Numerical Simulation of Flow Characteristics in CO2 Long-Term Storage in Bedded Salt Cavern
Abstract
1. Introduction
2. Physical Model of Salt Cavern
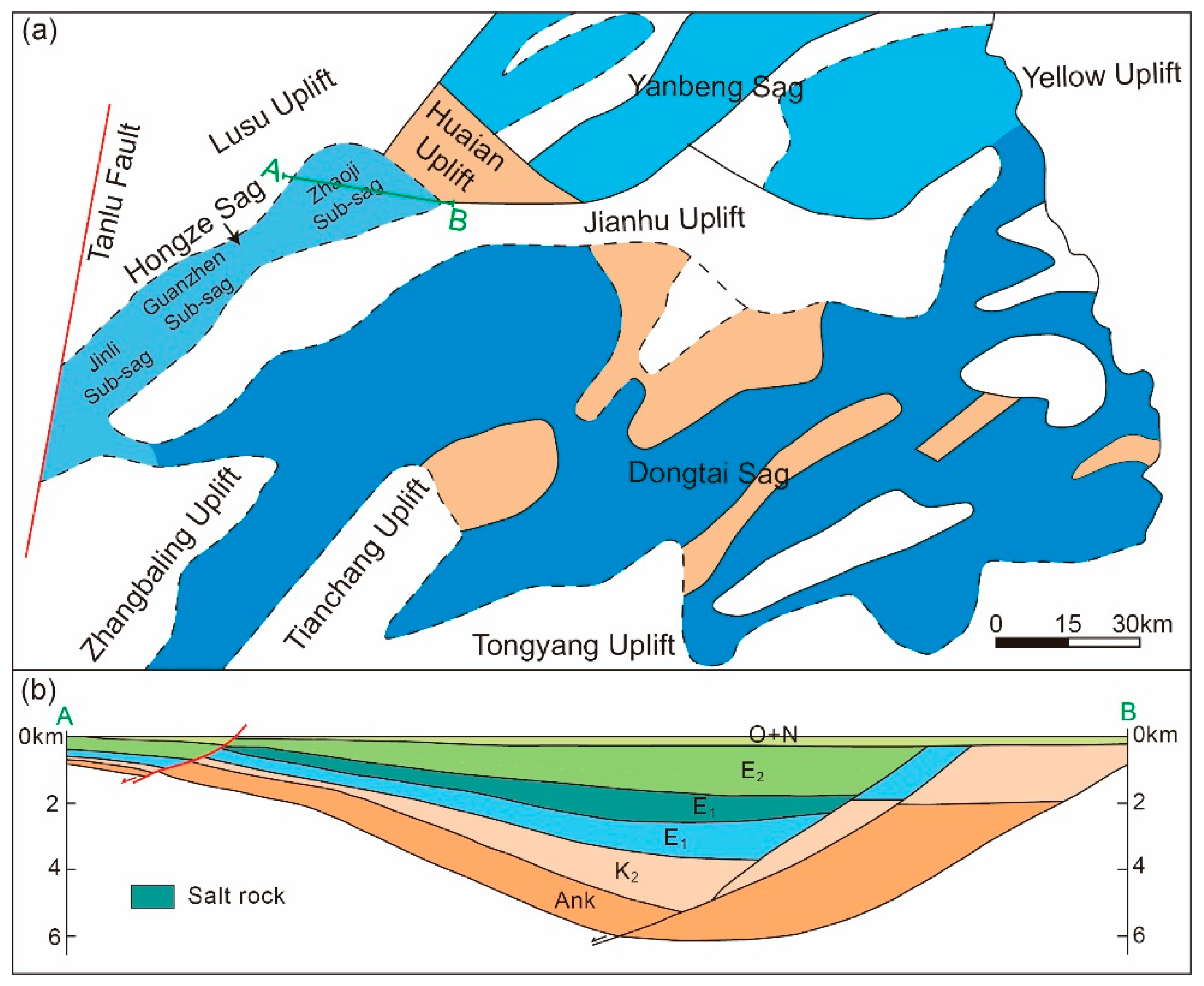
2.1. The Distribution of the Salt Rock
2.2. The Shape of the Cavern
2.3. Physical Model
3. The Mathematical Model of CO2 Storage in Salt Caverns
3.1. Model Assumptions
3.2. Governing Model of Deformation
3.3. Gas Transport in Salt Rock and Mudstone Interlayer
3.4. Governing Equation of Thermal Field
3.5. Governing Equation of Porosity and Permeability
3.6. The Coupling Model of the THM Fields
- (1)
- The changes in the mechanical fields of the salt layers and mudstone interlayers affect their porosity and permeability, subsequently leading to the variations in the hydraulic field. Conversely, the alterations in the hydraulic field influence the CO2 seepage velocity, pore pressure, and effective stress within the salt layers and mudstone interlayers, thereby modifying the mechanical fields.
- (2)
- The variations in the thermal fields of the salt layers and mudstone interlayers induce changes in the thermal stress, which in turn cause the changes in the mechanical fields. Reciprocally, the changes in the mechanical fields impact the heat transfer and strain energy within the salt layers and mudstone interlayers, subsequently resulting in the variations in the thermal fields.
- (3)
- On the one hand, the changes in the hydraulic fields of the salt layers and mudstone interlayers affect the heat transfer and thermal conduction, thereby causing the variations in the thermal fields. On the other hand, the changes in the thermal fields influence the density of CO2, subsequently leading to the alterations in the hydraulic fields.
