Microplastic Identification in Domestic Wastewater-Treating Constructed Wetlands and Its Potential Usage in a Circular Economy
Abstract
1. Introduction
- To identify the presence of microplastics in domestic wastewater treated by horizontal subsurface flow constructed wetlands with ornamental plants using FTIR spectroscopy and visual characterization;
- To assess constructed wetland-treated wastewater reuse for Phaseolus vulgaris irrigation as a circular economy strategy.
2. Materials and Methods
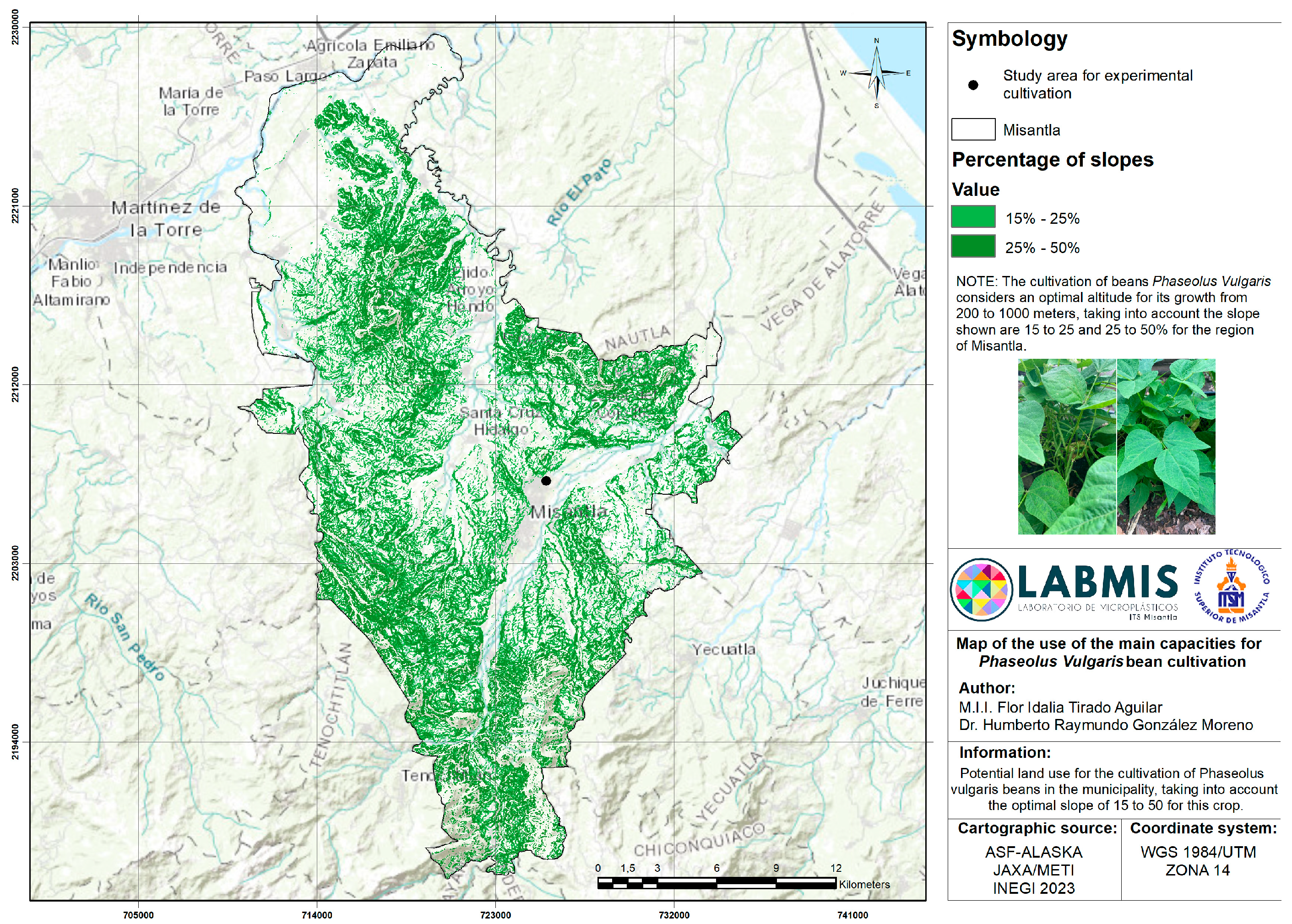
- Stage 1. Selection of crop and study area.
- Study area.
- Stage 2. Setting up of the horizontal subsurface flow constructed wetland system (HSSF-CW).
