Abstract
This study explores the intensification of molybdenite concentrate processing through a synergistic hydrometallurgical approach using sulfuric acid, nitric acid, and their combination to enhance leaching efficiency while minimizing environmental impact. Molybdenum, a strategic metal widely used in advanced engineering and catalytic systems, presents extraction challenges due to the refractory nature of molybdenite (MoS2). The experimental approach incorporated oxygen sparging and mechanoactivation to improve dissolution kinetics and molybdenum availability. A central composite design (CCD) of response surface methodology (RSM) was employed to develop a predictive model for optimizing the leaching parameters. Acid concentration, temperature, and leaching time were systematically varied, allowing for the identification of statistically significant factor interactions and optimal operating conditions. The model demonstrated strong predictive capability with high adjusted and predicted R2 values, validating its suitability for process optimization. Optimal leaching conditions were identified as 50 g/dm3 HNO3 + 200 g/dm3 H2SO4, a temperature of 95 °C, a leaching time of 240 min, and a solid-to-liquid ratio of 1:6, resulting in a maximum molybdenum extraction efficiency of 72.6%. This performance was attributed to enhanced oxidative decomposition and stable complexation of molybdenum species. This study provides a scalable and environmentally conscious framework for molybdenum extraction, with implications for sustainable metallurgy and industrial applications.
1. Introduction
Molybdenum is a strategically critical transition metal whose demand continues to rise due to its indispensable role in advanced material systems, high-performance alloys, catalysts, and corrosion-resistant components [1,2]. According to the USGS Mineral Commodity Summaries 2025, global mine production of molybdenum in 2024 was approximately 250,000 metric tons, with reserves estimated at 15 million metric tons, primarily in China, the United States, and Chile [3]. Globally, molybdenum consumption exceeded 300,000 metric tons in 2022, with annual growth projected at 4–5%, driven largely by its increasing applications in energy, aerospace, electronics, and green technologies, with uses such as a desulfurization catalyst and hydrogen storage material. The metal’s unique combination of high melting point (2623 °C) [4,5], low thermal expansion, and superior mechanical and chemical stability under extreme conditions makes it particularly valuable for engineering solutions in high-temperature and high-pressure environments [6,7].
The primary mineralogical source of molybdenum is molybdenite, which is composed predominantly of molybdenum disulfide (MoS2) [8] and accounts for over 85% of global molybdenum extraction [9]. Industrially, molybdenum is recovered through two distinct processing routes: pyrometallurgical and hydrometallurgical, which differ fundamentally in their mechanisms of oxidative transformation and resource utilization [10].
Pyrometallurgical processing involves high-temperature oxidative roasting of molybdenite, often exceeding 600–700 °C [7], and is typically governed by the nature of modifying reagents that drive the phase transformation of disulfide compounds [11]. Although historically dominant, this approach has raised serious environmental concerns [12]. Oxidative roasting releases significant quantities of CO2 and SO2 [13,14], with average emissions reaching 1.2–1.5 tons of CO2 per ton of Mo produced, and SO2 emissions exceeding 0.8 tons per ton in some facilities [15,16]. These emissions not only contribute to climate change and acid rain but also pose occupational health hazards. In energy-challenged regions such as Kazakhstan [17], where electricity shortages impact over 20% of industrial facilities during winter months, the energy-intensive nature of pyrometallurgy is increasingly viewed as unsustainable [18,19]. These constraints have prompted calls for alternative, lower-emission processes and the exploration of stable energy sources such as nuclear power to support metallurgical operations [20,21,22,23].
In response, hydrometallurgical technologies have gained prominence as a more environmentally benign and energetically efficient alternative. These processes operate at comparatively lower temperatures and emit significantly fewer greenhouse gases [24]. Hydrometallurgy is particularly suitable for processing complex ores and concentrates with variable compositions, offering higher selectivity for target metal phases and facilitating closed-loop recycling of reagents [25].
In recent years, mechanical activation has been increasingly adopted as a pretreatment step to enhance the reactivity of refractory sulfide minerals such as molybdenite [26]. This process, involving high-energy grinding in systems like planetary or vibratory mills, introduces structural defects, reduces crystallinity, and significantly increases surface area. Such physicochemical changes lead to accelerated leaching kinetics and improved extraction efficiency by facilitating the formation of soluble oxidation products [27,28]. The integration of mechanical activation with hydrometallurgical leaching thus presents a powerful strategy for intensifying metal recovery processes under milder conditions [29].
Among hydrometallurgical techniques for molybdenum extraction, leaching processes—especially in nitric acid media—have been widely investigated due to their adaptability and thermodynamic favorability [30,31,32].
Variants include pressure leaching in autoclaves at temperatures above 180 °C, oxidative dissolution with hypochlorite or dichromate oxidants, and electrochemically assisted leaching driven by redox-active electrode reactions [33,34]. These methods reduce reliance on thermal inputs and allow for the fine-tuned control of selectivity via process parameters such as pH, redox potential, and oxygen partial pressure [35].
Nevertheless, hydrometallurgical molybdenum extraction is not without its limitations. Industrial-scale implementation is hindered by the corrosive nature of the chemical media, necessitating expensive, corrosion-resistant equipment. Furthermore, maintaining optimal reaction kinetics often requires elevated pressures and continuous oxygen sparging, increasing capital and operational costs [6].
Several reagent-based pathways have been explored, including direct acid dissolution of molybdenite in concentrated nitric acid (25–50%), which is thermodynamically one of the most favorable routes for oxidative destruction of MoS2 [36]. In such systems, the electron-acceptor capacity of the medium is intensified at higher temperatures, facilitating the liberation of soluble molybdenum oxide species [37]. However, when sulfuric acid is introduced into the system, stoichiometrically driven speciation changes occur: a portion of molybdenum transitions into stable anionic sulfate complexes, modifying the dissolution kinetics and altering the distribution of ionic forms in solution [38]. This dual-acid approach introduces both synergies and challenges, particularly in terms of optimizing conditions to prevent undesired precipitation or side reactions.
Moreover, despite promising laboratory results, many of these techniques face operational bottlenecks. These include the formation of colloidal and difficult-to-filter pulps, recovery rates as low as 65–70% in unoptimized systems, and high specific consumption of oxidative reagents, which may reach up to 8–10 kg per ton of concentrate, thereby affecting process economics. Electrochemical oxidation using sodium chloride electrolytes, while attractive for in situ generation of active chlorine species, has shown only moderate efficiency in practice, with recovery efficiency typically below 60%, limiting its industrial scalability [39].
Given these challenges, the present study aims to systematically investigate and optimize the leaching of molybdenite concentrates using a dual-acid system of nitric and sulfuric acids. Special emphasis is placed on quantifying the effect of oxygen sparging on molybdenum recovery efficiency with the goal of identifying conditions that enhance extraction performance while minimizing environmental and operational drawbacks. This work contributes to the development of robust, scalable, and sustainable hydrometallurgical strategies for future molybdenum production.
