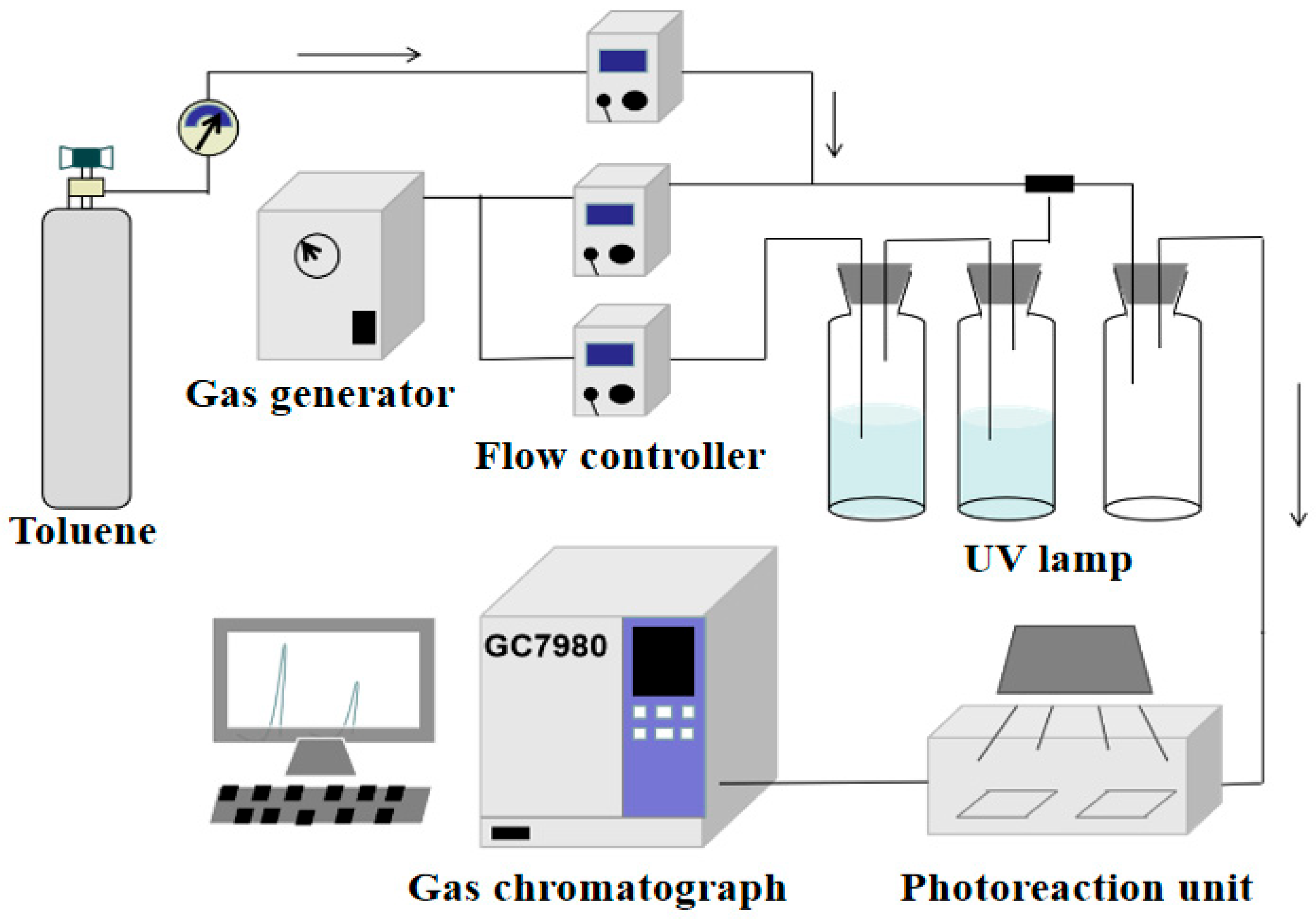
2.4. Photocatalyst Activity Evaluation Test
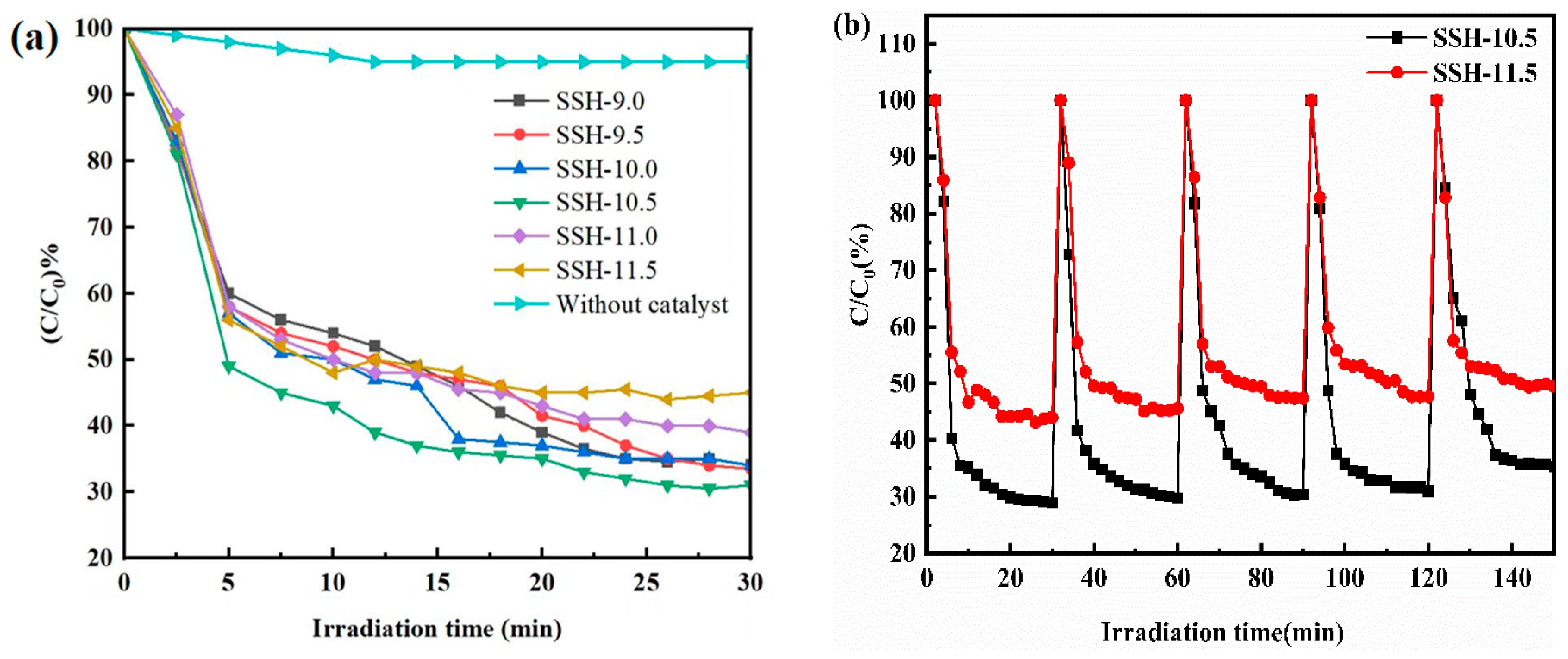
2.5. Stability of Photocatalyst Test
3. Results and Discussion
3.1. Effect of Process Parameters on Photocatalytic Activity of SrSn(OH)6
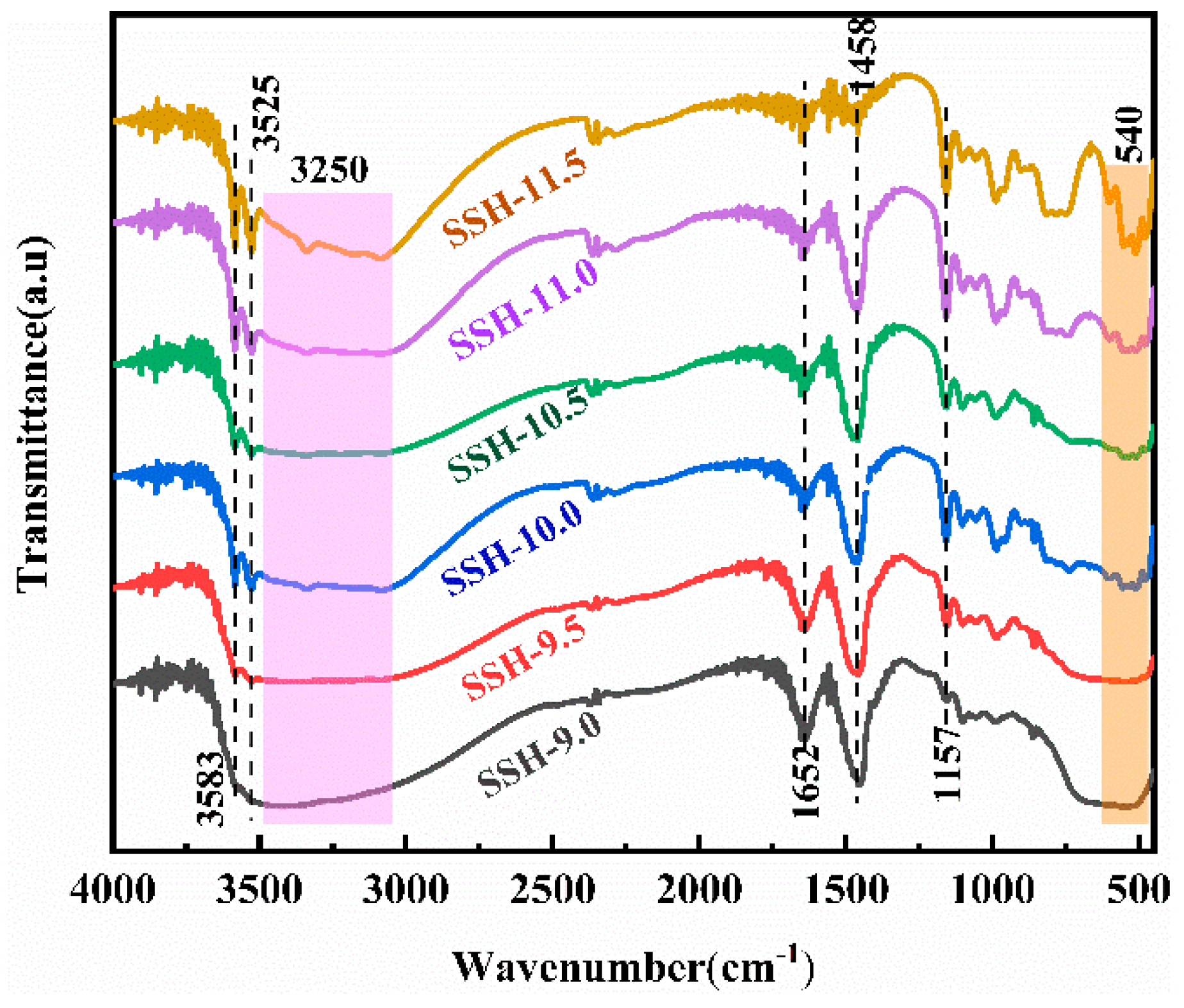
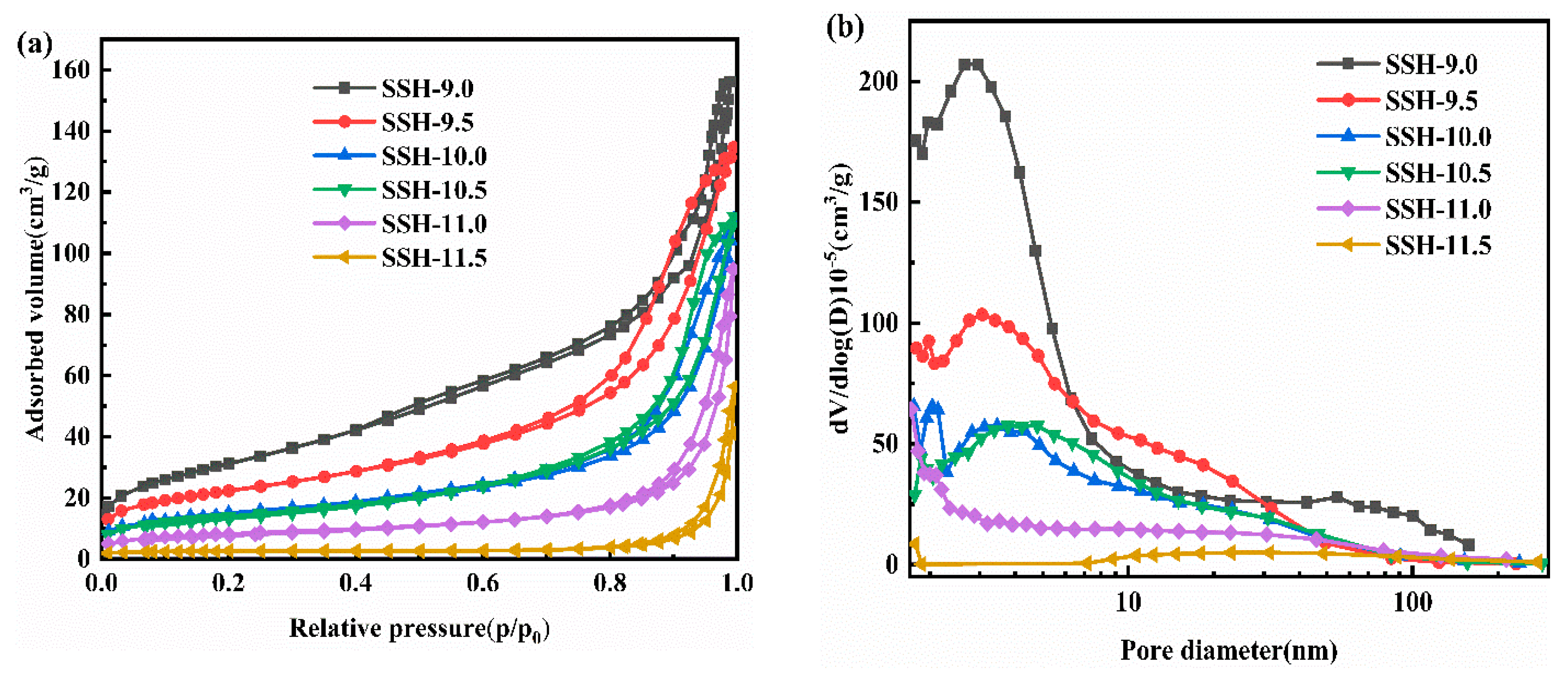
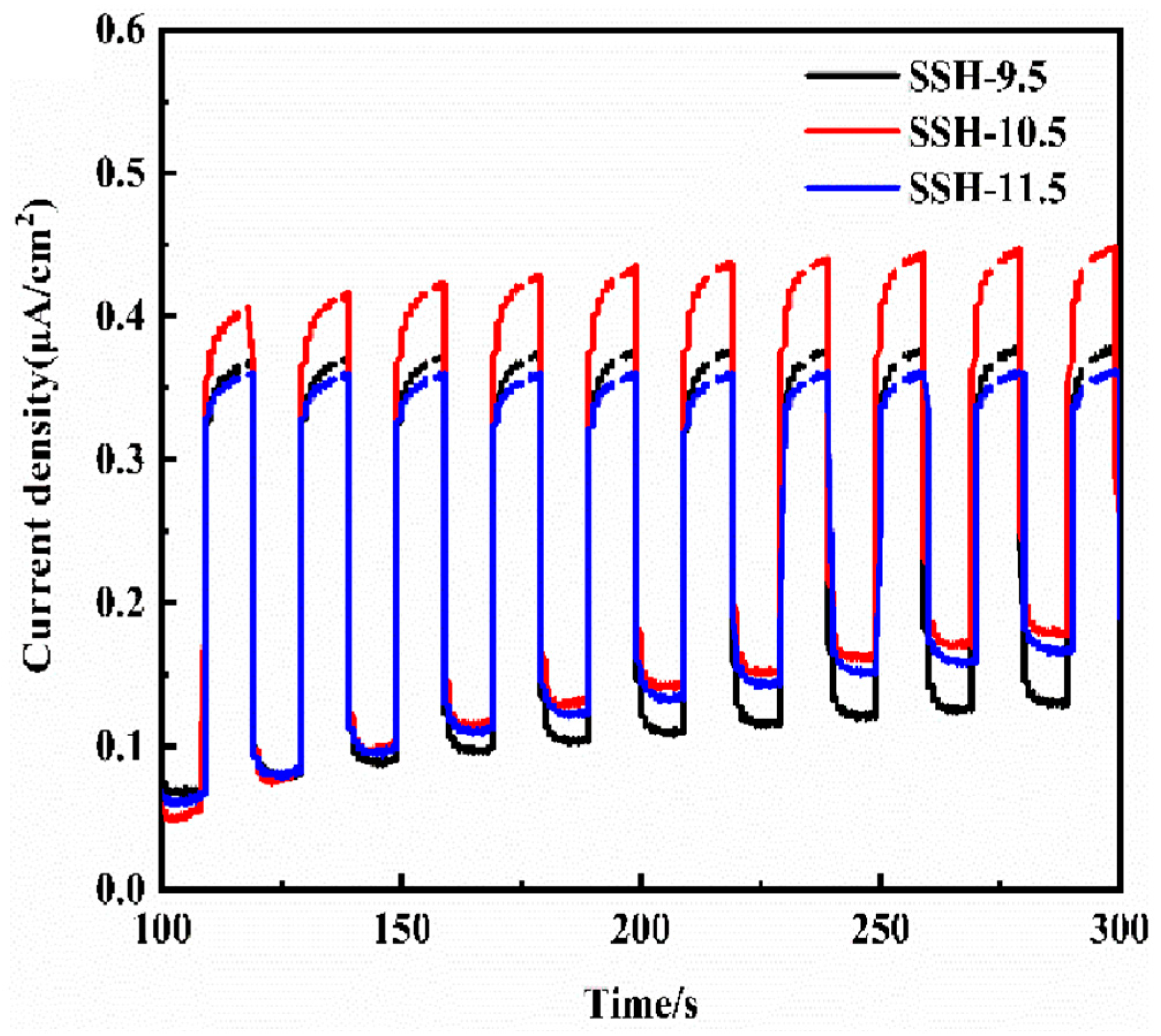
3.2. Characterization of Materials
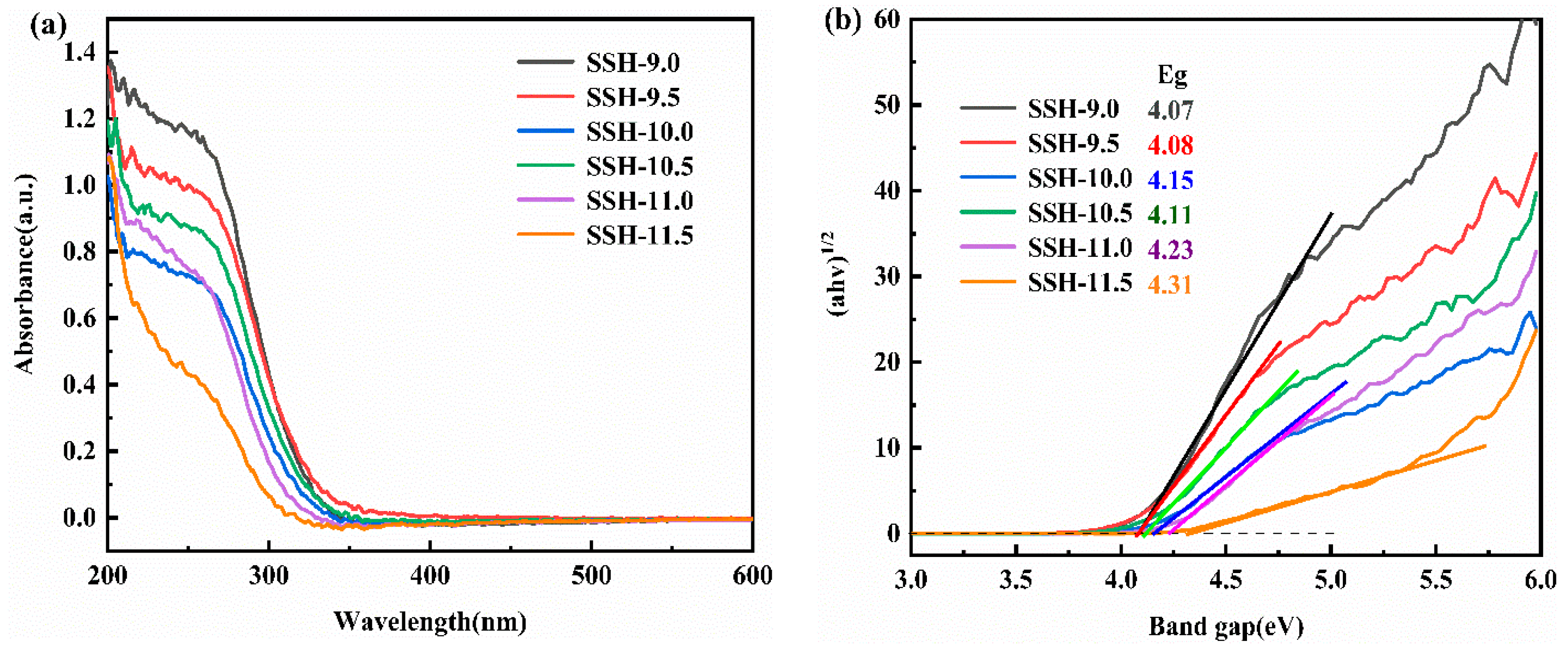
3.3. Optical Properties
3.4. Analysis of Photocatalytic Activity
3.5. Radical Analysis
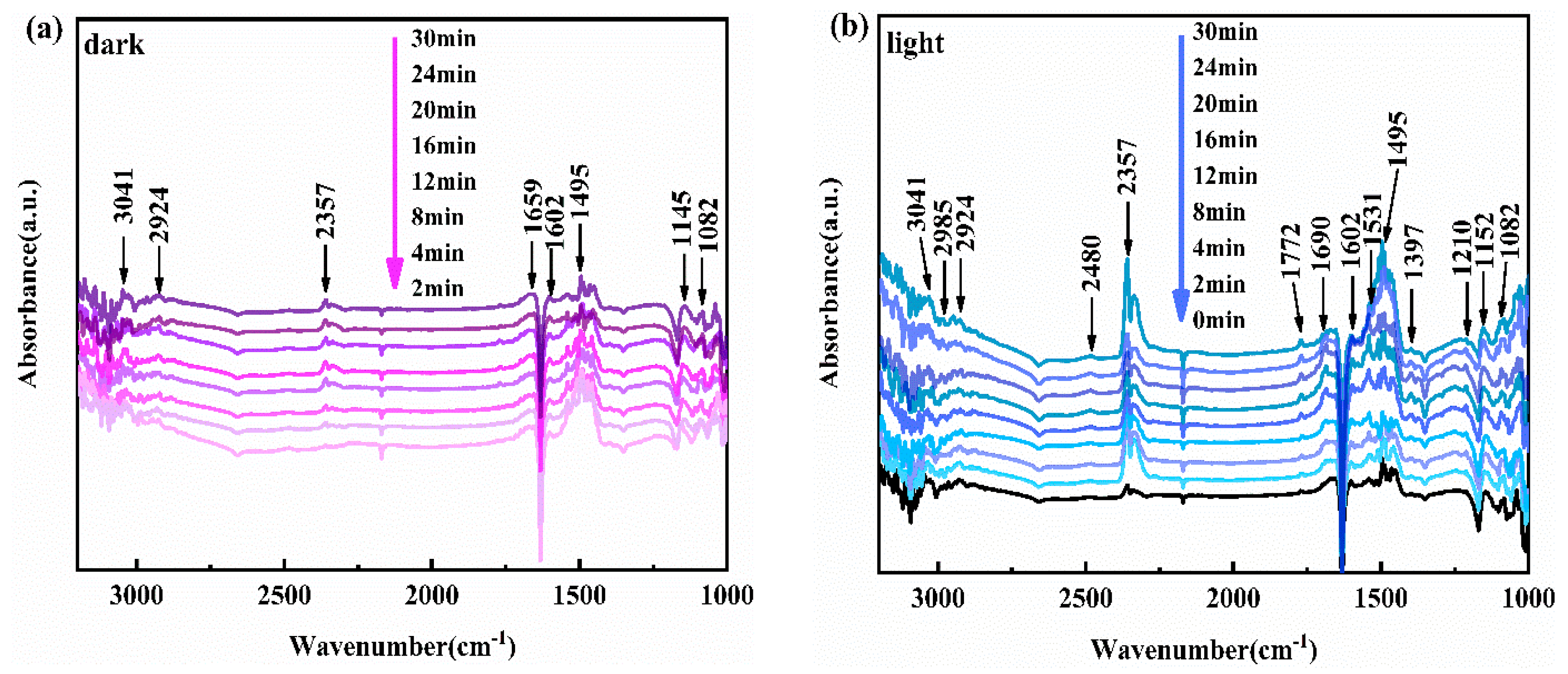
3.6. In Situ Infrared Analysis
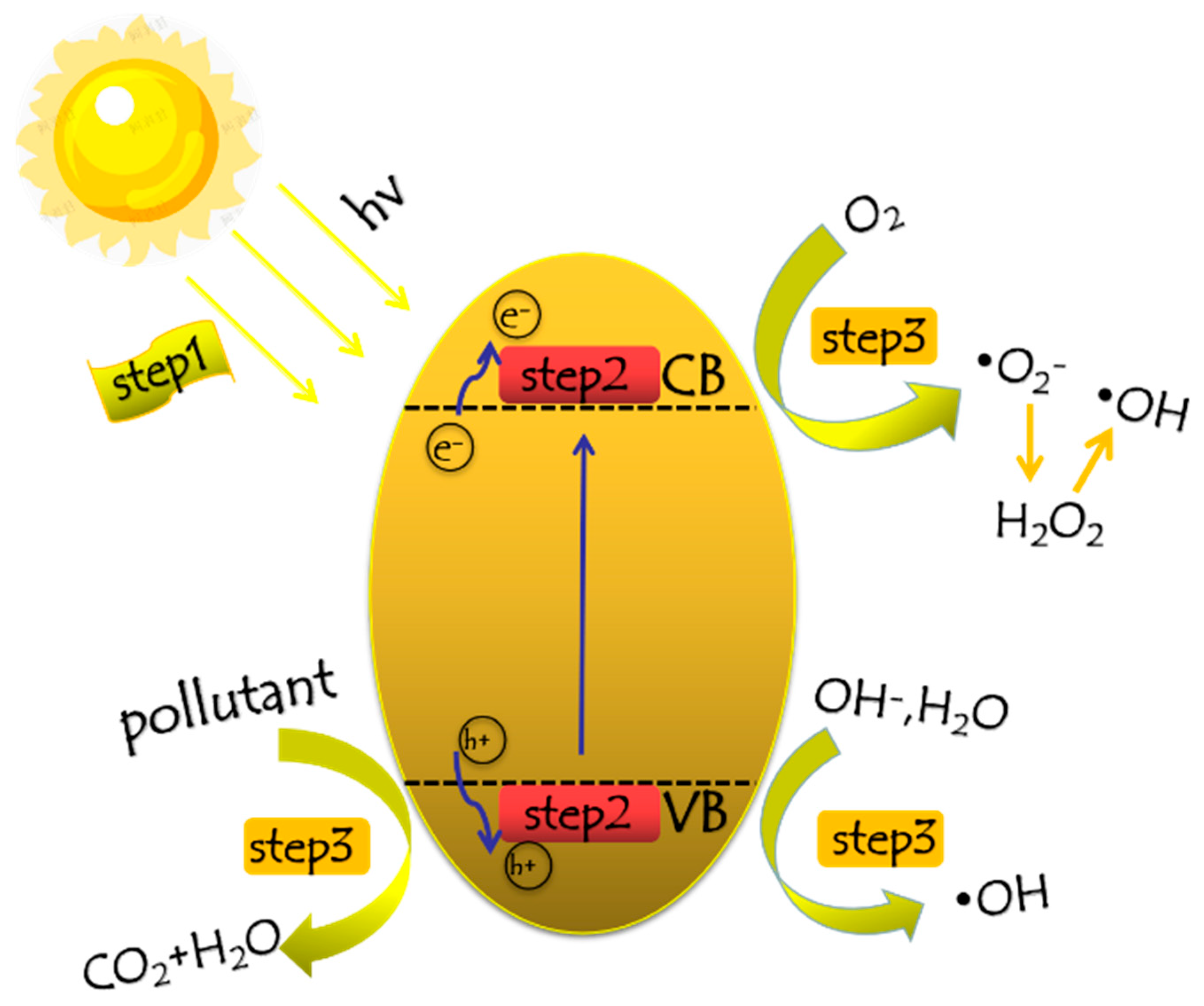
3.7. Mechanism of Photocatalytic Reaction
4. Conclusions
Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, S.; Shao, Z.; Jin, L.N.; Chen, L.; Zhang, X.; Fang, M.; Li, D.; Chen, J. Distinct baseline toxicity of volatile organic compounds (VOCs) in gaseous and liquid phases: Mixture effects and potential molecular mechanisms. J. Hazard. Mater. 2025, 485, 136890. [Google Scholar] [CrossRef]
- Qin, C.; Guo, M.; Jiang, C.; Yu, R.; Huang, J.; Yan, D.; Li, S.; Dang, X. Simultaneous oxidation of toluene and ethyl acetate by dielectric barrier discharge combined with Fe, Mn and Mo catalysts. Sci. Total Environ. 2021, 782, 146931. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Liang, Q. Electrothermal toluene oxidation by utilizing Joule heat from Pd/FeCrAl electrified metallic monolith catalyst. Appl. Surf. Sci. 2024, 658, 159827. [Google Scholar] [CrossRef]
- Zahed, M.A.; Salehi, S.; Khoei, M.A.; Esmaeili, P.; Mohajeri, L. Risk assessment of Benzene, Toluene, Ethyl benzene, and Xylene (BTEX) in the atmospheric air around the world: A review. Toxicol. Vitr. 2024, 98, 105825. [Google Scholar] [CrossRef]