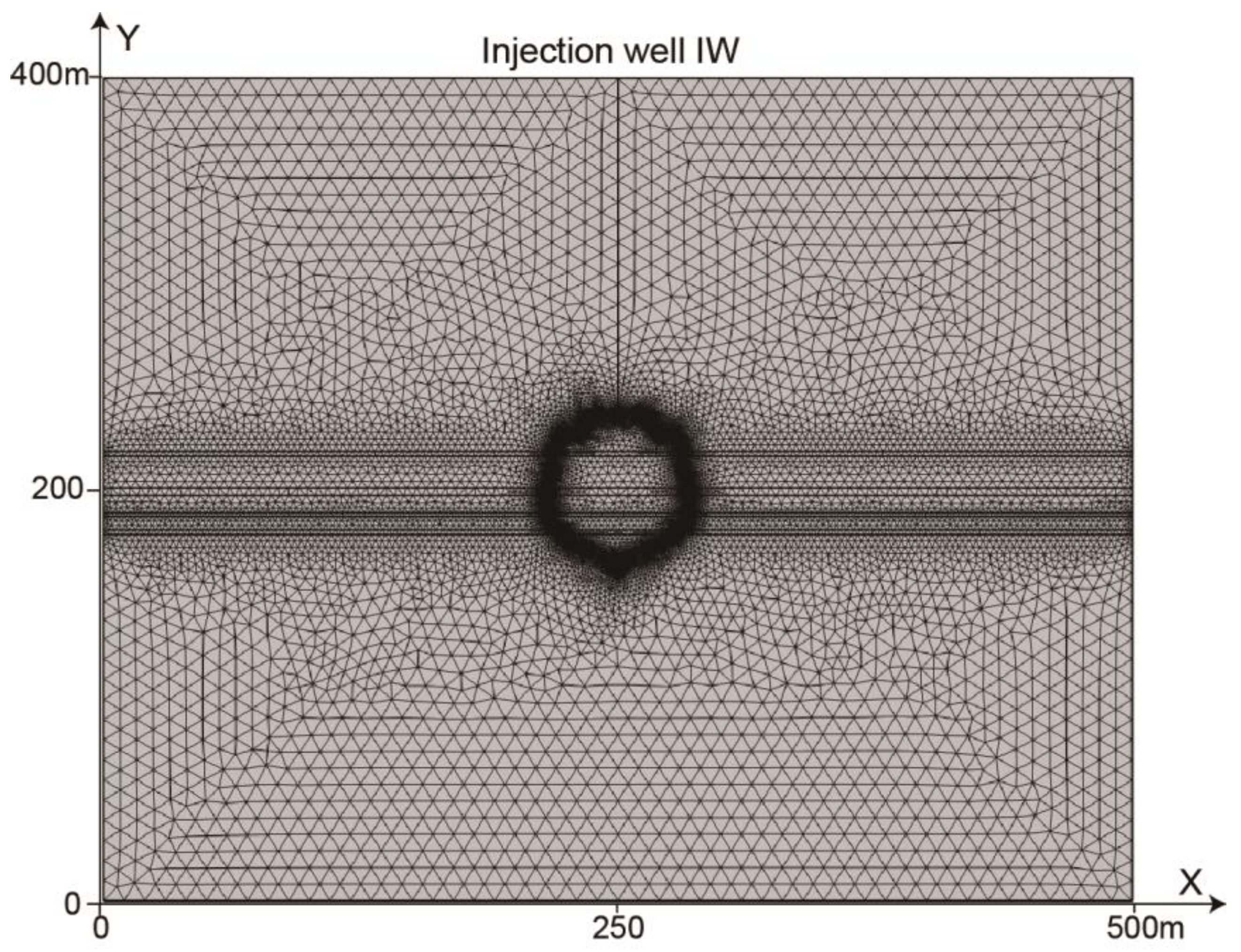
4. Numerical Parameter and Scheme
5. The Results of Numerical Simulations
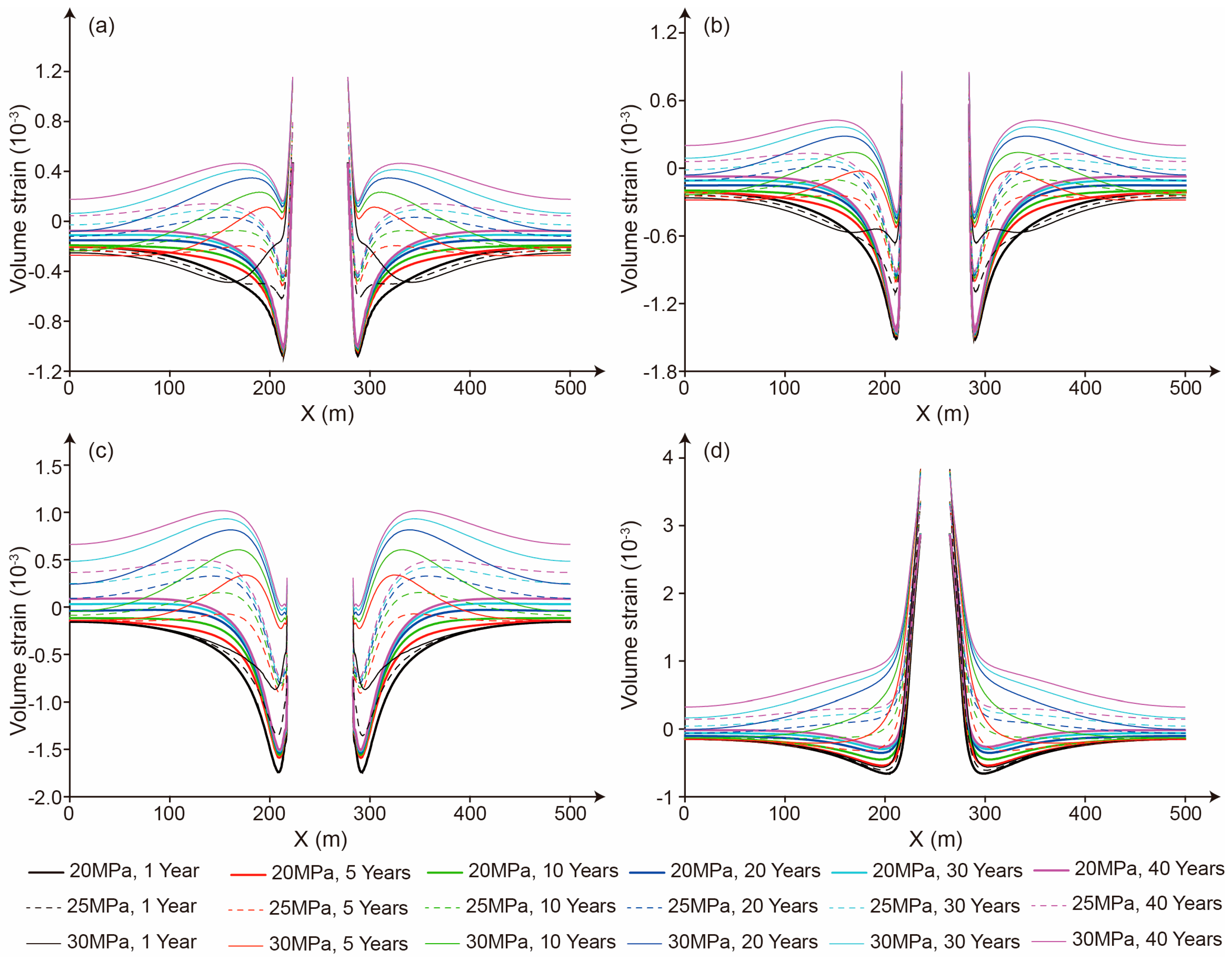
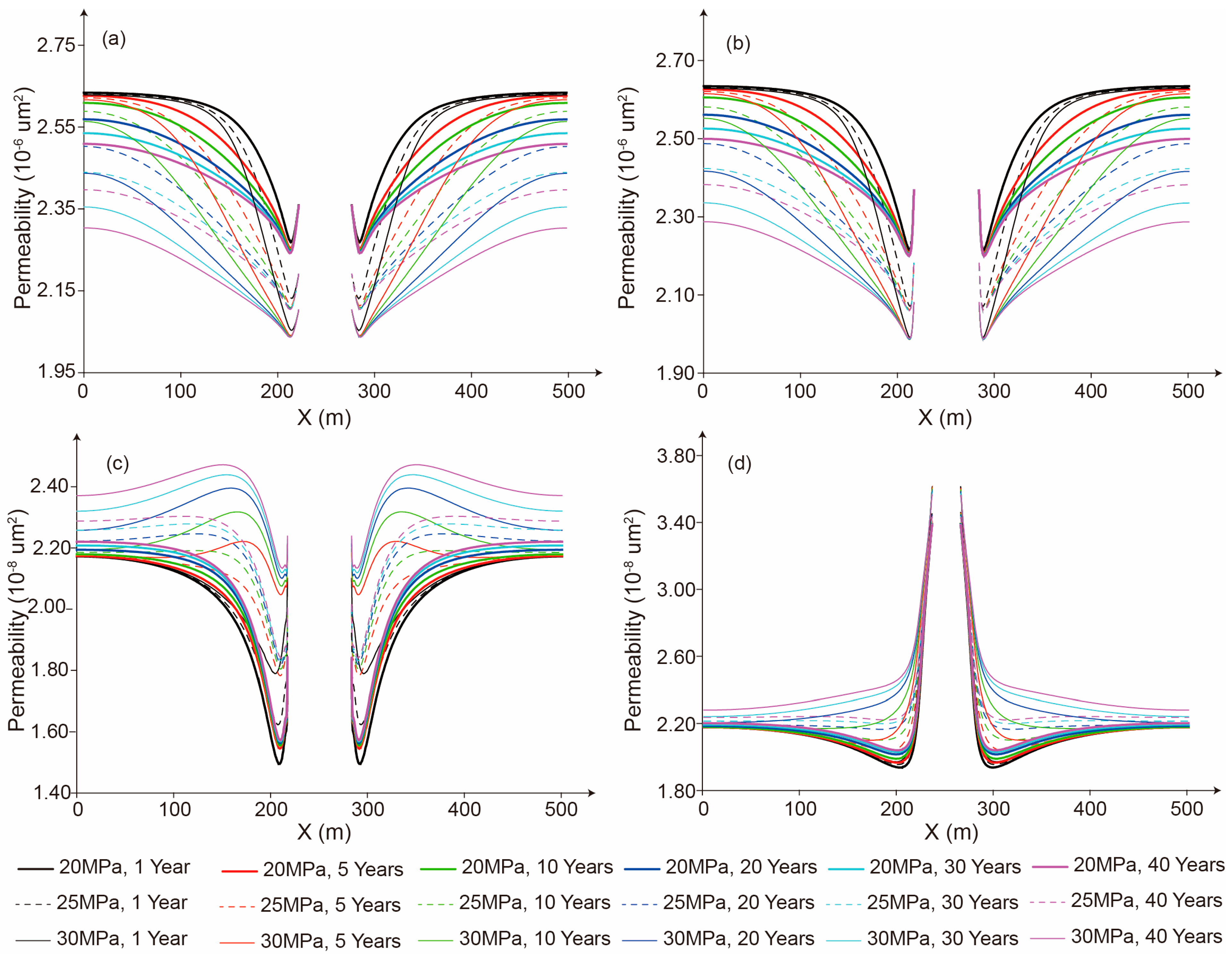
5.1. Strain Characteristics of the Salt Cavern and the Impact on Permeability
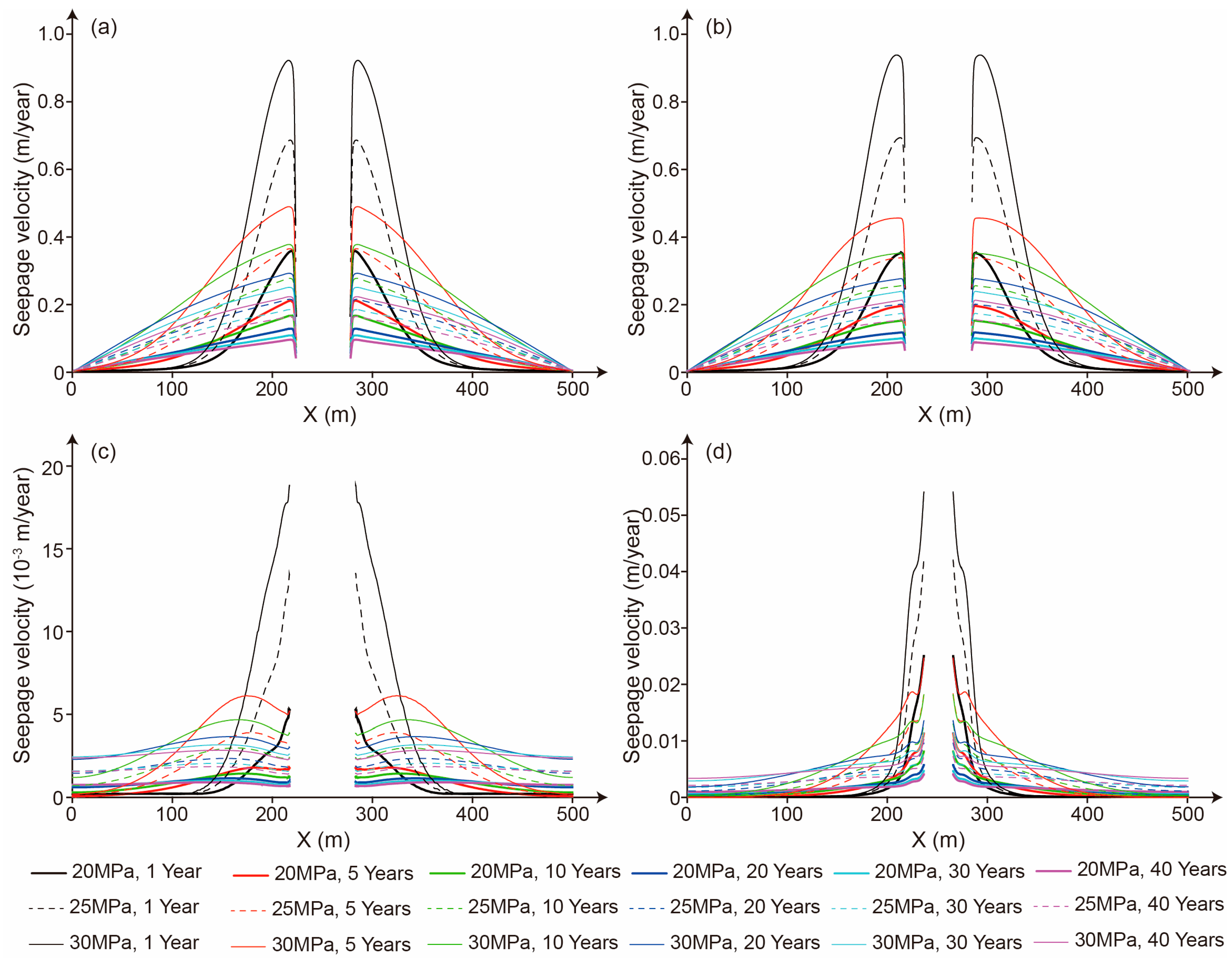
5.2. The Influence of the Mudstone Interlayer on Seepage Behavior
5.3. The Influence of Storage Time on the Seepage Character
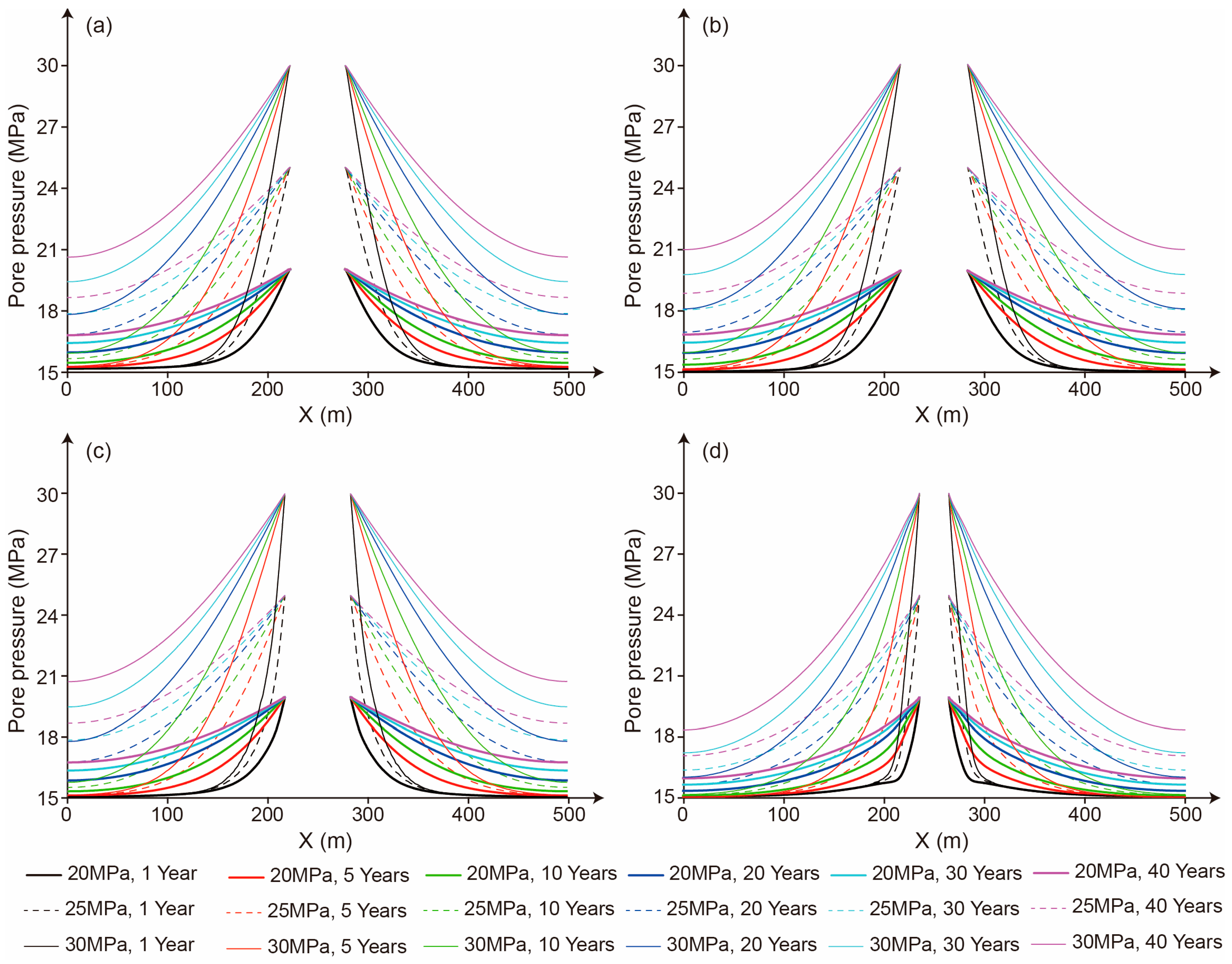
5.4. The Influence of Storage Pressure on Seepage Characteristics
6. Study Limitations and Safety Suggestions
