Molecularly Imprinted Polymers for Pollutant Capture and Degradation: A Snapshot Review
Abstract
1. Introduction
2. Methodology
2.1. Bibliometric Analysis
2.2. Systematic Review and Meta-Analysis
3. Results and Discussions
3.1. Bibliometric Trends
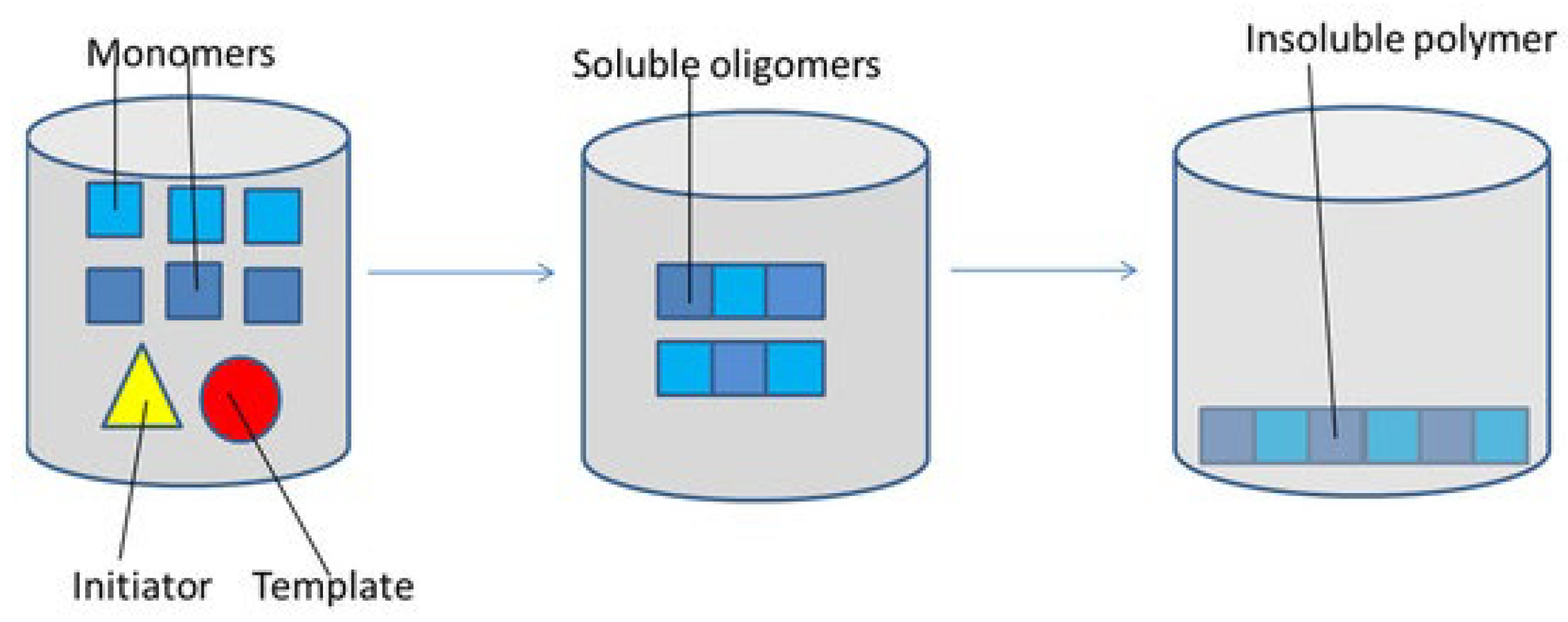
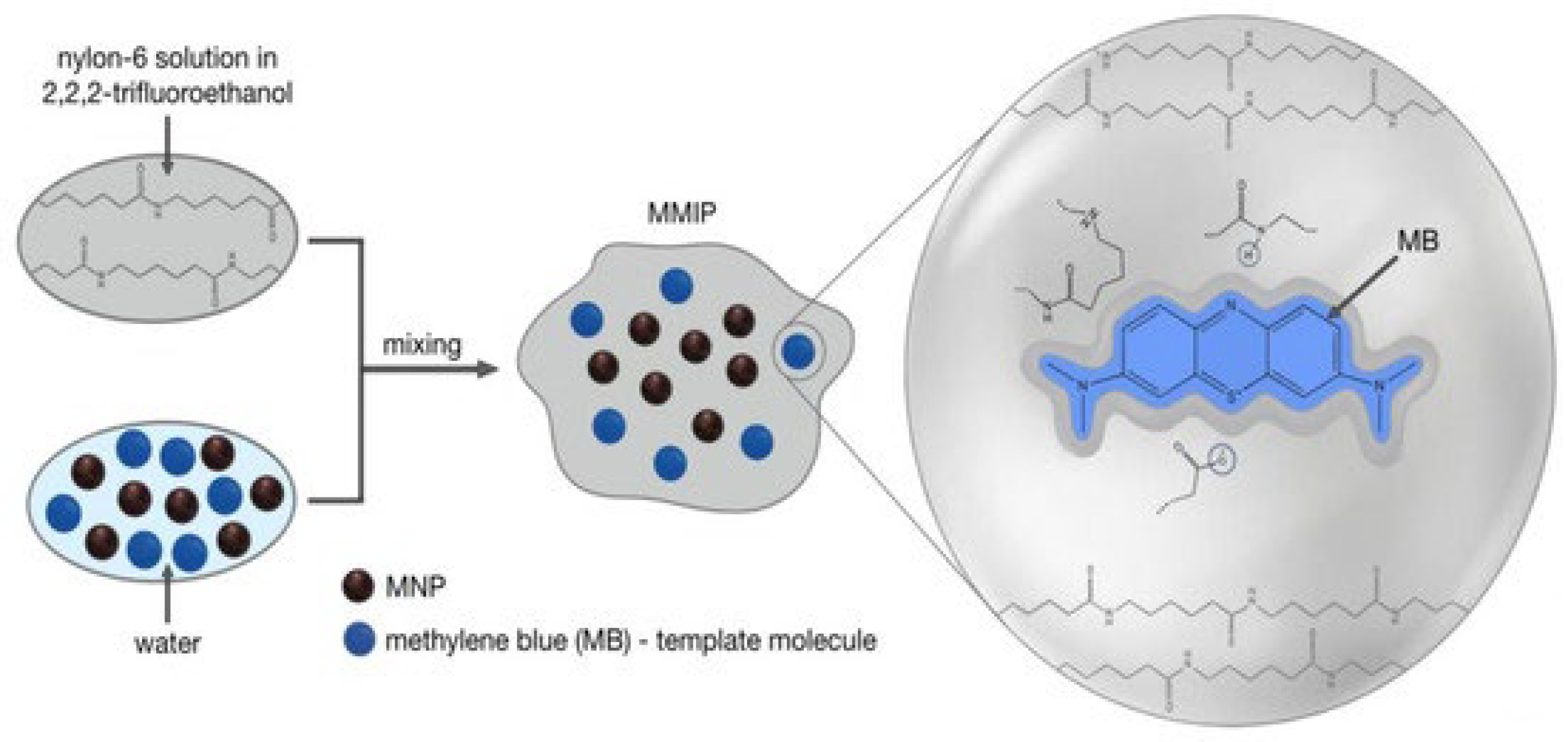
3.2. MIPs for Pollutant Uptake and Degradation
4. Main Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, N.; Poonia, T.; Siwal, S.S.; Srivastav, A.L.; Sharma, H.K.; Mittal, S.K. Challenges of water contamination in urban areas. Curr. Dir. Water Scarcity Res. 2022, 6, 173–202. [Google Scholar] [CrossRef]
- Hirai, M.; Graham, J. Toward Universal Access to Basic and Safely Managed Drinking Water: Remaining Challenges and New Opportunities in the Era of Sustainable Development Goals. In Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures, 2nd ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 3–16. [Google Scholar] [CrossRef]
- Rajapakse, J.; Otoo, M.; Danso, G. Progress in delivering SDG6: Safe water and sanitation. Camb. Prism. Water 2023, 1, e6. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Boczkaj, G.; Aubry, O.; Aravind, U.K.; Aravindakumar, C.T. Advanced Oxidation Processes for Degradation of Water Pollutants—Ambivalent Impact of Carbonate Species: A Review. Water 2023, 15, 1615. [Google Scholar] [CrossRef]
- Rosli, N.A.; Aziz, H.A.; Pueh, L.L.L.; Othman, I.B.; Zawawi, M.H.; Hung, Y.-T. Management of Various Sources of Hazardous Waste. In Waste Treatment in the Biotechnology, Agricultural and Food Industries; Springer: Cham, Switzerland, 2024; Volume 2, pp. 19–64. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Aramesh, N.; Khan, A.A.; Gul, I.; Ghotekar, S.; Bilal, M. Molecularly imprinted polymers-based adsorption and photocatalytic approaches for mitigation of environmentally-hazardous pollutants─A review. J. Environ. Chem. Eng. 2021, 9, 104879. [Google Scholar] [CrossRef]
- Khan, I.; Liu, W.; Zada, A.; Raziq, F.; Ali, S.; Shah, M.I.A.; Ateeq, M.; Khan, M.; Alei, D.; Fazil, P.; et al. Recent progress in emerging materials and hybrid nanocomposites for peroxymonosulfate and peroxydisulfate activation towards solar light-driven photocatalytic degradation of emerging pollutants. Coord. Chem. Rev. 2023, 499, 215466. [Google Scholar] [CrossRef]