- Liu, Y.; Zang, X.; Zhao, S.; Wang, Z.; Wang, W.; Xu, J.; Wang, S.; Ye, Z. Degradation of toluene by DBD plasma catalytic technology with CoMn-BTC/Ti3C2Tx composites. Appl. Catal. A Gen. 2025, 695, 120180. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Y.; Gao, Y.; Han, D.; Wang, Z.; Shen, B.; Wang, X. Recent advances in catalysts for toluene elimination via catalytic oxidation. Chemosphere 2024, 368, 143720. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, X.; Chen, J.; Shen, B. The removal of toluene by thermoscatalytic oxidation using CeO2-based catalysts:a review. Chemosphere 2024, 351, 141253. [Google Scholar] [CrossRef]
- Xu, L.; Guo, P.; Xu, J.; Shen, B.; Zhao, Z. Regulation of TiO2/ZSM-5 catalyst for enhanced photocatalytic toluene oxidation: Intensified light absorption, charge separation and toluene adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2024, 690, 133832. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, J.; Lovely, B.; Li, Y.; Ponder, M.; Waterman, K.; Kim, Y.-T.; Shuai, D.; Yin, Y.; Huang, H. Visible light-activated dye-sensitized TiO2 antibacterial film: A novel strategy for enhancing food safety and quality. J. Hazard. Mater. 2024, 480, 136296. [Google Scholar] [CrossRef]
- Manikanta, P.; Mohanty, J.; Mounesh; Nikam, R.R.; Sandeep, S.; Santhosh, A.S.; Pramoda, K.; Nagaraja, B.M. Recent advances and current status of semiconductor stannate-based electrode materials for electrochemical sensing and energy applications. J. Alloys Compd. 2025, 1020, 179370. [Google Scholar] [CrossRef]