- Stage 3. Experimental design of the wetland system.
- Stage 4. Set up of two grow beds (GBs) for planting the crop and a drip irrigation system.
- Stage 5. Planting, harvesting, and food safety analysis of Phaseolus vulgaris crops.
- Stage 6. Fourier transform infrared spectroscopy (FTIR) analysis and visual characterization of microplastics.
- Sampling: a sterile, 500 mL glass container with a metal lid was used.
- Digestion of organic matter: using potassium hydroxide and a thermo-stirrer for 24 h at a temperature of 50 °C, digestion of the organic matter in each water sample was achieved.
- Sedimentation: forty-eight hours after organic matter digestion, the samples were transferred to 60 mL glass test tubes with lids, using a glass pipette previously marked with an identification label.
- Filtration supplies: a filter holder, cellulose or glass fiber filters, fine-tipped forceps, beaker, 60 mL syringes, Petri dish, yellow isolating tape, black oil pen, optical microscope, and stereoscope.
- Filtration process: the water sample is poured into a beaker, the filter is placed in the filter holder, and the filter holder is placed in the sample container; to supply the water sample with the help of the syringe, the filter is removed with the forceps and placed in a Petri dish and marked with insulating tape.
- Drying: the drying time of the filter is 24 h.
- Visualization: the filter is visualized by means of an optical microscope and a model VE S-1, VELAB-brand stereoscopic microscope, with different lens objectives (magnifications, 2× and 4×).
3. Results
3.1. Cropping of Phaseolus vulgaris
3.2. The Wetland System
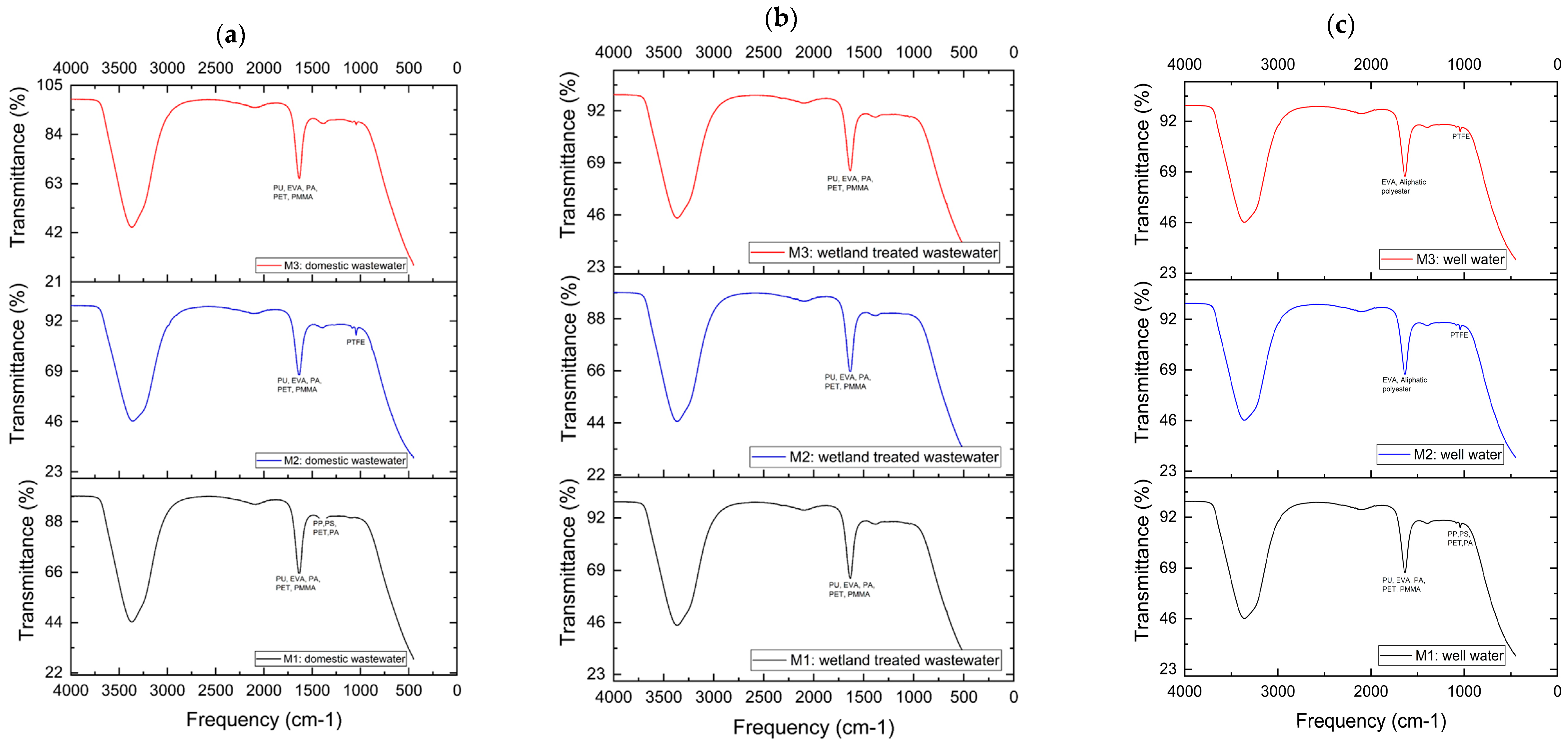
3.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
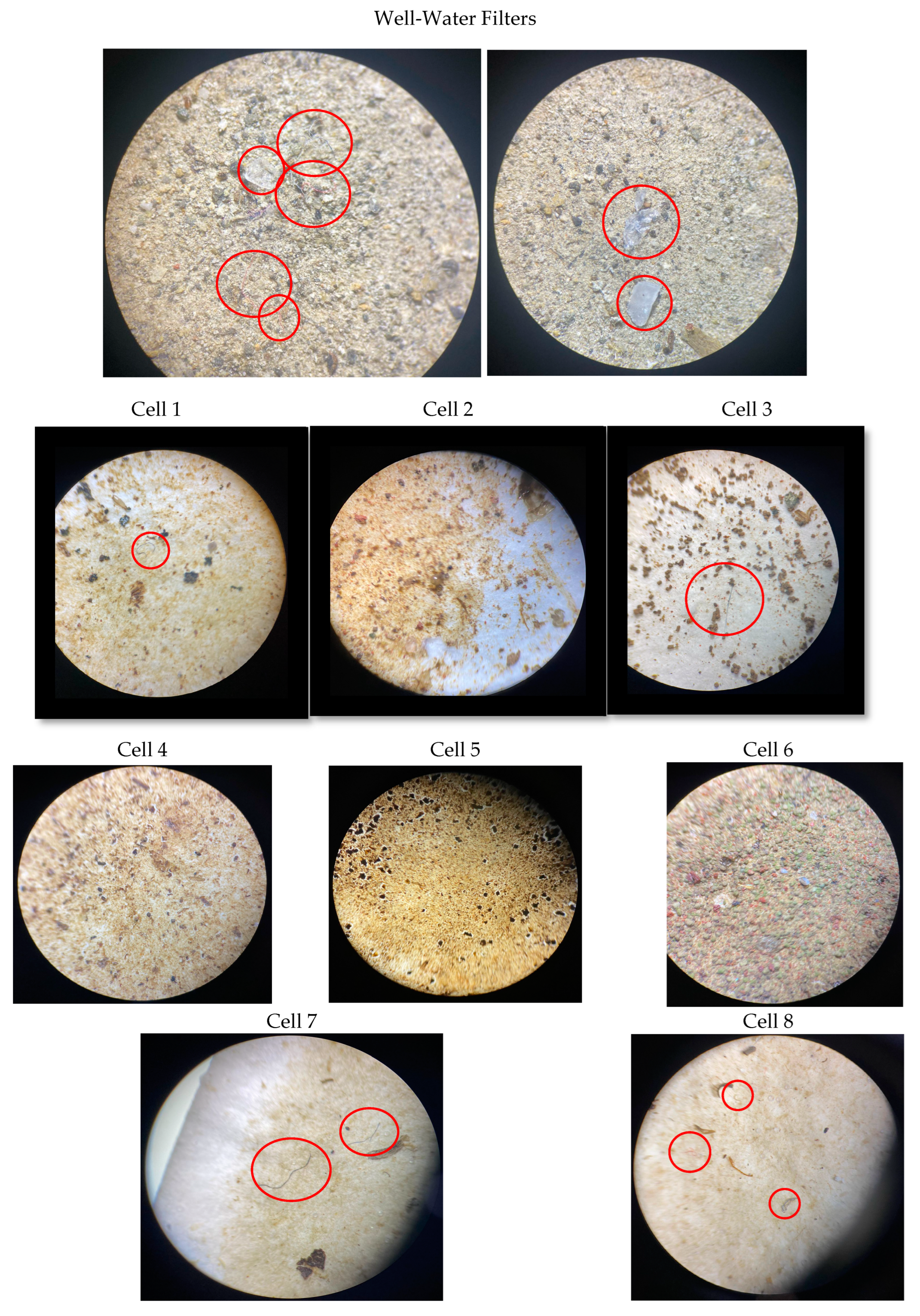
3.4. Visual Characterization of Microplastics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balakrishnan, V.S. Microplastics: An underestimated and underregulated crisis. In The Lancet Respiratory Medicine; Elsevier: Amsterdam, The Netherlands, 2025; pp. 2213–2600. [Google Scholar] [CrossRef]
