1. Introduction
Agriculture plays a pivotal role in feeding the growing global population. Yield losses of up to 33% are estimated in areas impacted by weeds for crops such as maize, wheat, and soybean [
1,
2,
3]. Herbicides account for approximately 60% of all pesticides used worldwide, with most large-scale agricultural practices relying on these chemical compounds for weed control [
4]. Although herbicides effectively control weeds, concerns about their adverse environmental impacts are increasing [
3]. The development of resistance in weeds to various active ingredients used for their control is a major driver in the search for alternatives to current herbicides [
5]. Globally, more than 500 cases of resistant weeds have been reported. Of the 31 commercially used herbicide sites of action, 21 have encountered at least one resistant weed species [
6]. Given the externalities associated with synthetic herbicides, identifying and testing new products is imperative to overcome these challenges.
In this way, the use of bioherbicides is an alternative to becoming more environmentally friendly for weed control. Although these bioherbicides are promising, their efficacy remains a challenge, as evidenced by their limited commercial and industrial production, mainly when using conventional biological control (living cells as active ingredients), since the microorganisms are highly host-specific, limiting a broad spectrum. Other limiting aspects are related to stabilization for a long shelf-life and performance in the field since the control level is much dependent on the environmental factors [
7].
Due to the limitations of biological control, microbial metabolites have garnered growing interest in scientific research due to their potential either for direct application in weed control or as templates for developing synthetic analogs [
7]. These compounds exhibit a wide structural diversity and unique mechanisms of action, often distinct from conventional chemical herbicides [
8,
9]. Such features make them promising for addressing herbicide resistance issues while reducing synthetic herbicides’ environmental impacts. Furthermore, investigating microbial metabolites enables the discovery of novel biochemical pathways and molecular targets, fostering innovation in sustainable crop management, as highlighted by Schmaltz et al. [
10]. These molecules may act by altering physiological and biochemical processes in plant metabolism or even causing direct damage to plant tissues [
11,
12]. In some cases, phytotoxins can serve as substitutes for the pathogen itself [
11,
12,
13].
The herbicidal activity of various phytotoxic compounds has been well documented in the literature, as recently reviewed by Macías-Rubalcava and Garrido-Santos [
2].
Phoma is a genus that encompasses species of phytopathogens, endophytes, and saprophytes [
14,
15,
16,
17]. Species within this genus produce a wide range of metabolites with diverse biological activities. The production of herbicidal compounds by fungi of the
Phoma genus has been investigated and reported by several authors [
14,
18,
19,
20,
21,
22,
23,
24,
25]. The two most common forms of fungal cultivation are solid-state fermentation and submerged fermentation. When compared, submerged fermentation offers notable advantages, including ease of process parameter control, larger volume processing, shorter cultivation time, and greater scalability. These characteristics make submerged fermentation the preferred method for the production of fungal metabolites. However, despite these advantages, the recovery and purification processes of metabolites can be more complex and require a greater number of unit operations, as well as larger volumes of solvents. In these conditions, the yield of metabolites in a liquid medium is usually lower, making the optimization of cultivation conditions essential to maximize production.
In previous studies, our group already demonstrated the potential of biomolecules from
Phoma dimorpha as bioherbicides [
18,
21,
22,
23,
24]. However, all the studies focused on the use of a crude extract free of cells. For this reason, the present study focuses on the isolation of metabolites produced by
Phoma dimorpha in submerged fermentation and the evaluation of the herbicidal potential on
Amaranthus retroflexus and
Raphanus sativus. From this paper, it will be possible to advance the knowledge frontier for the development of a product.
2. Materials and Methods
2.1. Microorganism and Culture Conditions
The Phoma dimorpha strain (NRRL 43879) was obtained from the culture collection of the Agricultural Research Service (ARS, Washington, DC, USA). For long-term storage, the strain was lyophilized and kept at 4 °C. As an alternative, ready-to-use storage method, the strain was preserved on rice grains under the same conditions. The strain was reactivated by culturing it on potato dextrose agar (PDA) plates (Kasvi, São José dos Pinhais, Brazil), incubated at 25 °C for 15 days. The inoculum was prepared by scraping the mycelial surface to obtain a conidial suspension adjusted to a final concentration of 1 × 108 conidia mL−1. For culture preparation, 1 mL of the inoculum was transferred to 250 mL Erlenmeyer flasks containing 99 mL of potato dextrose broth (PDB) (HiMedia Laboratories, Thane, India). The cultures were incubated in an orbital shaker at 28 °C, 150 rpm, for 7 days without light. The mycelium was separated from the broth by filter paper filtration, followed by microbial cell removal using sequential filtration with 0.45 µm and 0.22 µm pore size membranes (Filtrilo, Colombo, Brazil). The cell-free broth obtained after filtration was designated as cell-free filtrate and subjected to compound extraction and purification processes.