- Yang, L.; Shi, C.-l. Effect of Zinc Hydroxystannate Coated M-HOS Whisker on Flame Retardant Properties of Flexible PVC. Procedia Eng. 2018, 211, 901–905. [Google Scholar] [CrossRef]
- Yang, L.; Yu, Y.; Yang, W.; Li, X.; Zhang, G.; Shen, Y.; Dong, F.; Sun, Y. Efficient visible light photocatalytic NO abatement over SrSn(OH)6 nanowires loaded with Ag/Ag2O cocatalyst. Environ. Res. 2021, 201, 111521. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, J.; Liu, J.; Shao, Y.; Li, X.; Li, D. Hydroxide SrSn(OH)6: A new photocatalyst for degradation of benzene and rhodamine B Author links open overlay panel. Appl. Catal. B Environ. 2016, 182, 533–540. [Google Scholar] [CrossRef]
- Ruilin, L.; Yee, K.Y.; Salleh, N.A.; Deghfel, B.; Zakaria, Z.; Yaakob, M.K.; Ong, H.R.; Rahiman, W.; Akbulut, H.; Wang, D.; et al. Hydrothermal synthesis of manganese doped zinc oxide wurtzite nanoparticles for supercapacitors—A brief review. Inorg. Chem. Commun. 2024, 170, 113311. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Wang, Y.; Liang, Y.; Dong, F. Highly efficient photocatalytic NO removal and in situ DRIFTS investigation on SrSn(OH)6. Chin. Chem. Lett. 2022, 33, 1259–1262. [Google Scholar] [CrossRef]
- Guo, P.; Xu, L.; Yu, T.; Zhao, P.; Xu, J.; Shen, B. Transition metal in-situ doped BiOBr: Introducing oxygen vacancies to enhance hydroxyl radical generation for improved photocatalytic degradation of toluene. Sep. Purif. Technol. 2025, 354, 129247. [Google Scholar] [CrossRef]
- Sun, Y.; Ahmadi, Y.; Kim, K.-H. The selection of a nitrogen precursor for the construction of N-doped titanium dioxide with enhanced photocatalytic activity for the removal of gaseous toluene. Environ. Res. 2024, 260, 119664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Long, Z.; Sun, Z.; Lv, L.; Liang, J.; Zhang, G.; Wang, P.; Gao, W. MnCe-based catalysts for removal of organic pollutants in urban wastewater by advanced oxidation processes-A critical review. J. Environ. Manag. 2024, 370, 122773. [Google Scholar] [CrossRef]