- Al-Azzawi, M.S.M.; Kefer, S.; Weißer, J.; Reichel, J.; Schwaller, C.; Glas, K.; Knoop, O.; Drewes, J.E. Validation of sample preparation methods for the analysis of microplastics in wastewater matrices: Reproducibility and standardization. Water 2020, 12, 2445. [Google Scholar] [CrossRef]
- Andrady, A.L. Plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Cedeño, R.; Villacis, J.J.; Nicole, K. Systematic review: Micro and nanoplastics and their influence on human health. Gac. Méd. Estud. 2024, 5. ISSN 2708-5546. [Google Scholar]
- Dick Vethaak, A.; Legler, J. Microplastics and human health: Knowledge gaps must be addressed to determine the health risks of microplastics. Science 2021, 371, 672–674. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Xia, R.; Liao, J.; Liu, J.; Yu, P. Microplastics and chemical leachates from plastic pipes are associated with increased virulence and antimicrobial resistance potential of microbial communities in drinking water. J. Hazard. Mater. 2024, 463, 132900. [Google Scholar] [CrossRef]
- Castañeta, G.; Gutiérrez, A.F.; Nacaratte, F.; Manzano, C.A. Microplastics: A contaminant growing in all environmental settings, its characteristics and possible risks to public health by exposure. Rev. Boliv. De Química 2020, 37, 160–175. [Google Scholar] [CrossRef]
- Vethaak, D.C.; Amsterdam, V.U. The Emerging Health Crisis of Microplastic Waste. Think Global Health. November 2022. Available online: https://www.thinkglobalhealth.org/article/emerging-health-crisis-microplastic-debris (accessed on 12 September 2024).
- Shaikh, I.V.; Shaikh, V.A.E. A comprehensive review on the assessment of plastic debris in aquatic environment and its prevalence in fish and other aquatic animals in India. Sci. Total Environ. 2021, 779, 146421. [Google Scholar] [CrossRef]
- Arshad, N.; Alam, M.M.; Su’ud, M.B.M.; Imran, S.; Siddiqui, T.; Saleem, K.; Bashir, A.; Batool, A. Microplastic contamination of surface waters and commercially valuable fishes of Karachi coast, Pakistan. Reg. Stud. Mar. Sci. 2023, 62, 102955. [Google Scholar] [CrossRef]