- (1)
- The relatively high permeability of mudstone interlayers significantly increases the CO2 seepage velocity. Therefore, during the site selection and cavern construction processes for salt cavern storage reservoirs, mudstone interlayers should be avoided as much as possible. If it is unavoidable to encounter mudstone interlayers, locations with fewer mudstone interlayers and lower permeability should be selected. Meanwhile, an appropriate storage pressure and perfect monitoring methods can enhance the safety of CO2 storage in salt caverns with mudstone interlayers.
- (2)
- The CO2 seepage velocity is significantly influenced by the storage pressure. A higher storage pressure will notably expand the flow distance of CO2 around the salt cavern. Therefore, an appropriate storage pressure should be designed based on the number and thickness of mudstone interlayers, as well as the permeability and mechanical properties of both the mudstone interlayers and salt rock, to meet the requirements for the safety and economic viability of CO2 storage.
- (3)
- In contrast to the short-term cyclic injection–production approach employed in salt cavern gas storage, long-term CO2 storage within salt caverns results in a significant increase in the pore pressure within the salt and mudstone and an extended migration distance of CO2. Long-term or even permanent storage demands higher requirements on the safety of CO2 storage in salt caverns. Thereby, a comprehensive underground CO2 leakage monitoring system should be established to conduct the real-time monitoring of parameters such as the pore pressure, salt rock creep displacement, CO2 pressure, and temperature within the salt cavern to ensure the safety of CO2 storage in salt caverns.