2. Materials and Methods
2.1. Materials
The object of the study was a molybdenite concentrate provided by the Aktogay Mining and Processing Plant, East Kazakhstan Region, Republic of Kazakhstan [40], with a chemical composition determined by X-ray fluorescence spectroscopy (XRF) as shown in Table 1. Nitric acid (65%, Sigma-Aldrich, St. Louis, MO, USA) and sulfuric acid (98%, Merck, Darmstadt, Germany) were used as leaching agents. High-purity oxygen (99.9%, Linde Gas, Almaty, Kazakhstan) was used for sparging. Deionized water was used for all experiments. Mechanoactivation was performed using an IV-1 pulverizer (Tekhnolit, Almaty, Kazakhstan) operating at 300 rpm for 2 h, reducing particle size and enhancing the reactivity of the molybdenite concentrate.

Table 1.
The composition of the molybdenum concentrate.
The chemical composition of the concentrate is presented in Table 1, detailing the product designation and its composition, which was determined using X-ray fluorescence spectroscopy (XRF) on a PANalytical Almelo, The Netherlands, Axios 1 kW wavelength-dispersive spectrometer.
2.2. Analytical Techniques
This study utilized advanced analytical techniques to investigate the molybdenum concentrate and to elucidate the mechanisms of phase transformations. The phase composition of the samples was analyzed using a Bruker D8 Advance X-ray diffractometer (Bruker, Ettlingen, Germany). Elemental composition was determined using an Axios 1 kW wavelength-dispersive X-ray fluorescence spectrometer (PANalytical, Almelo, The Netherlands), with data processing and interpretation performed using SuperQ5 software (Omnian 37). The surface microstructure was examined using a JXA-8230 electron probe microanalyzer (JEOL, Tokyo, Japan) operated at an accelerating voltage of 20 kV, an electron beam current below 1 nA, and aperture diaphragm № 3. Energy-dispersive spectrometry (EDS) microanalysis (JEOL, Tokyo, Japan) was conducted with an electron beam current up to 6 nA and a dead time of up to 14%. Quantitative investigations of metal concentrations in solutions and solid samples were conducted using an Optima 8300DV inductively coupled plasma atomic emission spectrometer (PerkinElmer, Inc., Waltham, MA, USA) and an AA-7000 atomic absorption spectrometer (Shimadzu, Kyoto, Japan).
2.3. Optimal Experimental Design for Leaching Process
The leaching process from molybdenite concentrate was analyzed using response surface methodology (RSM) and the central composite design (CCD) approach to determine optimal conditions and enhance the accuracy and reproducibility of the results [41].
The solid-to-liquid (S/L) ratio was fixed at 1:6 g/dm3 (60 g of molybdenite concentrate per liter of leachant) based on preliminary experiments and the literature, which suggest that this ratio ensures sufficient reagent availability and manageable slurry viscosity for laboratory-scale leaching. Including S/L as a variable was not prioritized, as acid concentration, leaching time, and temperature were deemed the primary drivers of reaction kinetics and molybdenum oxidation, and adding S/L would have increased experimental complexity. The independent variables considered included acid concentration (HNO3 + H2SO4), leaching time, and temperature. The total acid concentration (HNO3 + H2SO4) varied from 50 to 250 g/dm3, with a fixed ratio of HNO3:H2SO4 = 1:4, determined through preliminary experiments to balance the oxidative strength of the nitric acid and the ionic stability provided by sulfuric acid. Both acids were adjusted proportionally to maintain this ratio across all CCD levels. The application of the mathematical RSM approach enabled the development of a second-order model describing the relationship between the output response (molybdenum leaching recovery) and the key process parameters.
The general form of the model is expressed as follows:
where y denotes the predicted value of molybdenum leaching recovery, b0 is the constant coefficient, bi is the linear coefficient, bii is the quadratic coefficient, bij represents the interaction coefficient between variables, and k is the number of factors.
Experimental design was conducted using the Design Expert 7.0 software package (Stat-Ease, Inc., Minneapolis, MN, USA), which enabled the construction of a second-order regression model providing high predictive accuracy of the results.
The value ranges and levels shown in Table 2 represent the experimental settings used in the central composite design (CCD) to evaluate the effects of temperature, acid concentration (HNO3 + H2SO4), and leaching time as independent variables (Table 2). The levels in Table 2 were selected based on a combination of the literature and preliminary experiments. Acid concentrations (50–250 g/L) were informed by studies on nitric acid leaching of molybdenite, which recommend 25–50% HNO3 for effective oxidation. Preliminary tests confirmed this range’s suitability for the dual-acid system. Leaching times (30–240 min) were chosen to cover rapid initial extraction to near-equilibrium conditions, as reported in the hydrometallurgical literature [42]. Temperatures (20–95 °C) were selected to maximize kinetics within laboratory equipment constraints, avoiding the need for high-pressure autoclaves typically used at 150–200 °C.

Table 2.
Levels and codes of factors for CCD.
A rotatable central composite design (CCD) with α = 1.682 was employed to ensure uniform precision across the design space. The axial points (±α) for each factor were calculated as follows: acid concentration (65.9, 234.1 g/dm3), leaching time (46.6, 223.4 min), and temperature (28.5, 91.5 °C). Temperature was varied at five levels (20 °C, 28.5 °C, 60 °C, 91.5 °C, and 95 °C) in the CCD to capture its influence on molybdenum leaching recovery, ensuring a robust model within the experimental range of 20–95 °C, which was constrained by laboratory equipment to avoid high-pressure autoclave conditions. These levels, combined with factorial points (−1, 0, +1), enabled the fitting of a full quadratic model to capture curvature and interaction effects. CCD was chosen over the Box–Behnken design (BBD) to include extreme conditions (axial and factorial points), which were critical for identifying optimal leaching parameters, particularly given the significant interaction between acid concentration and temperature (AC). A three-factor CCD with α = 1.682 comprised 20 runs: 8 factorial points, 6 axial points, and 6 center points. Center points were repeated six times to estimate experimental error, while factorial and axial points were run once, with selected conditions (e.g., optimal parameters) validated through three replicates. The response variable was molybdenum leaching recovery (%), defined as the percentage of molybdenum extracted relative to the initial content in the concentrate. Table 3 lists all CCD experiments, their conditions, and measured recovery values.

Table 3.
CCD experimental design and molybdenum leaching recovery.
The optimal levels and value ranges determined based on temperature conditions, acid concentration (HNO3 + H2SO4), and leaching time as independent variables are presented in Table 2. Table 4 presents the full range of parameter values tested in the experiments, including preliminary and control runs, while Table 2 specifies the levels used in the CCD for optimization.