- Jáuregui Nolen, E.C.; Tello Medina, D.C.; Rivas García, M.P. Inequality and environmental policy in Mexico. Econ. Mex. Nueva Época 2012, 21, 251–275. Available online: http://hdl.handle.net/11651/3940 (accessed on 6 October 2024).
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M. Atmospheric microplastics: A review on current status and prospects. J. Earth Sci. 2020, 203, 103118. [Google Scholar] [CrossRef]
- Terzi, Y.; Öztürk, R.Ç.; Eryaşar, A.R.; Yandı, İ.; Şahin, A.; Yılmaz, F.; Gedik, K.; Gündogdu, S. Riverine microplastic discharge along the southern Black Sea coast of Türkiye. Environ. Res. Lett. 2025, 20, 024061. [Google Scholar] [CrossRef]
- Su, L.; Xiong, X.; Zhang, Y.; Wu, C.; Xu, X.; Sun, C.; Shi, H. Global transport of plastics and microplastics: A critical review of pathways and influences. Sci. Total Environ. 2022, 831, 154884. [Google Scholar] [CrossRef] [PubMed]
- Chanda, M.; Bathi, J.R.; Khan, E.; Katyal, D.; Danquah, M. Microplastics in ecosystems: Critical review of occurrence, distribution, toxicity, fate, fate, transport and advances in experimental and computational studies in surface and groundwater. J. Environ. Manag. 2024, 370, 122492. [Google Scholar] [CrossRef] [PubMed]
- Prasath, A.R.; Sudhakar, C.; Selvam, K. Microplastics in the environment: Types, sources and impact on human and aquatic systems. Bioresour. Technol. Rep. 2025, 29, 102055. [Google Scholar] [CrossRef]
- Mendoza-Retana, S.S.; Cervantes-Vázquez, M.G.; Valenzuela-García, A.A.; Guzmán-Silos, T.L.; Orona-Castillo, I.; Cervan-tes-Vázquez, T.J.Á. Potential use of wastewater in agriculture. Rev. Mex. De Cienc. Agropecu. 2021, 12, 115–126. [Google Scholar] [CrossRef]
- Sarria Villa, R.A.; Gallo-Corredor, J.A. The major environmental problem of plastic waste: Microplastics. J. Cienc. Ing. 2016, 8, 21–27. Available online: https://www.researchgate.net/publication/323200126_La_gran_problematica_ambiental_de_los_residuos_plasticos_Microplasticos (accessed on 6 October 2024).
