Abstract
Waste wheat straw (WS) is a common agricultural waste with a low acquisition cost and a high annual yield, making it a promising feedstock for a biorefinery. In this work, efficient co-production of reducing sugars and xylo-oligosaccharides (XOSs) from WS was realized through FeCl3-assisted p-toluene sulfonic acid (PTSA) pretreatment. The effects of reaction conditions (PTSA content, FeCl3 loading, pretreatment duration, and temperature) on lignin and xylan elimination and enzymolysis were analyzed. The results manifested that the enzymolysis of WS substantially elevated from 22.0% to 79.3% through the treatment with FeCl3-PTSA/water (120 °C, 60 min). The xylan removal and delignification were 79.7% and 66.6%, respectively. XOSs (4.0 g/L) were acquired in the pretreatment liquor. The linear fitting about LogR0 with enzymolysis, delignification, xylan elimination and XOSs content was investigated to explain the reasons for the elevated enzymolysis and to clarify the comprehensive understanding of WS enzymolysis through the FeCl3-PTSA/water treatment. In addition, the recycling test of FeCl3-PTSA/water manifested a good recycling ability for WS treatment, which would reduce the pretreatment cost and enhance the economic benefit. To sum up, FeCl3-assisted PTSA treatment of biomass for co-production of reducing sugars and XOSs is an alternative method of waste biomass valorization.
1. Introduction
To alleviate the shortage of fossil energy, the effective development and utilization of sustainable energy has become a research hotspot [1,2,3]. Lignocellulose (LC) is abundant in the world and has been successfully valorized into biofuels and biobased chemicals [4]. Waste wheat straw (WS) represents a significant potential source of raw material for manufacturing biobased chemicals. Currently, the majority of WS is discarded and incinerated, leading to resource waste and environmental pollution [5]. It is of great interest to employ an effectual strategy to transform WS into highly value-added chemicals. The pretreatment process is a vital step for valorization of LC [6,7,8]. Different pretreatment approaches are utilized to overcome the recalcitrance of LC and expedite its disintegration into individual components: hemicellulose, cellulose, and lignin [9,10,11,12,13].
Mineral and organic acids have been utilized for LC pretreatment in water, organic solvents, ionic liquids, etc. [14]. Compared to inorganic acids, the effect of organic acids on corrosion is relatively small, and the thermal decomposition of organic acids produces components that are relatively less toxic [10,15]. In recent years, as a cheap aromatic strong organic acid, PTSA has had good reusability and can quickly and efficiently dissolve lignin at low temperatures, which has aroused widespread concern [16]. p-Toluene sulfonic acid (PTSA) (pKa = −2.8) is a strong acid, and it almost entirely dissipates in the reaction system to provide a large amount of H+ [17]. Yang et al. found that treatment with PTSA effectively eliminated lignin and xylan [16]. Wu et al. isolated 48.5% hemicellulose from poplar trees with 2.0% PTSA at 120 °C for 2 h [18]. However, conventional treatments with single organic acids are difficult to effectively erode the cell wall and are limited in their ability to separate hemicellulose [19]. It is known that H+ generated by Lewis acids dissolved in water can effectively destroy the compact LC structure, enhancing enzymatic saccharification of the treated LC solid residues [20]. As a Lewis acid, FeCl3 can be used as a substitute for H2SO4 in GVL treatment [21], and inhibition products are not easily formed during treatment [22]. Meanwhile, the FeCl3-catalyzed GVL/water treatment can effectively degrade sugarcane leaves, improving reducing sugar yield and bioethanol productivity [23].
As mentioned above, this study aimed to develop an effective strategy to valorize WS though Lewis acid-assisted PTSA treatment. According to the chemical composition and enzymolysis of WS, the key parameters (PTSA concentration, type and amount of Lewis acids, temperature, time, and pretreatment severity factor) were tested and discussed. The reusability of the pretreatment media was also tested. The findings of this work will effectively realize the high-value utilization of WS and provide an important reference for the utilization of other biomasses.
2. Materials and Methods
2.1. Materials
Waste wheat straw (WS) was collected in the suburb of Changzhou (Jiangsu Province, China). p-toluene sulfonic acid (PTSA) and other reagents (KCl, NaCl, MgCl2, ZnCl2, AlCl3, and FeCl3) were purchased from Shanghai McLean Biochemical Co., Ltd. (Shanghai, China). Cellulase (Cellic CTec 2) was sourced from Novozymes (Franklinton, NC, USA).
2.2. Pretreatment of WS with Lewis Acid-Mediated PTSA
Waste WS (20–60 mesh) was treated with different Lewis acids (KCl, NaCl, MgCl2, ZnCl2, AlCl3, and FeCl3) (20–80 mM) and PTSA (5–80 g/L). Pretreatment was implemented in an oil bath equipped with a 250 mL round-bottom flask. A 30 g PTSA aqueous solution was mixed with 3 g dry WS in a round-bottom flask under a temperature of 40–140 °C for the designed pretreatment duration (30–90 min). The treated WS (solid) and collected filtrate (liquid) were used for subsequent analysis. The severity factor (LogR0) is defined as Equation (1) that evaluates the intensity of pretreatment [pretreatment temperature: T (°C); pretreatment duration: t (min)].
2.3. Reuse of PTSA and Lewis Acid in WS Pretreatment
In the reusability test, WS was soaked in FeCl3-PTSA/water. After FeCl3-PTSA/water treatment was completed, the flask immersed in a bath of oil was taken out, and the prepared filter bottle and Buchner funnel were used to filter the pretreated slurry. The filtrate was rapidly transferred to the next flask, and the fresh WS was added to the recovered pretreatment solution (FeCl3-PTSA/water) for the next round of reaction. The filter solid (FeCl3-PTSA/water-treated WS) was used for subsequent enzymolysis. Since the recovered FeCl3-PTSA/water had a certain loss during the transfer process, WS with a reinforcement liquid ratio of 1:10 (wt/wt) was supplemented for each reuse according to the quality of the recovered FeCl3-PTSA/water solution.
2.4. Chemical Composition Analysis
The LC composition measurement protocol published by the National Renewable Energy Laboratory was used to analyze the main chemical composition of biomass before and after pretreatment [13]. The determination of monosaccharides and XOSs was implemented by HPLC (Agilent 1260, Agilent Technologies, Santa Clara, CA, USA) containing an Aminex HPX-87H column. The samples were eluted by using 5 mM sulfuric acid as the eluent (0.6 mL/min) at 65 °C. Lignin removal, xylan elimination and the recovery of solids and glucan were calculated as previously reported [20].