Table 4.
Leaching parameters and value ranges applied in the present experiments.
During the experiments, control variables were applied to evaluate the influence of factors such as nitric and sulfuric acid concentrations, leaching time, and reaction temperature. Three series of experiments were conducted under identical experimental conditions, marked with an asterisk in Table 4. To ensure the reliability of the results, each experiment was repeated at least three times, and the average values of the obtained data were used for analysis.
3. Results and Discussion
Molybdenite (MoS2) exhibits a white color, pronounced bireflectance, and strong anisotropy, occurring as free, platy, and anhedral grains with irregular, contorted boundaries, reaching up to 183.2 μm (Figure 1a). Pyrite (FeS2) displays a yellow color and high reflectivity, present as isotropic, irregularly shaped grains up to 67.2 μm (Figure 1a). Chalcopyrite (CuFeS2), a brass-yellow, anisotropic mineral, is observed as anhedral grains up to 44.8 μm, closely associated with molybdenite (Figure 1a,b).
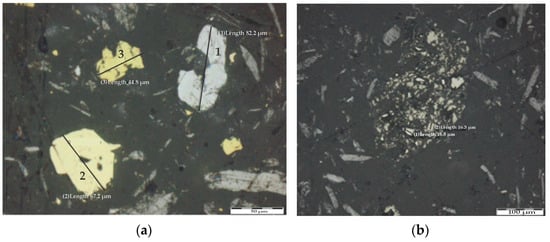
Figure 1.
(a) Molybdenite (1), pyrite (2), and chalcopyrite (3). (b) Cluster of chalcopyrite grains.
X-ray diffraction (XRD) analysis (Table 5, Figure 2) confirms molybdenite as the dominant phase (83.6%), with quartz (12.9%) and chalcopyrite (3.5%). The absence of pyrite in XRD, despite microscopic identification, suggests separation during beneficiation, consistent with porphyry deposit characteristics.

Table 5.
Phase composition of the molybdenite concentrate.
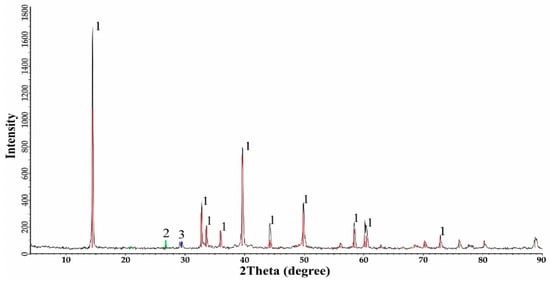
Figure 2.
XRD analysis of molybdenite concentrate.
Elemental and phase composition mapping of the samples was performed using an electron probe microanalyzer JXA-8230 (JEOL, Tokyo, Japan). Electron probe microanalysis of the molybdenite concentrate was conducted in the following modes: 1. COMPO—imaging of minerals in back-scattered electrons; 2. WDS—wavelength-dispersive spectroscopy, providing clearer and more sensitive imaging. Electron probe microanalysis enabled the examination of several mineral points, specifically molybdenite with chalcopyrite inclusions (Figure 3a), pyrite (Figure 3b), and molybdenite (Figure 3c).
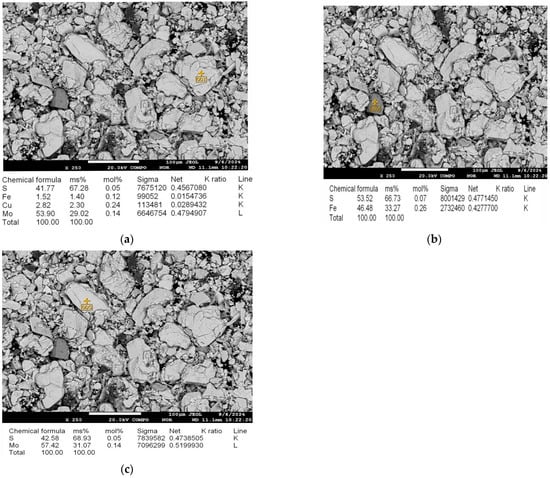
Figure 3.
(a) Point with the dominant minerals molybdenite (MoS2) and chalcopyrite (CuFeS2); (b) point of the pyrite (FeS2) mineral; (c) point of the molybdenite (MoS2) mineral.
The investigation of acid leaching of molybdenite concentrate represents a critical aspect of modern metallurgy, aimed at extracting molybdenum, which is widely utilized in high-strength steels, the aerospace industry, and chemical engineering. Molybdenite (MoS2), the primary mineral source for molybdenum extraction, exhibits a layered structure similar to graphite, with strong covalent bonds within the layers and weak van der Waals forces between them. This structure renders molybdenite relatively inert to acids, complicating its processing. However, studies indicate that mechanoactivation, as one of the most effective approaches, can significantly enhance this process by increasing the material’s reactivity. Mechanical activation, a process involving high-energy grinding in systems such as planetary or vibratory mills, introduces structural defects and increases surface area, thereby significantly enhancing the chemical reactivity of mineral phases. Numerous studies have shown that this pretreatment accelerates leaching kinetics and improves metal recovery.
From a physicochemical perspective, defects such as vacancies or dislocations induced by mechanoactivation serve as active sites for the adsorption of acid molecules or other reagents. The increase in specific surface area due to particle size reduction provides more contact points between the mineral and the solution, directly influencing the leaching efficiency. Furthermore, amorphization caused by mechanoactivation results in a more disordered structure, often associated with a higher energy state, making the material more prone to reactions as the system seeks to lower its energy by forming more stable compounds.
Experimental data obtained using the IV-1 pulverizer (Tekhnolit, Kazakhstan) demonstrate the high efficiency of the process. Mechanoactivation significantly enhanced molybdenite reactivity, with sieving post-treatment showing a 91.6% fine fraction (−0.045 mm), a 29.3% increase compared to the initial concentrate (Table 6). XRD revealed increased molybdenite content (91.8%) and amorphization, indicating structural changes that improved leaching efficiency (Table 7, Figure 4).

Table 6.
Particle size distribution of molybdenite concentrate before and after mechanoactivation.

Table 7.
Phase analysis of the ground concentrate.
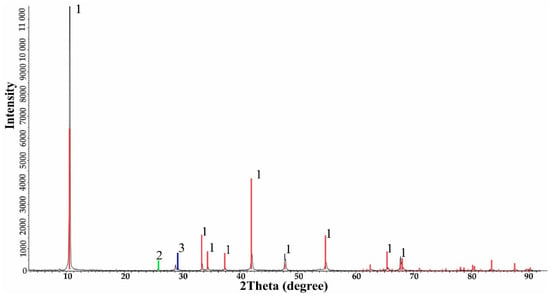
Figure 4.