- Ashrafy, A.; Liza, A.A.; Islam, M.N.; Billah, M.M.; Arafat, S.T.; Rahman, M.M.; Rahman, S.M. Microplastic contamination: A brief review of their origin and abundance in different aquatic ecosystems. J. Hazard. Mater. Adv. 2023, 9, 100215. [Google Scholar] [CrossRef]
- Arredondo-Navarro, A.; Flores-Cervantes, D.X. Microplastics in water and sediments: Sampling, detection, characterization methods and quality control: A review. Water Technol. Sci. 2023, 14, 474–522. [Google Scholar] [CrossRef]
- Sandoval-Herazo, L.; Alvarado-Lassman, A.; Marín-Muñiz, J.; Méndez-Contreras, J.; Zamora-Castro, S.A. Effects of the use of ornamental plants and different substrates on the removal of pollutants from wastewater through artificial wetland microcosms. Sustainability 2018, 10, 1594. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, L.; Cizdziel, J.; Huang, Y. Progress of microplastics research in wastewater treatment plants: A holistic review. J. Environ. Manag. 2023, 325, 116411. [Google Scholar] [CrossRef]
- Cabrera, D.C.; Wang, Q.; Martin, M.; Rajadel, N.O.; Rousseau, D.P.L.; Hernandez-Crespo, C. Occurrence and fate of microplastics in large-scale treatment wetlands. Water Res. 2023, 240, 120106. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Luo, H.; Zou, J.; Chen, J.; Pan, X.; Rousseau, D.P.L.; Li, J. Characteristics and removal of microplastics in rural domestic wastewater treatment facilities in China. Sci. Total Environ. 2020, 739, 139935. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Methods for Assessing the Condition of Wetlands: Vegetation-Based Indicators of Wetland Nutrient Enrichment; EPA-822-R-02-024; Office of Water, U.S. Environmental Protection Agency: Washington, DC, USA, 2002. Available online: https://www.epa.gov/sites/default/files/documents/wetlands_16indicators.pdf (accessed on 3 March 2024).
- Komorowska-Kaufman, M.; Marciniak, W. Removal of microplastic particles during municipal wastewater treatment: A current review. Desalination Water Treat. 2024, 317, 100006. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Hu, H.; Ao, H.; Xiong, X.; Shi, H.; Wu, C. Transport and fate of microplastics in artificial wetlands: A microcosm study. J. Hazard. Mater. 2021, 415, 125615. [Google Scholar] [CrossRef] [PubMed]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Donald Huisingh, Water scarcity in agriculture: An overview of causes, impacts and approaches to reduce risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef]
- Gonzalez, M. Phaseolus vulgaris L. (Common bean); Agricultural Sciences; Waliszewski, W., Ed.; Bolivar College, Cali, Valle del Cauca, Colombia: 2019–2020. Available online: https://www.colegiobolivar.edu.co/garden/wp-content/uploads/2019/10/D-Mariana-Gonzalez-Phaseolus-vulgaris-Pinto-Group.pdf (accessed on 3 March 2024).
- Chávez-Mendoza, C.; Sánchez, E. Bioactive Compounds from Mexican Varieties of the Common Bean (Phaseolus vulgaris): Implications for Health. Molecules 2017, 22, 1360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Viveros, J.A.F.; Martínez-Reséndiz, G.; Zurita, F.; Marín-Muñiz, J.L.; Méndez, M.C.L.; Zamora, S.; Sandoval Herazo, L.C. Partially Saturated Vertical Constructed Wetlands and Free-Flowing Vertical Constructed Wetlands for Pilot-Scale Municipal Wastewater/Porcine Treatment using Heliconia latispatha. Water 2022, 14, 3860. [Google Scholar] [CrossRef]
- Witczak, A.; Przedpełska, L.; Pokorska-Niewiada, K.; Cybulski, J. Microplastics as a threat to aquatic ecosystems and human health. Toxics 2024, 12, 571. [Google Scholar] [CrossRef]
- García-Ávila, F.; Avilés-Añazco, A.; Cabello-Torres, R.; Guanuchi-Quito, A.; Cadme-Galabay, M.; Gutiérrez-Ortega, H.; Alvarez-Ochoa, R.; Zhindón-Arévalo, C. Application of ornamental plants in artificial wetlands for wastewater treatment: A scientometric analysis. Case Stud. Chem. Environ. Eng. 2023, 7, 100307. [Google Scholar] [CrossRef]
- NOM-043-SSA2-2005; Norma Oficial Mexicana NOM-043-SSA2-2005, para la Prevención y Control de la Contaminación del Agua por Microorganismos Patógenos. Secretaría de Salud: Mexico City, Mexico, 2005.
