Abstract
Heavy metal pollution severely affects soil and rice quality in China. In a one-year field experiment conducted in Hg-Cd co-contaminated farmland in Tongren, Guizhou, we examined the effects of low-accumulation rice cultivars, soil amendments (Fupei (D1), Wansan (D2), Shengwujun (D3), and Shigou (D4)) and foliar barrier agents on Hg and Cd transport and uptake. The rice cultivar Longliangyouhuanglizhan (P1) showed lower Hg and Cd accumulation. When combined with amendments, P1 further reduced health risks. All amendments effectively reduced non-carcinogenic health risks, with Fupei reducing Hg and Cd accumulation in rice by 65.16% and 97.54%, respectively, achieving a 91.74% reduction in health risks. Foliar barrier agents further decreased heavy metal content in rice. Additionally, D1 was the most cost-effective option. Soil assessments showed amendments reduced available Hg content by 66.67–70.51%, while Cd content increased by 3.51–16.67%. Mechanistic analysis indicated that D1 and D2 mainly immobilized heavy metals through adsorption and precipitation, while D3 facilitated removal via microbial reduction, and D4 relied on adsorption. Overall, D1 was most effective in mitigating heavy metal risks and improving soil quality, providing a comprehensive strategy for managing contamination in rice production with important implications for food safety and sustainable agriculture.
Keywords:
mercury; cadmium; soil amendments; health risk assessment; plant uptake; rice quality; translocation 1. Introduction
Rice (Oryza sativa L.) is one of the most important staples in the world. In a major food-producing country like China, about 65% of the population consumes rice as their staple food []. When discharged into the environment, trace metals like mercury (Hg) and cadmium (Cd) are extremely poisonous, persistent, and non-biodegradable, and they may be detrimental to ecosystems and living things [,]. Once Hg and Cd accumulate in food crops, they can cause changes in DNA strands, genetic material, and dysfunctions in the normal physiological functions of food crops []. Heavy metals entering the human body through migration through the food chain can be hazardous to human health. It is well known that excessive consumption of Hg and Cd can seriously damage the human kidney, immunological system, and brain system []. Therefore, finding appropriate techniques to remediate farmland contaminated with Hg and Cd and to lessen the accumulation of soil heavy metals by rice is therefore essential.
Heavy metal pollution affects rice primarily in terms of growth, quality, and safety. During the growth process, heavy metals such as Cd and Hg enter plants through the soil and are transferred at varying rates through the rice roots, ultimately impacting the growth and development of rice []. The accumulation of heavy metals inhibits photosynthesis, respiration, and nutrient absorption, leading to symptoms such as stunted growth, yellowing, browning of roots, and even death [], thereby affecting rice yield and quality. In addition, heavy metal contamination directly influences the edible safety of rice. As heavy metals continue to accumulate, rice becomes a potential source of contamination in the food chain. Long-term consumption of rice containing heavy metals can introduce these toxic substances into the human body, posing serious health risks. When Cd and Hg accumulate in the human body, they can induce various epigenetic changes in mammalian cells, leading to pathogenic risks and the development of various types of cancer [,]. The accumulation of heavy metals not only affects organ function but may also cause chronic poisoning, genetic mutations, and other health issues.
Screening of rice cultivars with low accumulation of heavy metals, agronomic measures, and remediation techniques are effective ways to decrease heavy metal accumulation in rice plants, improve rice quality, and prevent heavy metals from entering the food chain []. Xiao [] found that the root system of rice variety HH61 had a lower Cd uptake capacity than that of other varieties and that the variety–water condition interaction (CWI) had a lower root radial oxygen loss (ROL) and a lower root–grain Cd translocation rate. Chen [] found that, in high-Cd areas, hybridized rice varieties are more likely to accumulate Cd levels in their bodies than conventional varieties, and Cd content in rice was significantly negatively correlated with yield. Ding [] showed that arsenic uptake and root-to-stem translocation rates were much higher in high-accumulating varieties than in low-accumulating varieties.
The primary approaches for remediating heavy metal-contaminated soils are as follows: (1) removal or reduction of the total amount of harmful forms of heavy metals in the soil; (2) regulation of the mobility and bioavailability of heavy metals in the soil. Bioavailability refers to the ability of pollutants to be absorbed by organisms, while immobilization focuses on reducing the mobility of heavy metals in the soil, thus limiting plant uptake and preventing groundwater contamination [,]. Based on the different remediation mechanisms, these technologies can be classified into physical, chemical, and biological remediation. Among them, in situ chemical stabilization/immobilization is considered a promising remediation technology. It has many advantages, such as reduced soil disturbance, reduced worker exposure to contaminants, low cost, and rapid implementation [,]. In recent years, several studies have systematically evaluated the remediation effects of commonly used soil conditioners through field experiments and mechanism analyses. The effects of biochar at different pyrolysis temperatures on soil remediation were assessed. Biochar produced at 300 °C significantly reduced the mobility of Pb and Cu (>93%) in alkaline soils, while biochar produced at 700 °C completely immobilized Pb and Zn (100%) in acidic soils. These findings indicate significant remediation effects, although practical application measures need optimization []. The effect of lime application on improving soil pH and reducing Cd accumulation in crops was also evaluated. The results showed that lime significantly increased soil pH and reduced Cd accumulation in crops, with CaCO3 proving to be the most effective. However, soil background values and crop types influenced the efficiency of the remediation []. The stability of Ca-Mg-Si soil conditioners (SC) in long-term immobilization of Cd in soil was also assessed. The results indicated that continuous high-rate SC application increased soil pH and reduced Cd activity, although the effectiveness was influenced by soil pH, application rate, and climatic factors []. The remediation effect of granite and marble waste (GMWA) as soil amendments was evaluated. The study found that the application of 3% GMWA significantly reduced the mobility of Cu, Pb, Zn, and Cd in soil, improved soil nutrients and pH, and provided a novel strategy for heavy metal contamination remediation []. Soil conditioners can not only reduce the effective content of heavy metals in soil but also improve soil quality and soil environment. Therefore, to enhance soil quality and lessen the accumulation of heavy metals in crops, the best management measure is to combine low-accumulating crop varieties with soil conditioners.
Therefore, this study aims to explore effective strategies for remediating Hg and Cd co-contaminated farmland through field experiments involving four soil conditioners and foliar barrier agents. The investigation focuses on changes in soil properties, metal bioavailability, and the accumulation of heavy metals in seven major rice cultivars, in order to identify suitable soil conditioner–rice cultivar combinations for safe crop production. Additionally, to assess potential environmental side effects, concentrations of arsenic (As), lead (Pb), and chromium (Cr) were also monitored. The hypothesis of this study is that specific soil conditioners can significantly reduce the bioavailability of heavy metals in the soil, and when combined with low-accumulating rice varieties, they can enable the safe utilization of contaminated farmland. This research provides theoretical support and practical solutions for the remediation of heavy metal-contaminated farmland, with significant implications for environmental safety and sustainable agricultural development.
2. Materials and Methods
2.1. Experimental Site and Characterization
The test field was located in a typical karst region, which belongs to the subtropical monsoon humid climate zone, with high temperature and precipitation in summer and low temperature and precipitation in winter []. The field experiment was located in farmland (27°32′18″–27°52′40″ N, 108°56′13″–109°28′20″ E) in Tongren City, Guizhou, China, in a typical karst area, belonging to the Hg and Cd composite contaminated soil, with sampling depths of 0–20 cm. The physicochemical properties, including pH, organic matter (OM), available phosphorus (AP), available potassium (AK), total Hg, and total Cd concentrations, are summarized in Table 1. The geographic locations of the soils are shown in Figure 1. The levels of Hg and Cd in the test soil were higher than the standard limit values in GB15618-2018 (1.0 mg·kg−1 for Hg; 0.8 mg·kg−1 for Cd) []. Based on the assessment of soil environmental quality, including indicators such as pH, heavy metal concentrations (specifically Hg and Cd), and OM content, both Hg and Cd concentrations exceeded the standard limits, indicating that the soil is contaminated with a combined Hg-Cd pollution. The comprehensive evaluation classified the tested soil as a strictly controlled category in terms of environmental quality.