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Lu, B.; Sun, J.; Jiang, R.; Liu, Z.; Zhang, Z.; Naguib, H.M.; Hou, G. A novel approach to accelerate the carbonation of γ-C2S under atmospheric pressure: Increasing CO2 dissolution and promoting calcium ion leaching via triethanolamine. Constr. Build. Mater. 2024, 450, 138719. [Google Scholar] [CrossRef]
- Hou, G.; Yan, Z.; Sun, J.; Naguib, H.M.; Lu, B.; Zhang, Z. Microstructure and mechanical properties of CO2-cured steel slag brick in pilot-scale. Constr. Build. Mater. 2021, 271, 121581. [Google Scholar] [CrossRef]
- Cao, B.; Fu, X.; Kang, J.; Lu, J.; Tang, P.; Xu, H.; Huang, M. Mini-Review on Influence of CO2-Enhanced Coalbed Methane Recovery and CO2 Geological Storage on Physical Properties of Coal Reservoir. Energy Fuel 2024, 38, 23268–23280. [Google Scholar] [CrossRef]
- Jun, Y.S.; Zhang, L.; Min, Y.; Li, Q. Nanoscale chemical processes affecting storage capacities and seals during geologic CO2 sequestration. Acc. Chem. Res. 2017, 50, 1521–1529. [Google Scholar] [CrossRef]
- Lin, Z.; Kuang, Y.; Li, W.; Zheng, Y. Research status and prospects of CO2 geological sequestration technology from onshore to offshore: A review. Earth-Sci. Rev. 2024, 258, 104928. [Google Scholar] [CrossRef]
- Sampath, K.H.S.M.; Ranjith, P.G.; Perera, M.S.A. A Comprehensive Review of Structural Alterations in CO2-Interacted Coal: Insights into CO2 Sequestration in Coal. Energy Fuels 2020, 34, 13369–13383. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, J.; Xian, X.; Zhang, C.; Gan, Q.; Dong, Z. Effect of supercritical CO2-water-shale interaction on mechanical properties of shale and its implication for carbon sequestration. Gas Sci. Eng. 2023, 111, 204930. [Google Scholar] [CrossRef]
- Zong, S.; Liu, S.Q.; Xu, H.; Wang, W.K.; Cao, B.; Huang, F.S. Numerical simulation of CO2 storage in bedded salt rock storage cavern in Subei basin. Coal Geol. Explor. 2023, 51, 27–36, (In Chinese with English abstract). [Google Scholar]
- Leiby, P.; Bowman, D. The Value of Expanding the U.S. Strategic Petroleum Reserve 1. ORNL/TM-2000/179; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2000. [Google Scholar]
- Davis, R.M. National Strategic Petroleum Reserve. Science 1981, 213, 618–622. [Google Scholar] [CrossRef]
- Langer, M. Use of solution-mined caverns in salt for oil and gas storage and toxic waste disposal in Germany. Eng. Geol. 1993, 35, 183–190. [Google Scholar] [CrossRef]
- Chen, J.S.; Li, J.J.; Jing, G.; Ao, H.B.; Liu, C.; Wang, Y.G.; Fu, Y.P. Research progress of geomechanical evaluation system used for Jintan salt cavern gas storage. Oil Gas Storage Transp. 2018, 37, 1088–1096, (In Chinese with English abstract). [Google Scholar]
- Yang, C.H.; Wang, G.B.; Shi, X.L.; Zhu, S.J.; Zheng, Z.Y.; Liu, W.; Fan, H.Y. Demands and challenges of large-scale salt cavern hydrogen storage in China. Rock Soil. Mech. 2024, 45, 1–19, (In Chinese with English abstract). [Google Scholar]
- Cheng, W.B.; Wang, K.F.; Fu, X.H.; He, P. Brief analysis of carbon dioxide storage in salt caverns. China Well Rock Salt 2023, 54, 16–18, (In Chinese with English abstract). [Google Scholar]
- Mwakipunda, G.C.; Mgimba, M.M.; Ngata, M.R.; Yu, L. Recent advances on carbon dioxide sequestration potentiality in salt caverns: A review. Int. J. Greenh. Gas Control 2024, 133, 104109. [Google Scholar] [CrossRef]
- Bachu, S.; Dusseault, M.B. Underground Injection of Carbon Dioxide in Salt Beds. In Developments in Water Science; Tsang, C., Apps, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 52, pp. 637–648. [Google Scholar]
- Costa, Á.M.D.; Costa, P.; Miranda, A.C.O.; Goulart, M.B.R.; Bergsten, A.; Meneghini, J.R.; Nishimoto, K.; Assi, G.R.S.; Sampaio, C.M.P.; Ebecken, N.F.F. Well Design for the Construction and Operation of an Experimental Salt Cavern Built Offshore in Ultra-Deep Water for CCS in Brasil. In Proceedings of the 54th U.S. Rock Mechanics/Geomechanics Symposium, Golden, CO, USA, 28 June–1 July 2020. [Google Scholar]
- Zhou, B. The Jintan gas storage of the natural gas branch—Building an “underground granary” for the Yangtze river economic belt. Sinopec 2023, 11, 38. [Google Scholar]
- Ministry of Ecology and Environment. Annual Report on Carbon Dioxide Capture, Utilization and Storage (CCUS) in China (2024); Ministry of Ecology and Environment: Beijing, China, 2024. [Google Scholar]
- Ba, J.H.; Kang, Y.P.; Jiang, H.T.; Zhou, D.L.; Jiao, Y.J.; Li, R.M.; Ren, Z.X.; Qi, D.S.; Jing, G.; Yan, F.L. Present situation and prospect of the utilization of old cavity in domestic salt cavern gas storage. Petrochem. Ind. Appl. 2020, 39, 1–5, (In Chinese with English abstract). [Google Scholar]
- Zhang, X.; Liu, W.; Chen, J.; Jiang, D.; Fan, J.; Daemen, J.J.K.; Qiao, W. Large-scale CO2 disposal/storage in bedded rock salt caverns of China: An evaluation of safety and suitability. Energy 2022, 249, 123727. [Google Scholar] [CrossRef]