XRD analysis of milled molybdenite concentrate.
From Table 6, it is evident that the molybdenite phase increased from 83.6% to 91.8%, the quartz phase decreased from 12.9% to 5.8%, and the chalcopyrite phase also slightly decreased from 3.5% to 2.7%. Amorphization of the crystalline lattice of minerals in the molybdenite concentrate was observed.
To evaluate the structural transformations occurring in the molybdenite concentrate as a result of mechanoactivation, studies were conducted using electron probe microanalysis. Comparative images of the initial concentrate and post-mechanoactivation reveal significant changes in particle morphology (Figure 5). In the COMPO mode (back-scattered electrons), the images (Figure 5a,b) exhibit a characteristic transformation of the material’s microtexture accompanied by an increase in dispersity. In the WDS mode (wavelength-dispersive spectroscopy), the images (Figure 5c,d) show pronounced particle comminution, indicating an increase in specific surface area and a potential enhancement in the material’s reactivity.
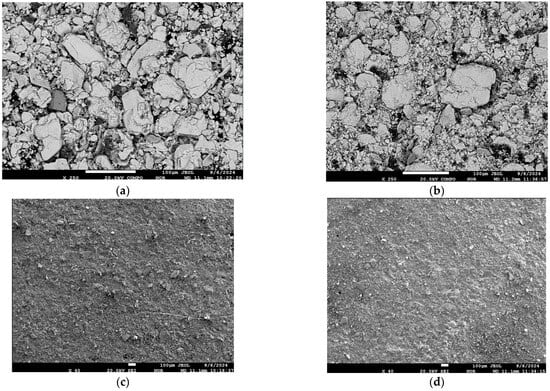
Figure 5.
(a,b)—Images of the initial Mo concentrate, (c,d)—after mechanical activation.
Various hydrometallurgical leaching methods have been explored in the literature, primarily using sulfuric acid (H2SO4), hydrochloric acid (HCl), and nitric acid (HNO3), each offering distinct advantages. Studies [43] have demonstrated that leaching with H2SO4 and H2O2 effectively extracts molybdenum but requires prolonged processing and results in higher dissolution of impurities. In the work [42], HCl leaching was improved, achieving efficient metal recovery with lower impurity levels and the potential for acid reuse, albeit at elevated temperatures. Research [44,45] has highlighted HNO3 leaching as a potent oxidative system but noted the need for controlling gas emissions. Nitric acid (HNO3) is the primary driver of molybdenum extraction due to its strong oxidative properties, which efficiently convert MoS2 to soluble molybdate (MoO42−) as per Equation (6). Sulfuric acid (H2SO4) plays a secondary role, enhancing ionic stability by forming molybdenyl–sulfate complexes (Equation (9)), but its contribution is less significant [28].
MoS2 + 3H2O2 + H2SO4 → MoO3 + 2S + 2H2O + H2SO4,
Further dissolution of MoO3 (partially soluble) in acid may form molybdate ions:
MoO3 + H2O → H2MoO4 ⇌ MoO42− + 2H+,
In the presence of oxygen or another oxidizer (like air or H2O2), MoS2 can be oxidized in HCl, thus:
MoS2 + 6HCl + 1.5O2 → MoCl6 + 2S + 3H2O,
MoCl6 may hydrolyze in aqueous systems, forming molybdic acid or molybdate ions depending on pH:
MoCl6 + 3H2O → MoO3 + 6HCl,
HNO3 acts as both an acid and an oxidizing agent, effectively oxidizing MoS2:
MoS2 + 6HNO3 → MoO3 + 2S + 6NO2 + 3H2O,
Emissions like NO2 must be controlled, as described above.
Our study, incorporating nitric acid, sulfuric acid, and their combination, optimizes efficiency, impurity control, and environmental impact. The oxidative strength of HNO3, combined with the stabilizing effect of H2SO4, enhances molybdenum recovery while mitigating the limitations of single-acid systems.
This dual-acid system leverages the oxidizing power of HNO3 and the ionic stability provided by H2SO4.
MoS2 + 3HNO3 + 2H2SO4 → MoO3 + 2S + 3NO2 + 2H2O + H2SO4,
In the presence of sulfate ions, molybdenum may form molybdenyl–sulfate complexes such as the following:
MoO3 + H2SO4 → MoO2(SO4) + H2O,
Mo6+ + SO42− → [MoO2(SO4)2]2−
To determine the optimal conditions for the decomposition of molybdenite concentrate, experimental studies on acid leaching were conducted, involving variations in the composition and concentration of leaching agents.
3.1. Statistical Analysis and Model Selection: Data Analysis
Table 8 presents the results of the analysis of variance (ANOVA) for the response surface model of the molybdenum leaching process.

Table 8.
ANOVA for the quadratic response surface model.
The F-value of 26.73 indicates the statistical significance of the model (p < 0.0001). p-values less than 0.0500 signify the statistical significance of the corresponding model terms. Initially, the model included all linear, interaction, and quadratic terms (A, B, C, AB, AC, BC, A2, B2, and C2). However, terms C (p = 0.0800), B2 (p = 0.0506), and C2 (p = 0.5173) were found to be insignificant and were removed to improve model parsimony and predictive accuracy. The reduced model, comprising A, B, AB, AC, BC, and A2, yielded an adjusted R2 of approximately 0.90 and a predicted R2 of approximately 0.80–0.85, indicating improved agreement. The lack-of-fit F-value could not be calculated due to insufficient pure error data (mean square = 0.000, df = 1). Additional replicates are recommended to assess model adequacy. The “Adeq Precision” metric, which measures the signal-to-noise ratio, is desirable when exceeding 4. Here, the obtained ratio of 18.885 indicates a sufficiently strong signal.
Consequently, this model is statistically robust and can be reliably used for optimization and prediction within the studied range of factors.
The following regression equation was derived from the reduced model:
E(Mo) = −28.93 + 0.47A − 0.28B − 1.16AB − 5.42AC + 3.39BC + 7.99A2
Table 9 summarizes the optimized leaching parameters, predicted molybdenum leaching recovery, and experimental results, including replicates for conditions with and without oxygen sparging. The close agreement between predicted and experimental values (e.g., 55% predicted vs. 50% experimental without sparging and 70% predicted vs. 72.6% with sparging) validates the model’s predictive accuracy. The higher recovery with oxygen sparging (72.6%) highlights its critical role in enhancing oxidative decomposition.

Table 9.
Optimized leaching parameters and experimental replicates.