- Khanna, R.; Gupta, S. Agrochemicals as a potential cause of ground water pollution: A review. Int. J. Chem. Stud. 2018, 6, 985–990. Available online: https://www.researchgate.net/publication/350874038 (accessed on 1 March 2025).
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Ngole-Jeme, V.M.; Momba, M.N.B. Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water: A review. J. Environ. Manag. 2021, 277, 111485. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Akhtar, N.; Ishak, M.I.S.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Peters, N.E.; Meybeck, M. Water Quality Degradation Effects on Freshwater Availability: Impacts of Human Activities. Water Int. 2000, 25, 185–193. [Google Scholar] [CrossRef]
- Wang-Erlandsson, L.; Tobian, A.; van der Ent, R.J.; Fetzer, I.; Wierik, S.T.; Porkka, M.; Staal, A.; Jaramillo, F.; Dahlmann, H.; Singh, C.; et al. A planetary boundary for green water. Nat. Rev. Earth Environ. 2022, 3, 380–3922. [Google Scholar] [CrossRef]
- Ng, B.; Quinete, N.; Maldonado, S.; Lugo, K.; Purrinos, J.; Briceño, H.; Gardinali, P. Understanding the occurrence and distribution of emerging pollutants and endocrine disruptors in sensitive coastal South Florida Ecosystems. Sci. Total. Environ. 2021, 757, 143720. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Khan, Z.A.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Chaudhary, Emerging pollutants in aquatic environment: Source, effect, and challenges in biomonitoring and bioremediation—A review. Pollution 2020, 6, 99–113. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef]
- Silodia, K.; Bhardwaj, L.; Kumar, A.; Nigam, S.; Yadav, P.; Singh, R.P.; Anza; Singh, R.K.; Hasem, A.; Abd_Allah, E.F.; et al. Strategies for bioremediation of emerging pollutants: A green and sustainable environment. Adv. Chem. Pollut. Environ. Manag. Prot. 2025, 10, 484. [Google Scholar] [CrossRef]
- WHO; UNICEF. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2020: Five Years into the SDGs; World Health Organization: Geneva, Switzwerland, 2021; Available online: http://apps.who.int/bookorders (accessed on 1 March 2025).