- Shabani, M.; Haghighi, M.; Haghighi, A.; Mohseni, N. DC-glow plasma-assisted precipitation synthesis of S-scheme 1D nanostick-like SrSn(OH)6 decorated with AgBr nanophotocatalyst: Sono-sunlight photocatalytic hybrid system for destruction of anti-coronavirus and antibiotics. J. Clean. Prod. 2025, 502, 145384. [Google Scholar] [CrossRef]
- Patel, D.K.; Nuwad, J.; Rajeswari, B.; Vishwanadh, B.; Sudarsan, V.; Vatsa, R.K.; Kadam, R.M.; Pillai, C.G.S.; Kulshreshtha, S.K. Blue light emitting SrSn(OH)6 nano-rods doped with lanthanide ions (Eu3+, Tb3+ and Dy3+). Mater. Res. Bull. 2013, 48, 566–573. [Google Scholar] [CrossRef]
- Fu, X.; Huang, D.; Qin, Y.; Li, L.; Jiang, X.; Chen, S. Effects of preparation method on the microstructure and photocatalytic performance of ZnSn(OH)6. Appl. Catal. B Environ. 2014, 148–149, 532–542. [Google Scholar] [CrossRef]
- Tang, Y.; Fan, C.; Zang, Z.; Cheng, Y.; Li, L.; Yu, X.; Yang, X.; Lu, Z.; Zhang, X.; Liu, H. Non-metallic surface-modified X-Cu (X = F, Cl, Br) metal catalysts for all-pH hydrogen evolution reaction with high performance. J. Colloid Interface Sci. 2025, 683, 312–321. [Google Scholar] [CrossRef]
- Uematsu, M.; Ishii, K.; Samitsu, S.; Ismail, E.B.; Ichinose, I.; Ohashi, N.; Berthebaud, D.; Halet, J.-F.; Ishigaki, T.; Uchikoshi, T. Fabrication and characterization of zeolite bulk body containing mesopores and macropores using starch as pore-forming agent. Adv. Powder Technol. 2022, 33, 103626. [Google Scholar] [CrossRef]