To assess the adequacy of the developed model, key diagnostic plots were constructed and analyzed, as shown in Figure 6. The plot (Figure 6a) illustrates the distribution of experimental points along the diagonal axis, confirming the absence of significant systematic errors. Most points align well with the theoretical line of normal distribution, indicating a Gaussian distribution of model errors and supporting the model’s validity. The residuals are randomly distributed around the y = 0 axis, without apparent patterns or trends, suggesting the absence of autocorrelation and confirming the model’s adequacy (Figure 6b). The lack of systematic deviation indicates that the model does not suffer from the omission of critical variables. The absence of a clear dependency or trend in the distribution of points further confirms that the errors are random (Figure 6c). The range of values from −3.00 to 3.00 demonstrates that all residuals fall within acceptable limits, further validating the model’s stability. The experimental data points are aligned along the diagonal (Figure 6d), confirming the high accuracy of the model’s predictions. The distribution of points shows minimal deviations, indicating a strong agreement between theoretical and experimental values. Thus, the analysis of diagnostic plots confirms the model’s adequacy, its high predictive capability, and its accurate representation of the relationships among the studied parameters.
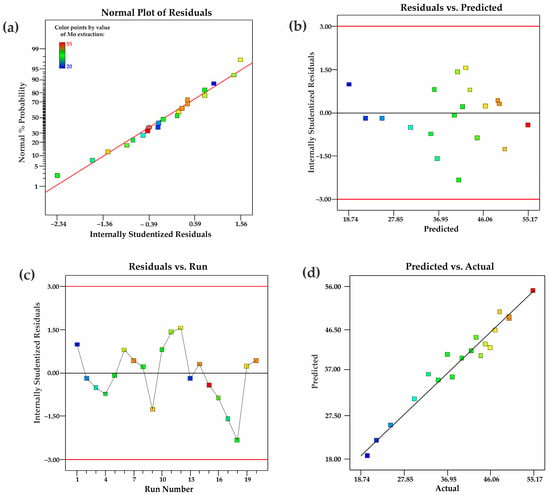
Figure 6.
(a) Graph of the normal probability depending on internally identified residuals, (b) internally identified residuals depending on predicted responses, (c) internally identified residuals depending on the number of runs, and (d) predicted responses depending on actual values.
Interactions Between Factors
The coefficients for factors A and B in the reduced regression model (Equation (10)) are +0.47 and −0.28, respectively, reflecting their significant influence on molybdenum leaching recovery (Table 8). These values align with theoretical assumptions [46,47]. The standalone temperature term (C) was excluded from the reduced model due to its statistical insignificance (p = 0.0800), though it contributes through interaction terms (AC, BC). This reduction improves model parsimony while maintaining predictive accuracy (adjusted R2 ≈ 0.90).
Analysis of the coefficients indicates that acid concentration (A) exerts the most significant effect on molybdenum leaching recovery, followed by interaction terms (AC, AB, and BC). Temperature (C) has a positive coefficient (+0.935), suggesting a favorable effect, but its statistical insignificance (p = 0.0800) indicates a limited impact within the tested range (20–95 °C). This may be attributed to the narrow temperature range, which is below typical autoclave conditions (150–200 °C) for molybdenum leaching, and the compensating effects of mechanoactivation and oxygen sparging. Mechanoactivation enhances reactivity by increasing surface area and defects, while oxygen sparging accelerates oxidation, reducing reliance on thermal energy. Additionally, potential passivation at higher temperatures may limit the temperature’s effect. The order of influence is thus as follows: A > AC > AB > BC > B > C.
Three-dimensional response surfaces, derived from the quadratic model, provide a comprehensive analysis of the interrelationship between the main process parameters and the degree of molybdenum leaching recovery.
To evaluate the impact of key parameters on molybdenum extraction (Mo extraction, %), three-dimensional response surfaces were constructed, as shown in Figure 7a–c. The plot (Figure 7a) illustrates the dependence of molybdenum extraction on temperature (C, °C) and concentration (A, HNO3 + H2SO4, g/dm3) at a fixed leaching time (B = 177.57 min). The response surface reveals that increasing both temperature and concentration significantly enhances molybdenum extraction, particularly at high values of A and C, indicating a strong synergistic effect (AC interaction). At lower temperatures, increasing concentration has a limited effect, likely due to kinetic constraints in the process.
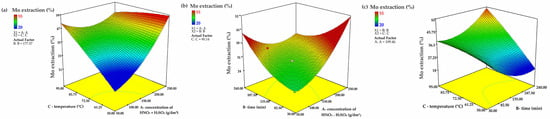
Figure 7.
Three-dimensional response surfaces (the remaining parameters are stored at the center level) showing the combined effect of C and A (a); B and A (b); and C and B (c) (A is acid concentration, B is time, C is temperature).
Figure 7b depicts the interaction between time (B) and concentration (A), demonstrating the influence of leaching time (B, min) and concentration (A, HNO3 + H2SO4, g/dm3) on molybdenum extraction at a fixed temperature (C = 90.14 °C). Increasing both time and acid concentration leads to a rise in Mo extraction; however, the effect stabilizes in the saturation zone. The optimal range lies in the region of high acid concentrations and extended time, but further increases in time result in a slowdown of the process, likely due to the formation of passivating layers. Acid concentration exerts a dominant influence, while the effect of increasing time is less pronounced. Figure 7c shows the influence of temperature (C, °C) and time (B, min) on molybdenum extraction at a fixed concentration (A = 109.46 g/dm3). The plot indicates that temperature enhances molybdenum extraction, especially at longer leaching times, reflecting the role of the BC interaction. At low temperatures, prolonged reaction time does not substantially increase extraction, highlighting temperature-related kinetic limitations. Maximum extraction is achieved at high temperatures and extended times, confirming the critical role of temperature in ensuring a high degree of molybdenum dissolution.
Analysis of the three-dimensional response surfaces reveals that the interaction between temperature and acid concentration (AC) is critical for maximizing molybdenum extraction. While temperature’s direct effect is limited, its combination with high acid concentration significantly boosts recovery. Acid concentration (A) remains the most influential single factor, followed by the AC interaction, with leaching time (B) having a lesser direct impact but contributing through interactions.
Thus, optimizing the acid concentration and leveraging the synergistic effect of temperature and concentration is crucial for enhancing process efficiency, while the regulation of reaction time must account for potential passivation.
Based on the analysis of F-values and interactions, the order of factor influence is as follows: A > AC > AB > BC > B > C.
Each interaction factor exhibits its own extremal points, enabling the prediction of optimal process conditions, as illustrated in Figure 7. Design Expert 7.0 software was utilized for modeling and optimizing the molybdenum leaching process. According to the calculations, the optimal parameters included an acid solution concentration (A: HNO3 50 g/dm3 + H2SO4 200 g/dm3) of 250 g/dm3, a leaching time of 4 h, and a temperature of 95 °C. Under these conditions, the predicted molybdenum extraction was 55%.