- Arora, N.K.; Mishra, I. Sustainable development goal 6: Global Water Security. Environ. Sustain. 2022, 5, 271–275. [Google Scholar] [CrossRef]
- Andriamahefazafy, M.; Touron-Gardic, G.; March, A.; Hosch, G.; Palomares, M.; Failler, P. Sustainable development goal 14: To what degree have we achieved the 2020 targets for our oceans? Ocean Coast. Manag. 2022, 227, 106273. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Noutsopoulos, C.; Mapelli, F.; Fenyvesi, É.; Vieira, M.G.A.; Picos-Corrales, L.A.; Moreno-Piraján, J.C.; et al. Removal of emerging contaminants from wastewater using advanced treatments. A review. Environ. Chem. Lett. 2022, 20, 1333–1375. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Wang, S. Recent advances in the removal of emerging contaminants from water by novel molecularly imprinted materials in advanced oxidation processes—A review. Sci. Total. Environ. 2023, 883, 163702. [Google Scholar] [CrossRef]
- Gamal, M.; Imam, M.S.; Albugami, A.S.; Hunjur, S.A.; Aldhalmi, A.K.; AbdElrahman, M.; Ghoneim, M.M.; Ali, H.M.; Abdelwahab, N.S.; Eissa, M.S. Current advances in the implementation of magnetic molecularly imprinted polymers tailored for enrichment of target analytes in different environmental samples: An overview from a comprehensive perspective. Trends Environ. Anal. Chem. 2024, 43, e00236. [Google Scholar] [CrossRef]
- Metwally, M.G.; Benhawy, A.H.; Khalifa, R.M.; El Nashar, R.M.; Trojanowicz, M. Application of Molecularly Imprinted Polymers in the Analysis of Waters and Wastewaters. Molecules 2021, 26, 6515. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, J.; Wei, C. Molecularly imprinted polyaniline immobilized on Fe3O4/ZnO composite for selective degradation of amoxycillin under visible light irradiation. Appl. Surf. Sci. 2023, 609, 155324. [Google Scholar] [CrossRef]
- Guo, M.; Hu, Y.; Wang, R.; Yu, H.; Sun, L. Molecularly imprinted polymer-based photocatalyst for highly selective degradation of methylene blue. Environ. Res. 2021, 194, 110684. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Passas, I. Bibliometric Analysis: The Main Steps. Encyclopedia 2024, 4, 1014–1025. [Google Scholar] [CrossRef]
- Arya, S.; Kaji, A.H.; Boermeester, M.A. PRISMA Reporting Guidelines for Meta-analyses and Systematic Reviews. JAMA Surg. 2021, 156, 789–790. [Google Scholar] [CrossRef]
- Parlapiano, M.; Akyol, Ç.; Foglia, A.; Pisani, M.; Astolfi, P.; Eusebi, A.L.; Fatone, F. Selective removal of contaminants of emerging concern (CECs) from urban water cycle via Molecularly Imprinted Polymers (MIPs): Potential of upscaling and enabling reclaimed water reuse. J. Environ. Chem. Eng. 2021, 9, 105051. [Google Scholar] [CrossRef]
- Dong, C.; Shi, H.; Han, Y.; Yang, Y.; Wang, R.; Men, J. Molecularly imprinted polymers by the surface imprinting technique. Eur. Polym. J. 2021, 145, 110231. [Google Scholar] [CrossRef]
- Garnier, M.; Sabbah, M.; Ménager, C.; Griffete, N. Hybrid Molecularly Imprinted Polymers: The Future of Nanomedicine? Nanomaterials 2021, 11, 3091. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Bai, L.; Han, C.; Lv, X.; Sun, X.; Wang, T. Hybrid amino-functionalized TiO2/sodium lignosulfonate surface molecularly imprinted polymer for effective scavenging of methylene blue from wastewater. J. Clean. Prod. 2022, 337, 130457. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. Chemengineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Li, F.; Yue, S.; Zhao, Z.; Liu, K.; Wang, P.; Zhan, S. Application of molecularly imprinted polymers in the water environmental field: A review on the detection and efficient removal of emerging contaminants. Mater. Today Sustain. 2024, 27, 100904. [Google Scholar] [CrossRef]