Table 1.
Physicochemical properties of tested farmland soils.
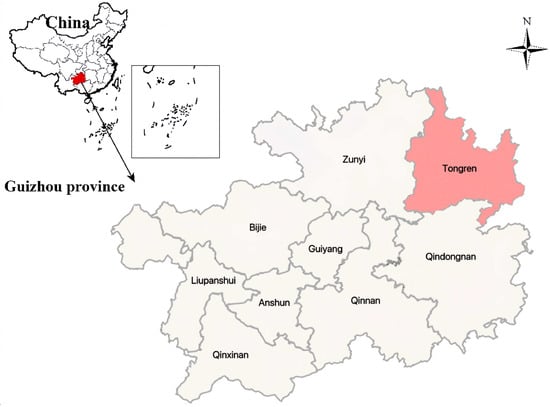
Figure 1.
The specific location of the experimental field.
Soil conditioners used in the field trials included Fupei conditioner (D1), Wansan conditioner (D2), Shengwujun conditioner (D3), and Shigou conditioner (D4). Based on the soil conditioner, foliar barrier agents were additionally applied in each corresponding treatment (Y1, Y2, Y3, and Y4). The treatments, including the types of soil conditioner ingredients, application rates, costs, and additional notes, are detailed in Table 2. The selection of soil conditioner components and their corresponding application rates was based on widely used commercial products, with reference to the specifications and recommended usage provided in their respective product information sheets.

Table 2.
Conditioner ingredients and dosage.
2.2. Plot Experiment
Based on the preliminary investigation, a one-year field experiment was conducted in a farmland in Tongren City in May 2023. The field experiment employed six rice varieties with varying capacities for Hg and Cd accumulation, selected from the Tongren area: Longliangyouhuanglizhan (P1), Jingliangyouhuazhan (P2), Jingliangyou 7818 (P3), Longliangyou 1988 (P4), Yixiangyou 62 (P5), and Yixiangyou 800 (P6). Detailed information regarding their variety types, assigned numbers, and whole growth periods is presented in Table 3. The rice varieties used in this study were provided by an agricultural company in Tongren City, Guizhou Province, and the relevant data for the rice varieties were obtained from the China Rice Data Center. According to the results of the preliminary investigation, P1 and P2 belonged to the positive list of rice varieties with low Hg accumulation (Table 4). Therefore, the rice variety P1 was selected as the field trial variety.

Table 3.
Basic Information of Different Rice Varieties.

Table 4.
Positive list of rice cultivars with low Hg accumulation.
In this experiment, a total of multiple plots was established (Table 5). Specifically, five treatments of soil amendments were set up, namely CK, D1 (150 kg/mu), D2 (150 kg/mu), D3 (150 kg/mu), and D4 (150 kg/mu), with three replicates each, resulting in a total of 15 plots. Similarly, five treatments of foliar regulators were implemented, including CK, Y1, Y2, Y3, and Y4, also with three replicates each, totaling 15 plots. For low heavy metal-accumulation rice varieties, seven treatments (CK, P1, P2, P3, P4, P5, and P6) were arranged, with three replicates per treatment, resulting in a total of 21 plots. Additionally, for the screening of amendment concentrations, five treatments (CK, T1, T2, T3, and T4) were set up, with three replicates each, summing up to 15 plots.

Table 5.
The experimental design.
Prior to the experiment, the farmland was divided into small plots and irrigated to maintain soil moisture and prevent desiccation. The soil amendments were applied and thoroughly mixed into the soil 5–7 days before transplanting (Figure 2). The experimental area was managed under a standardized protocol, including uniform seedling cultivation, transplanting, fertilization, pesticide application, harvesting, yield measurement, and analytical testing methods. The growth status of rice seedlings in the field was monitored regularly, and water was supplemented as needed. During the seedling, panicle initiation, and full heading stages, special attention was given to the prevention of rice blast, while during the tillering and booting stages, measures were taken to control rice planthoppers and stem borers. Upon maturation, the rice was harvested for subsequent determination of relevant indicators.

Figure 2.
Field planting experiment photograph.
2.3. Sample Collection and Preservation
The sample collection was conducted during the rice maturity stage in the plot experiment farmland. Following standard sampling protocols, rice plant samples and corresponding rhizosphere soil samples (sampling depth: 0–20 cm) were collected. Soil sampling points were selected using the five-point sampling method, and five subsamples were combined to form a representative sample. All biological samples were sealed and stored using a double-layer interlocking polyethylene bag system to ensure isolation. Soil samples were transported under a continuous cold chain (maintained at 4 °C with ice packs) and transferred to the laboratory on the same day of sampling.
In the laboratory, wet soil samples were first passed through 50-mesh and 200-mesh sieves to remove large particles, such as stones and plant residues, followed by homogenization using a blender. Before use, the blender was sequentially cleaned with tap water, acid-washed, thoroughly rinsed with ultrapure water, and dried with an air blower to prevent cross-contamination. Rice samples were washed three times with ultrapure water and dried at 40 °C until a constant weight was achieved. After dehusking, rice grains were separated using a hulling machine, while rice bran was removed using a precision milling machine. Throughout the entire process, all necessary precautions were taken to prevent cross-contamination. Before processing the next batch of samples, the milling equipment was rinsed with ultrapure water [].
2.4. Analysis of Rice and Soil
2.4.1. Heavy Metal Content of Rice
Husked brown rice was digested using a mixture of HNO3 and HClO4 (v/v 5:2). Hg was determined using an atomic fluorescence spectrometer (AFS-933, Jitian Instruments, Beijing, China); arsenic (As) was measured using an atomic fluorescence spectrometer (AFS-8220, Jitian Instruments, Beijing, China); Cd, Pb, and Cr were analyzed using an inductively coupled plasma-mass spectry (ICAP RQ, Thermo Fisher, Waltham, MA, USA). The detection limits for Hg, Cd, As, Pb, and Cr were 0.003 mg·kg−1, 0.002 mg·kg−1, 0.002 mg·kg−1, 0.02 mg·kg−1, and 0.05 mg·kg−1, respectively.
2.4.2. Soil Physicochemical Characteristics
Soil pH was measured with water as a leaching agent at a water–soil ratio of 2.5:1 and determined by a pH []. Based on the study by Zhang [], the content of soil OM, AP, and AK was determined. The available Hg content in soil was determined by thioglycolic acid-dibasic Sodium Phosphate extraction-atomic Fluorescence Spectrometry []. According to Lyu [], available Cd in soil was extracted with diethylenetriaminepentaacetic acid-CaCl2-triethanolamine (DTPA-CaCl2-TEA) buffer solution and determined by inductively coupled plasma-mass spectrometry (Avio 200, PerkinElmer, Waltham, MA, USA).
2.5. Assessment of Health Risk Reduction
2.5.1. Bio-Concentration Factor (BCF)
The BCF of heavy metals in rice is known as the ratio of rice heavy metal content to soil heavy metal content, and the larger value indicates that rice is more capable of enriching heavy metals and vice versa. The formula is as follows:
where: Crice is the heavy metal content of rice (mg·kg−1); Csoil is the heavy metal content of soil (mg·kg−1).