2.2. Compound Extraction and Fractionation
In a separation funnel, the cell-free filtrate was subjected to successive extractions using equivalent volumes of ethyl acetate (two extractions). The organic phases were combined, dehydrated with anhydrous magnesium sulfate, and concentrated under reduced pressure at 40 °C. The resulting extract (162.3 mg) was fractionated and purified by column chromatography (CC). The chromatographic column, with a 2.0 cm diameter, was packed with silica gel 60 Å (h = 15 cm; 70–230 mesh) (Sigma Aldrich, São Paulo, Brazil). The solvent system used for elution consisted of a hexane–isopropyl alcohol mixture (hexane–IPrOH), varying from 100:0 to 70:30 (v/v). A total of 50 mL of each solvent combination (100:0; 90:10, and so on) was used, and 30 fractions were collected. These fractions were analyzed by TLC on aluminum chromatoplates coated with silica gel 60 Å (0.5 mm) with a fluorescence indicator F254 (Sigma Aldrich, São Paulo, Brazil). The mobile phase for TLC was hexane–IPrOH (80:20 v/v). The compounds were visualized under UV light at 254 nm and 365 nm using a UV chamber. Three homogeneous fractions (Fr1: 34.3 mg, Fr2: 41.0 mg, and Fr3: 24.7 mg) were obtained. Fraction 1, which consisted of at least three different compounds, was subsequently purified.
To purify the compounds present in Fraction 1, 3.0 L of cell-free filtrate was prepared and extracted as described above. The compounds were purified using a column with an internal diameter of 3.5 cm packed with silica gel 60 Å (230–400 mesh, h = 12 cm). The mobile phase consisted of a hexane–iPrOH solvent system (90:10 v/v). A total of 115 fractions, each 0.5–0.7 mL, were collected. Fractions 47–70 were combined as they contained the first purified compound (245 mg). The purification of the other compounds was not achieved.
2.3. Bioassays
2.3.1. Effect of Cell-Free Broth on Germination and Emergence of Amaranthus retroflexus
The phytotoxicity of the cell-free filtrate, obtained after 7 days of microorganism cultivation, was evaluated based on its capacity to inhibit the germination and seedling emergence of
Amaranthus retroflexus. The experiment was conducted in acrylic germination boxes (11 × 11 × 3.5 cm) filled with commercial substrate (MecPlant, Telêmaco Borba, Brazil). Each box was seeded with 1.0 g of
A. retroflexus seeds; then, 5 mL of the corresponding treatment sample was applied. The controls included sterile unmetabolized culture medium (T2) and sterile distilled water (T3). The bioassay was performed using three treatments in triplicate. After seeding and treatment application, the boxes were kept in a greenhouse under controlled conditions (25 °C, 12 h photoperiod) for 10 days. The treatments were manually irrigated daily. The results were evaluated and expressed as germination control (%) based on the scale proposed by Frans and Crowley [
26], analyzed by ANOVA, and compared using Tukey’s test with a 5% significance level.
2.3.2. Screening for Herbicidal Potential of Fractions Obtained from the First Fractionation
This bioassay, divided into two parts, aimed to identify which of the three fractions obtained from the first fractionation had herbicidal potential against A. retroflexus, an important cosmopolitan weed.
First Part: A leaf puncture bioassay was conducted with intact
A. retroflexus plants following the methodology described by Tanaka et al. [
27]. Fractions from the first fractionation were evaluated by preparing 2.0 mg mL
−1 solutions, dissolving 2 mg of each sample in 1000 µL of 10% dimethyl sulfoxide (DMSO). The controls included a 10% DMSO solution (C1) and sterile distilled water (C2).