- Liu, J.; Shi, J.; Deng, H. Current status of research on BiOX-based heterojunction photocatalytic systems: Synthesis methods, photocatalytic applications and prospects. J. Environ. Chem. Eng. 2023, 11, 110311. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, H.; Cao, Y. Effect of In(OH)3 species modified ZnS on improved photocatalytic activity of photoreduction of CO2. J. Solid State Chem. 2021, 296, 121976. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, C.; Tayyab, M.; Wei, Z.; Zheng, X.; Shangguan, W.; Zhang, S.; Chen, S.; Meng, S. Regulating electron-hole pairs of g-C3N4 efficiently separated and fully utilized for photosynthesis of H2O2 under visible light. Chem. Eng. J. 2025, 509, 161409. [Google Scholar] [CrossRef]
- Jian, C.; Chen, P.; Cheng, Z.; Liu, L.; Yan, C.; Qiu, F. Hydrogenated red mud biochar as visible-light-driven peroxymonosulfate (PMS) activators for efficient degradation of antibiotic: Resource utilization, mechanism insights and toxicity assessment. Environ. Res. 2025, 273, 121233. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Cheng, F.; Wei, Z.; Cai, C.; Fu, Y. Enhanced heat retention and energy efficiency in photothermal membranes via sealed light-absorbing cavity engineering. Chem. Eng. J. 2025, 508, 161130. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Cheng, R.; Wang, M.; Li, M.; Zhou, Y.; Shi, J. Construction of Graphitic C3N4-Based Intramolecular Donor-Acceptor Conjugated Copolymers for Photocatalytic Hydrogen Evolution. ACS Catal. 2015, 5, 5008–5015. [Google Scholar] [CrossRef]