2.5. Enzymolysis of WS
In saccharification experiments, WS (50 g/L) was enzymatically hydrolyzed in 100 mL citrate buffer solution (pH 4.8, 50 mM) containing cellulase (15 FPU/g glucan) at 50 °C for 4–72 h. Enzymatic digestibility was calculated according to the following equation:
3. Results and Discussion
3.1. Investigation of Lignin and Xylan Elimination Under Factors of Different Severity
To improve the enzymolysis of LC, effective elimination of hemicellulose and lignin should be implemented in the LC pretreatment process [24,25]. In this research, Lewis acid and PTSA were combined to enhance WS pretreatment efficacy. The raw material WS contained 38.2% glucan, 20.2% xylan, and 15.4% lignin. To better destroy the dense structure of LC and eliminate the lignin that hinders saccharification, it is necessary to acquire the related conditions affecting the pretreatment efficacy [26]. The effects of PTSA content, Lewis acid types and loading, pretreatment duration, and pretreatment temperature on the pretreatment efficacy were investigated (Figure 1). In the case of control (water), only 11.6% of xylan and 7.0% of lignin were eliminated from WS. However, the supplementation of PTSA resulted in a decrease in lignin and xylan content. Upon raising PTSA content from 5 g/L to 40 g/L, the xylan elimination increased from 20.3% to 59.6%, and lignin removal increased from 7.2% to 39.6% (Figure 1a), implying that the increased acid loading promoted the breaking of ether bonds among lignin macromolecules and further enhanced the elimination of lignin. It is known that PTSA can eliminate lignin through the proton-catalyzed hydrolysis of polysaccharides, lignin, and ether or ester bonds in aqueous solution [27]. Through pretreatment with 40 g/L PTSA, high lignin removal was obtained. Upon raising PTSA to 80 g/L, lignin removal dropped to 30.4%, and the xylan elimination was not significantly increased. This was associated with the fact that the high concentration of acid might favor damage to the LC structure and increase the dissolution of cellulose. While the dissolved lignin might be repolymerized and attached to the solid surface [28], the lignin content in pretreated solid WS can increase. Consequently, 40 g/L was chosen as the optimal concentration of PTSA.
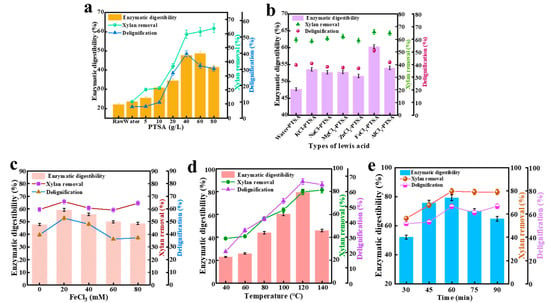
Figure 1.
Investigating PTSA’s (5–80 g/L) influence on enzymatic digestibility, xylan elimination and lignin removal [100 °C, 60 min, solid–liquid ratio 1:10] (a). Examining Lewis acids’ (KCl, NaCl, MgCl2, ZnCl2, AlCl3, FeCl3) influence on enzymatic digestibility, xylan elimination and lignin removal [100 °C, 60 min, solid–liquid ratio = 1:10, 40 g/L PTSA, 20 mM Lewis acid] (b). Evaluation of FeCl3 loading (0–80 mM) in influencing enzymatic digestibility, xylan elimination and lignin removal [100 °C, 60 min, solid–liquid ratio 1:10, 40 g/L PTSA] (c). Assessment of pretreatment temperatures (40–140 °C) in influencing enzymatic digestibility, xylan elimination and lignin removal [60 min, solid–liquid ratio 1:10, 40 g/L PTSA, 20 mM FeCl3] (d). Investigation of pretreatment duration (30–90 min) in influencing enzymatic digestibility, xylan elimination and lignin removal [120 °C, 40 g/L PTSA, 20 mM FeCl3, solid–liquid ratio 1:10] (e).
It is known that Lewis acid can be used to enhance the acidity of the pretreatment medium so that the xylan in LC may be decomposed into small molecular fragments [29]. Different types of Lewis acids with diverse acid dissociation constants can lead to distinct pretreatment effects [30]. In this research, six Lewis acids (KCl, NaCl, MgCl2, ZnCl2, AlCl3, and FeCl3) were selected to individually mix with PTSA for combined pretreatment of WS. The pretreatment capacity of six pretreatment systems (KCl-PTSA/water, NaCl-PTSA/water, MgCl2-PTSA/water, ZnCl2-PTSA/water, AlCl3-PTSA/water, and FeCl3-PTSA/water) was evaluated, and the xylan and lignin removal results are listed in Figure 1b. Distinct from other systems, the FeCl3-PTSA/water manifested the highest pretreatment efficacy under the same pretreatment conditions (40 g/L PTSA, 20 mM Lewis acid, 100 °C, 60 min, solid–liquid 1:10 wt/wt). Different from the PTSA/water treatment, the existence of 20 mM FeCl3 in PTSA/water could elevate the xylan elimination from 59.6% to 65.8% and enhance the lignin removal from 39.6% to 51.9%. The supplementation of FeCl3 might promote the formation of an acidic environment, resulting in the increased depolymerization of carbohydrate polymers. In PTSA/water, FeCl3 showed better delignification than AlCl3. It is known that FeCl3 has a stronger acidity compared with AlCl3 [31]. Consequently, FeCl3 was selected to mix with PTSA for combined pretreatment of WS in this research. However, the loading of Lewis acid might directly influence the elimination of xylan and lignin, and extreme pretreatment conditions degrade glucan and other soluble components to produce other inhibitors [30], which will severely affect subsequent enzymolysis. Accordingly, it is very important to choose the appropriate loading of FeCl3. As showcased in Figure 1c, upon adding FeCl3 to PTSA/water from 0 to 20 mM (100 °C, 60 min, 40 g/L PTSA, solid–liquid 1:10 wt/wt), the strengthening effect of FeCl3 increased xylan elimination from 59.6% to 65.8% and promoted lignin removal significantly. However, no significant alteration in LC chemical composition was detected when FeCl3 was increased from 20 mM to 80 mM, implying that the addition of excessive Fe3+ cannot significantly improve lignin and xylan elimination. This finding was similar to results previously reported [32]. Consequently, the addition of 20 mM FeCl3 as Lewis acid was a good strategy for enhancing WS pretreatment. As a Lewis acid, FeCl3 may effectively cleave the linkages between carbohydrates and lignin [20], as well as enhancing enzymolysis of xylan to monomeric and oligomeric sugars. PTSA can selectively cleave β-O-4, benzyl ether, and benzyl ester bonds among lignin units, thereby hindering lignin condensation. FeCl3 and PTSA can work together to efficiently eliminate lignin and hamper the condensation of lignin, achieving the highly effective saccharification of treated biomass with cellulases.