2.5.2. Health Risk Reduction
The health risk assessment was conducted according to the procedures of Beinat and van Drunen []. Since the type of soil utilization was agricultural, this study only looked at the health concerns associated with consuming agricultural products orally. According to Equations (2) and (3), only the non-carcinogenic risk of oral rice was calculated as a risk index:
where: EDI is the average daily intake of pollutants via crop intake (mg·kg−1·d−1); Crice is the Hg/Cd content in rice (mg·kg−1); IR is the daily human consumption of crops (kg·d−1), which was determined to be 0.389 kg·d−1 for adult rice intake in Guizhou []: EF is the exposure frequency (d·a−1); ED is the exposure time (a); BW is body weight of the receptor (kg); and LE is life expectancy (a). The parameters used in the health risk assessment are summarized in Table 6.

Table 6.
Health risk parameters for heavy metals in soil.
The health risk index (RI) for a single heavy metal is calculated as:
where RI is the health risk index; RfD is the heavy metal exposure reference dose [mg·(kg·d)−1]; RI > 1 indicates that the pollutant can cause health risk to human beings, and the larger the health risk index, the greater the health risk of the pollutant to human beings; RI < 1 indicates that the pollutant does not cause health risk to human beings.
Calculate the risk reduction according to Equation (4) []:
where RI0 is the RI without conditioner treatment and RIAi is the RI after conditioner treatment.
2.6. Data Analysis
Analysis of variance and significance was performed using IBM SPSS Statistics 26. The values were plotted using OriginPro 2023b (OriginLab Corporation, Northampton, MA, USA), and the significance of the differences between the mean values was assessed using least significant difference (LSD) tests at the 5% level of probability.
3. Results
3.1. Effects of Different Varieties on the Accumulation of Heavy Metal in Rice
As shown in Figure 3a, replacing rice varieties significantly reduced the accumulation of Hg and Cd in rice. The Hg content in CK-treated rice was 0.0287 mg·kg−1, and the Cd content was 0.5167 mg·kg−1, both exceeding the Chinese food safety standard limits (Hg ≤ 0.02 mg·kg−1; Cd ≤ 0.2 mg·kg−1). Compared to CK, changing rice varieties significantly reduced (p < 0.05) Hg accumulation, with reductions ranging from 78.05% to 94.77%. The ranking of Hg accumulation among the rice varieties followed the order: P1 < P6 < P5 < P4 < P3 < P2 < CK, with no significant differences observed among the improved varieties (p > 0.05). Similarly, Cd content in rice was reduced by 97.23% to 99.32%, indicating a significant decrease (p < 0.05).
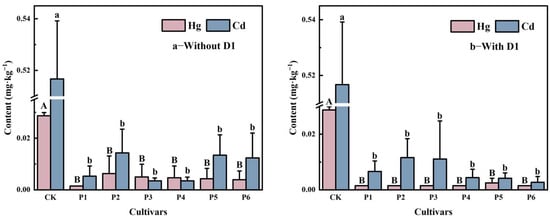
Figure 3.
The Hg and Cd accumulation capacity of different rice varieties, where (a) without application of the D1 soil conditioner; (b) with application of the D1 soil conditioner (D1, 150 kg/mu). Note: Different lowercase and uppercase letters indicate significant differences in Hg and Cd accumulation among different rice varieties (p < 0.05).
As presented in Table 7, the application of the D1 soil conditioner significantly reduced the accumulation of As in rice grains across different rice varieties. Moreover, varietal differences also contributed to mitigating As uptake, keeping its concentration within the safe limit for consumption. However, replacing rice varieties alone did not significantly reduce the accumulation of Cr or Pb (p > 0.05). Nevertheless, after switching to improved rice cultivars, the concentrations of five heavy metals remained well below the safety thresholds for Chinese food standards (Hg ≤ 0.02 mg·kg−1; Cd ≤ 0.2 mg·kg−1; As ≤ 0.35 mg·kg−1; Pb ≤ 0.2 mg·kg−1; Cr ≤ 1.0 mg·kg−1) [].

Table 7.
Effects of different soil environments on reducing As, Cr, and Pb uptake by rice varieties.
As illustrated in Figure 3b, the combined application of D1 soil conditioner and rice variety replacement further reduced Hg and Cd levels. The Hg content decreased by 91.29% to 94.77% (p < 0.05), while Cd content was reduced by 97.75% to 99.19% (p < 0.05). However, no significant changes (p > 0.05) were observed for As, Cr, or Pb accumulation. The reduction effect was most pronounced for Hg, where all rice varieties, except P5, exhibited Hg concentrations below the detection limit (0.003 mg·kg−1).
Overall, the levels of all five heavy metals in the six rice varieties did not exceed the standard limits (Hg: 0.02 mg·kg−1, As: 0.35 mg·kg−1, Cd: 0.2 mg·kg−1, Cr: 1.0 mg·kg−1, Pb: 0.2 mg·kg−1). Notably, for Hg and Cd, which exceeded the safety limits in CK-treated rice, their concentrations were reduced to acceptable levels after replacing rice varieties. Furthermore, with the additional application of D1 soil conditioner, Hg concentrations in all rice varieties—except P5—fell below the detection limit. These findings indicate that the combined approach of varietal replacement and soil conditioner application is highly effective in mitigating Hg and Cd bioaccumulation in rice, thereby improving food safety in heavy metal-contaminated agricultural areas.
3.2. Effect of Application of Soil Conditioners on Heavy Metal Level and Soil Quality in Rice
3.2.1. Impact of Conditioners on the Field Experiment’s Heavy Metal Level of Longliangyouhuanglizhan
As illustrated in Figure 4, the application of soil conditioners significantly reduced the accumulation of heavy metals (Hg, As, Cd, and Cr) in rice to varying degrees. Among these, the concentrations of Hg and Cd exhibited the most pronounced reductions (Figure 4a,b). Compared to the CK group, Hg content in rice decreased by 64.46–73.52%, while Cd content was reduced by 97.54–98.95%, both showing significant differences (p < 0.05). Regarding Hg accumulation, among the different soil conditioners, D4 demonstrated the most significant reduction (p < 0.05) compared to D1, D2, and D3, with a 73.52% decrease. In contrast, for Cd accumulation, no significant differences (p > 0.05) were observed among the different soil conditioner treatments. The concentrations of As, Pb, and Cr in rice exhibited varying degrees of fluctuation (Figure 4c–e). Although a slight increase in lead concentration was observed, all three elements remained within safe utilization thresholds (As ≤ 0.35 mg·kg−1; Pb ≤ 0.2 mg·kg−1; Cr ≤ 1.0 mg·kg−1), suggesting that the application of soil conditioners did not result in excessive lead accumulation in rice.
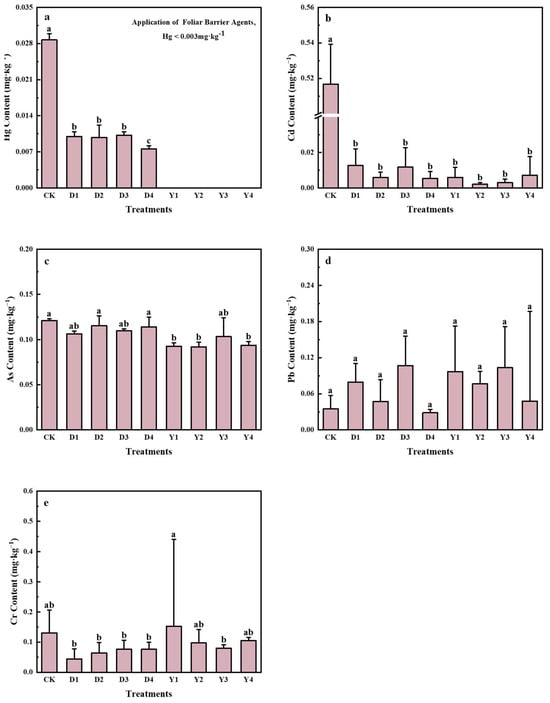
Figure 4.