- Zhan, C.; Cao, X.; Xu, B.; Yan, P.; Yang, T.; Ye, Z.; Chen, X. Visible light induced molecularly imprinted Dawson-type heteropoly acid cobalt (II) salt modified TiO2 composites: Enhanced photocatalytic activity for the removal of ethylparaben. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124244. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, Z.; Dong, J.; Song, M.; Liu, Y.; Liu, X.; Ma, Z.; Su, H.; Yan, Y.; Huo, P. Facile microwave synthesis of a Z-scheme imprinted ZnFe2O4/Ag/PEDOT with the specific recognition ability towards improving photocatalytic activity and selectivity for tetracycline. Chem. Eng. J. 2018, 337, 228–241. [Google Scholar] [CrossRef]
- Azizi, A.; Bottaro, C.S. A critical review of molecularly imprinted polymers for the analysis of organic pollutants in environmental water samples. J. Chromatogr. A 2020, 1614, 460603. [Google Scholar] [CrossRef]
- Sohrabi, N.; Mohammadi, R.; Ghassemzadeh, H.R.; Heris, S.S.S. Design and synthesis of a new magnetic molecularly imprinted polymer nanocomposite for specific adsorption and separation of diazinon insecticides from aqueous media. Microchem. J. 2022, 175, 107087. [Google Scholar] [CrossRef]
- Mumtaz, F.; Atif, M.; Naz, F.; Li, B.; Wang, K.; AlShehhi, M.R. Advanced Hybrid Molecular Imprinted Polymers for Antibiotics Remediation from Wastewater. ChemBioEng Rev. 2024, 11, 495–512. [Google Scholar] [CrossRef]
- Guo, J.; Yu, M.; Wei, X.; Huang, L. Preparation of Core–Shell Magnetic Molecularly Imprinted Polymer with Uniform Thin Polymer Layer for Adsorption of Dichlorophen. J. Chem. Eng. Data 2018, 63, 3068–3073. [Google Scholar] [CrossRef]
- Liu, X.; Yu, D.; Yu, Y.; Ji, S. Preparation of a magnetic molecularly imprinted polymer for selective recognition of rhodamine B. Appl. Surf. Sci. 2014, 320, 138–145. [Google Scholar] [CrossRef]
- Parisi, O.I.; Francomano, F.; Dattilo, M.; Patitucci, F.; Prete, S.; Amone, F.; Puoci, F. The Evolution of Molecular Recognition: From Antibodies to Molecularly Imprinted Polymers (MIPs) as Artificial Counterpart. J. Funct. Biomater. 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Haller, P.; Machado, I.; Torres, J.; Vila, A.; Veiga, N. Fe(III)-Complex-Imprinted Polymers for the Green Oxidative Degradation of the Methyl Orange Dye Pollutant. Polymers 2021, 13, 3127. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.H.; Ben Aissa, A.; Bessegato, G.G.; Fajardo, L.M.; Zanoni, M.V.B.; Pividori, M.I.; Sotomayor, M.D.P.T. Assessment of molecularly imprinted polymers (MIPs) in the preconcentration of disperse red 73 dye prior to photoelectrocatalytic treatment. Environ. Sci. Pollut. Res. 2017, 24, 4134–4143. [Google Scholar] [CrossRef]
- Sedelnikova, A.; Poletaeva, Y.; Golyshev, V.; Chubarov, A.; Dmitrienko, E. Preparation of Magnetic Molecularly Imprinted Polymer for Methylene Blue Capture. Magnetochemistry 2023, 9, 196. [Google Scholar] [CrossRef]
- Cantarella, M.; Di Mauro, A.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Privitera, V.; Impellizzeri, G. Selective photodegradation of paracetamol by molecularly imprinted ZnO nanonuts. Appl. Catal. B Environ. 2018, 238, 509–517. [Google Scholar] [CrossRef]
- Amiri, M.; Dashtian, K.; Ghaedi, M.; Mosleh, S. A dual surface inorganic molecularly imprinted Bi2WO6-CuO/Ag2O heterostructure with enhanced activity-selectivity towards the photocatalytic degradation of target contaminantst. Photochem. Photobiol. Sci. 2020, 19, 943–955. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Lv, Z.; Wang, B. Ultrahigh ciprofloxacin accumulation and visible-light photocatalytic degradation: Contribution of metal organic frameworks carrier in magnetic surface molecularly imprinted polymers. J. Colloid Interface Sci. 2022, 616, 872–885. [Google Scholar] [CrossRef]
- Huo, P.; Lu, Z.; Liu, X.; Wu, D.; Liu, X.; Pan, J.; Gao, X.; Guo, W.; Li, H.; Yan, Y. Preparation photocatalyst of selected pho-todegradation antibiotics by molecular imprinting technology onto TiO2/fly-ash cenospheres. Chem. Eng. J. 2012, 189–190, 75–83. [Google Scholar] [CrossRef]