The effects of pretreatment temperature and duration on the xylan elimination and delignification were also tested (Figure 1d,e). To determine the effect of pretreatment temperature, WS was treated with the mixture of FeCl3 (20 mM) and PTSA (40 g/L) for 60 min at 40–140 °C (solid–liquid 1:10 wt/wt). Upon implementing pretreatment from 40 °C to 120 °C, lignin removal increased greatly, and the xylan elimination elevated significantly, showing that the lignin and xylan were substantially eliminated by FeCl3-PTSA/water treatment. The apparent alteration in the removal of xylan and lignin was associated with strong molecular motion [13]. By raising the temperature from 120 °C to 140 °C, there was a slight enhancement in lignin elimination. It is likely that some dissolved lignin remained attached or re-adsorbed on the residual solid, increasing lignin content. The duration of pretreatment might substantially impact the elimination of lignin and xylan. Upon elevating pretreatment duration from 30 to 90 min, the lignin and xylan elimination initially rose before declining. For a pretreatment time of 60 min, the lignin and xylan elimination rates were 66.6% and 79.7%, respectively. This phenomenon might be attributed to the enhanced penetration of acids into the cell walls of WS due to the sufficient pretreatment duration, thereby facilitating lignin dissociation and hemicellulose hydrolysis [33]. However, once protons saturate their attack on LC within the system, additional acids might be unable to further degrade WS, resulting in weakened elimination [26]. Based on the above analysis and results, the optimized pretreatment conditions were as follows: pretreatment temperature of 120 °C, pretreatment time of 60 min, PTSA dose of 40 g/L, and FeCl3 loading of 20 mM. The xylan removal and delignification reached 79.7% and 66.6%, respectively.
LogR0 is an important index for evaluating pretreatment intensity [32]. During the optimization process, there were certain fittings about LogR0–delignification (R2 = 0.91) and LogR0–xylan elimination (R2 = 0.89) (Figure 2). Chen et al. also reported a correlation between LogR0 and xylan elimination (R2 = 0.90) and between LogR0 and delignification (R2 = 0.85) [13]. These results showed that high LogR0 was beneficial to the preferential dissolution and elimination of lignin and xylan during pretreatment, which effectively promoted the destruction of the natural physical barrier structure of WS. These alterations will contribute to the subsequent biotransformation of cellulase [34].
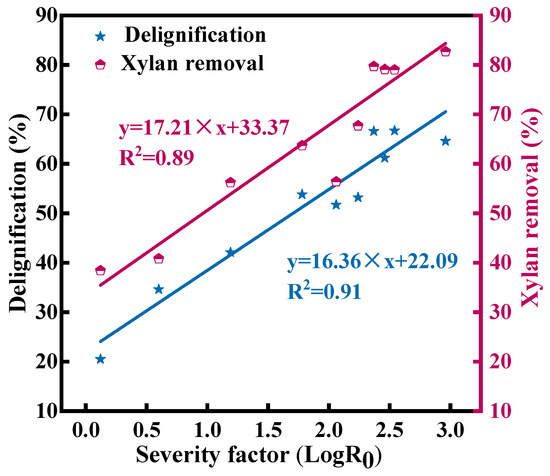
Figure 2.
The fitting of lignin and xylan elimination with LogR0.
3.2. Investigation of XOS Formation by FeCl3-PTSA/Water Treatment
Xylo-oligosaccharide (XOS) is derived from hemicellulose in LC, which has multiple prebiotic effects, antioxidant capacities and anticancer activities [35,36] for wide utilization in animal feed, healthcare, the food industry and other fields [37]. During pretreatment, an acidic environment can promote the decomposition of xylan, enhance the degradation of xylan’s skeleton structure, and produce XOSs with different molecular weights [38]. The presence of XOSs in the filtrate can also be used as an indicator of hemicellulose dissolution levels [39]. Therefore, the pretreatment filtrate from the above optimization process was studied in this work.
The effects of PTSA content, FeCl3 loading, pretreatment duration, and pretreatment temperature on the formation of XOSs were analyzed. In this research, high yield of XOSs was acquired in the presence of 40 g/L PTSA. However, further increasing PTSA content could weaken the formation of XOSs. This might be due to further depolymerization or condensation of the resulting cyst-like sugars to form degradation products [40]. Compared with other cations, Fe3+ could counteract the acid-buffering effect of ash in the pretreatment medium, which was the key factor for Fe3+ to increase the XOS concentration [20]. The dosage of FeCl3 had a crucial role in XOS formation (Figure 3a). WS samples were treated with 0–80 mM FeCl3 plus 40 g/L PTSA for 60 min at 100 °C. Upon increasing the FeCl3 loading from 0 to 20 mM, the content of XOSs elevated from 2.9 g/L to 3.4 g/L and then decreased gradually as FeCl3 surpassed 20 mM. This might be associated with the fact that FeCl3 as a Lewis acid catalyzed the hydrolysis of hemicellulose. To obtain XOSs at low energy cost, we can reduce the temperature, and a high quantity of XOSs (3.4 g/L) can be acquired at 100 °C.
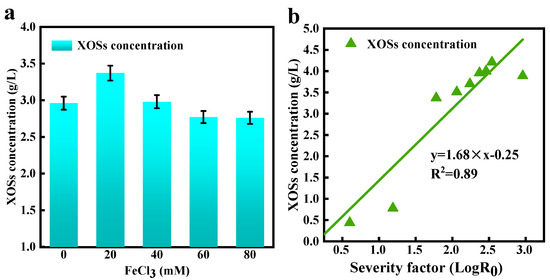
Figure 3.