Effects of different soil amendments on the concentrations of heavy metals Hg (a), Cd (b), As (c), Pb (d), and Cr (e) in rice. Note: CK represents the untreated control, D1–D4 correspond to amendments D1, D2, D3, and D4, respectively, while Y1–Y4 denote their combinations with a foliar regulator. Different lowercase letters indicate significant differences in Hg and Cd content in rice at p < 0.05.
Furthermore, when foliar barrier agents were combined with four soil conditioners, Hg levels in rice dropped below the detection limit (0.003 mg·kg−1), indicating that foliar treatments further suppressed Hg accumulation. Meanwhile, Cd content was further reduced by 98.63–99.59% (p < 0.05). Notably, in the combined treatment group, except for Y4, Cd levels in rice were lower than those in the single soil conditioner treatments, implying that the foliar barrier agents enhanced the Cd-reducing effect of soil conditioners.
3.2.2. Impact of Conditioners on Available Hg and Cd Content
As shown in Figure 5, the available Hg content in each treatment was as follows: D1 (0.023 mg·kg−1) ≈ D2 (0.023 mg·kg−1) < D3 (0.026 mg·kg−1) ≈ D4 (0.026 mg·kg−1) < CK (control, 0.078 mg·kg−1). The effective Hg content in all treatments was lower than the safety threshold of 0.04 mg·kg−1 specified in the heavy metal pollution grading index for agricultural soils. These findings indicate that the four soil conditioners (D1, D2, D3, and D4) were effective in reducing the available Hg content in the soil. This suggests that these conditioners can adsorb heavy metals, particularly Hg, thereby reducing its bioavailability and limiting the transfer of Hg from soil to the food chain.
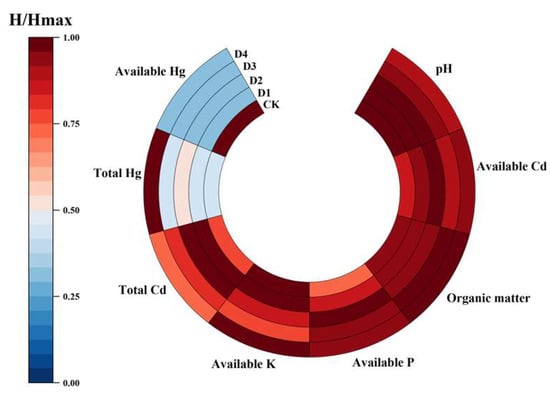
Figure 5.
Soil characteristics under different soil amendment treatments. Note: H represents the input value, and Hmax represents the maximum input value.
For effective Cd content, as shown in Figure 5, the soil Cd content ranged from 1.18 to 1.33 mg·kg−1 following the application of the conditioners, with no significant difference between the treatments and the control group (CK: 1.14 mg·kg−1). The increase in Cd was slight, ranging from 3.51% to 16.67%, with D4 showing the smallest increase (3.51%) and D2 showing the largest (16.67%). This slight increase may be attributed to various factors, including soil physicochemical properties (e.g., pH, OM, and soil texture), as well as plant–root interactions.
3.2.3. Impact of Conditioners on Soil Physicochemical Characteristics
Based on Figure 5, the impact of soil conditioner treatments on soil pH was as follows: D2 (8.03) > D1 (7.90) > CK (7.74) > D4 (7.40) > D3 (7.10). Compared with the control (CK, pH = 7.74), treatments D3 (pH = 7.10) and D4 (pH = 7.40) led to a reduction in soil pH by 0.64 and 0.34 units, respectively, indicating a mild acidifying effect. In contrast, D1 and D2 treatments increased soil pH by 0.16 to 0.29 units, with D2 resulting in the highest pH value. These results suggest that D1 and D2 significantly increased soil alkalinity, while D3 and D4 caused mild acidification of the soil.
As shown in Figure 5 and Figure 8, treatments D3 and D4 increased both soil OM and AP contents. Specifically, soil OM increased by 7.99% and 4.42%, while AP increased by 28.07% and 28.95% under D3 and D4 treatments, respectively.
3.2.4. Effect of Conditioner Content on Heavy Metal Content in Rice
From Figure 6, it can be seen that different application rates of soil conditioners can reduce the heavy metal level of rice to varying degrees. The reductions of heavy metals Hg, As, Cd, and Cr contents in rice ranged from 60.63% to 62.72%, 11.49% to 22.56%, 98.41% to 99.42%, and 34.74% to 54.06%, respectively. The changes of Pb contents ranged from −54.44% to +60.46%, and the Pb contents of T1 and T2 treatments slightly increased, but none of them were above the standard limit (Pb ≤ 0.2 mg·kg−1). The variation of heavy metal content between different concentrations was not significant. The results of the study through different application rates of soil conditioners showed that the heavy metal contents of rice Hg, As, and Cd were significantly reduced in contrast to CK after the use of conditioners, and the effect of changes among different application rates was not significant. In a comprehensive analysis, different application rates of soil conditioners had positive effects on rice agronomic traits. Considering the changes in the soil environment after the application of conditioner, 100 kg/mu of conditioner was selected as the optimal application rate.
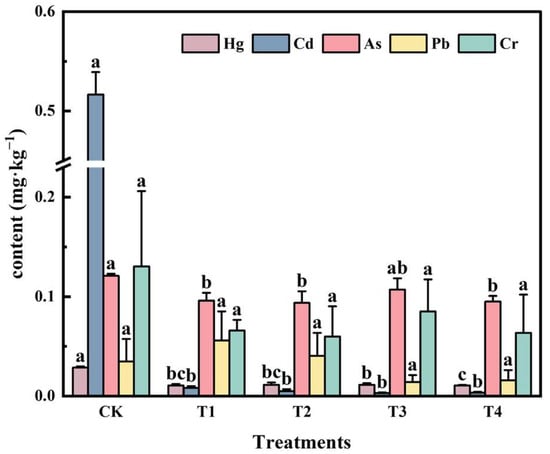
Figure 6.
Effect of different application rates of soil conditioners on heavy metal content in rice. Note: The control group (CK) received no soil conditioner treatment; T1, T2, T3, and T4 correspond to different application rates of the soil conditioners, with T1 representing 50 kg/mu, T2 representing 100 kg/mu, T3 representing 150 kg/mu, and T4 representing 300 kg/mu. Different lower case letters indicate significant differences (p < 0.05) in heavy metal accumulation between treatments.
The results of ANOVA showed that (1) the application of varying doses of conditioner treatments decreased the Hg and Cd content of rice substantially better than the CK group (p < 0.05), while neither Cr nor Pb content had a significant (p > 0.05) reducing effect compared to the CK group. (2) In terms of the As content of rice, T1, T2, and T4 treatments were substantially (p < 0.05) less than the CK group, except that the difference between the T3 treatment and other treatments was not significant (p > 0.05). (3) For Hg and Cd contents with high background values, the effect of applying different concentrations of conditioners was significant (p < 0.05) for both heavy metal contents compared to the CK group, but the changes in heavy metal contents of rice between different application rates were not significant (p > 0.05).