- Huang, X.; Xiong, J. Numerical simulation of gas leakage in bedded salt rock storage cavern. Procedia Eng. 2011, 12, 254–259. [Google Scholar] [CrossRef]
- Ge, X.; Huang, J.; Zhou, K.; Su, K.; Wang, W.; Li, Y.; Shi, X. Research of interlayer dip angle effect on stability of salt cavern energy and carbon storages in bedded salt rock. Geoenergy Sci. Eng. 2024, 243, 213291. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Z.; Meng, X.; Shi, X.; Ma, H.; Yang, C.; Li, H. Inversion of creep parameters and their application in underground salt cavern energy storage systems for enhancing wind power integration. Int. J. Electr. Power 2024, 162, 110237. [Google Scholar] [CrossRef]
- Fourmeau, M.; Liu, W.; Li, Z.; Nelias, D.; Fan, J.; Tian, H.; Liu, W. Research status of creep–fatigue characteristics of salt rocks and stability of compressed air storage in salt caverns. Earth Energy Sci. 2025, 1, 98–116. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Yang, C.; Daemen, J.J.K. Stability and tightness evaluation of bedded rock salt formations for underground gas/oil storage. Acta Geotech. 2014, 9, 161–179. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Wang, T.; Zhang, H.; Wang, Z.; Zhao, C.; Chen, X. Pillar stability of salt caverns used for gas storage considering sedimentary rhythm of the interlayers. J. Energy Storage 2021, 43, 103229. [Google Scholar] [CrossRef]
- Cyran, K. Insight into a shape of salt storage caverns. Arch. Min. Sci. 2020, 65, 363–398. [Google Scholar]
- Wang, T.; Yang, C.; Chen, J.; Daemen, J.J.K. Geomechanical investigation of roof failure of China’s first gas storage salt cavern. Eng. Geol. 2018, 243, 59–69. [Google Scholar] [CrossRef]
- Wang, T.; Yan, X.; Yang, H.; Yang, X.; Jiang, T.; Zhao, S. A new shape design method of salt cavern used as underground gas storage. Appl. Energ. 2013, 104, 50–61. [Google Scholar] [CrossRef]
- Liu, W.; Duan, X.; Li, Q.; Wan, J.; Zhang, X.; Fang, J.; Jiang, D.; Chen, J. Analysis of pressure interval/injection and production frequency on stability of large-scale supercritical CO2 storage in salt caverns. J. Clean. Prod. 2023, 433, 139731. [Google Scholar] [CrossRef]
- Pajonpai, N.; Bissen, R.; Pumjan, S.; Henk, A. Shape design and safety evaluation of salt caverns for CO2 storage in northeast Thailand. Int. J. Greenh. Gas Control 2022, 120, 103773. [Google Scholar] [CrossRef]
- Fan, J.; Chen, J.; Jiang, D.; Ren, S.; Wu, J. Fatigue properties of rock salt subjected to interval cyclic pressure. Int. J. Fatigue 2016, 90, 109–115. [Google Scholar] [CrossRef]
- Chen, W.Z.; Tan, X.J.; Wu, G.J.; Yang, J.P. Research in gas seepage law in laminated salt rock gas storage. Chin. J. Rock Mech. Eng. 2009, 28, 1297–1304, (In Chinese with English abstract). [Google Scholar]
- Xiong, J.; Huang, X.; Ma, H. Gas leakage mechanism in bedded salt rock storage cavern considering damaged interface. Petroleum 2015, 1, 366–372. [Google Scholar] [CrossRef]
- Soubeyran, A.; Rouabhi, A.; Coquelet, C. Thermodynamic analysis of carbon dioxide storage in salt caverns to improve the Power-to-Gas process. Appl. Energy 2019, 242, 1090–1107. [Google Scholar] [CrossRef]
- Liu, Y.F. Pillar Stability of Salt Caverns Used for Gas Storage Considering Sedimentary Rhythm of Interlayers. Master’s Thesis, China University of Mining and Technology, Xuzhou, China, 2022. (In Chinese with English abstract). [Google Scholar]
- Guria, C. Pressure- and temperature-dependent klinkenberg slippage effect in porous media to non-ideal gases. Geoenergy Sci. Eng. 2023, 224, 211629. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, S.Y. Oil and gas accumulation characteristics of nearshore subaqueous fan in Hongze Sag. Lithol. Reserv. 2011, 23, 56–59, (In Chinese with English abstract). [Google Scholar]
- Zhao, Y.P.; Zhang, P.; Zhu, B.; Wu, J.F.; Che, Z.G.; Feng, L.W.; Xu, Y.s. Water inrush cause and resource exploitation in a salt exploration well in the Zhaoji Sub-sag, Hongze Sag. J. Geol. 2024, 48, 100–106, (In Chinese with English abstract). [Google Scholar]
- Wang, W.F.; Zhang, M. Study on deep water resedimented origin of saline sediments of E1f4 in Zhaoji Sag, Hongze Depression, Northern Jiangsu basin. Acta Sedimentol. Sin. 2015, 33, 242–253, (In Chinese with English abstract). [Google Scholar]
- Qi, D.S.; Li, J.J.; Ba, J.H.; Wang, Y.G.; Ao, H.B.; Li, S.P.; Wang, W.Q. Design of solution mining technology for thick-interlayer salt beds in Huai’an salt cavern gas storage. Oil Gas Storage Transp. 2020, 39, 947–952, (In Chinese with English abstract). [Google Scholar]
- Zhang, M. Study on the Origin and Distribution of Saline Sediments of E1f4 in Zhaoji Sag, Hongze Depression, Northern Jiangsu Basin. Master’s Thesis, China University of Petroleum (East China), Qingdao, China, 2014. (In Chinese with English abstract). [Google Scholar]