A. retroflexus seeds, obtained from a specialized seed bank (AGS Sementes, Paraná, Brazil) and stored at 4 °C to preserve viability, were cultivated in 200 mL pots containing commercial substrate (MecPlant). The plants were grown in a greenhouse under controlled conditions (25 °C, 12 h photoperiod) for 15 days and manually irrigated daily. Each pot contained a single plant, which was considered an experimental unit.
A sterile needle was used to puncture four fully developed leaves per plant. The treatments were applied directly to the punctures using a capillary tube, with 10 µL per application. After treatment application, the plants were kept under the same greenhouse conditions and evaluated after 24 h. Symptoms were characterized based on the phytotoxicity scale proposed by Frans and Crowley [
24], considering the percentage of foliar area affected by lesions. Each treatment was performed in sextuplicate, and the results were expressed as mean ± standard deviation (SD), analyzed by ANOVA, and compared using Tukey’s test with a 5% significance level.
Second Part: Fraction 1 was prepared for application to A. retroflexus plants within 15 days of development. It was diluted in sterile distilled water containing 0.1% Tween 80, resulting in a final concentration of 600 µg mL−1. A total of 6 mL of the formulation was produced and distributed among six experimental units. The controls included two treatments: sterile distilled water with 0.1% Tween 80 and 10% DMSO (C1) and pure sterile distilled water (C2), forming three experimental groups.
The formulations were applied manually using a spray bottle, ensuring complete foliar coverage. After application, the plants were kept under controlled greenhouse conditions (25 °C, 12 h photoperiod) and manually irrigated daily. Phytotoxic effects were assessed four days after application using the Frans and Crowley [
26] scale, classifying the percentage of damaged foliar area. The experiments were conducted in sextuplicate, with the results expressed as mean ± SD, analyzed by ANOVA, and the means compared using Tukey’s test with a 5% significance level.
2.3.3. Effect of Purified Compound on Germination and Emergence of R. sativus Cultivated in Substrate
The purified compound (PC), obtained as described in
Section 2.2, was evaluated for its inhibitory potential in pre-emergence bioassays using
Raphanus sativus (radish) as a bioindicator plant. This species was chosen because of its rapid and uniform germination, which reduces the experiment’s response time.
Seeds of radish (R. sativus L., Sparker variety), free of agrochemicals, were purchased locally in Santa Maria-RS and stored at 4 °C to maintain viability. Before the bioassay, the seeds underwent an asepsis protocol: immersion for 1 min in 70% (v/v) ethanol, followed by 1 min in 1% sodium hypochlorite solution, and two subsequent washes in sterile distilled water (1 min each).
The PC sample was dissolved in DMSO (1%) with 0.877 g of Tween 80, resulting in a final concentration of 24.50 mg mL−1 of PC. A treatment with a concentration of 12.25 mg mL−1 of PC was included, while the controls consisted of sterile distilled water and a solution of DMSO (1%) with Tween 80.
The bioassays were conducted in transparent acrylic boxes (5 × 5 cm) filled with 7 g of peat-based fertile commercial substrate (Carolina Soil®-Garden, Carolina Soil, Rio de Janeiro, Brazil) moistened with 7 mL of sterile distilled water. Four replicates, each containing four seeds, were used. For the treatment, 130 µL of the sample was applied directly onto the seeds covered by the substrate. The boxes were sealed and incubated in a germination chamber (POL-EKO-APARATURA, Wodzisław Śląski, Poland) at 20 °C with a 12 h photoperiod for 7 days.
At the end of the incubation period, the following parameters were evaluated: percentage of emergence, calculated using Equation (1), and total seedling length, expressed in centimeters. The data were subjected to analysis of variance (ANOVA), compared using Tukey’s test with a 5% significance level, and presented as mean ± SD.
2.3.4. Statistical Analysis
Before performing ANOVA, the normality of the data was verified using the Shapiro–Wilk test, the results of which indicated that the data were normally distributed (p > 0.05). After finding significant differences between the treatments using ANOVA (p < 0.05), the Tukey test was used as a method for multiple comparisons. The Tukey test was chosen because it is suitable when all means are to be compared with each other, controlling type I error in analyses with multiple comparisons. In addition, the test assumes homogeneity of variances and normality of residuals, criteria that were previously met.