Assessing FeCl3 loading (0–80 mM) in influencing the generation of XOSs [60 min, 100 °C] (a); correlation between LogR0 and XOS content (b).
During pretreatment with metal salts, hydroxides or oxides might form and the Lewis acid characteristic is lost. Thus, XOSs are protected from further catalytic degradation, thereby facilitating their accumulation in the system. In the presence of high concentrations of FeCl3, XOS formation might be hindered due to extensive destruction by Lewis acids, resulting in decreased XOS concentration [20,36]. In Table 1, it could be observed that a small amount of XOS was produced when the temperature was lower than 100 °C. As the temperature was raised to 120 °C, the XOS content reached 4.0 g/L (120 °C, 60 min; LogR0 = 2.37). The acidic reaction environment further promoted the dissociation of xylan to produce XOSs and xylose. At 140 °C, the XOS content showed no significant change, because some XOSs might be further hydrolyzed to xylose at excessive temperatures. However, the xylose is at least partially converted into furfural at that temperature (140 °C) [36,41,42]. At high temperatures, the xylans in biomass can be hydrolyzed to pentoses (mainly xylose) in an acidic system. As these pentoses are further dehydrated, furfural is manufactured. SC-GCa-800 (50 mg) dehydrated xylose into furfural at 140 °C in 40 min [43]. A furfural yield of 75% from 3 g/L xylose was achieved through catalysis with 0.1 mol/L of [Bmim]Cl/FeCl3 as a chemocatalyst in a butanone/water (4:1, v/v) system at 140 °C for 1.5 h [44]. The reaction duration had a crucial influence on the formation of XOSs. The content of XOSs increased from 3.5 g/L to 4.2 g/L with a successively increasing reaction duration from 30 to 90 min (LogR0 1.7–3.0). The fitting between LogR0 and XOS production is illustrated in Figure 3b. The content of XOSs tended to increase when raising severity factors (LogR0); 4.0 g/L of XOSs was produced through catalysis with FeCl3-PTSA/water under optimal pretreatment conditions. Therefore, efficient production of XOSs could be acquired in the process of WS pretreatment using FeCl3-PTSA/water. Single-factor ANOVA and data significance analysis tests for different treatment temperature conditions were carried out, and a significant difference was found (p < 0.050) (Table S1, in Supplementary Materials).

Table 1.
Effects of reaction temperature on the formation of XOSs.
3.3. Investigation of Pretreatment Conditions in Influencing Enzymolysis
Enzymolysis ability is a key index to evaluate the effect of pretreatment [45]. The enzymatic saccharification results under each optimization condition are illustrated in Figure 1. Firstly, the impact of PTSA content (0, 5, 10, 20, 40, 60 and 80 g/L) on enzymolysis was tested (Figure 1a). The digestibility of the raw material was only 22.0%. In the PTSA/water pretreatment of WS (100 °C, 60 min, solid–liquid ratio 1:10), the enzymolysis ability first elevated and then decreased with increasing PTSA content. The high content of PTSA might intensify the destruction of lignocellulose and promote the dissolution of hemicellulose and lignin. The dissolved lignin will depolymerize and adhere to the sample surface, thus increasing the lignin content in the obtained solid sample, which is not conducive to the subsequent enzymolysis [46]. Accordingly, 40 g/L of PTSA was the optimal concentration for this reaction.
Upon adding different species of Lewis acid to the PTSA/water system, the content of xylan and lignin in WS residue was further reduced, and the ability of enzymatic hydrolysis varied greatly with the species of Lewis acid (Figure 1b). It was observed that FeCl3 and AlCl3 had positive effects on the degradation of xylan and lignin, implying that trivalent Lewis acids had a significantly positive effect on the enzymatic saccharification of WS compared to divalent and monovalent Lewis acids [32]. FeCl3 showed a more efficient enzymolysis ability because FeCl3 was more acidic and better able to break the ester bond and ether bonds in the lignin–hemicellulose complex compared with AlCl3, showing a better delignification effect and reducing the possibility of an unproductive combination of cellulase and lignin [47]. In addition, the dose of FeCl3 also had an important effect on the ability of enzymatic hydrolysis (Figure 1c). Combined with the results of component analysis, when the loading of FeCl3 increased from 0 to 20 mM (100 °C, 60 min, 40 g/L PTSA, solid–liquid ratio 1:10), the enzymolysis ability elevated from 47.7% to 59.3%. Over 20 mM, high loading of FeCl3 (Lewis acid) might condense lignin, resulting in reduced lignin elimination. The presence of condensed lignin acted as a physical barrier to prevent enzymolysis of cellulose components, which negatively affected the enzymatic hydrolysis of pretreated biomass [48]. Accordingly, the supplementation of excess Lewis acid did not result in a higher enzymolysis ability. Thus, the optimal amount of FeCl3 added was determined to be 20 mM.
The pretreatment temperature could influence enzymolysis ability greatly (Figure 1d). By raising pretreatment temperature from 40 °C to 120 °C, the enzymolysis efficacy gradually increased. As the temperature was elevated to 140 °C, the enzymatic digestibility dropped (Figure 1d). This was ascribed to the fact that higher temperature might lead to more glucan loss, and some dissolved lignin would attach or reprecipitate on the surface of the residual solid, preventing enzymolysis [30]. Accordingly, 120 °C was selected as the appropriate temperature for pretreatment. With the extension of pretreatment duration from 30 to 60 min, the enzymolysis efficacy also increased. However, the efficacy showed a decreasing trend when the pretreatment duration continued to be extended to 75 min (Figure 1e). In combination with the analysis of WS chemical components, a too-long pretreatment duration might lead to a decrease in cellulose retention, and the formation of condensed lignin will not only reduce delignification but also hinder enzymatic hydrolysis [49].