3.3. Effects of Conditioners on Enrichment Differences in Heavy Metal Uptake and Health Risks in Rice
3.3.1. Effect of Application of Different Soil Conditioners on Bio-Concentration Factor
In the control group (CK), the BCF of five heavy metals in rice were as follows: Hg (0.0023), Cd (0.1717), As (0.0063), Pb (0.0003), and Cr (0.0030). As shown in the Figure 7, the application of different treatments significantly reduced the BCF of five heavy metals (Hg, Cd, As, Pb, and Cr) in rice. After the application of different soil conditioners, the reductions in BCF for Hg, Cd, and Cr ranged from 17.48% to 62.05%, 97.54% to 99.06%, and 44.25% to 74.66%, respectively. The changes in As ranged from −32.80% to +7.45%. Following the addition of foliar passivators, the BCF for Hg and Cd were further reduced, while the changes in As, Pb, and Cr were minimal. For treatments involving both soil conditioners and foliar passivators, the BCF of Pb in rice ranged from 0.0006 to 0.0027, which were higher than those in the control group, but still well below 1.
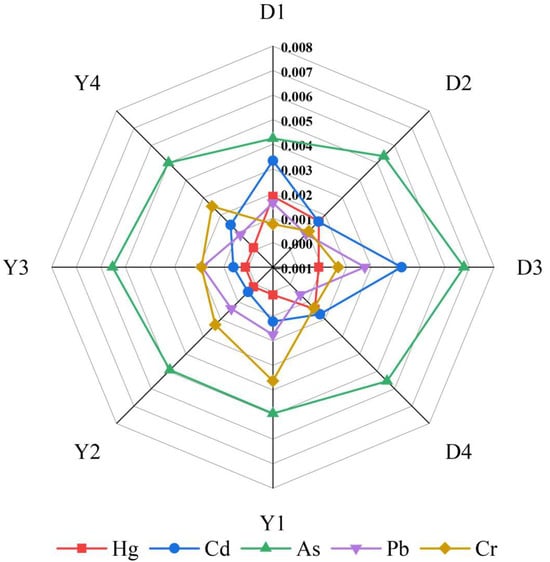
Figure 7.
Effects of different treatments on the bioconcentration factor (BCF).
3.3.2. Impact of Soil Conditioners on Reducing Health Risks
As shown in Table 8, after the application of soil conditioners and foliar barrier agents, the average daily intake of pollutants via crop consumption (EDI) for all five heavy metals was lower than the reference doses (RfD) established by the United States Environmental Protection Agency (EPA) (RfD(Hg) = 3.00 × 10−4 mg·(kg·d)−1; RfD(Cd) = 1.00 × 10−3 mg·(kg·d)−1; RfD(As) = 3.00 × 10−4 mg·(kg·d)−1; RfD(Pb) = 3.50 × 10−3 mg·(kg·d)−1; RfD(Cr) = 3.00 × 10−3 mg·(kg·d)−1), indicating that the heavy metals in the treated soils posed no significant health risk to humans. In addition, except for a slight increase in the non-carcinogenic risk index (RI) of Cr under the foliar passivator treatment, the RI values for the other four heavy metals were reduced compared to the control (CK), and all RI values remained below 1, suggesting that the treated contaminated soils did not pose a health risk to humans.

Table 8.
Effects of different treatments on the RI and EDI.
3.3.3. Economic Analysis of Conditioner Applications
Determine the unit price of various amendments while accounting for their actual use. The cost of treatment using D1 was calculated as 1350 yuan·t−1, D2 as 1380 yuan·t−1, D3 as 50,000 yuan·t−1, and D4 as 2200 yuan·t−1. The result showed that the cost of D1 treatment was below the other conditioners due to the low cost of the corresponding conditioner, thus demonstrating more advantages.
4. Discussion
Adjustment of rice varieties and the application of soil conditioners have shown promise in remediating heavy metal pollution in agricultural fields. While many studies have focused on single-metal contamination, research on co-contaminated farmland remains limited. This study, conducted in the typical karst region of Guizhou, comprehensively evaluates the effects of different soil conditioners in reducing heavy metal accumulation in rice, decreasing bioavailable fractions in soil, and minimizing remediation costs. Although As, Pb, and Cr were also measured to assess potential unintended effects, no significant changes were observed across all treatments, indicating that the applied soil conditioners and foliar barrier agents did not affect the uptake of these metals. Therefore, this paper focuses primarily on Hg and Cd, where distinct treatment-related differences were detected. The study further explores the mechanisms by which soil conditioners influence metal dynamics, providing insights into efficient and safe strategies for managing co-contaminated agricultural land.
4.1. Effect of Different Cultivars on the Accumulation of Hg and Cd in Rice
Through several defense mechanisms, including binding to the cell wall, active exocytosis, and transit through the plasma membrane, crops like rice can lessen the transfer of heavy metals within the body. The mechanism of Hg absorption by rice roots is the formation of iron-containing gel membranes on the roots, which effectively sequester total Hg, thus blocking the pathway of Hg transfer from the rice roots to the plant []. It was found that differences in the uptake of heavy metals in rice varieties of distinct genotypes were related to genes, environmental factors, or interactions [,]. Longliangyouhuanglizhan belongs to indica-type two-line hybrid rice, the Hg content in rice was below the detection limit (0.003 mg·kg−1), and the Cd level was 0.0053 mg·kg−1, which were both significantly lower than the CK. Early planting of rice varieties for Yuxiang 203, belong to the indica three-line hybrid rice. Some studies have shown that concentrations of Pb and Cd in the brown rice f are significantly higher than those in two-line hybrid rice [], and the conclusions of this paper are in line with the findings of earlier research. It showed that there were differences in the uptake of heavy metals by rice varieties of different genotypes, which may be due to different genes for the ability of rice to absorb and transport heavy metals, or soil microorganisms may have altered the biological activity of rice [].
4.2. Adsorption Mechanism of Soil Heavy Metals Hg-Cd
Soil amendments have a significant effect on improving soil physicochemical properties and reducing the bioaccumulation of heavy metals (such as Hg and Cd) in rice. The application of soil amendments significantly reduced the available Hg content in the soil (Figure 5), while also inhibiting the accumulation of heavy metals in rice (Figure 4). After the D1 treatment, the available Hg content in the soil decreased from 0.078 mg·kg−1 to 0.023 mg·kg−1, and the Hg bioaccumulation factor (BCF Rice Hg) in rice decreased from 0.0023 to 0.0018, indicating that D1 has a passivating effect on Hg in the soil. Similarly, after the application of D2, D3, and D4 treatments, the effective Hg content in the soil also decreased, and the BCF Rice Hg showed varying degrees of reduction. Notably, the D3 treatment reduced the BCF Rice Hg to 0.0009, demonstrating the best passivation effect on Hg.
The D1 amendment is rich in selenium (Se) at concentrations of 40–60 mg·kg−1. Under high pH conditions (10–12) and the presence of silicon and sulfur components, it may form insoluble compounds with heavy metals in the soil through coprecipitation or ligand exchange reactions, thereby reducing their solubility and mobility in the soil. Studies have found that Hg in the soil readily combines with sulfur to form inert HgS complexes []. Se and sulfur are adjacent elements in Group VIA, and their similar atomic structures (such as radius and outermost electrons) allow Se to easily replace some of the sulfur in HgS, forming less soluble HgSe precipitates or HgS–HgSe solid solution series precipitates []. This is because the bonding strength between Se and Hg (1045) is much higher than that between sulfur and Hg (1039), and the solubility product constant of HgSe (approximately 10−60) is significantly lower than that of HgS (approximately 10−52) [,]. HgSe is poorly soluble in water and is not easily utilized by plants and microorganisms; thus, the formation of HgSe can reduce the bioavailability of Hg in the soil and its translocation in plants.
