Phytochemical Characterization and Antifungal Potential of Opuntia ficus-indica Cladode Extracts Against Tomato Pathogens
Abstract
1. Introduction
2. Materials and Methods
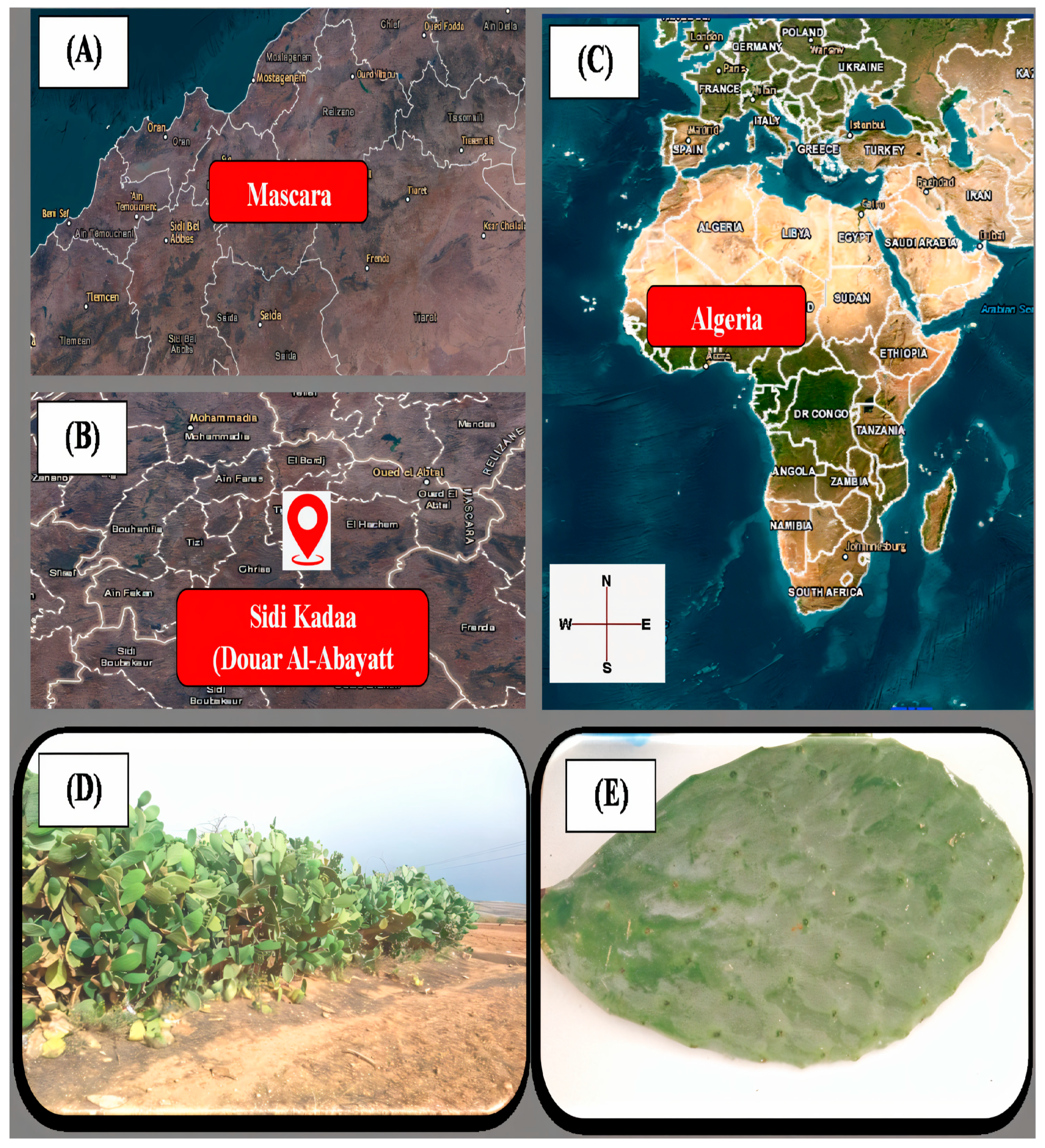
2.1. Sampling of Plant Material
2.2. Isolation and Identification of Fungal Pathogens
2.2.1. Isolation and Purification
2.2.2. Morphological Identification
2.2.3. Methanolic Extract Preparation
2.2.4. Bioactive Compound Quantification
2.2.5. Antioxidant Activity (DPPH Essay)
2.3. Antifungal Activity
2.3.1. Hydroethanolic Extract Preparation
2.3.2. Preparation of PDA Medium with Different Concentrations of Cladode Hydroethanolic Extract
2.3.3. Cladode’s Hydroethanolic Effect on Mycelial Growth
2.3.4. Cladode’s Hydroethanolic Effect on Sporulation
2.4. Statistical Analysis
3. Results
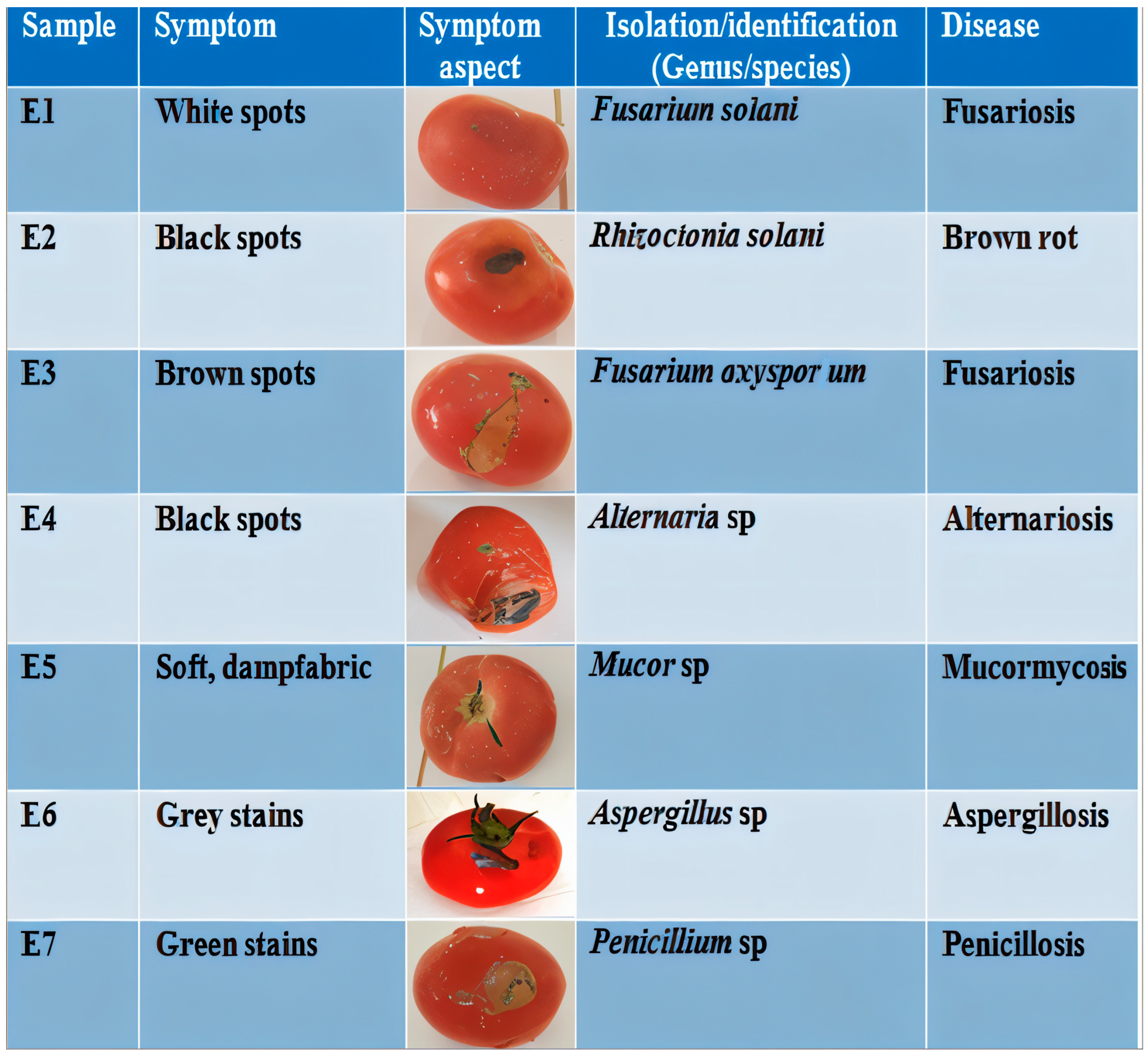