It was also found that the enzymatic digestibility changed significantly with the increase in LogR0. Figure 4a shows that the efficiency of enzymatic hydrolysis was positively correlated with LogR0 [Y(enzymatic digestibility efficiency) = 22.96 × X(LogR0) + 16.68, R2 = 0.87], and high LogR0 was conducive to the improvement of reducing sugar yield. The biggest obstacle to the valuable utilization of biomass is lignin, and the effect of delignification on enzymolysis is worth exploring [34]. There were significant positive fittings about lignin removal–enzymolysis efficacy [Y(delignification) = 0.63 × X(enzymatic digestibility efficiency) + 11.73, R2 = 0.89] and xylan elimination–enzymolysis efficacy [Y(xylan removal) = 0.71 × X(enzymatic digestibility) + 13.05, R2 = 0.95] (Figure 4b). Clearly, high enzymolysis ability was attributed to the removal of xylan as a barrier for cellulases [50]. The efficient contact of cellulose with the cellulases would result in the enhancement of enzymolysis.
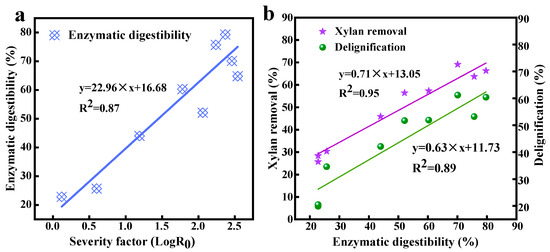
Figure 4.
The fitting about LogR0 and enzymolysis ability (a); the fitting about delignification and xylan elimination with enzymolysis ability (b).
3.4. Recycling of FeCl3-PTSA/Water for Pretreatment of WS
The reusability of pretreatment solvents is an important advantage, allowing them to potentially reduce production cost [32]. Here, FeCl3-PTSA/water treatment filtrate was recovered and reused five times [120 °C, 60 min, solid–liquid ratio 1:10 (wt/wt), 40 g/L PTSA, 20 mM FeCl3] to explore whether it has good reusability. Afterwards, we performed analysis of the chemical composition of the reused WS pretreated with FeCl3-PTSA/water (Table 2). When FeCl3-PTSA/water was used for the fourth time, FeCl3-PTSA/water (4th) still exhibited good lignin removal ability. However, the eliminations of lignin and xylan were gradually reduced as FeCl3-PTSA/water was reused a fifth time. Compared to the first run, the solid recovery elevated from 62.0% to 71.1%, xylan elimination decreased from 75.3% to 63.8%, and lignin removal decreased from 66.2% to 58.4%. After five cycles, the efficiency of enzymatic hydrolysis declined from 79.7% to 69.7% (Table 2). Accumulation of too many lignin monomers and other hydrophobic substances in recovered FeCl3-PTSA/water may have weakened the pretreatment ability, and the H+ in the pretreatment system was consumed in a large amount during the pretreatment process for attacking the β-glucoside bond and ether bond in LC [51,52]. The formation of metal complexes of Fe3+ with hemicellulose and lignin caused the loss of Fe3+ (Lewis acid) [32]. Single-factor ANOVA and data significance analysis tests for reuse times regarding XOS formation were measured, and a significant difference was observed (p < 0.050) (Table S2, in Supplementary Materials).

Table 2.
Effect of reuse times on treated WS with FeCl3-PTSA/water treatment [120 °C, 40 g/L PTSA, 20 mM FeCl3, solid–liquid ratio 1:10].
As mentioned above, the pretreatment ability of FeCl3-PTSA/water was gradually weakened after its recovery and reuse. In addition, the composition analysis of each recovered filtrate was performed and the amount of XOSs in the filtrate was detected to increase with the number of cycles (Table 1). The percentage of the solvent mixture could be recovered in the range of 90–92% after each reuse. The effective degradation of xylan during each cycle resulted in the accumulation of XOSs in the pretreatment slurry, which increased the concentration of XOSs. After four cycles of reuse, FeCl3-PTSA/water’s treatment capacity was relatively high, indicating that it had good reusability. The reusability of FeCl3-PTSA/water held significant promise to reduce production costs, thereby increasing economic efficiency and achieving sustainable development.
3.5. Mass Balance
The pretreatment and enzymolysis processes of WS are illustrated in Figure 5; 1000 g of crude WS containing 382 g glucan, 202 g xylan, 154 g lignin, and 262 g of other substances was put into the pretreatment reactor. After the crude WS was treated at 120 °C for 60 min (solid–liquid ratio 1:10), 615 g WS residue was recovered, and the treated WS was composed of 324 g glucan, 41 g xylan, 51 g lignin, and 199 g of other materials. In the pretreatment liquor, 40 g XOSs formed. The remaining glucan in treated WS was enzymatically hydrolyzed with cellulases (15 FPU/g), while the xylan was also attacked to release part of xylose, yielding 281 g glucose, 35 g xylose, and 5 g cellobiose.
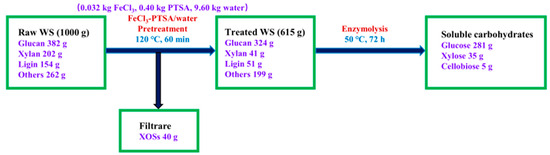
Figure 5.
Mass flow through the treatment with FeCl3-PTSA/water.
The construction of an efficient pretreatment process is crucial to enhancing the enzymatic saccharification of lignocellulosic biomass [53,54,55,56]. ChCl:p-TsOH (1:1, mol:mol) was used to pretreat WS at 80 °C for 60 min, and only 36.1% of glucan was kept in the solid residue. The delignification and xylan removal reached 72.4% and 90.5%, respectively. The enzymatic digestibility reached 89.3%, and XOSs were obtained at 1.4 g/L [12]. Through the pretreatment with TTAB/LCA/Fe3+ (1:4:0.0111, mol:mol:mol) (162.5 °C, 61.7 min), 79.8% of glucan remained. Lignin (89.2%) and xylan (77.9%) were eliminated, acquiring an enzymolysis efficiency of 92.5%. Additionally, XOSs were obtained at 3.64 g/L [20]. CTAC:LA (1:4, mol/mol) was utilized to treat wheat stalk at 160 °C for 60 min), acquiring 50.8% glucan recovery, 89.0% xylan removal and 88.3% delignification (88.3%). XOSs were obtained at 2.3 g/L [57]. Lewis acid-assisted organic acid pretreatment is an alternative method of biomass valorization [21]. In this research, an FeCl3-PTSA/water pretreatment system was constructed for co-producing reducing sugars and xylo-oligosaccharides (XOSs) from WS; high glucan recovery was obtained at 84.8%. The enzymolysis efficiency and XOS concentration could reach 79.3% and 4.0 g/L, respectively. Compared to ChCl:p-TsOH, TTAB/LCA/Fe3+, and CTAC:LA, the FeCl3-PTSA/water system is easy to construct. FeCl3, PTSA and water are readily available; FeCl3-PTSA/water pretreatment has potential in the valorization of biomass on an industrial scale. The obtained reducing sugars could be fermented or have the potential to be fermented into biofuels and biobased compounds. How to effectively separate lignin and realize the conversion of lignin into high value-added chemicals is an attractive issue [58,59,60] that deserves further study in the biorefinery process involving FeCl3-PTSA/water treatment. The established FeCl3-PTSA/water treatment can be considered in future work to promote the effective valorization of various biomasses.