Silicon (SiO2) components may also enhance the negative charge on the surface of soil colloids, increasing the electrostatic adsorption capacity of heavy metal ions, thereby further reducing their bioavailability. The results of Liang [] revealed that Cd in silica (Si)-amended soils is mainly converted to the Fe–Mn oxide-bound state. The form of Si in D1, D2, and D4 is SiO2, and the silicate will be attached to the surface of the iron oxide in the form of a polymer. During the complexation of Si and Fe, numerous negatively charged functional groups are formed, which provide a significant number of adsorption sites for Cd2+ []. Meanwhile, the increase of SiO2 in the soil will promote the precipitation process of Si–Cd hydroxyl compounds and reduce the availability of Cd [].
The D3 amendment contains microorganisms, which may alter the speciation of heavy metals in the soil through metabolic activities, thereby reducing their bioavailability. For instance, microorganisms may convert water-soluble heavy metal ions into insoluble compounds through reduction reactions, thus decreasing the bioavailability of heavy metals and their absorption by rice []. The lower Se content (10–30 mg·kg−1) in the D4 amendment may also reduce the absorption of Hg and Cd through similar mechanisms.
In summary, soil amendments effectively reduce the bioavailability of heavy metals in the soil and inhibit the absorption of Hg and Cd by rice through various mechanisms, such as coprecipitation of Se with heavy metals, adsorption of silicates, and microbial-mediated speciation transformations.
4.3. Effect of Conditioners on Soil Quality
Some studies have found [] that soil amendments can affect the physicochemical properties of soil. Soil quality includes pH, OM, available Hg, and Cd, as well as nutrition (OM, AP, AK, etc.). Soil amendments can improve the physicochemical properties of the soil; therefore, the mechanisms by which soil amendments fix heavy metals in the soil are a key focus of this study. The fixation mechanisms are discussed below in relation to soil pH, AP, and AK content (Figure 8).
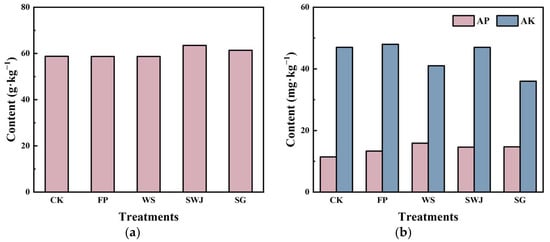
Figure 8.
Effect of conditioners on soil OM (a), AP, and AK content (b).
The change in soil pH is a key indicator that directly reflects the regulatory effect of amendments on soil acidity and alkalinity. After the application of D1 and D2 treatments, the soil pH values were 7.90 and 8.03, respectively, compared to 7.74 in the CK control group, indicating an increase in soil pH. This is primarily due to the high alkaline components, such as CaO and MgO, present in D1 and D2, which release OH− through hydrolysis, thereby increasing the alkalinity of the soil. The soil pH after the D3 and D4 treatments decreased to varying degrees, suggesting that the regulatory capacity of these two amendments is relatively weak. Heavy metals in high pH conditions are often precipitated as hydroxides, which reduces their mobility and bioavailability. This process is mainly governed by the precipitation mechanism.
The soil OM content showed little change after the application of D1 and D2, indicating that these two amendments did not have a significant effect on increasing soil OM. However, after the D3 and D4 treatments, the soil OM content increased to 63.5 g·kg−1 and 61.4 g·kg−1, respectively. The D3 amendment contains microorganisms, which can promote the degradation and transformation of soil OM, thereby increasing the soil OM content []. The negatively charged functional groups in OM (such as carboxyl and phenolic hydroxyl groups) can strongly adsorb heavy metal ions, reducing their bioavailability [,].
After the application of D1 and D2 treatments, the effective phosphorus content in the soil significantly increased. Following the D1 treatment, the effective phosphorus content in the soil was 15.9 mg·kg−1, which is approximately 39% higher than the 11.4 mg·kg−1 in the CK control group. Simultaneously, the soil AK content in the D1 treatment (48 mg·kg−1) was slightly higher than that in the CK treatment (47 mg·kg−1). After the D2 treatment, the effective phosphorus content in the soil was also 15.9 mg·kg−1, the same as that in the D1 treatment, indicating that both amendments significantly increased the effective phosphorus content in the soil. The D1 amendment contains 40–60 mg·kg−1 of Se, which can form complexes with phosphorus, reducing phosphorus loss and enhancing its availability in the soil. Silicate components (such as SiO2) may also play a role in regulating phosphorus availability. Silicates can enhance the adsorption capacity of phosphate ions (PO43−) by increasing the negative charge on the surface of soil particles, reducing phosphorus loss in the soil, and increasing its availability to plants []. Therefore, D1 and D2 amendments improve phosphorus availability through adsorption and complexation processes.
4.4. Effects of Conditioning Agents on Bio-Concentration Factor and Health Risks
The BCF of rice Cd was not significantly correlated with soil-effective Cd (p > 0.05), but the correlation coefficient was 0.633. Zhang [] found that soil pH and SOM had significantly negative relationships with the BCF, with coefficients of 0.281 (p < 0.05) and 0.212 (p < 0.05), respectively. Mu et al. [] have shown through their findings that soil pH, population density, and elevation had a significant impact on the migration and transformation of Cd, with 5.05%, 5.62%, and 5.50% to BCF. It can be seen that there are many factors affecting soil–rice Cd bioconcentration factors in field environments, which are influenced by other factors in addition to effective soil Cd.
The conditioner reduced the accumulation of Hg and Cd by immobilizing heavy metals in the soil, which significantly lowered the non-carcinogenic risk index (RI) (Table 8). The application of D1 led to a higher enrichment factor of P1 for Hg, which helped reduce the health risk. Additionally, the application of D4 increased soil OM, AP content, and soil biomass, leading to higher health risks in the D4 treatment.
5. Conclusions
A field experiment conducted in Tongren, Guizhou, investigated the safe utilization of Hg–Cd compound-contaminated soil. Among six rice varieties, Longliangyouhuanglizhan exhibited the lowest capacity for heavy metal accumulation. The combined application of conditioner D1 (Fupei) and foliar barrier agents effectively reduced the Hg concentration in rice to below the detection limit (0.003 mg·kg−1), and significantly decreased As and Cd levels compared with the control. Conditioners such as D1 and D2 reduced Hg absorption by increasing soil pH, while D1 and D4 mitigated Hg toxicity through selenium-mediated competition for binding sites. D3 immobilized Hg via adsorption and precipitation. The optimal conditioner application rate was determined to be 100 kg/mu.
This study demonstrates that combining low-accumulation rice varieties with soil conditioners and foliar barrier agents enables the safe cultivation of rice in Hg-contaminated soils. Future research should focus on optimizing conditioner combinations and application schedules, as well as assessing long-term impacts on soil health and ecosystem services.
Author Contributions
Conceptualization, H.H. and Z.S.; Data curation, Y.L. and J.D.; Formal analysis, Y.L.; Investigation, Z.S. and W.W.; Methodology, X.F. and W.W.; Project administration, H.H.; Supervision, H.H. and K.L.; Visualization, X.F., Y.L. and K.Z.; Writing—original draft, X.F. and Y.L.; Writing—review & editing, H.H., S.W., R.L., G.H., J.D. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
The Science and Technology Program of Guangdong Forestry Administration (2020-KYXM-08), National Social Science Fund (23ZDB020), the Major Science and Technology Program for Water Pollution Control and Treatment (2017ZX07101003), National Key Research and Development Project (2019YFC1804800), Youth Foundation of SCIES (PM-zx097-202304-147) and Pearl River S&T Nova Program of Guangzhou, China (No. 201710010065).