3. Results
3.1. Effect of Cell-Free Culture Filtrate on the Germination and Emergence of Amaranthus retroflexus
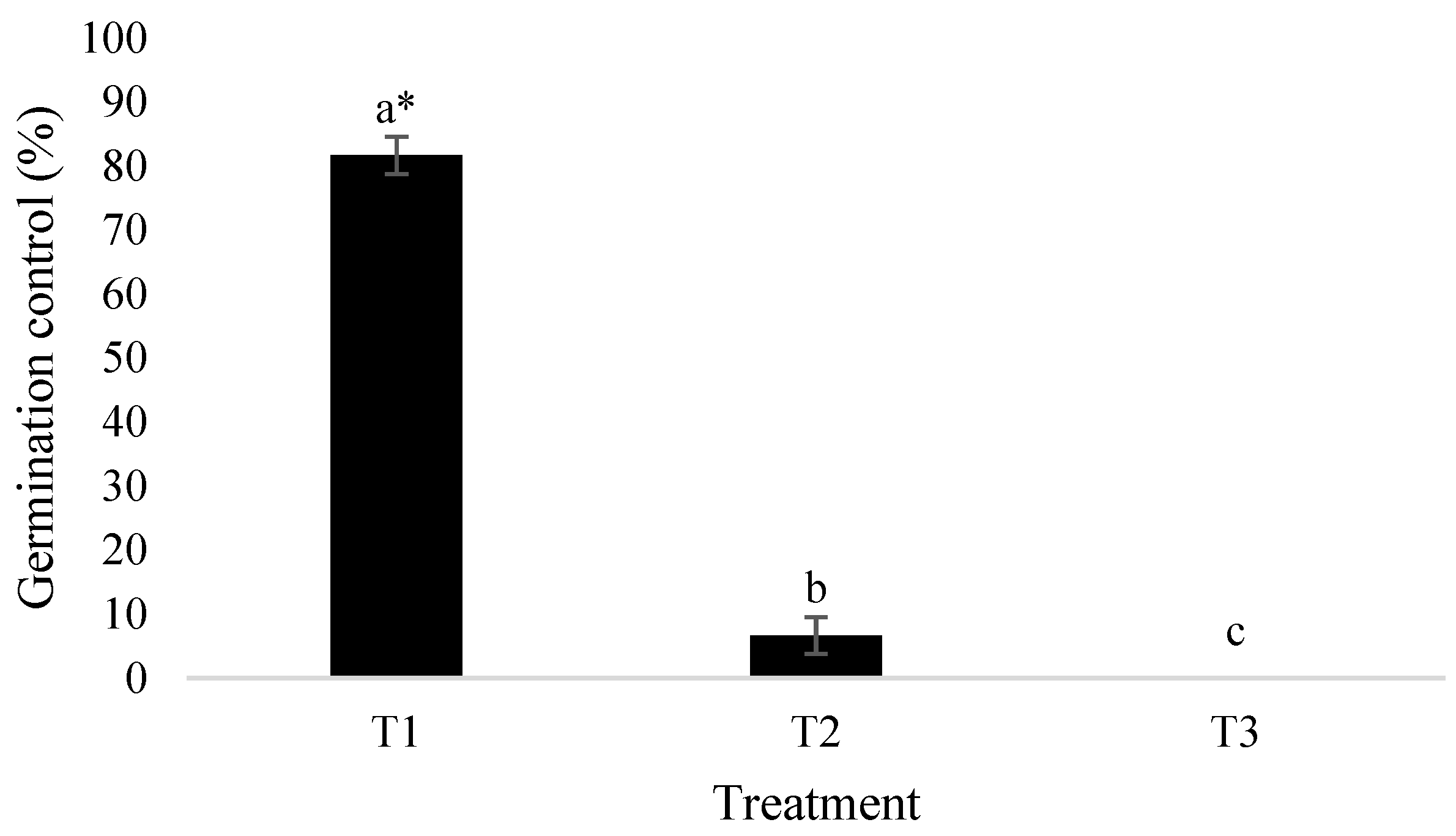
The data regarding seedling emergence, evaluated ten days after sowing, and the application of the cell-free filtrate obtained from
P. dimorpha culture are presented in
Figure 1. Treatment T1 (cell-free filtrate) showed a significant reduction in the germination and emergence of
A. retroflexus seedlings, achieving 82% germination control (±2.89), whereas control treatment T2 (sterile culture medium) resulted in 7% germination control (±2.89). Treatment 3 (distilled water) was considered 100% germination. These findings demonstrate that
P. dimorpha can produce metabolites with herbicidal activity, highlighting its potential for managing agriculturally important weeds, such as
A. retroflexus.
Figure 2 visually illustrates the differences between treatments T1 and T2, emphasizing the inhibitory effect of the filtrate on germination.