- INEGI. National Institute of Statistics and Geography. Encuesta Nacional de Ingresos y Gastos de los Hogares (ENIGH) 2020. Available online: https://www.inegi.org.mx (accessed on 3 September 2024).
- Ungureanu, N.; Vlăduț, V.; Voicu, G. Water scarcity and wastewater reuse in crop irrigation. Sustainability 2020, 12, 9055. [Google Scholar] [CrossRef]
- Plaimart, J. Nature-Based Solutions for Rural Wastewater and Agricultural Waste Management. Ph.D. Thesis, University of Newcastle, Callaghan, Australia, 2023. [Google Scholar]
- De Matos, M.P.; Von Sperling, M.; de Matos, A.T. Clogging in horizontal subsurface flow artificial wetlands: Influencing factors, investigation methods, and remediation techniques. Rev. Environ. Sci. Biotechnol. 2018, 17, 87–107. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Kim, B.S.; Lee, S.S.; Pandey, S.K.; Kim, K.H. Constructed wetlands for removal of organic micropollutants from wastewater: Current status, progress and challenges. Chemosphere 2024, 360, 142364. [Google Scholar] [CrossRef]
- FIRA. Fideicomiso Instituido en Relación con la Agricultura. Available online: https://www.fira.gob.mx (accessed on 12 September 2024).
- Madera-Parra, C.A.; Peña-Salamanca, E.J.; Peña, M.R.; Rousseau, D.P.L.; Lens, P.N.L. Phytoremediation of landfill leachate with Colocasia esculenta, Gynerum sagittatum and Heliconia psittacorum in artificial wetlands. Int. J. Phytoremediation 2014, 17, 16–24. [Google Scholar] [CrossRef]
- Shukla, R.; Gupta, D.; Singh, G.; Mishra, V.K. Performance of a horizontal flow constructed wetland for secondary treatment of domestic wastewater in a remote tribal area of central India. Sustain. Environ. Res. 2021, 31, 13. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Wasington, DC, USA, 2005. [Google Scholar]
- Guerra Hernández, G.; Marrón Manrique, O.; Melo Camaraza, B.; Hernández Rodríguez, A.; Martínez Montero, M.E.; Donis Almeida, E. Reuse of treated urban wastewater for irrigation of stabilization ponds. Revista Ciencias Técnicas Agropecuarias 2023, 32, e04. Available online: https://www.redalyc.org/journal/932/93276285004/html/ (accessed on 3 September 2024).
- OECD-FAO. Agricultural Outlook 2023–2032; OECD Publications, FAO: 2023. Available online: https://www.oecd.org/en/publications/oecd-fao-agricultural-outlook-2023-2032_08801ab7-en.html (accessed on 3 October 2024).
- Jaramillo Chamba, D.A.; Panchana González, W.V.; Cumbicos Calva, A.Y.; Escudero Molina, N.E. Intelligent irrigation system for the maintenance of green areas in an educational institution. Revista Científica y Tecnológica UPSE 2023, 10, 50–63. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef]
- Radford, F.; Zapata-Restrepo, L.M.; Horton, A.A.; Hudson, M.D.; Shaw, P.J.; Williams, I.D. Developing a systematic method for extraction of microplastics in soils. Methods 2021, 13, 1695–1705. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Microplasctic Pollutants; Elsevier Science: Amsterdam, The Netherlands, 2017; 330p, Available online: https://www.sciencedirect.com/book/9780128094068/microplastic-pollutants (accessed on 3 March 2024).