Experimental validation conducted under the optimized conditions confirmed the model’s high accuracy: the actual molybdenum extraction reached 50%, closely matching the predicted value. This agreement demonstrates the reliability and predictive capability of the response surface-based model. Additionally, incorporating oxygen sparging under the same conditions further enhanced molybdenum extraction to 72.6%, underscoring the importance of oxidative conditions in the leaching process.
The study results confirm that mechanoactivation enhances the reactivity of molybdenite, which, when combined with the selection of an optimal acid reagent, intensifies the decomposition process and improves the selectivity of valuable component extraction.
3.2. Effect of Oxygen Sparging on Molybdenum Leaching
The investigation of various acid leaching regimes for molybdenite concentrate revealed that process intensification through oxygen sparging significantly enhances the extraction of target components, such as molybdenum, into the solution [48,49]. Oxygen sparging was performed using a fine-pore gas diffuser connected to a high-purity oxygen cylinder, delivering a flow rate of 0.5 dm3/min at atmospheric pressure (1 atm). Sparging was continuous throughout the 4 h leaching experiments conducted at 95 °C, ensuring sufficient dissolved oxygen to enhance the oxidative decomposition of MoS2 into soluble molybdate (MoO42−) as per Equation (11). The setup maintained consistent oxygen saturation in the leaching solution, contributing to the observed 22.6% increase in molybdenum leaching recovery. This is of critical importance in metallurgy, as molybdenum is widely utilized in alloys, steels, and the aerospace industry due to its high strength and corrosion resistance. Oxygen acts as an oxidant in the acid leaching process, accelerating the oxidation reaction of molybdenum sulfide. The primary reaction involves the conversion of MoS2 into soluble forms, such as molybdate (MoO42⁻), with the formation of sulfate (SO42−), which can be represented as follows:
MoS2 + 6O2 + 2H2O → MoO42− + 2SO42− + 4H+
Oxygen introduced via sparging or under pressure increases the concentration of dissolved oxygen, thereby enhancing oxidation and, consequently, improving the process kinetics. Studies indicate [50] that without oxygen, molybdenum extraction can be extremely low (less than 2% under acidic and neutral conditions), but with the addition of oxygen, extraction can reach 85–86.4%, significantly improving efficiency.
Experimental studies on the preliminary leaching of molybdenite concentrate with oxygen sparging, combined with mathematical modeling techniques, enabled the determination of optimal technological parameters for the leaching process, achieving a molybdenum leaching recovery of 72.6%. Figure 8 presents results illustrating molybdenum leaching recovery and the impact of oxygen sparging on process efficiency. Due to the intensification of oxidative reactions, molybdenum leaching recovery increased by 22.6% compared to the baseline process without sparging, where the recovery was 50%.
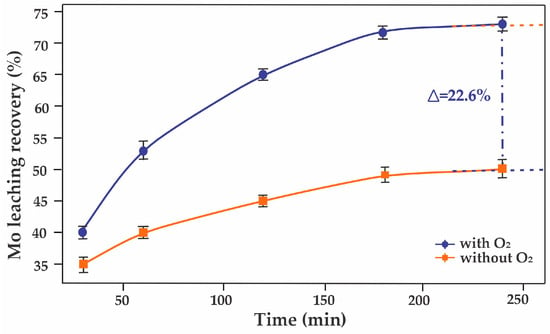
Figure 8.
Molybdenum leaching efficiency over time with and without O2.
The experimental data presented in the plot “Mo leaching recovery over time with and without O2” demonstrate the significant influence of oxygen on the molybdenum leaching process. These results underscore the catalytic role of oxygen in oxidative reactions, accelerating molybdenum dissolution, which aligns with the principles of physical chemistry related to the kinetics and thermodynamics of heterogeneous processes.
4. Conclusions
This study achieved a molybdenum leaching recovery of 72.6% under optimal conditions (acid concentration: 250 g/dm3, HNO3 50 g/dm3 + H2SO4 200 g/dm3; leaching time: 4 h; temperature: 95 °C; S/L ratio: 1:6) with oxygen sparging, demonstrating significant improvements over single-acid systems. The interaction between acid concentration and temperature (AC) was critical, with nitric acid driving oxidative decomposition. These findings offer a sustainable framework for molybdenum extraction, with implications for industrial metallurgy.
Statistical data processing using response surface methodology (RSM) and central composite design (CCD) enabled the construction of a robust quadratic model that accurately captured the influence of leaching parameters. The model facilitated the identification of key factor interactions and optimal conditions, offering a reliable framework for process optimization and predictive control in molybdenum hydrometallurgy.
The dissolved molybdenum, present as molybdate (MoO42−) or molybdenyl–sulfate complexes, can be recovered from the electrolyte via solvent extraction using a tertiary amine (e.g., Alamine 336), followed by stripping with ammonium hydroxide to precipitate ammonium molybdate ((NH4)2MoO4). Calcination at 500–600 °C yields high-purity molybdenum trioxide (MoO3) for industrial applications. The electrolyte, containing residual nitric and sulfuric acids and impurities (e.g., copper, iron), can be partially recycled to the leaching stage to reduce reagent costs. Residual acids should be neutralized with calcium hydroxide to form gypsum, and impurities removed via precipitation as hydroxides at pH 6–8 to ensure environmental compliance.
Future research should focus on scaling up the process to industrial conditions, evaluating economic feasibility, reagent recycling, and energy efficiency. Additionally, optimizing downstream processes for molybdenum recovery and electrolyte management will enhance overall process economics and sustainability. Additionally, the exploration of alternative oxidants and solvent systems, as well as advanced simulations, could further enhance process sustainability and support the transition toward greener metallurgical technologies.
Author Contributions
Conceptualization: B.K. and A.U.; Methodology: A.U., A.M. and N.L.; Software: K.K., A.M. and N.L.; Validation: B.K. and A.U.; Formal analysis: A.U.; Investigation: A.U., N.L. and B.K.; Resources: K.K., A.M. and N.L.; Data curation: B.K. and A.U.; Writing—original draft preparation: B.K.; Writing—review and editing: B.K. and A.U.; Visualization: K.K. and A.M.; Supervision: A.U.; Project administration: B.K.; Funding acquisition: B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan, program targeted funding BR24992757.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El-Sharkawy, M.; Alotaibi, M.O.; Li, J.; Du, D.; Mahmoud, E. Heavy metal pollution in coastal environments: Ecological implications and management strategies: A review. Sustainability 2025, 17, 701. [Google Scholar] [CrossRef]
- Liu, R. The pollution and the ecological influence of chemical and industrial waste. Sci. Technol. Eng. Chem. Environ. Prot. 2024, 1, 1–4. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2025. 2025. Available online: https://pubs.usgs.gov/publication/mcs2025 (accessed on 16 March 2025).