4. Conclusions
This work explored the effects of Lewis acid on the pretreatment of WS in the PTSA system. The FeCl3-PTSA/water was chosen as an optimum medium to pretreat waste WS. The relationship of severity factor (LogR0) with the changes of chemical components and enzymolysis efficiency was fitted. After pretreatment at 120 °C for 60 min, the xylan removal and delignification reached 79.7% and 66.6%, respectively. Enzymolysis efficiency could reach 79.3%. After four cycles of reuse, FeCl3-PTSA/water’s treatment ability was still high. The constructed pretreatment with FeCl3-PTSA/water realized the efficient co-production of reducing sugars and XOSs from WS. To sum up, this constructed FeCl3-PTSA/water treatment has potential application in the sustainable conversion of LC into valuable biobased molecules.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13051615/s1, Table S1: Single factor analysis (The effects of reaction temperature on the XOS formation). Table S2: Single factor analysis (The effects of reuse times on the XOS formation).
Author Contributions
Conceptualization, methodology and writing—original draft, X.H.; conceptualization, data curation, software, and writing—original draft, Q.G.; supervision, review and revising manuscript, Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the Analysis and Testing Center (Changzhou University) for analysis of biomass samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhou, S.; Xue, Y.; Cai, J.; Cui, C.; Ni, Z.; Zhou, Z. An understanding for improved biomass pyrolysis: Toward a systematic comparison of different acid pretreatments. Chem. Eng. J. 2021, 411, 128513. [Google Scholar] [CrossRef]
- Dharmalingam, B.; Tantayotai, P.; Panakkal, E.J.; Cheenkachorn, K.; Kirdponpattara, S.; Gundupalli, M.P.; Cheng, Y.-S.; Sriariyanun, M. Organic acid pretreatments and optimization techniques for mixed vegetable waste biomass conversion into biofuel production. BioEnergy Res. 2023, 16, 1667–1682. [Google Scholar] [CrossRef]
- Eda, S.; Kota, B.J.; Thella, P.K.; Bankupalli, S.; Bhargava, S.K.; Parthasarathy, R. Regeneration of levulinic acid from loaded-organic phase: Equilibrium, kinetic studies and process economics. Chem. Pap. 2017, 71, 1939–1951. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Townsend, T.J.; Sparkes, D.L.; Ramsden, S.J.; Glithero, N.J.; Wilson, P. Wheat straw availability for bioenergy in England. Energy Policy 2018, 122, 349–357. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, W.; Fan, B.; He, Y.C.; Ma, C. Implementing efficient and sustainable pretreatment of Sorghum stalks for delignification and xylan separation with a ternary deep eutectic solvent under mild conditions. Int. J. Biol. Macromol. 2025, 303, 140417. [Google Scholar] [CrossRef]
- Alam, A.; Zhang, R.; Liu, P.; Huang, J.; Wang, Y.; Hu, Z.; Madadi, M.; Sun, D.; Hu, R.; Ragauskas, A.J.; et al. A finalized determinant for complete lignocellulose enzymatic saccharification potential to maximize bioethanol production in bioenergy Miscanthus. Biotechnol. Biofuels 2019, 12, 99. [Google Scholar] [CrossRef]
- Beig, B.; Riaz, M.; Raza Naqvi, S.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.E.; Thuy Lan Chi, N. Current challenges and innovative developments in pretreatment of lignocellulosic residues for biofuel production: A review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Abbas, A.; Adesina, A.Y.; Suleiman, R.K. Influence of organic acids and related organic compounds on corrosion behavior of stainless steel—A critical review. Metals 2023, 13, 1479. [Google Scholar] [CrossRef]
- Jiang, C.-X.; He, Y.-C.; Chong, G.-G.; Di, J.-H.; Tang, Y.-J.; Ma, C.-L. Enzymatic in situ saccharification of sugarcane bagasse pretreated with low loading of alkalic salts Na2SO3/Na3PO4 by autoclaving. J. Biotechnol. 2017, 259, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Tang, Z.; He, Y.C. Valorization of wheat straw through enhancement of cellulose accessibility, xylan elimination and lignin removal by choline chloride:p-toluenesulfonic acid pretreatment. Int. J. Biol. Macromol. 2025, 301, 140335. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, C.; Tang, W.; He, Y.-C. Comprehensive understanding of enzymatic saccharification of Betaine:Lactic acid-pretreated sugarcane bagasse. Bioresour. Technol. 2023, 386, 129485. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, L.; Jiang, Y.; Wang, X.; Xu, J.; Wang, Q.; Jiang, S. Recent advances in the acid-catalyzed conversion of lignin. Biomass Convers. Biorefinery 2023, 13, 519–539. [Google Scholar] [CrossRef]
- Javed, F.; Aslam, M.; Rashid, N.; Shamair, Z.; Khan, A.L.; Yasin, M.; Fazal, T.; Hafeez, A.; Rehman, F.; Rehman, M.S.U.; et al. Microalgae-based biofuels, resource recovery and wastewater treatment: A pathway towards sustainable biorefinery. Fuel 2019, 255, 115826. [Google Scholar] [CrossRef]
- Yang, M.; Gao, X.; Lan, M.; Dou, Y.; Zhang, X. Rapid Fractionation of Lignocellulosic Biomass by p-TsOH Pretreatment. Energy Fuels 2019, 33, 2258–2264. [Google Scholar] [CrossRef]