- Fei, H.; Zhao, T.; Guo, W.; Wang, X.; Zhang, J.; Fei, Z.; Feng, Z.; Liu, G. Strategies for enhancing activities of typical piezo-photocatalytic material and its applications in environmental remediation: A review. J. Environ. Chem. Eng. 2024, 12, 111650. [Google Scholar] [CrossRef]
- Yi, G.; Wang, B.; Lu, S.; Zhang, L.; Liu, W.; Chen, Z.; Yang, L.; Luo, X.; Wang, A.-J. Identification and quantification of electron-generated atomic hydrogen through in-situ electron spin resonance and density functional theory. Chem. Eng. J. 2024, 483, 149226. [Google Scholar] [CrossRef]
- Sun, P.; Zhong, K.; Huang, X.; Wu, G.; Miao, Z.; Chen, Z.; Mo, Z.; Yang, J.; Xu, H. Synergistic utilization of photogenerated electrons and holes in carbon nitride nanosheet assembly for enhanced photocatalytic H2O2 production. Appl. Catal. B Environ. Energy 2025, 366, 124998. [Google Scholar] [CrossRef]
- Li, J.; Na, H.; Zeng, X.; Zhu, T.; Liu, Z. In situ DRIFTS investigation for the oxidation of toluene by ozone over Mn/HZSM-5, Ag/HZSM-5 and Mn-Ag/HZSM-5 catalysts. Appl. Surf. Sci. 2014, 311, 690–696. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Pan, L.; Wang, Z.; Zhang, X.; Zou, J.-J.; Mi, W.; Zhang, X.; Wang, L. Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution. Nano Energy 2015, 12, 646–656. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Z.; Li, Y.; Ho, W.-K.; Lee, S.C. Immobilization of polymeric g-C3N4 on structured ceramic foam for efficient visible light photocatalytic air purification with real indoor illumination. Environ. Sci. Technol. 2014, 48, 10345–10353. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, X.; Zhao, Q.; Li, H.; Shen, Y.; Chen, G. Porous “brick-like” NiFe2O4 nanocrystals loaded with Ag species towards effective degradation of toluene Author links open overlay panel. Chem. Eng. J. 2010, 165, 64–70. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Zhao, Q.; Shi, Y.; Zhang, F.; Liu, B.; Ke, J.; Wang, L. Photocatalytic degradation of gaseous toluene over bcc-In2O3 hollow microspheres. Appl. Surf. Sci. 2015, 337, 27–32. [Google Scholar] [CrossRef]