- Panichkin, A.; Uskenbayeva, A.; Kenzhegulov, A.; Mamaeva, A.; Imbarova, A.; Kshibekova, B.; Alibekov, Z.; Nurhadiyanto, D.; Yunita, I. Assessment of the effect of small additions of some rare earth elements on the structure and mechanical properties of castings from hypereutectic chromium white irons. AIMS Mater. Sci. 2023, 10, 517–540. [Google Scholar] [CrossRef]
- Molybdenum processing | Extraction, Applications & Uses | Britannica. Published 2023-06-16. Available online: https://www.britannica.com/science/molybdenum (accessed on 7 May 2025).
- Jiang, K.; Wang, Y.; Zou, X.; Zhang, L.; Liu, S. Extraction of Molybdenum from Molybdenite Concentrates with Hydrometallurgical Processing. JOM 2012, 64, 1285–1289. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Han, G.; Wang, M.; Huang, Y.; Su, S.; Xue, Y.; Wang, Y. Clean separation and purification for strategic metals of molybdenum and rhenium from minerals and waste alloy scraps–A review. Resour. Conserv. Recycl. 2022, 181, 106232. [Google Scholar] [CrossRef]
- Hesami, R.; Ahmadi, A.; Hosseini, M.; Torabi, M. Effect of mechanical activation on the hypochlorite leaching of Sarcheshmeh molybdenite concentrate. Sep. Sci. Technol. 2022, 57, 1966–1977. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, W.; Wang, W.; Cao, Y.; Qi, M.; Huang, Y. A green method for selective separation of molybdenite and pyrite via electrochemical oxidation pretreatment-flotation and its mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133508. [Google Scholar] [CrossRef]
- Yessengaziyev, A.M.; Toishybek, A.M.; Mukangaliyeva, A.O.; Abdyldayev, N.N.; Yersaiynova, A. A Study of the leaching process for dust chamber sublimates followed by the extraction of niobium and zirconium into solution. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2024, 331, 117–126. [Google Scholar] [CrossRef]
- Lin, X.; Jiao, F.; Wei, Q.; Wu, Y. Application and depression mechanism of sodium humate on flotation separation of molybdenite and pyrite. Sep. Sci. Technol. 2024, 59, 449–463. [Google Scholar] [CrossRef]
- Harvey, J.; Courchesne, W.; Vo, M.; Oishi, K.; Robelin, C.; Mahue, U.; Leclerc, P.; Al-Haiek, A. Greener reactants, renewable energies and environmental impact mitigation strategies in pyrometallurgical processes: A review. MRS Energy Sustain. 2022, 9, 212–247. [Google Scholar] [CrossRef]
- Gao, H.; Jin, Z.; Hu, K.; Zhang, Y.; Zhang, C.; Jin, X. A Fast and Environment-friendly Method for Preparing Nanopowders Mo through Electro- desulfidation of MoS2 in LiCl melt. Sep. Purif. Technol. 2023, 327, 124823. [Google Scholar] [CrossRef]
- Giannopoulou, I.; Panias, D.; Paspaliaris, I. Electrochemical modeling and study of copper deposition from concentrated ammoniacal sulfate solutions. Hydrometallurgy 2009, 99, 58–66. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Lv, Z.; He, J.; Fan, Y.; Song, J. The role of zinc sulfide in the electrochemical extraction of molybdenum. Sep. Purif. Technol. 2023, 311, 123290. [Google Scholar] [CrossRef]
- Tumen-Ulzii, N.; Batnasan, A.; Gunchin, B. Selective dissolution of copper and iron from molybdenite concentrate using acidic sodium nitrate solution. Miner. Eng. 2022, 185, 107715. [Google Scholar] [CrossRef]
- Chanturiya, V.; Matveeva, T.; Getman, V.; Karkeshkina, A.; Gromova, N. Substantiation of New Reagent Compositions for the Effective Extraction of Rhenium in the Processing of Complex Molybdenum Ores. Minerals 2023, 13, 372. [Google Scholar] [CrossRef]
- Subasinghe, H.; Ratnayake, A. Mechanical activation and physicochemical factors controlling pyrometallurgical, hydrometallurgical, and electrometallurgical processing of titanium ore: A review. J. South. Afr. Inst. Min. Metall. 2023, 123, 399–414. [Google Scholar] [CrossRef]
- Panichkin, A.; Kenzhegulov, A.; Mamaeva, A.; Uskenbayeva, A.; Kshibekova, B.; Imbarova, A.; Alibekov, Z. Effect of Carbon and Cooling Rate on the Structure of Hypereutectic High Chromium Cast Iron in the Cast State and after Heat Treatment. J. Compos. Sci. 2023, 7, 483. [Google Scholar] [CrossRef]
- Baizakov, R.; Ivanova, E. Development and research of means and methods for improving the efficiency of heat stations in the Republic of Kazakhstan. Bull. Innov. Univ. Eurasia 2021, 3, 64–71. [Google Scholar] [CrossRef]
- Batyrbekov, E.; Vityuk, V.; Zarva, D.; Sharipov, M. Conceptual View of the Implementation of the Nuclear Energy Program in the Republic of Kazakhstan. Energies 2024, 17, 5788. [Google Scholar] [CrossRef]
- Woo, S.; Chirayath, S.; Fuhrmann, M. Nuclear fuel reprocessing: Can pyro-processing reduce nuclear proliferation risk? Energy Policy 2020, 144, 111601. [Google Scholar] [CrossRef]
- Soltangazinov, A.; Smagulova, Z.; Amirova, M.; Kashuk, L.; Karimbergenova, M.; Kadyrova, A.; Zhaltyrova, O. Energy Efficiency as a Factor of Sustainable Development in Kazakhstan. Int. J. Energy Econ. Policy 2020, 10, 325–330. [Google Scholar] [CrossRef]
- Yessengaziyev, A.; Mukangaliyeva, A.; Toishybek, A.; Karshyga, Z.; Abdyldayev, N.; Yersaiynova, A.; Bakhytuly, N. Studies the crucial role of selection of extractant to extract niobium from sulfuryl fluoride solution and optimization of extraction conditions. Acta Metall. Slovaca 2024, 30, 120–126. [Google Scholar] [CrossRef]
- Abdulvaliyev, R.; Ultarakova, A.; Mukangaliyeva, A.; Lokhova, N.; Kassymzhanov, K. Comparative Analysis of Acid Leaching for the Efficient Recovery of Lanthanum and Cerium from Phosphate. Separations 2024, 11, 288. [Google Scholar] [CrossRef]