- Nani, G.; Sandoval-Herazo, M.; Martínez-Reséndiz, G.; Marín-Peña, O.; Zurita, F.; Sandoval Herazo, L.C. Influence of Bed Depth on the Development of Tropical Ornamental Plants in Subsurface Flow Treatment Wetlands for Municipal Wastewater Treatment: A Pilot-Scale Case. Plants 2024, 13, 1958. [Google Scholar] [CrossRef]



| Irrigation Type | Water Supply | Water Need | Water Saving % | Production Increase % | Energy Cost | Crop Type | |
|---|---|---|---|---|---|---|---|
| Surface irrigation | Ridge | Irregular | Abundant | S-N | 30% | High | Rice, corn, grasses, vineyards, and cotton |
| Furrow | Irregular | Moderate | S-N | NR | High | Sugarcane | |
| Pressurized irrigation | Drip irrigation | Continual | Limited | 95–100% | 40–85% | Moderate | Limes, lemons, tangerines, oranges, grapefruits, corn, soybeans, herbs, bananas, sugarcane, cotton, beans, potatoes, pumpkin, melons, peanuts, and chilis |
| Sprinkler irrigation | Regular | Medium | 80–85% | 25–35% | Medium | Corn, soybeans, wheat, cotton, peanuts, potatoes, sunflowers, alfalfa, sorghum, sugar beets, and vegetables | |
| Micro-sprinkler irrigation | Regular | Medium–high | 46–82% | 27% | Medium | Nursery, frost protection, garden irrigation, horticulture, fruit growing, flowers, greenhouses, and cocoa | |
| Item | Description |
|---|---|
| Crop | Ejotero Bean |
| Variety | Phaseolus vulgaris |
| Distance between seeds | 30 cm |
| Seeds per vine | 4 |
| Total plant number | 42 |
| Total number of sown seeds | 126 |
| Sowing depth | 4 cm |
| Days to germination | 7 to 15 days |
| Vegetative development | 2 to 3 weeks |
| Sowing depth | 2–3 cm |
| Germination % | >91% |
| Blossoming | 1 to 2 weeks |
| Pod formation | 2 to 3 weeks |
| Seed/crop maturity | 50 to 70 days |
| Phaseolus vulgaris | |
|---|---|
| Seeding date | 23 November 2023 |
| Sprouting date | 27 November 2023 |
| Measurement start date | 30 November 2023 |
| Irrigation type | Drip |
| Requirement/Day | |
| Irrigation | 2 times a day |
| Harvest date | 14 February 2024 |
| Total days | 84 days |
| Hippeastrum hybridum Hort | Heliconia bihai marginata | Polyculture | Control | ||
|---|---|---|---|---|---|
| COD (mg/L) | IC | 908.17 ± 17.45 | |||
| EC | 105.10 ± 1.64 | 88.4 ± 0.190 | 10.59 ± 0.35 | 536 ± 9.10 | |
| % of removal | 88.4 ± 0.190 | 93.64 ± 0.161 | 98.83 ± 0.04 | 40.77 ± 1.16 | |
| NH4++ (mg/L) | IC | 35.91 ± 1.30 | |||
| EC | 7.47 ± 0.33 | 3.36 ± 0.11 | 0.40 ± 0.05 | 23.40 ± 1.25 | |
| % of removal | 78.99 ± 1.012 | 90.54 ± 0.334 | 98.89 ± 0.13 | 33.01 ± 4.50 | |
| TN (mg/L) | IC | 87.56 ± 3.60 | |||
| EC | 14.72 ± 0.42 | 8.71 ± 0.18 | 3.38 ± 0.10 | 50 ± 1.56 | |
| % of removal | 82.80 ± 0.736 | 89.85 ± 0.363 | 96.05 ± 0.18 | 42.06 ± 2.14 | |
| NO2−− (mg/L) | IC | 184.74 ± 4.77 | |||
| EC | 2.97 ± 0.13 | 11.30 ± 0.32 | 1.77 ± 0.05 | 107 ± 2.41 | |
| % of removal | 88.94 ± 0.377 | 93.85 ± 0.183 | 99.03 ± 0.04 | 41.24 ± 1.96 | |
| TP (mg/L) | IC | 20.64 ± 0.76 | |||
| EC | 2.97 ± 0.13 | 1.43 ± 0.04 | 0.19 ± 0.01 | 12 ± 0.49 | |
| % of removal | 85.53 ± 0.709 | 93.04 ± 0.218 | 99.05 ± 0.04 | 41.12 ± 2.71 | |
| PO43−− (mg/L) | IC | 41.00 ± 0.88 | |||
| EC | 5.63 ± 0.19 | 2.71 ± 0.08 | 0.40 ± 0.0038 | 23 ± 1.53 | |
| % of removal | 86.23 ± 0.480 | 93.36 ± 0.213 | 99.03 ± 0.02 | 43.66 ± 3.83 | |