- Hernández-Alonso, M.D.; Tejedor-Tejedor, I.; Coronado, J.M.; Anderson, M.A. Operando FTIR study of the photocatalytic oxidation of methylcyclohexane and toluene in air over TiO2–ZrO2 thin films: Influence of the aromaticity of the target molecule on deactivation. Appl. Catal. B Environ. 2011, 101, 283–293. [Google Scholar] [CrossRef]
- Yu, Z.; Chuang, S.S.C. In situ IR study of adsorbed species and photogenerated electrons during photocatalytic oxidation of ethanol on TiO2. J. Catal. 2007, 246, 118–126. [Google Scholar] [CrossRef]
- Kaur, G.; Kamal; Sharma, A.; Sud, D.; Rai, R. Enriched photocatalytic degradation of chlorophenols and mineralization of industrial effluents using DUT-8(Zn/Ni)@CeO2 composites. J. Mol. Struct. 2025, 1319, 139417. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.; Huang, Y.; Du, X.; He, Z.; Wang, D.; Song, S. Hydroxylated SiO2-modified {001}-TiO2 nanosheets as a surface multifunctional photocatalyst for enhanced degradation of gaseous toluene. Chem. Eng. J. 2024, 499, 156055. [Google Scholar] [CrossRef]

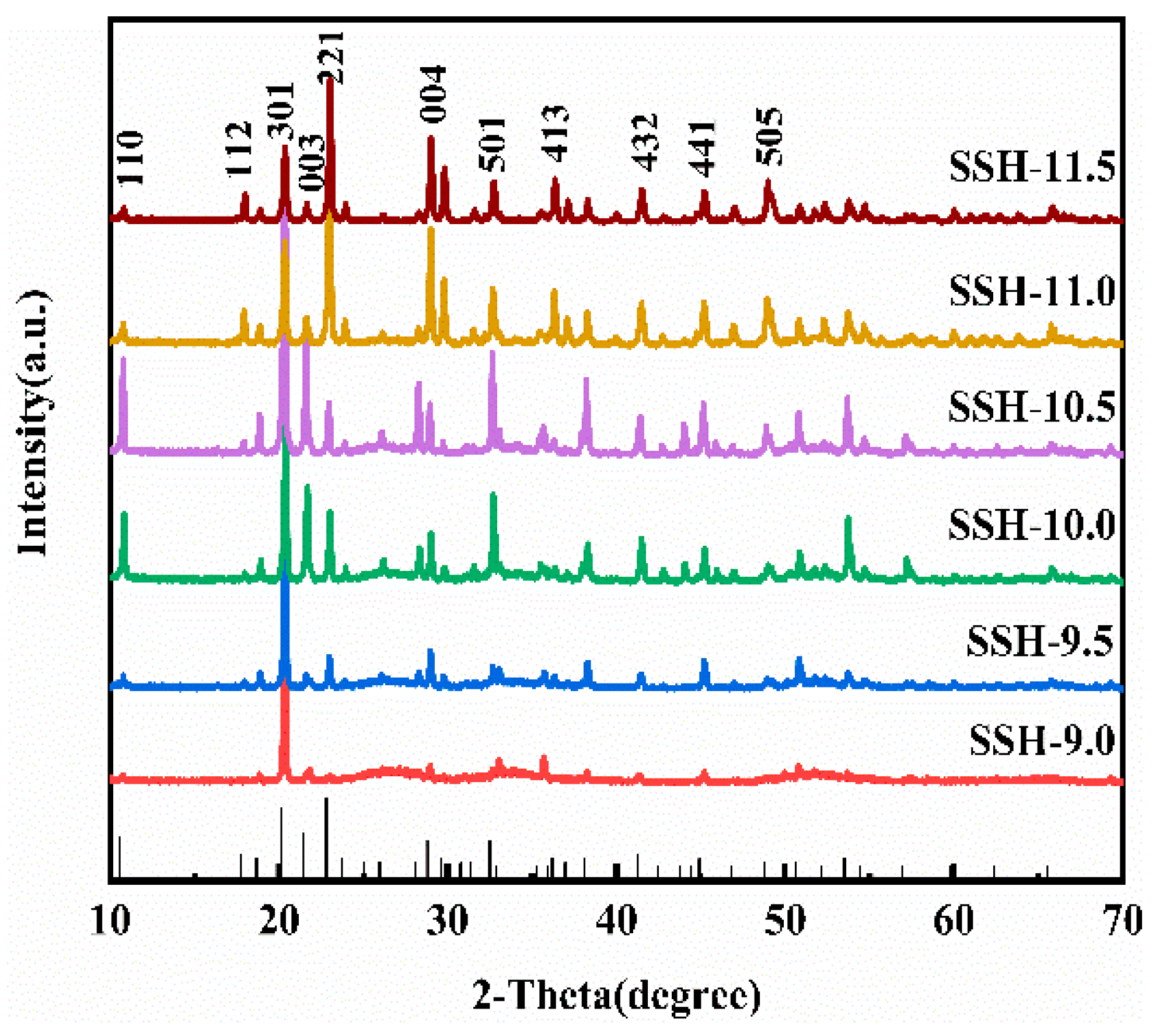


| Sample | D (nm) | |
|---|---|---|
| (301) | (221) | |
| SSH-9.5 | 43.876 | 42.441 |
| SSH-10.5 | 55.070 | 46.097 |
| SSH-11.5 | 35.492 | 49.815 |
| Sample | SBET (m2·g−1) | Total Pore Volume (cm3·g−1) | Peak Pore Size (nm) |
|---|---|---|---|
| SSH-9.0 | 114.25 | 0.39 | 140.25 |
| SSH-9.5 | 78.59 | 0.21 | 105.99 |
| SSH-10.0 | 50.13 | 0.17 | 137.18 |