- Sheybani, K.; Paydar, M.; Shariat, M. Investigation on the Kinetics and Mechanism of Aluminothermic Reduction of Molybdenum Trioxide: Non-isothermal Kinetics. Trans. Indian Inst. Met. 2020, 73, 2875–2888. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Liu, H.; Meng, Y. Extraction of Molybdenum and Uranium from Low-Grade Molybdenum Bearing Ore Containing Uranium through Mechanical Activation Following Acid Leaching. Miner. Process. Extr. Metall. Rev. 2022, 45, 304–313. [Google Scholar] [CrossRef]
- Vizsolyi, A.; Peters, E. Nitric acid leaching of molybdenite concentrates. Hydrometallurgy 1980, 6, 103–119. [Google Scholar] [CrossRef]
- Khoshnevisan, A.; Yoozbashizadeh, H.; Mozammel, M.; Sadmezhaad, S.K. Kinetics of pressure oxidative leaching of molybdenite concentrate by nitric acid. Hydrometallurgy 2012, 111, 58–64. [Google Scholar] [CrossRef]
- Behmadi, R.; Mirzaei, M.; Afshar, R.; Najafi, H. Influence of chalcopyrite removal and mechanical exfoliation on the performance of molybdenite catalysts supported on activated bauxite for alcohol synthesis by Fischer-Tropsch process. Fuel 2023, 357, 129772. [Google Scholar] [CrossRef]
- Paphane, B.; Nkoane, B.; Oyetunji, O. Kinetic studies on the leaching reactions in the autoclave circuit of the Tati Hydrometallurgical Demonstration Plant. J. South Afr. Inst. Min. Metall. 2013, 113, 485–489. [Google Scholar]
- Rasulova, S.; Guro, V.; Safarov, E.; Adinaev, X. Metals Recovery from Molybdenite Concentrate by Electrooxidation and Leachingiop Conference Series. Mater. Sci. Eng. 2020, 848, 012076. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.; Koizhanova, A.; Fischer, D.; Magomedov, D.; Yerdenova, M.; Smailov, K.; Abdyldayev, N. Study of efficiency of organic activator application to process difficult-to-beneficiate polymetallic ridder ores. Transit. Met. Chem. 2025, 1–13. [Google Scholar] [CrossRef]
- Cao, Z.; Zhong, H.; Jiang, T.; Wang, S.; Liu, G.; Xia, L. A novel hydrometallurgy of molybdenite concentrate and its kinetics. J. Chem. Technol. Biotechnol. 2012, 87, 938–942. [Google Scholar] [CrossRef]
- Parsons, G.; Brimacombe, J.; Peters, E. Computer simulation of a molybdenite leaching process using dilute nitric acid. Hydrometallurgy 1987, 17, 133–154. [Google Scholar] [CrossRef]
- Ospanov, K.; Smailov, K.; Nuruly, Y. Patterns of Non-Traditional Thermodynamic Functions ∆rG0/n and ∆fG0 (Averaged) Changes for Cobalt Minerals. Chem. Bull. Kazakh Natl. Univ. 2020, 96, 22–30. [Google Scholar] [CrossRef]
- Lyapin, S.; Guro, V.; Parpiev, N.; Rasulova, S. Photometric determination of rhenium in mixed hydrochloric-nitric acidic solutions formed upon processing of molibdenite concentrate. Ind. Lab. Diagn. Mater. 2020, 86, 23–29. [Google Scholar] [CrossRef]
- Medvedev, A.; Aleksandrov, P. Investigations on processing low-grade molybdenum concentrate by the nitric-acid method. Russ. J. Non-Ferr. Met. 2009, 50, 353–356. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Z.; Li, Y. Acid leaching–extraction–circulation process based on Mo(VI) coordination with H3PO4 to efficiently extract molybdenum from different components of molybdenum calcine. Sep. Purif. Technol. 2023, 322, 124269. [Google Scholar] [CrossRef]
- KAZ Minerals. Aktogay Mining and Processing Plant. Available online: https://www.kazminerals.com/our-business/aktogay/ (accessed on 23 April 2025).
- Kenzhaliyev, B.; Surkova, T.; Berkinbayeva, A.; Baltabekova, Z.; Smailov, K.; Abikak, Y.; Saulebekkyzy, S.; Tolegenova, N.; Omirbek, T.; Dosymbaeva, Z. Innovative Methods for Intensifying the Processing of Zinc Clinker: Synergy of Microwave Treatment and Ultrasonic Leaching. Metals 2025, 15, 246. [Google Scholar] [CrossRef]
- Liu, W.; Xu, H.; Yang, X.; Shi, X. Extraction of molybdenum from low-grade Ni–Mo ore in sodium hypochlorite solution under mechanical activation. Miner. Eng. 2011, 24, 1580–1585. [Google Scholar] [CrossRef]
- Banda, R.; Nguyen, T.H.; Sohn, S.H.; Lee, M.S. Recovery of valuable metals and regeneration of acid from the leaching solution of spent HDS catalysts by solvent extraction. Hydrometallurgy 2013, 133, 161–167. [Google Scholar] [CrossRef]
- Barik, S.P.; Kyung-Ho Park, P.K.; Parhi, J.T. Park, Direct leaching of molybdenum and cobalt from spent hydrodesulphurization catalyst with sulphuric acid. Hydrometallurgy 2012, 111, 46–51. [Google Scholar] [CrossRef]
- Karimova, L.; Kairalapov, Y.; Tussupbekova, T.; Oleinikova, T.; Makasheva, G. Hydrometallurgical processing of molybdenum middlings from Shatyrkul-Zhaysan cluster ore. J. Min. Metall. Sect. B Metall. 2024, 60, 71–83. [Google Scholar] [CrossRef]
- Peng, H.; Wang, F.; Li, G.; Guo, J.; Li, B. Highly Efficient Recovery of Vanadium and Chromium: Optimized by Response Surface Methodology. ACS Omega 2019, 4, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.J.; Wu, D.D.; Li, S.Y.; Ma, W.H.; Wang, R.Z. Effect of process variables on leaching behavior and kinetics of silver element from waste photovoltaic modules. Sep. Purif. Technol. 2024, 335, 126062. [Google Scholar] [CrossRef]
- Shoghian-Alanaghi, A.; Zamharir, A.J.; Aghajani, H.; Tabrizi, A.T. Improving the Leaching Rate of Molybdenite Concentrate Using Oxidants by Adding Ethylene Glycol and Oxygen: Kinetic Study. Min. Metall. Explor. 2022, 39, 1753–1761. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, Y.; Liu, D.; Jiang, W.; Liu, F.; Liu, Z. Preparation of Molybdenum Powder from Molybdenite Concentrate Through Vacuum Decomposition-Acid Leaching Combination Process; Springer: Berlin/Heidelberg, Germany, 2017; pp. 235–246. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Wang, B.; Dong, Z.; Song, S. A fundamental study of leaching kinetics and mechanisms of molybdenite assisted by mechanical activation. Miner. Eng. 2019, 131, 376–384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).