- Zhao, Y.J.; Ma, J.W.; Zheng, Y.L.; Wang, Z.Y.; Zhang, H.B. Determination of operating pressure limit for injection-production cycle of salt-cavern gas storage in Huai’an City. Oil Gas Storage Transp. 2013, 32, 526–531, (In Chinese with English abstract). [Google Scholar]
- Wei, D.H. Engineering Technology of Cavity Making in Jintan Salt Caverns Underground Gas Storage. Master’s Thesis, China University of Petroleum (East China), Qingdao, China, 2008. (In Chinese with English abstract). [Google Scholar]
- Li, W.; Liu, J.; Zeng, J.; Leong, Y.; Elsworth, D.; Tian, J. A fully coupled multidomain and multiphysics model considering stimulation patterns and thermal effects for evaluation of coalbed methane (CBM) extraction. J. Petrol. Sci. Eng. 2022, 214, 110506. [Google Scholar] [CrossRef]
- Fang, H.; Sang, S.; Liu, S. Establishment of dynamic permeability model of coal reservoir and its numerical simulation during the CO2-ECBM process. J. Petrol. Sci. Eng. 2019, 179, 885–898. [Google Scholar] [CrossRef]
- Fan, Y.; Deng, C.; Zhang, X.; Li, F.; Wang, X.; Qiao, L. Numerical study of CO2-enhanced coalbed methane recovery. Int. J. Greenh. Gas Control 2018, 76, 12–23. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, L.; Zhang, T.; He, W.; Hu, W.; Yin, H.; Ma, L.; Hong, X.; Zhang, W.; Zhang, B. Numerical simulation of CO2-ECBM for deep coal reservoir with strong stress sensitivity. Heliyon 2024, 10, e34818. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, K.; Jiang, Y.; Wang, S. Reservoir permeability evolution during the process of CO2 -enhanced coalbed methane recovery. Energies 2018, 11, 2996. [Google Scholar] [CrossRef]
- Peng, D.Y.; Robinson, D.B. New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 3069–3078. [Google Scholar] [CrossRef]
- Tan, X.J.; Chen, W.J.; Yang, J.P.; Yang, C.H. Study of THM-damage coupling model of gas storage in salt rock with interlayer. Rock Soil. Mech. 2009, 30, 3633–3641, (In Chinese with English abstract). [Google Scholar]
- Kong, X.; Wang, E.; Liu, Q.; Li, Z.; Li, D.; Cao, Z.; Niu, Y. Dynamic permeability and porosity evolution of coal seam rich in CBM based on the flow-solid coupling theory. J. Nat. Gas Sci. Eng. 2017, 40, 61–71. [Google Scholar] [CrossRef]
- Liu, S.Q.; Fang, H.H.; Sang, S.X.; Wu, J.G.; Zhang, S.R.; Zhang, B. Numerical simulation study on coal seam CO2-ECBM based on multi-physics fields coupling solution. Coal Sci. Technol. 2019, 47, 51–59, (In Chinese with English abstract). [Google Scholar]
- Liu, Z.; Hu, Z.; Zhu, W.; Zhao, T.; Liu, S.; Guo, Z.; Sun, C.; Bai, G. Effect of coal permeability evolution on CO2 storage capacity under phase partial pressure in ScCO2-ECBM processes. Energy 2024, 297, 131298. [Google Scholar] [CrossRef]
- Klinkenberg, L. The Permeability of Porous Media To Liquids And Gases. Drill. Prod. Pract. 1941, 108, 200–213. [Google Scholar] [CrossRef]
- Yang, S.Y. Experimental Study on Isobaric Specific Heat Capacity and Density of Carbon Dioxide Under Supercritical and Subcritical Pressure. Master’s Thesis, Dalian University of Technology, Dalian, China, 2020. [Google Scholar]


| Parameter (Unit) | Value |
|---|---|
| Overburden pressure (MPa) | 15 |
| Initial reservoir temperature (K) | 338.15 |
| Initial reservoir pressure (MPa) | 12 |
| Initial porosity of salt rock (%) | 1 |
| Initial porosity of mudstone (%) | 6 |
| Initial permeability of salt rock (10−6 μm2) | 1 × 10−3 |
| Initial permeability of mudstone (10−6 μm2) | 1 |
| CO2 diffusion coefficient of mudstone (10−12 m2/s) | 0.5 |
| CO2 diffusion coefficient of salt rock (10−12 m2/s) | 0.2 |
| Elastic modulus of salt rock (GPa) | 5.16 |
| Elastic modulus of mudstone (GPa) | 10 |
| Heat conductivity of salt rock (W/(m K)) | 2.5 |
| Heat conductivity of mudstone (W/(m K)) | 2.7 |
| Specific heat capacity of salt rock (J/(kg K)) | 837 |
| Specific heat capacity of mudstone (J/(kg K)) | 850 |
| Permeability of the interfaces between salt rock and mudstone (10−6 μm2) | 1 × 10−2 |
| CO2 heat conductivity (W/(m K)) | 0.1 |
| CO2 specific heat capacity (J/(kg K)) | 10,000 |
| Temperature of injection gas (K) | 318.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, B.; Fu, X.; Kang, J.; Tang, P.; Xu, H.; Zhang, Y. Numerical Simulation of Flow Characteristics in CO2 Long-Term Storage in Bedded Salt Cavern. Processes 2025, 13, 1563. https://doi.org/10.3390/pr13051563
Cao B, Fu X, Kang J, Tang P, Xu H, Zhang Y. Numerical Simulation of Flow Characteristics in CO2 Long-Term Storage in Bedded Salt Cavern. Processes. 2025; 13(5):1563. https://doi.org/10.3390/pr13051563
Chicago/Turabian StyleCao, Bo, Xuehai Fu, Junqiang Kang, Pan Tang, Hui Xu, and Yuanyuan Zhang. 2025. "Numerical Simulation of Flow Characteristics in CO2 Long-Term Storage in Bedded Salt Cavern" Processes 13, no. 5: 1563. https://doi.org/10.3390/pr13051563
APA StyleCao, B., Fu, X., Kang, J., Tang, P., Xu, H., & Zhang, Y. (2025). Numerical Simulation of Flow Characteristics in CO2 Long-Term Storage in Bedded Salt Cavern. Processes, 13(5), 1563. https://doi.org/10.3390/pr13051563