- Sajid, M.; Rizwan Dilshad, M.; Saif Ur Rehman, M.; Liu, D.; Zhao, X. Catalytic conversion of xylose to furfural by p-toluenesulfonic acid (pTSA) and chlorides: Process optimization and kinetic modeling. Molecules 2021, 26, 2208. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Liu, N.; Zhao, Y.; Tian, G.; Wang, Z. Sequential extraction of hemicelluloses and lignin for wood fractionation using acid hydrotrope at mild conditions. Ind. Crops Prod. 2020, 145, 112086. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, C.; Yin, J.; Fan, B.; He, Y.-C.; Ma, C. Valorization of rapeseed straw through the enhancement of cellulose accessibility, lignin removal and xylan elimination using an n-alkyltrimethylammonium bromide-based deep eutectic solvent. Int. J. Biol. Macromol. 2025, 301, 140151. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Yuan, H.; Lyu, G.; Xie, J. FeCl3-catalyzed ethanol pretreatment of sugarcane bagasse boosts sugar yields with low enzyme loadings and short hydrolysis time. Bioresour. Technol. 2017, 249, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Xie, X.; Shi, Q. Improving enzymatic saccharification of Chinese silvergrass by FeCl3-catalyzed γ-valerolactone/water pretreatment system. Renew. Energy 2021, 177, 853–858. [Google Scholar] [CrossRef]
- Palliprath, S.; Poolakkalody, N.J.; Ramesh, K.; Mangalan, S.M.; Kabekkodu, S.P.; Santiago, R.; Manisseri, C. Pretreatment of sugarcane postharvest leaves by γ-valerolactone/water/FeCl3 system for enhanced glucan and bioethanol production. Ind. Crops Prod. 2023, 197, 116571. [Google Scholar] [CrossRef]
- Wang, Q.; Su, Y.; Gu, Y.; Lai, C.; Ling, Z.; Yong, Q. Valorization of bamboo shoot shell waste for the coproduction of fermentable sugars and xylooligosaccharides. Front. Bioeng. Biotechnol. 2022, 10, 1006925. [Google Scholar] [CrossRef]
- Selvakumar, P.; Adane, A.A.; Zelalem, T.; Hunegnaw, B.M.; Karthik, V.; Kavitha, S.; Jayakumar, M.; Karmegam, N.; Govarthanan, M.; Kim, W. Optimization of binary acids pretreatment of corncob biomass for enhanced recovery of cellulose to produce bioethanol. Fuel 2022, 321, 124060. [Google Scholar] [CrossRef]
- Yildirim, O.; Tunay, D.; Ozkaya, B.; Demir, A. Optimization of oxalic and sulphuric acid pretreatment conditions to produce bio-hydrogen from olive tree biomass. Int. J. Hydrogen Energy 2022, 47, 26316–26325. [Google Scholar] [CrossRef]
- Madadi, M.; Elsayed, M.; Sun, F.; Wang, J.; Karimi, K.; Song, G.; Tabatabaei, M.; Aghbashlo, M. Sustainable lignocellulose fractionation by integrating p-toluenesulfonic acid/pentanol pretreatment with mannitol for efficient production of glucose, native-like lignin, and furfural. Bioresour. Technol. 2023, 371, 128591. [Google Scholar] [CrossRef]
- Wei, N.; Qi, S.; Wang, G.; Ge, J.; Sui, W.; Sun, H.; Parvez, A.M.; Jia, H.; Si, C. Acid-promoted lignin reductive depolymerization under mild conditions via a condensation minimizing approach: From organosolv lignin to woody biomass. Fuel 2023, 338, 127311. [Google Scholar] [CrossRef]
- Wang, Z.-K.; Hong, S.; Wen, J.-l.; Ma, C.-Y.; Tang, L.; Jiang, H.; Chen, J.-J.; Li, S.; Shen, X.-J.; Yuan, T.-Q. Lewis acid-facilitated deep eutectic solvent (DES) pretreatment for producing high-purity and antioxidative lignin. ACS Sustain. Chem. Eng. 2020, 8, 1050–1057. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, W.; Ma, C.; He, Y.-C. Efficient co-production of xylooligosaccharides, furfural and reducing sugars from yellow bamboo via the pretreatment with biochar-based catalyst. Bioresour. Technol. 2023, 387, 129637. [Google Scholar] [CrossRef]
- Tomifuji, R.; Maeda, K.; Takahashi, T.; Kurahashi, T.; Matsubara, S. FeCl3 as an ion-pairing Lewis acid catalyst. Formation of highly Lewis acidic FeCl2+ and thermodynamically stable FeCl4– to catalyze the Aza-Diels–Alder reaction with high turnover frequency. Org. Lett. 2018, 20, 7474–7477. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Fan, B.; Tang, W.; He, Y.-C.; Ma, C. Comprehensive understanding of co-producing fermentable sugar, furfural, and xylo-oligosaccharides through the pretreatment with CTAB-based deep eutectic solvent containing Brønsted and Lewis acid. Chem. Eng. J. 2024, 488, 150637. [Google Scholar] [CrossRef]
- Varilla-Mazaba, A.; Raggazo-Sánchez, J.A.; Calderón-Santoyo, M.; Gómez-Rodríguez, J.; Aguilar-Uscanga, M.G. Optimization of lignin extraction by response surface methodology from sugarcane bagasse using deep eutectic solvents (DES). Ind. Crops Prod. 2022, 184, 115040. [Google Scholar] [CrossRef]
- Sun, D.; Lv, Z.-W.; Rao, J.; Tian, R.; Sun, S.-N.; Peng, F. Effects of hydrothermal pretreatment on the dissolution and structural evolution of hemicelluloses and lignin: A review. Carbohydr. Polym. 2022, 281, 119050. [Google Scholar] [CrossRef]
- Hong, C.; Corbett, D.; Venditti, R.; Jameel, H.; Park, S. Xylooligosaccharides as prebiotics from biomass autohydrolyzate. LWT 2019, 111, 703–710. [Google Scholar] [CrossRef]
- Martins, M.; Tramontina, R.; Squina, F.M.; Dinamarco, T.M.; Goldbeck, R. Synergism for xylo-oligosaccharides, ρ-coumaric and ferulic acid production, and thermostability modulation of GH 62 α-l-arabinofuranosidase. Biocatal. Agric. Biotechnol. 2022, 44, 102469. [Google Scholar] [CrossRef]
- Palaniappan, A.; Antony, U.; Emmambux, M.N. Current status of xylooligosaccharides: Production, characterization, health benefits and food application. Trends Food Sci. Technol. 2021, 111, 506–519. [Google Scholar] [CrossRef]