- Lai, C.; Wang, M.-M.; Zeng, G.-M.; Liu, Y.-G.; Huang, D.-L.; Zhang, C.; Wang, R.-Z.; Xu, P.; Cheng, M.; Huang, C.; et al. Synthesis of surface molecular imprinted TiO2/graphene photocatalyst and its highly efficient photocatalytic degradation of target pollutant under visible light irradiation. Appl. Surf. Sci. 2016, 390, 368–376. [Google Scholar] [CrossRef]
- Li, D.; Yuan, R.; Zhou, B.; Chen, H. Selective photocatalytic removal of sulfonamide antibiotics: The performance differences in molecularly imprinted TiO2 synthesized using four template molecules. J. Clean. Prod. 2023, 383, 135470. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Li, X.; Liu, C.; Wang, H.; Huo, P.; Yan, Y.S. Molecularly imprinted Ag/Ag3VO4/g-C3N4 Z-scheme photocatalysts for enhanced preferential removal of tetracycline. J. Colloid Interface Sci. 2019, 552, 271–286. [Google Scholar] [CrossRef]
- Peng, K.; Liu, X.; Wu, X.; Yu, H.; He, J.; Chen, K.; Zhu, L.; Wang, X. Study on the preparation of molecularly imprinted ZrO2-TiO2 photocatalyst and the degradation performance of hydroquinone. Environ. Sci. Pollut. Res. 2023, 30, 83575–83586. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Wang, X.; Meng, X. Evaluation of photocatalytic selectivity of Ag/Zn modified molecularly imprinted TiO2 by multiwavelength measurement. Sci. Total. Environ. 2020, 703, 134732. [Google Scholar] [CrossRef]
- Liu, R.; Han, X.; Liu, R.; Qi, Z.; Ren, B.; Sun, Y. Molecularly imprinted Fe3O4/g-C3N4/TiO2 catalyst for selective photodegradation of chlorotetracycline. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 680, 132691. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.-W.; Yang, X.-T.; Zhu, Y.-Z.; Wang, H.-F. Afterglow-catalysis and molecular imprinting: A promising union for elevating selectivity in degradation of antibiotics. Appl. Catal. B Environ. 2022, 305, 121025. [Google Scholar] [CrossRef]
- Li, X.; Yang, B.; Xiao, K.; Duan, H.; Wan, J.; Zhao, H. Targeted degradation of refractory organic compounds in wastewaters based on molecular imprinting catalysts. Water Res. 2021, 203, 117541. [Google Scholar] [CrossRef]
- Xie, Y.; Wan, J.; Yan, Z.; Wang, Y.; Xiao, T.; Hou, J.; Chen, H. Targeted degradation of sulfamethoxazole in wastewater by molecularly imprinted MOFs in advanced oxidation processes: Degradation pathways and mechanism. Chem. Eng. J. 2022, 429, 132237. [Google Scholar] [CrossRef]
- Ding, S.; Wan, J.; Ma, Y.; Wang, Y.; Li, X.; Sun, J.; Pu, M. Targeted degradation of dimethyl phthalate by activating persulfate using molecularly imprinted Fe-MOF-74. Chemosphere 2021, 270, 128620. [Google Scholar] [CrossRef]
- Chai, S.; Zhao, G.; Zhang, Y.-N.; Wang, Y.; Nong, F.; Li, M.; Li, D. Selective Photoelectrocatalytic Degradation of Recalcitrant Contaminant Driven by an n-P Heterojunction Nanoelectrode with Molecular Recognition Ability. Environ. Sci. Technol. 2012, 46, 10182–10190. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yang, R.; Li, J.; Qu, L. A highly sensitive and selective electrochemical sensor based on polydopamine functionalized graphene and molecularly imprinted polymer for the 2,4-dichlorophenol recognition and detection. Talanta 2019, 195, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Foroughirad, S.; Arabzadeh, N.; Mohammadi, A.; Khosravi, A. Synthesis and characterization of novel water-compatible magnetic molecularly imprinted polymer for tartrazine. J. Chin. Adv. Mater. Soc. 2018, 6, 706–721. [Google Scholar] [CrossRef]
- Fang, L.; Tang, K.; Wei, D.; Zhang, Y.; Zhou, Y. Photocatalytic degradation of norfloxacin by magnetic molecularly imprinted polymers: Influencing factors and mechanisms. Environ. Technol. 2021, 44, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Miao, Y.; Wei, D.; Zhang, Y.; Zhou, Y. Efficient removal of norfloxacin in water using magnetic molecularly imprinted polymer. Chemosphere 2021, 262, 128032. [Google Scholar] [CrossRef]