3.2. Herbicidal Potential of Fractions
The three homogeneous fractions obtained from the first fractionation process in this study were tested in a leaf puncture bioassay on intact
A. retroflexus plants to identify which fraction(s) contained metabolites with herbicidal potential.
Table 1 presents the phytotoxic effects caused by the tested fractions.
T1, composed of Fraction 1, demonstrated the ability to cause irreversible damage to A. retroflexus leaves and led to plant death, evidencing the presence of herbicidal compounds in this fraction. The control efficacy obtained in this treatment was 96%, indicating that Fraction 1 contains compounds with high potential for A. retroflexus control.
Subsequently, Fraction 1, preformulated with Tween 80 at 0.1%, was tested through a foliar application on
A. retroflexus plants after 15 days of cultivation. The resulting formulation yielded a final concentration of 600 µg mL
−1 of the fraction, and 1 mL was applied per plant. The results obtained four days after treatment (DAT) are shown in
Table 2.
Figure 3 compares an experimental unit immediately after T1 application and at the end of the experiment. These findings corroborate the evidence from the leaf puncture bioassay, confirming that Fraction 1 contains one or more molecules with herbicidal activity against
A. retroflexus.
3.3. Purification of the Herbicidal Compound
Thin-layer chromatography (TLC) analysis of Fraction 1 revealed three distinct fluorescent bands under UV light (at 254 nm), each with a specific retention factor, indicating that this fraction contained at least three chemical compounds. The purification process was conducted using column chromatography (CC), allowing the separation and isolation of the compound with the shortest retention factor. However, the remaining two compounds exhibited chemical instability and degraded during the chromatographic process, precluding their purification by this method.
For the purification step, 3.0 L of cell-free filtrate was processed, yielding 1.2 g of crude extract through ethyl acetate extraction (twice, 1:1 v/v). From this crude extract, 245 mg of the purified compound (PC) was isolated, corresponding to a yield of 20.4% relative to the crude extract. With an oily texture and brown coloration, the PC was subsequently subjected to bioassays to evaluate its biological activity and potential application as a bioherbicide.
3.4. Effect of the Purified Compound on the Emergence of Raphanus sativus Cultivated in Substrate
Table 3 presents the emergence (%) and seedling lengths (cm) of
R. sativus grown in acrylic boxes filled with commercial substrate (Carolina Soil
®–Garden). In treatment 1 (T1), where the purified compound (PC) was applied at a concentration of 24.5 mg mL
−1, germination was reduced to 18.88%. In contrast, the control treatment with DMSO (1%) and Tween 80 (T3) exhibited significantly higher emergence rates, reaching 87.50%. Compared to T4 (pure water), T1 caused an 80% reduction in radish seedling emergence. These results clearly demonstrate the inhibitory effect of PC on the germination of the bioindicator plant. At an intermediate concentration of 12.25 mg mL
−1 (T2), the PC resulted in a 26% reduction in the emergence rate compared to T4. Statistical analysis revealed that only T1 showed significant differences compared to the other treatments.
Regarding the average seedling length, T1 also exhibited the lowest value, with a mean of 3.20 cm, representing a 4.88-fold reduction compared to the highest length recorded, i.e., 15.60 cm, observed in the control treatment with water (T4). This result reinforces the impact of the PC, particularly at the highest tested concentration, on the seedlings’ emergence and initial development.
Figure 4 illustrates the visual effects of the treatments on the emerged seedlings. In
Figure 4a, the overall experimental setup is shown, highlighting the significant impact of the PC at a concentration of 24.5 mg mL
−1 (fourth column of boxes), as evidenced by the low emergence rate and reduced seedling development.
Figure 3b provides a comparison of the average lengths of representative seedlings from each treatment, clearly demonstrating the differences between the group treated with the PC (T1 and T2) and the controls (T3 and T4). These findings support the potential of the PC as a candidate for bioherbicide development.