- Xie, X.; Chen, M.; Tong, W.; Song, K.; Wang, J.; Wu, S.; Hu, J.; Jin, Y.; Chu, Q. Comparative study of acid- and alkali-catalyzed 1,4-butanediol pretreatment for co-production of fermentable sugars and value-added lignin compounds. Biotechnol. Biofuels Bioprod. 2023, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, C.; Song, M.; Fan, R. Xylo-oligosaccharides preparation through acid hydrolysis of hemicelluloses isolated from press-lye. Grain Oil Sci. Technol. 2019, 2, 73–77. [Google Scholar] [CrossRef]
- Maroušek, J.; Stehel, V.; Vochozka, M.; Maroušková, A.; Kolář, L. Postponing of the intracellular disintegration step improves efficiency of phytomass processing. J. Clean. Prod. 2018, 199, 173–176. [Google Scholar] [CrossRef]
- Nowicki, J.; Stanek, N. Conversion of selected carbohydrates into furan aldehydes in aqueous media. Effect of cation structure of imidazolium ionic liquids on the selectivity phenomena. Biomass Bioenergy 2021, 154, 106252. [Google Scholar] [CrossRef]
- Liu, C.; Wei, L.; Yin, X.; Wei, M.; Xu, J.; Jiang, J.; Wang, K. Selective conversion of hemicellulose into furfural over low-cost metal salts in a γ-valerolactone/water solution. Ind. Crops Prod. 2020, 147, 112248. [Google Scholar] [CrossRef]
- Yang, T.; Li, W.; Su, M.; Liu, Y.; Liu, M. Production of furfural from xylose catalyzed by a novel calcium gluconate derived carbon solid acid in 1,4-dioxane. New J. Chem. 2020, 44, 7968–7975. [Google Scholar] [CrossRef]
- Hua, D.; Ding, H.; Liu, Y.; Li, J.; Han, B. Dehydration of xylose to furfural over imidazolium-based ionic liquid with phase separation. Catalysts 2021, 11, 1552. [Google Scholar] [CrossRef]
- Wu, M.; Di, J.; Gong, L.; He, Y.-C.; Ma, C.; Deng, Y. Enhanced adipic acid production from sugarcane bagasse by a rapid room temperature pretreatment. Chem. Eng. J. 2023, 452, 139320. [Google Scholar] [CrossRef]
- Panakkal, E.J.; Cheenkachorn, K.; Chuetor, S.; Tantayotai, P.; Raina, N.; Cheng, Y.-S.; Sriariyanun, M. Optimization of deep eutectic solvent pretreatment for bioethanol production from Napier grass. Sustain. Energy Technol. Assess. 2022, 54, 102856. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Liu, Y.; Wang, Y.; Huo, F.; Fan, M.; Adidharma, H.; Li, X.; Zhang, S. Recent progress in theoretical and computational studies on the utilization of lignocellulosic materials. Green Chem. 2019, 21, 9–35. [Google Scholar] [CrossRef]
- Shinde, S.D.; Meng, X.; Kumar, R.; Ragauskas, A.J. Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem. 2018, 20, 2192–2205. [Google Scholar] [CrossRef]
- Liu, M.; Zuo, S.; Liang, Y.; Sheng, Y.; Ge, S.; Wu, J.; Ma, H.; Sun, F.; Ahamad, T.; Le, Q.V.; et al. The influence of 3-hydroxy-2-naphthoic acid on agricultural wastes extracted sugar production used as energy sources. Fuel 2022, 323, 124235. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, Q.; Wang, W.; Zhuang, X.; Deng, Y.; Yuan, Z. Comparison study of organosolv pretreatment on hybrid pennisetum for enzymatic saccharification and lignin isolation. Fuel 2019, 249, 334–340. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Y.; Meng, J.; Cheng, W.; Chen, W.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem. 2018, 20, 2711–2721. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Li, W.; Wu, W.; Tang, B.; Zhu, C. Efficient pretreatment of cornstalks for lignin valorization using p-toluene sulfonic acid coupling ethylene glycol. Biomass Convers. Biorefinery 2024, 14, 18707–18720. [Google Scholar] [CrossRef]
- Ying, W.; Yang, J.; Zhang, J. In-situ modification of lignin in alkaline-pretreated sugarcane bagasse by sulfomethylation and carboxymethylation to improve the enzymatic hydrolysis efficiency. Ind. Crops Prod. 2022, 182, 114863. [Google Scholar] [CrossRef]
- Lee, I.; Yu, J.-H. The production of fermentable sugar and bioethanol from acacia wood by optimizing dilute sulfuric acid pretreatment and post treatment. Fuel 2020, 275, 117943. [Google Scholar] [CrossRef]
- Morán-Aguilar, M.G.; Calderón-Santoyo, M.; de Souza Oliveira, R.P.; Aguilar-Uscanga, M.G.; Domínguez, J.M. Deconstructing sugarcane bagasse lignocellulose by acid-based deep eutectic solvents to enhance enzymatic digestibility. Carbohydr. Polym. 2022, 298, 120097. [Google Scholar] [CrossRef]
- Yang, D.; Kong, L.; He, Y. Demonstrating effectual catalysis of corncob with solid acid Sn-NUS-BH in cyclopentyl methyl ether–water for co-producing reducing sugar, furfural, and xylooligosaccharides. Processes 2024, 14, 821. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Z.; Tang, W.; Ma, C.; He, Y.C. Exploration of biomass fractionation and lignin removal for enhancing enzymatic digestion of wheat-stalk through deep eutectic solvent Cetyl trimethyl ammonium chloride:Lactic acid treatment. Int. J. Biol. Macromol. 2025, 306, 141460. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, M.; Jiang, B.; Zhang, M.; Miao, Q.; Fu, H.; Clark, J.H.; Fan, J. A hemicellulose and lignin-first process for corn stover valorization catalyzed by aluminum sulfate in γ-butyrolactone/water co-solvent. Green Chem. 2022, 24, 7429–7441. [Google Scholar] [CrossRef]
- Patel, R.; Dhar, P.; Babaei-Ghazvini, A.; Nikkhah Dafchahi, M.; Acharya, B. Transforming lignin into renewable fuels, chemicals, and materials: A review. Bioresour. Technol. Rep. 2023, 22, 101463. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).