Data Availability Statement
Data will be made available on request.
Acknowledgments
This work acknowledges the support of the above funds.
Conflicts of Interest
Authors Xiaohua Fu, Yingqi Liang, Zhihua Sun and Wei Wang were employed by the Hunan Kaidi Engineering Technology Co., Ltd.; Author Guiqiong Houd was employed by the Chenzhou Tungsten Products Branch of Hunan Shizhuyuan Nonferrous Metals Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Hg | Mercury |
| Cd | Cadmium |
| As | Arsenic |
| Pb | Lead |
| Cr | Chromium |
| Se | Selenium |
| OM | Organic Matter |
| AP | Available Phosphorus |
| AK | Available Potassium |
References
- Xiang, S.; Zheng, Y.; Zhou, Q.; Jin, M.; Fu, L.; Wu, W. nZVI/BC as a Soil Amendment and Its Effects on Potted Rice Growth and Soil Properties. Agronomy 2024, 14, 1710. [Google Scholar] [CrossRef]
- Yoon, D.-H.; Choi, W.S.; Hong, Y.K.; Lee, Y.B.; Kim, S.C. Effect of chemical amendments on reduction of bioavailable heavy metals and ecotoxicity in soil. Appl. Biol. Chem. 2019, 62, 53. [Google Scholar] [CrossRef]
- Sun, T.; Gao, G.; Yang, W.; Sun, Y.; Huang, Q.; Wang, L.; Liang, X. High-efficiency remediation of Hg and Cd co-contaminated paddy soils by Fe–Mn oxide modified biochar and its microbial community responses. Biochar 2024, 6, 57. [Google Scholar] [CrossRef]
- Moutcine, A.; Laghlimi, C.; Ziat, Y.; Smaini, M.A.; Qouatli, S.E.E.; Hammi, M.; Chtaini, A. Preparation, characterization and simultaneous electrochemical detection toward Cd (II) and Hg(II) of a phosphate/zinc oxide modified carbon paste electrode. Inorg. Chem. Commun. 2020, 116, 107911. [Google Scholar] [CrossRef]
- Peera Sheikh Kulsum, P.G.; Khanam, R.; Das, S.; Nayak, A.K.; Tack, F.M.G.; Meers, E.; Vithanage, M.; Shahid, M.; Kumar, A.; Chakraborty, S.; et al. A state-of-the-art review on cadmium uptake, toxicity, and tolerance in rice: From physiological response to remediation process. Environ. Res. 2023, 220, 115098. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Spiller, H.A.; Hays, H.L.; Casavant, M.J. Rethinking treatment of mercury poisoning: The roles of selenium, acetylcysteine, and thiol chelators in the treatment of mercury poisoning: A narrative review. Toxicol. Commun. 2021, 5, 19–59. [Google Scholar] [CrossRef]
- Chen, L.; Wu, W.; Han, F.; Li, J.; Ye, W.; Fu, H.; Yan, Y.; Ma, Y.; Wang, Q. Agronomic Management and Rice Varieties Controlling Cd Bioaccumulation in Rice. Int. J. Environ. Res. Public Health 2019, 16, 2376. [Google Scholar] [CrossRef]
- Xiao, A.; Chi, Y.; Huang, L.; Li, W.C.; Ye, Z. Effects of cultivar, water condition and their interactions on Cd accumulation in rice grains. Ecotoxicol. Environ. Saf. 2023, 262, 115168. [Google Scholar] [CrossRef]
- Ding, Y.; Di, X.; Norton, G.J.; Beesley, L.; Yin, X.; Zhang, Z.; Zhi, S. Selenite Foliar Application Alleviates Arsenic Uptake, Accumulation, Migration and Increases Photosynthesis of Different Upland Rice Varieties. Int. J. Environ. Res. Public Health 2020, 17, 3621. [Google Scholar] [CrossRef] [PubMed]
- Senila, M.; Cadar, O.; Senila, L.; Angyus, B.S. Simulated Bioavailability of Heavy Metals (Cd, Cr, Cu, Pb, Zn) in Contaminated Soil Amended with Natural Zeolite Using Diffusive Gradients in Thin-Films (DGT) Technique. Agriculture 2022, 12, 321. [Google Scholar] [CrossRef]
- Zhu, Q.; Ji, J.; Tang, X.; Wang, C.; Sun, H. Bioavailability Assessment of Heavy Metals and Organic Pollutants in Water and Soil Using DGT: A Review. Appl. Sci. 2023, 13, 9760. [Google Scholar] [CrossRef]
- Hseu, Z.-Y.; Su, S.-W.; Lai, H.-Y.; Guo, H.-Y.; Chen, T.-C.; Chen, Z.-S. Remediation techniques and heavy metal uptake by different rice varieties in metal-contaminated soils of Taiwan: New aspects for food safety regulation and sustainable agriculture. Soil Sci. Plant Nutr. 2010, 56, 31–52. [Google Scholar] [CrossRef]
- Xiao, R.; Huang, Z.; Li, X.; Chen, W.; Deng, Y.; Han, C. Lime and Phosphate Amendment Can Significantly Reduce Uptake of Cd and Pb by Field-Grown Rice. Sustainability 2017, 9, 430. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Lee, S.E.; Al-Wabel, M.I.; Tsang, D.C.W.; Ok, Y.S. Biochar-induced changes in soil properties affected immobilization/mobilization of metals/metalloids in contaminated soils. J. Soils Sediments 2017, 17, 717–730. [Google Scholar] [CrossRef]
- He, L.-L.; Huang, D.-Y.; Zhang, Q.; Zhu, H.-H.; Xu, C.; Li, B.; Zhu, Q.-H. Meta-analysis of the effects of liming on soil pH and cadmium accumulation in crops. Ecotoxicol. Environ. Saf. 2021, 223, 112621. [Google Scholar] [CrossRef]
- Jin, Y. Dynamic response of cadmium immobilization to a Ca-Mg-Si soil conditioner in the contaminated paddy soil. Sci. Total Environ. 2024, 908, 168394. [Google Scholar] [CrossRef]
- Chen, T.; Duan, L.; Cheng, S.; Jiang, S.; Yan, B. The preparation of paddy soil amendment using granite and marble waste: Performance and mechanisms. J. Environ. Sci. 2023, 127, 564–576. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Wang, Z.; Yang, S.; Li, P. Mercury concentration and speciation in mine wastes in Tongren mercury mining area, southwest China and environmental effects. Appl. Geochem. 2019, 106, 112–119. [Google Scholar] [CrossRef]
- GB15618-2018; National Standard Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment (MEE): Beijing, China, 2018.