- Lu, Y.C.; Mao, J.H.; Zhang, W.; Wang, C.; Cao, M.; Wang, X.D.; Wang, K.Y.; Xiong, X.H. A novel strategy for selective removal and rapid collection of triclosan from aquatic environment using magnetic molecularly imprinted nano−polymers. Chemosphere 2020, 238, 124640. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Yan, R.; Fu, X.; Wang, G.; Wang, Y.; Li, Z.; Zhang, X.; Hou, J. Facile fabrication of snowman-like magnetic molecularly imprinted polymer microspheres for bisphenol A via one-step Pickering emulsion polymerization. React. Funct. Polym. 2021, 164, 104911. [Google Scholar] [CrossRef]
- Wahab, A.; Minhas, M.A.; Shaikh, H.; Xiao, H.-M.; Malik, M.I. Enhancement in photocatalytic selectivity of TiO2-based nano-catalyst through molecular imprinting technology. Environ. Sci. Pollut. Res. Int. 2023, 30, 121929–121947. [Google Scholar] [CrossRef]
- Wang, J.; Bai, Z. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017, 312, 79–98. [Google Scholar] [CrossRef]
- Manikandan, R.; Kim, M.-J.; Jang, H.-G.; Mugunthan, A.; Kim, C.-S.; Yoon, J.-H.; Lee, J.; Chung, K.W.; Chang, S.-C. Fabrication of bio-mimic nanozyme based on Mxene@AuNPs and molecular imprinted poly(thionine) films for creatinine detection. Biosens. Bioelectron. 2025, 271, 117075. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Yarman, A.; Zhang, X.; Scheller, F.W. Electrochemical MIP Sensors for Environmental Analysis. In Biosensors for the Marine Environment; Springer: Cham, Switzerland, 2023; pp. 139–164. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in Drinking Water—A Review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Muthwa, S.F.; Chimuka, L. Determination of triclosan and ketoprofen in river water and wastewater by solid phase extraction and high performance liquid chromatography. S. Afr. J. Chem. 2014, 67, 143–150. [Google Scholar]
- Yin, X.; Long, J.; Xi, Y.; Luo, X. Recovery of Silver from Wastewater Using a New Magnetic Photocatalytic Ion-Imprinted Polymer. ACS Sustain. Chem. Eng. 2017, 5, 2090–2097. [Google Scholar] [CrossRef]
- Le Minh Tri, N.; Kim, J.; Giang, B.L.; Al Tahtamouni, T.; Huong, P.T.; Lee, C.; Viet, N.M.; Trung, D.Q. Ag-doped graphitic carbon nitride photocatalyst with remarkably enhanced photocatalytic activity towards antibiotic in hospital wastewater under solar light. J. Ind. Eng. Chem. 2019, 80, 597–605. [Google Scholar] [CrossRef]
- Zhang, P.; Mo, Z.; Han, L.; Wang, Y.; Zhao, G.; Zhang, C.; Li, Z. Magnetic recyclable TiO2/multi-walled carbon nanotube nanocomposite: Synthesis, characterization and enhanced photocatalytic activity. J. Mol. Catal. A Chem. 2015, 402, 17–22. [Google Scholar] [CrossRef]
- Khan, S.; Wong, A.; Zanoni, M.V.B.; Sotomayor, M.D.P.T. Electrochemical sensors based on biomimetic magnetic molecularly imprinted polymer for selective quantification of methyl green in environmental samples. Mater. Sci. Eng. C 2019, 103, 109825. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, Y.; Liu, S.; Luo, M.; Du, J.; Fan, J.; Xiong, H.; Peng, H. A novel magnetic fluorescent molecularly imprinted sensor for highly selective and sensitive detection of 4-nitrophenol in food samples through a dual-recognition mechanism. Food Chem. 2021, 348, 129126. [Google Scholar] [CrossRef]
- Afzali, M.; Mostafavi, A.; Shamspur, T. A novel electrochemical sensor based on magnetic core@shell molecularly imprinted nanocomposite (Fe3O4@graphene oxide@MIP) for sensitive and selective determination of anticancer drug capecitabine. Arab. J. Chem. 2020, 13, 6626–6638. [Google Scholar] [CrossRef]
- Han, S.; Yao, A.; Ding, Y.; Leng, Q.; Teng, F. A molecularly imprinted polymer based on MOF and deep eutectic solvent for selective recognition and adsorption of bovine hemoglobin. Anal. Bioanal. Chem. 2021, 413, 5409–5417. [Google Scholar] [CrossRef]