4. Discussion
The results presented in this study highlight the promising potential of microbial metabolites for developing bioherbicides. In the first experiment, the cell-free filtrate (T1) demonstrated a significant inhibitory effect on the germination of
Amaranthus retroflexus, resulting in a drastic reduction in the number of emerged seedlings compared to the control treatments (T2 and T3). This inhibition indicates that the culture broth contains metabolites with high phytotoxic activity capable of interfering with the germination process of the target plant. Studies have reported similar findings with microbial metabolites acting as germination inhibitors in typical weed species, underscoring their relevance for integrated weed management. Todero et al. [
24] reported that broth obtained from
Phoma sp. cultivation and concentrated through membranes (70% permeate and 30% retentate) completely inhibited the germination of
Bidens pilosa and
A. retroflexus in experiments performed on germination paper. Greenhouse experiments under conditions similar to this study demonstrated that higher broth concentrations resulted in greater control over
A. retroflexus germination. Similarly, Neto et al. [
18] evaluated the potential of cell-free fermented broth from
P. dimorpha in pre-emergence experiments with
Senna obtusifolia. Using the germination paper method, these authors concluded that the microorganism’s culture broth could inhibit the germination of the target weed by 100%.
In the second experiment, the screening of fractions revealed that Fraction 1 exhibited high efficacy in controlling A. retroflexus plants through a leaf puncture assay on intact plants, achieving a phytotoxicity of 96%. The foliar application of this fraction, formulated at 600 µg mL−1, resulted in plant death within just four days. These findings demonstrate the high efficacy of the fraction in post-emergence applications, highlighting its potential for practical use as a bioherbicide. The lethal activity observed can be attributed to the compounds present in the fraction, although only one compound was successfully purified, suggesting the need for further studies to identify other compounds degraded during the purification process.
The third experiment further reinforced the herbicidal potential of the purified compound (PC). The application of the PC at two different concentrations to
R. sativus significantly reduced seedling emergence and length, particularly at the higher concentration (24.5 mg mL
−1). The percentage of emergence in treatment T1 was statistically lower than in the controls, and the reduced average seedling length indicates a direct impact of the compound on the initial development processes of the plant. These effects are consistent with the action of microbial phytotoxins, which can alter fundamental physiological or biochemical processes in plants [
11,
12,
28].
The data obtained are promising but also reveal challenges for developing the compound as a bioherbicide. Firstly, further studies with the PC on different weed species, both in pre- and post-emergence applications, will be necessary to evaluate its spectrum of activity. Additionally, strategies to enhance its yield should be explored, as large-scale production is critical for commercial application. The degradation of other compounds in the fraction during the purification process suggests that adjustments in the extraction or purification methods are required to ensure greater stability and recovery of the metabolites.
Overall, the results obtained are of great relevance to the research and development of new herbicides based on microbial metabolites. In general, microbial metabolites exhibit mechanisms of action distinct from conventional chemical herbicides and may, in some cases, present more than one mode of action. This feature can contribute to overcoming issues, such as weed resistance and the negative environmental impacts associated with synthetic agrochemicals [
8,
29,
30]. Thus, advancements in the characterization, production, and formulation of these compounds may represent a significant step forward for sustainable agriculture.
5. Conclusions
This study explored the herbicidal metabolite production by P. dimorpha and confirmed its potential as a promising source for the development of novel bioherbicides. Among the tested extracts, Fraction 1, obtained during the initial fractionation, demonstrated significant efficacy against Amaranthus retroflexus, highlighting its potential as a reservoir of bioactive compounds. Furthermore, the purified compound (PC) isolated from Fraction 1 showed marked phytotoxic effects on Raphanus sativus, notably reducing both the emergence rate and seedling growth. Interestingly, the superior herbicidal activity of Fraction 1 compared to the purified compound suggests that additional components—possibly lost or degraded during purification—may synergistically contribute to the overall bioactivity. This highlights the importance of maintaining compound integrity during the purification process.
Looking ahead, future research will focus on optimizing purification protocols and conducting detailed structural characterization of active compounds, with an emphasis on identifying molecules with enhanced herbicidal potential. Moreover, advances in bioprocess engineering will be pursued to increase the yield of key metabolites in fermentation systems, paving the way for scalable production. These developments are essential steps toward the creation of environmentally friendly bioherbicides that can mitigate the ecological footprint of conventional chemical herbicides and contribute to global goals for sustainable agriculture, aligned with frameworks like the United Nations Sustainable Development Goals (SDGs), particularly SDG 2 (Zero Hunger), SDG 12 (Responsible Consumption and Production), and SDG 15 (Life on Land).