- Kong, X.; Liu, T.; Yu, Z.; Chen, Z.; Lei, D.; Wang, Z.; Zhang, H.; Li, Q.; Zhang, S. Heavy Metal Bioaccumulation in Rice from a High Geological Background Area in Guizhou Province, China. Int. J. Environ. Res. Public Health 2018, 15, 2281. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; You, L.-C.; Lyu, H.-H.; Liu, Y.-X.; He, L.-L.; Hu, Y.-D.; Luo, F.-C.; Yang, S.-M. Role of biochar–mineral composite amendment on the immobilization of heavy metals for Brassica chinensis from naturally contaminated soil. Environ. Technol. Innov. 2022, 28, 102622. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Gao, J.; Zhou, B.; Hao, W.; Feng, D.; Sun, X. Effect of biochar on biochemical properties of saline soil and growth of rice. Heliyon 2024, 10, e23859. [Google Scholar] [CrossRef]
- Xu, T.; Wang, G.; Yin, Q.; Zhou, Z.; Deng, N. Sulfur/zinc co-doped biochar for stabilization remediation of mercury-contaminated soil: Performance, mechanism and ecological risk. Ecotoxicol. Environ. Saf. 2024, 281, 116601. [Google Scholar] [CrossRef]
- Lyu, H.-H.; Cheng, K.; He, L.-L.; Yang, S.-M.; Liu, Y.-X.; You, L.-C.; Wang, Y.-Y. Efficiency of talcum-biochars in immobilization of heavy metals and promotion of the growth of Brassica chinensis in contaminated agricultural soil. Plant Stress 2025, 16, 100836. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Yang, J.; Chen, T. Three-year field experiment on the risk reduction, environmental merit, and cost assessment of four in situ remediation technologies for metal(loid)-contaminated agricultural soil. Environ. Pollut. 2020, 266, 115193. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D.; Xiao, D.; Xiang, Z.; Yang, X.; Xiao, Y.; Xiao, X.; Cheng, J.; Lu, Q.; Zhang, Q. Co-exposure of heavy metals in rice and corn reveals a probabilistic health risk in Guizhou Province, China. Food Chem. X 2023, 20, 101043. [Google Scholar] [CrossRef]
- Yu, G.; Chen, F.; Zhang, H.; Wang, Z. Pollution and health risk assessment of heavy metals in soils of Guizhou, China. Ecosyst. Health Sustain. 2021, 7, 1859948. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Exposure Factors Handbook; United States Environmental Protection Agency: Washington, DC, USA, 2011. [Google Scholar]
- Jiang, Y.; Chao, S.; Liu, J.; Yang, Y.; Chen, Y.; Zhang, A.; Cao, H. Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere 2017, 168, 1658–1668. [Google Scholar] [CrossRef]
- GB 2762-2022; National Food Safety Standard for Maximum Levels of Contaminants in Foods. NHC/SAMR: Beijing, China, 2022.
- Zhang, H.; Feng, X.; Larssen, T.; Shang, L.; Li, P. Bioaccumulation of Methylmercury versus Inorganic Mercury in Rice (Oryza sativa L.) Grain. Environ. Sci. Technol. 2010, 44, 4499–4504. [Google Scholar] [CrossRef]
- Li, W.; Xu, B.; Song, Q.; Liu, X.; Xu, J.; Brookes, P.C. The identification of ‘hotspots’ of heavy metal pollution in soil–rice systems at a regional scale in eastern China. Sci. Total Environ. 2014, 472, 407–420. [Google Scholar] [CrossRef]
- Zhou, H.; Zeng, M.; Zhou, X.; Liao, B.-H.; Peng, P.-Q.; Hu, M.; Zhu, W.; Wu, Y.-J.; Zou, Z.-J. Heavy metal translocation and accumulation in iron plaques and plant tissues for 32 hybrid rice (Oryza sativa L.) cultivars. Plant Soil 2015, 386, 317–329. [Google Scholar] [CrossRef]
- Cao, M.; Zhu, W.; Hong, L.; Wang, W.; Yao, Y.; Zhu, F.; Hong, C.; He, S. Assessing Pb-Cr Pollution Thresholds for Ecological Risk and Potential Health Risk in Selected Several Kinds of Rice. Toxics 2022, 10, 645. [Google Scholar] [CrossRef]
- Powell, K.J.; Brown, P.L.; Byrne, R.H.; Gajda, T.; Hefter, G.; Sjöberg, S.; Wanner, H. Chemical Speciation of Hg(II) with Environmental Inorganic Ligands. Aust. J. Chem. 2004, 57, 993. [Google Scholar] [CrossRef]
- Tran, T.A.T.; Dinh, Q.T.; Zhou, F.; Zhai, H.; Xue, M.; Du, Z.; Bañuelos, G.S.; Liang, D. Mechanisms underlying mercury detoxification in soil–plant systems after selenium application: A review. Env. Sci. Pollut. Res. 2021, 28, 46852–46876. [Google Scholar] [CrossRef]
- Björnberg, A.; Håkanson, L.; Lundbergh, K. A theory on the mechanisms regulating the bioavailability of mercury in natural waters. Environ. Pollut. 1988, 49, 53–61. [Google Scholar] [CrossRef]
- Syversen, T.; Kaur, P. The toxicology of mercury and its compounds. J. Trace Elem. Med. Biol. 2012, 26, 215–226. [Google Scholar] [CrossRef]
- Liang, Y.; Wong, J.W.C.; Wei, L. Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 2005, 58, 475–483. [Google Scholar] [CrossRef]
- Xiao, Z.; Peng, M.; Mei, Y.; Tan, L.; Liang, Y. Effect of organosilicone and mineral silicon fertilizers on chemical forms of cadmium and lead in soil and their accumulation in rice. Environ. Pollut. 2021, 283, 117107. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, H.; Qin, S.; Pan, P.; Tang, S.; Chen, L.; Wang, X.; Tang, F.; Tan, Z.; Wen, R.; et al. Soil conditioners improve Cd-contaminated farmland soil microbial communities to inhibit Cd accumulation in rice. J. Integr. Agric. 2023, 22, 2521–2535. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Liu, K.; Fang, L.; Li, F.; Hou, D.; Liu, C.; Song, Y.; Ran, Q.; Pang, Y.; Du, Y.; Yuan, Y.; et al. Sustainability assessment and carbon budget of chemical stabilization based multi-objective remediation of Cd contaminated paddy field. Sci. Total Environ. 2022, 819, 152022. [Google Scholar] [CrossRef]
- Raza, T.; Qadir, M.F.; Khan, K.S.; Eash, N.S.; Yousuf, M.; Chatterjee, S.; Manzoor, R.; Rehman, S.U.; Oetting, J.N. Unraveling the potential of microbes in decomposition of organic matter and release of carbon in the ecosystem. J. Environ. Manag. 2023, 344, 118529. [Google Scholar] [CrossRef]
- Su, J.; Weng, X.; Luo, Z.; Huang, H.; Wang, W. Impact of Biochar on Soil Properties, Pore Water Properties, and Available Cadmium. Bull. Env. Contam. Toxicol. 2021, 107, 544–552. [Google Scholar] [CrossRef]
- Song, J.; Jin, X.; Wang, X.C.; Jin, P. Preferential binding properties of carboxyl and hydroxyl groups with aluminium salts for humic acid removal. Chemosphere 2019, 234, 478–487. [Google Scholar] [CrossRef]
- Schaller, J.; Faucherre, S.; Joss, H.; Obst, M.; Goeckede, M.; Planer-Friedrich, B.; Peiffer, S.; Gilfedder, B.; Elberling, B. Silicon increases the phosphorus availability of Arctic soils. Sci. Rep. 2019, 9, 449. [Google Scholar] [CrossRef]
- Zhang, J.; Mu, G.; Zhang, Z.; Huang, X.; Fang, H. Speciation Variation and Bio-Activation of Soil Heavy Metals (Cd and Cr) in Rice-Rape Rotation Lands in Karst Regions. Int. J. Environ. Res. Public Health 2021, 18, 1364. [Google Scholar] [CrossRef]
- Mu, Y.; Cui, J.; Liu, A.; Wang, S.; Shi, Q.; Wang, J.; Wei, S.; Zhang, J. Interactions and quantification of multiple influencing factors on cadmium accumulation in soil-rice systems at a large region. Sci. Total Environ. 2023, 881, 163392. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).