- Samy, M.; Mensah, K.; Alalm, M.G. A review on photodegradation mechanism of bio-resistant pollutants: Analytical methods, transformation products, and toxicity assessment. J. Water Process. Eng. 2022, 49, 103151. [Google Scholar] [CrossRef]
- Klementová, Š.; Poncarová, M.; Langhansová, H.; Lieskovská, J.; Kahoun, D.; Fojtíková, P. Photodegradation of fluoroquinolones in aqueous solution under light conditions relevant to surface waters, toxicity assessment of photoproduct mixtures. Environ. Sci. Pollut. Res. 2022, 29, 13941–13962. [Google Scholar] [CrossRef]
- Li, X.; Wei, H.; Song, T.; Lu, H.; Wang, X. A review of the photocatalytic degradation of organic pollutants in water by modified TiO2. Water Sci. Technol. 2023, 88, 1495–1507. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.R.; Prasasti, A.; Islamiah, S.; Firdaus, A.N.; Marita, A.W.; Fajriyah, S.; Noviyanti, A.R.; Eddy, D.R. Degradation of Ciprofloxacin by Titanium Dioxide (TiO2) Nanoparticles: Optimization of Conditions, Toxicity, and Degradation Pathway. Bull. Chem. React. Eng. Catal. 2021, 16, 752–762. [Google Scholar] [CrossRef]
- Bikerchalen, S.; Akhsassi, B.; Bakiz, B.; Villain, S.; Taoufyq, A.; Guinneton, F.; Gavarri, J.-R.; Benlhachemi, A. Photocatalytic degradation of Rhodamine B dye over oxygen-rich bismuth oxychloride Bi24O31Cl10 photocatalyst under UV and Visible light irradiation: Pathways and mechanism. J. Phys. Chem. Solids 2024, 196, 112342. [Google Scholar] [CrossRef]
- Haleem, A.; Ullah, M.; Rehman, S.U.; Shah, A.; Farooq, M.; Saeed, T.; Ullah, I.; Li, H. In-Depth Photocatalytic Degradation Mechanism of the Extensively Used Dyes Malachite Green, Methylene Blue, Congo Red, and Rhodamine B via Covalent Organic Framework-Based Photocatalysts. Water 2024, 16, 1588. [Google Scholar] [CrossRef]
- Chi, H.; Li, C.; Huang, M.; Wan, J.; Zhou, X.; Yan, B. Targeted accumulation and spatial confinement effect of Fe(II)-MOFs@MIP for efficiently removing low concentration dibutyl phthalate. Chem. Eng. J. 2021, 424, 130367. [Google Scholar] [CrossRef]
- Aoulad El Hadj Ali, Y.; Azzouz, A.; Ahrouch, M.; Lamaoui, A.; Raza, N.; Lahcen, A.A. Molecular imprinting technology for next-generation water treatment via photocatalysis and selective pollutant adsorption. J. Environ. Chem. Eng. 2024, 12, 112768. [Google Scholar] [CrossRef]
- Del Sole, R.; Mele, G.; Bloise, E.; Mergola, L. Green Aspects in Molecularly Imprinted Polymers by Biomass Waste Utilization. Polymers 2021, 13, 2430. [Google Scholar] [CrossRef]
- ZLiu, Z.; Xu, Z.; Wang, D.; Yang, Y.; Duan, Y.; Ma, L.; Lin, T.; Liu, H. A Review on Molecularly Imprinted Polymers Preparation by Computational Simulation-Aided Methods. Polymers 2021, 13, 2657. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Obare, S.O.; Wei, J. Molecularly imprinted polymers as chemosensors for organophosphate pesticide detection and environmental applications. TrAC Trends Anal. Chem. 2023, 167, 117231. [Google Scholar] [CrossRef]
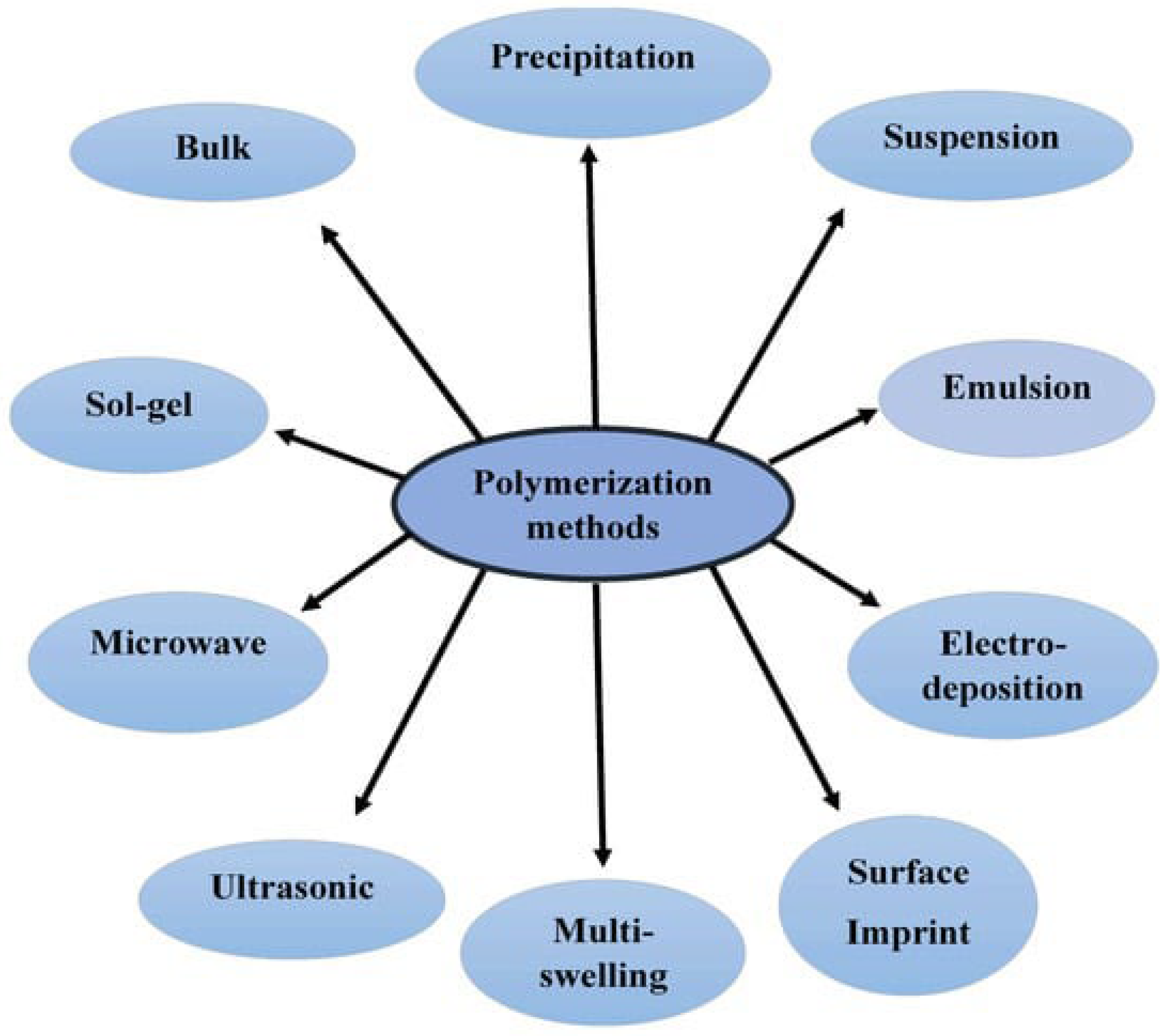
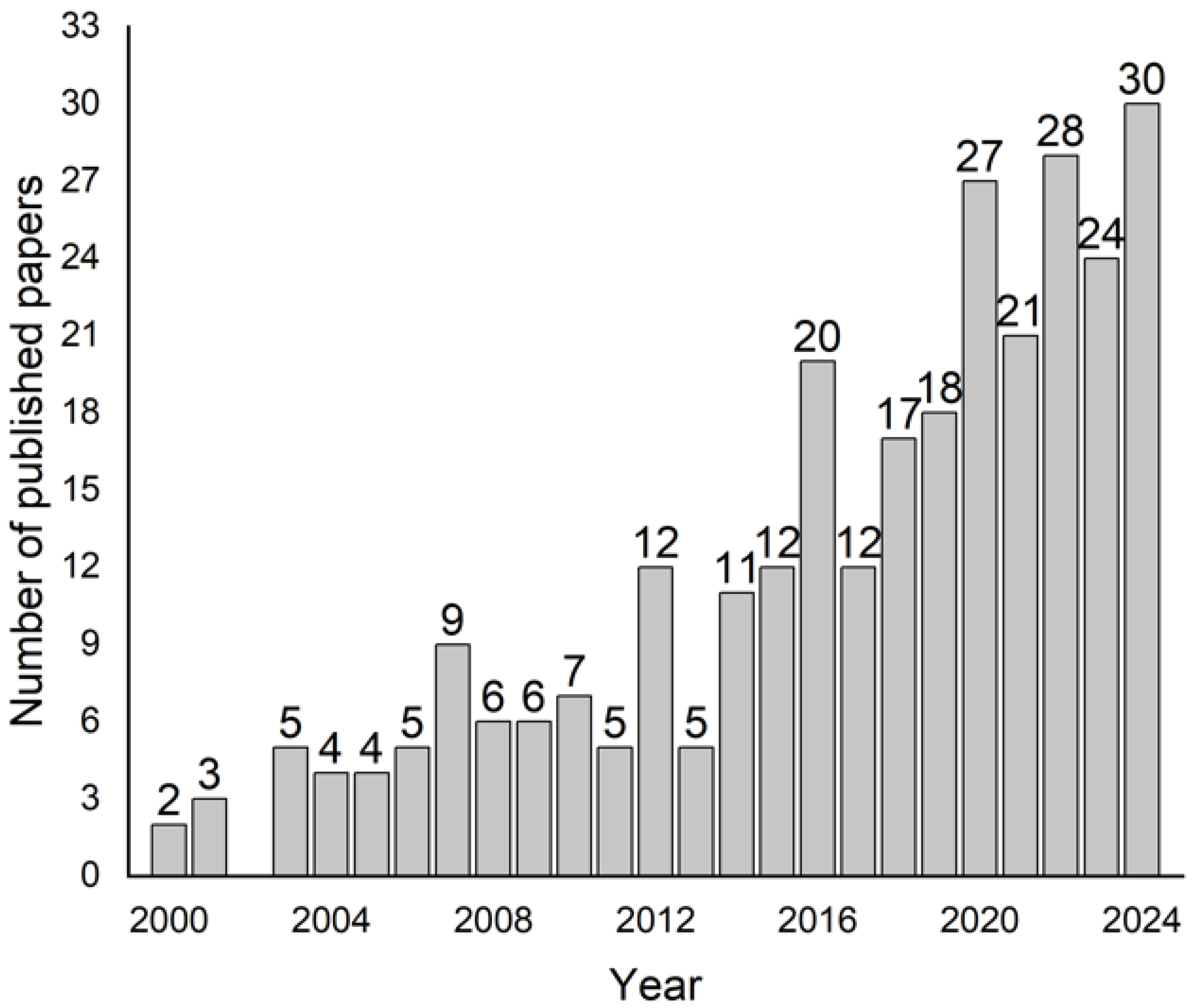



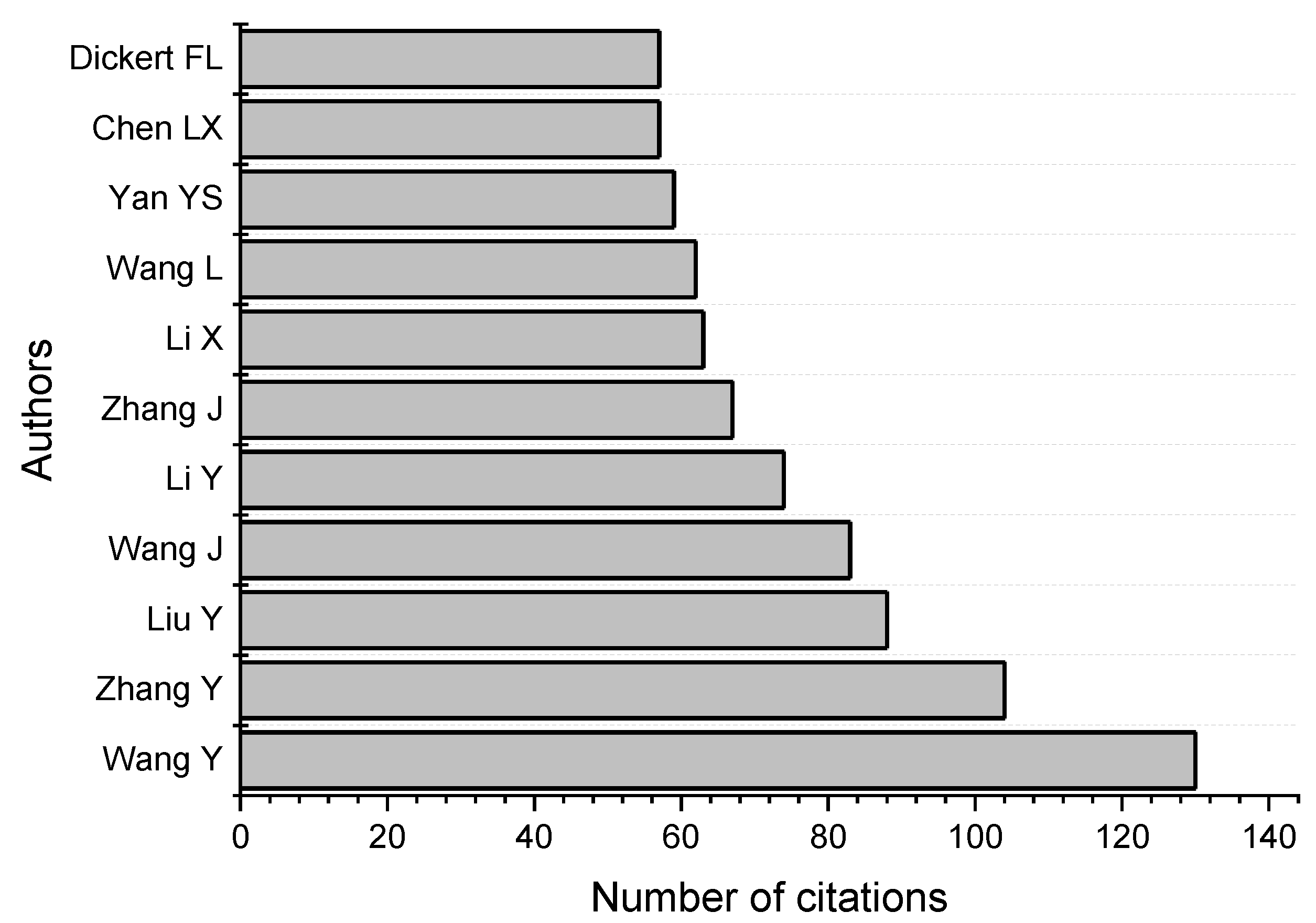
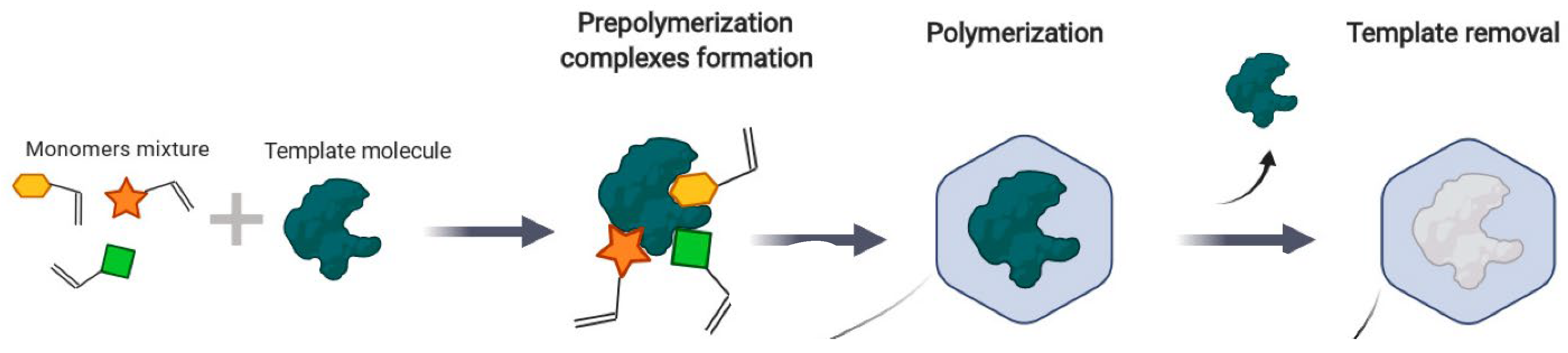
| Top | Publication Titles | Published Papers |
|---|---|---|
| 1 | Analytica Chimica Acta | 9 |
| 1 | Journal of Chromatography A | 9 |
| 2 | Analytical and Bioanalytical Chemistry | 8 |
| 2 | Environmental Science and Pollution Research | 8 |
| 2 | RSC Advances | 8 |
| 3 | Journal of Separation Science | 7 |
| 4 | Chemical Engineering Journal | 6 |
| 4 | Journal of Hazardous Materials | 6 |
| 4 | Talanta | 6 |
| 5 | Journal of Environmental Chemical Engineering | 5 |
| 5 | Molecules | 5 |
| 5 | Sensors and Actuators B Chemical | 5 |
| Top | Authors | Published Papers |
|---|---|---|
| 1 | Dickert F.L. | 10 |