| TSS (mg/L) | IC | 111.36 ± 4.56 | |||
| EC | 21.94 ± 0.46 | 13.29 ± 0.22 | 5.67 ± 0.10 | 59 ± 1.65 | |
| % of removal | 80.21 ± 0.435 | 87.97 ± 0.300 | 94.84 ± 0.16 | 46.41 ± 1.65 | |
| (a) Domestic Wastewaters | ||
| Sample No. | Range | Polymer |
| M1 | 1633 | PU, EVA, PA, PET, and PMMA |
| 1400–1480 | PP, PS, PET, and PA | |
| M2 | 1634 | PU, EVA, PA, PET, and PMMA |
| 1044 | PTFE | |
| M3 | 1634 | PU, EVA, PA, PET, and PMMA |
| (b) Constructed Wetland-treated Domestic Wastewaters | ||
| Sample No. | Range | Polymer |
| M1 | 1633 | PU, EVA, PA, PET, and PMMA |
| M2 | 1633 | PU, EVA, PA, PET, and PMMA |
| M3 | 1633 | PU, EVA, PA, PET, and PMMA |
| (c) Well Water | ||
| Sample No. | Range | Polymer |
| M1 | 1634 | PU, EVA, PA, PET, and PMMA |
| 1044 | PP, PS, PET, and PA | |
| M2 | 1634 | EVA and Aliphatic polyester |
| 1044 | PTFE | |
| M3 | 1634 | EVA and Aliphatic polyester |
| 1044 | PTFE | |
| Sample | Amount | Color | Shape |
|---|---|---|---|
| Domestic wastewater | 1 | Purple | Fiber |
| Domestic wastewater | 4 | Pale blue | Fragment |
| Domestic wastewater | 10 | Deep blue | Fiber |
| Domestic wastewater | 1 | Transparent | Fragment |
| Well water | 2 | Red | Fiber |
| Well water | 1 | Pale blue | Fiber |
| Well water | 1 | Deep blue | Fiber |
| Well water | 5 | Transparent | Fiber |
| Well water | 2 | White | Fragment |
| Cell 8 | 1 | Pale blue | Fragment |
| Cell 8 | 2 | Red | Fragment |
| Cell 7 | 2 | Deep blue | Fragment |
| Cell 6 | 0 | NF | NF |
| Cell 5 | 0 | NF | NF |
| Cell 4 | 0 | NF | NF |
| Cell 3 | 1 | Deep blue | Fiber |
| Cell 2 | 0 | NF | NF |
| Cell 1 | 1 | Deep blue | Fiber |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirado Aguilar, F.I.; Peña Montes, C.; Borroto Pentón, Y.; López Méndez, M.C.; Castellanos Rivera, J.; Martínez Castellanos, G.; González Moreno, H.R.; Monzón Reyes, B.L. Microplastic Identification in Domestic Wastewater-Treating Constructed Wetlands and Its Potential Usage in a Circular Economy. Processes 2025, 13, 1499. https://doi.org/10.3390/pr13051499
Tirado Aguilar FI, Peña Montes C, Borroto Pentón Y, López Méndez MC, Castellanos Rivera J, Martínez Castellanos G, González Moreno HR, Monzón Reyes BL. Microplastic Identification in Domestic Wastewater-Treating Constructed Wetlands and Its Potential Usage in a Circular Economy. Processes. 2025; 13(5):1499. https://doi.org/10.3390/pr13051499
Chicago/Turabian StyleTirado Aguilar, Flor Idalia, Carolina Peña Montes, Yodaira Borroto Pentón, María Cristina López Méndez, Jesús Castellanos Rivera, Gustavo Martínez Castellanos, Humberto Raymundo González Moreno, and Brenda Lizeth Monzón Reyes. 2025. "Microplastic Identification in Domestic Wastewater-Treating Constructed Wetlands and Its Potential Usage in a Circular Economy" Processes 13, no. 5: 1499. https://doi.org/10.3390/pr13051499
APA StyleTirado Aguilar, F. I., Peña Montes, C., Borroto Pentón, Y., López Méndez, M. C., Castellanos Rivera, J., Martínez Castellanos, G., González Moreno, H. R., & Monzón Reyes, B. L. (2025). Microplastic Identification in Domestic Wastewater-Treating Constructed Wetlands and Its Potential Usage in a Circular Economy. Processes, 13(5), 1499. https://doi.org/10.3390/pr13051499