| SSH-10.5 | 48.91 | 0.17 | 141.80 |
| SSH-11.0 | 28.05 | 0.15 | 209.02 |
| SSH-11.5 | 7.80 | 0.09 | 447.87 |
| Catalyst Types | Representative Materials | T90 (Toluene, °C) | Activation Energy (Eₐ, kJ/mol) | Key Active Site |
|---|---|---|---|---|
| Precious metal catalysts | Pt/Al2O3 | 160–200 | 50–80 | Pt0/PtOx nanoparticles |
| Transition metal oxides | MnO2-Co3O4 | 200–250 | 80–120 | Mn4+/Co3 4+ redox pair |
| Perovskite catalyst | LaMnO3 | 220–280 | 90–130 | Surface oxygen vacancies (Oᵥ) |
| Hydroxystannate | SrSn(OH)6 | 150–180 | 40–60 | Surface hydroxyl group (-OH)/Sn4+ -O− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Q.; Li, J.; Pan, R.; Yang, X.; Zeng, Y.; Shi, C.; Bao, H.; Li, P.; Fu, M.; Tian, S. Preparation of Strontium Hydroxystannate by a Hydrothermal Method and Its Photocatalytic Performance. Processes 2025, 13, 1654. https://doi.org/10.3390/pr13061654
Liang Q, Li J, Pan R, Yang X, Zeng Y, Shi C, Bao H, Li P, Fu M, Tian S. Preparation of Strontium Hydroxystannate by a Hydrothermal Method and Its Photocatalytic Performance. Processes. 2025; 13(6):1654. https://doi.org/10.3390/pr13061654
Chicago/Turabian StyleLiang, Qiao, Junke Li, Rui Pan, Xianxu Yang, Yufeng Zeng, Chao Shi, Hao Bao, Peng Li, Min Fu, and Shichao Tian. 2025. "Preparation of Strontium Hydroxystannate by a Hydrothermal Method and Its Photocatalytic Performance" Processes 13, no. 6: 1654. https://doi.org/10.3390/pr13061654
APA StyleLiang, Q., Li, J., Pan, R., Yang, X., Zeng, Y., Shi, C., Bao, H., Li, P., Fu, M., & Tian, S. (2025). Preparation of Strontium Hydroxystannate by a Hydrothermal Method and Its Photocatalytic Performance. Processes, 13(6), 1654. https://doi.org/10.3390/pr13061654