| 2 | Lieberzeit P.A. | 8 |
| 2 | Wang Y. | 8 |
| 3 | Pan J.M. | 7 |
| 4 | Xu Y. | 6 |
| 4 | Yan Y.S. | 6 |
| 5 | Chen L.X. | 5 |
| 5 | Li Y. | 5 |
| 5 | Shen X.T. | 5 |
| 5 | Wang J. | 5 |
| 5 | Wang N. | 5 |
| 5 | Wang X. | 5 |
| 5 | Zhang Y. | 5 |
| Catalyst Type | Synthesis Method | Light Source | Pollutant | pH | Degradation Time (h) | Degradation Efficiency (%) | Adsorption Capacity (mg/g) | Enhancement Factor vs. NIP | Ref |
|---|---|---|---|---|---|---|---|---|---|
| ZnO/CuFe2O4 MIP | Surface imprinting | Simulated natural light of a xenon lamp | Methylene Blue | - | 2.0 | 95.8 | - | 1.37× | [28] |
| TiO2 MIP | Liquid phase deposition | Xenon lamp | Refractory Organics | - | 2.0 | 60% higher than NIP | - | 1.60× | [60] |
| NH2-MIL-53(Fe) MIP | Surface imprinting | - | Sulfamethoxazole | 7.0 | 4.0 | - | 38.04 | - | [61] |
| Fe-MOF/MIP | In situ growth | - | Dimethyl Phthalate | - | 0.5 | - | - | 1.8× | [62] |
| MIP-BDD Electrode | Liquidphase deposition | Mercury lamp with 365 nm | Bisphenol A | - | 2.0 | - | - | 2.5× | [63] |
| MIP-Graphene Electrode | Electropolymerization | - | 2,4-Dichlorophenol | 2.0–7.0 | - | - | - | BOD5/CODCr ratio ↑ from 0.186 to 0.412 | [64] |
| Fe3O4@ SiO2 @MIPs | Surface radical polymerization | - | Tartrazine | 1.0–8.0 | 0.03 | - | 101 | - | [65] |
| CoFe2O4@TiO2@MIP | - | UV irradiation | Norfloxacin | - | 2.5 | 84.2 | - | - | [66] |
| CoFe2O4@TiO2-MMIP | Improved liquid phase deposition | UV irradiation | Norfloxacin | 3.0–13.0 | 1.0 | 99.4 | 14.26 | - | [67] |
| Fe3O4@ SiO2 @MIPs | Coprecipitation | - | Rhodamine B | - | 3.0 | - | 2.35 | - | [44] |
| Fe3O4@SiO2@MIPs | Magnetic polymerization | - | Triclosan | 2.0–7.0 | 0.17 | 94.2 | 0.053 | - | [68] |
| Fe3O4@MIPs | Pickering emulsion polymerization | - | Bisphenol A | - | 1.0 | - | 0.0162 | - | [69] |
| Technology | Selectivity | Adsorption Capacity | Degradation Efficiency | Cost | Environmental Impact |
|---|---|---|---|---|---|
| MIPs | High | Moderate to High | Moderate to High | Moderate | Low to Moderate |
| Nanomaterials | Moderate to High | High | High | High | Potentially toxic |
| Bio-adsorbents | Moderate | High | Low to Moderate | Low | Biodegradable |
| Activated Carbon | Moderate | High | Low | Moderate | Low |
| MOFs | High | Very High | High | High | Potential toxicity |
| Enzyme-Based Systems | High | Moderate | High | High | Biodegradable |
| Photocatalysts | Moderate to High | Moderate to High | Very High | High | Potential toxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Fernández, L.A.; Mizaikoff, B.; Medellín-Castillo, N.A.; Vilasó-Cadre, J.E.; Reyes-Domínguez, I.A.; Díaz de León-Martínez, L.; Huber, A.; Sánchez-Polo, M. Molecularly Imprinted Polymers for Pollutant Capture and Degradation: A Snapshot Review. Processes 2025, 13, 1086. https://doi.org/10.3390/pr13041086
González-Fernández LA, Mizaikoff B, Medellín-Castillo NA, Vilasó-Cadre JE, Reyes-Domínguez IA, Díaz de León-Martínez L, Huber A, Sánchez-Polo M. Molecularly Imprinted Polymers for Pollutant Capture and Degradation: A Snapshot Review. Processes. 2025; 13(4):1086. https://doi.org/10.3390/pr13041086
Chicago/Turabian StyleGonzález-Fernández, Lázaro Adrián, Boris Mizaikoff, Nahum Andrés Medellín-Castillo, Javier Ernesto Vilasó-Cadre, Iván A. Reyes-Domínguez, Lorena Díaz de León-Martínez, Amelie Huber, and Manuel Sánchez-Polo. 2025. "Molecularly Imprinted Polymers for Pollutant Capture and Degradation: A Snapshot Review" Processes 13, no. 4: 1086. https://doi.org/10.3390/pr13041086
APA StyleGonzález-Fernández, L. A., Mizaikoff, B., Medellín-Castillo, N. A., Vilasó-Cadre, J. E., Reyes-Domínguez, I. A., Díaz de León-Martínez, L., Huber, A., & Sánchez-Polo, M. (2025). Molecularly Imprinted Polymers for Pollutant Capture and Degradation: A Snapshot Review. Processes, 13(4), 1086. https://doi.org/10.3390/pr13041086