3.1. Characterization of Tomato Fungal Diseases
3.2. Identification of Fungal Agents
3.2.1. Macroscopic Identification
3.2.2. Microscopic Identification
3.3. Bioactive Compound Screening
3.4. Antifungal Activity
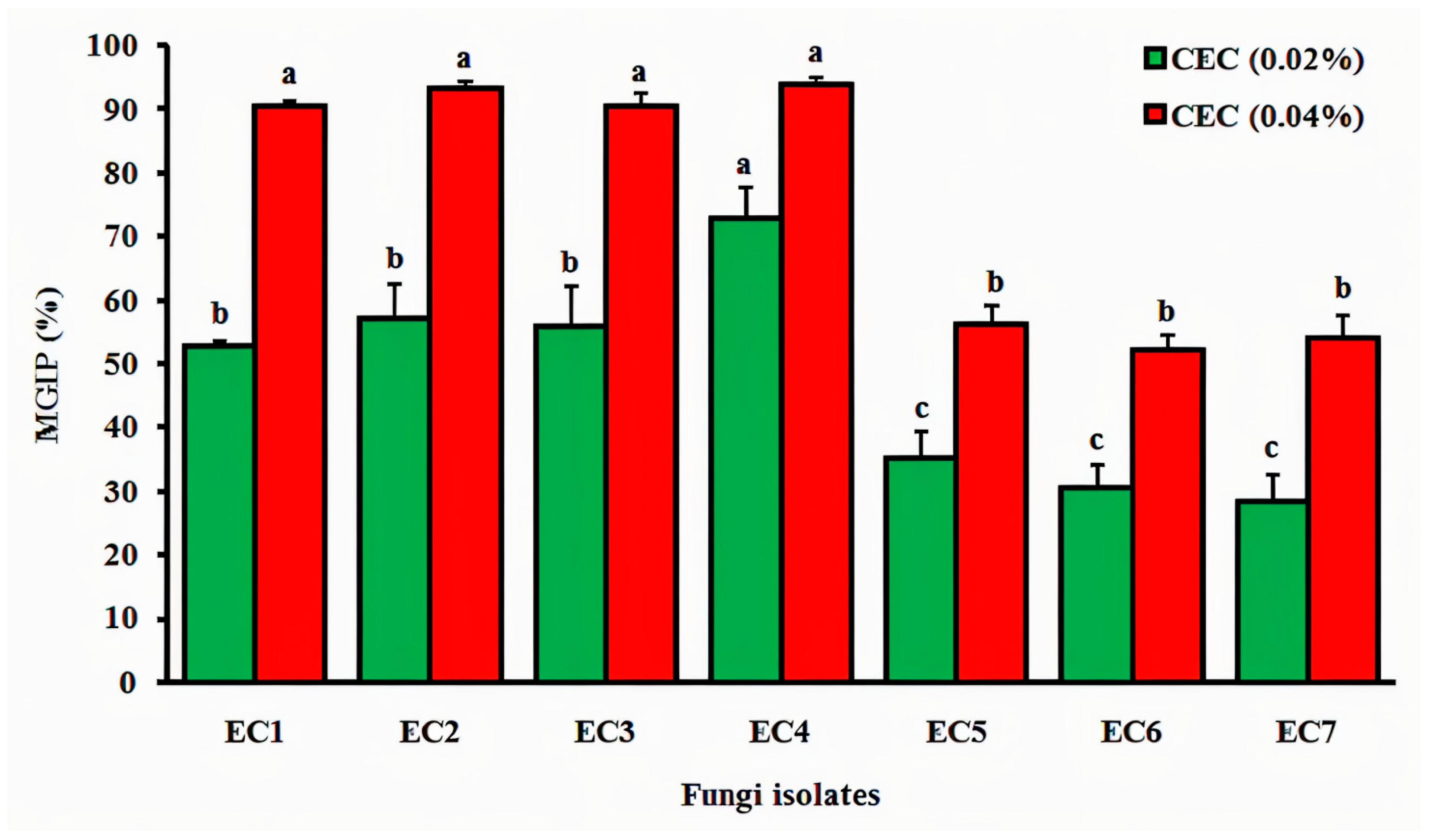
3.4.1. Cladode’s Hydroethanolic Effect on Mycelial Growth
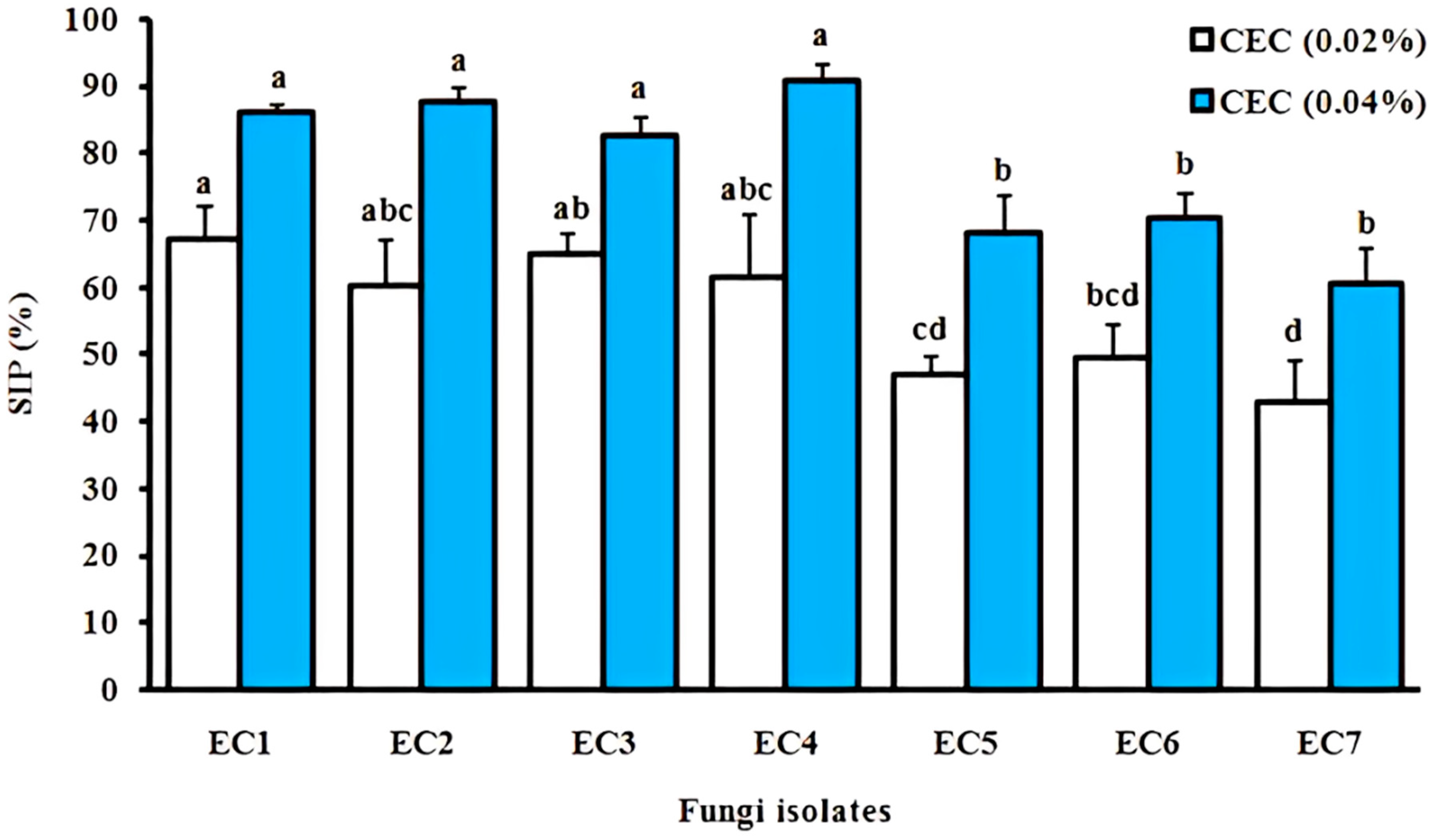
3.4.2. Cladode’s Hydroethanolic Effect on Sporulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González, M.; Cid, M.C.; Lobo, M.G. Usage of Tomato (Lycopersicum Esculentum Mill.) Seeds in Health. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Elsevier: San Diego, CA, USA, 2011; pp. 1123–1132. [Google Scholar]
- Willcox, J.K.; Catignani, G.L.; Lazarus, S. Tomatoes and cardiovascular health. Crit. Rev. Food Sci. Nutr. 2003, 43, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Singh, U.B.; Ilyas, T.; Malviya, D.; Vishwakarma, S.K.; Shafi, Z.; Yadav, B.; Singh, H.V. Bacterial inoculants for control of fungal diseases in Solanum lycopersicum L. (tomatoes): A comprehensive overview. In Rhizosphere Microbes. Microorganisms for Sustainability; Singh, U.B., Sahu, P.K., Singh, H.V., Sharma, P.K., Sharma, S.K., Eds.; Springer: Singapore, 2022; pp. 311–339. [Google Scholar]
- Doyle, M.P.; Beuchat, L.R.; Montville, T.J. Food Microbiology: Fundamentals and Frontiers; ASM Press: Washington DC, USA, 1998. [Google Scholar]
- Merghid, M.; Debbache, M.; Foughali, I. Impacts des pesticides utilisés dans la plasticulture sur la santé humaine En Algérie. Etude de cas la wilaya de Constantine. Master’s Thesis, Université des Frères Mentouri, Constantine, Algeria, 2017. [Google Scholar]
- Alleche, N. Activité Antifongique de Quelques Extraits D’une Plante Endémique Sur Des Moisissures du Blé Stocké. Master’s Thesis, Université des Frères Mentouri, Constantine, Algeria, 2017. [Google Scholar]
- Elkady, W.M.; Raafat, M.M.; Abdel-Aziz, M.M.; Al-Huqail, A.A.; Ashour, M.L.; Fathallah, N. Endophytic fungus from Opuntina fiscus-indica: A source of potential bioactive antimicrobial compounds against multidrug-resistant bacteria. Plants 2022, 11, 1070. [Google Scholar] [CrossRef]
- Bruneton, J. Pharmacognosie, Phytochimie, Plantes Médicinales, 3rd ed.; Éditions Tec & Doc Lavoisier: Paris, France, 1999. [Google Scholar]
- Msaddak, L. Propriétés Techno-Fonctionnelles et Substances Bioactives de Deux Ingrédients Alimentaires: Cladodes du Figuier de Barbarie et Feuilles de Vigne. Ph.D. Thesis, Université de Gabès, Tunis, Tunisia, 2018. [Google Scholar]
- Salem, H.B.; Nefzaoui, A.; Salem, L.B. Supplementing spineless cactus (Opuntia ficus-indica f. inermis) based diets with urea-treated straw or old man saltbush (Atriplex nummularia). Effects on intake, digestion and sheep growth. J. Agric. Sci. 2002, 138, 85–92. [Google Scholar] [CrossRef]
- Yahia, E.M.; Sáenz, C. Cactus pear (Opuntia species). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahiya, E.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 290–329. [Google Scholar]
- Felker, P.; Rodriguez, S.; Del, C.; Casoliba, R.M.; Filippini, R.; Medina, D.; Zapata, R. Comparison of Opuntia ficus-indica varieties of Mexican and Argentine origin for fruit yield and quality in Argentina. J. Arid. Environ. 2005, 60, 405–422. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Bonina, F. The polysaccharide and low molecular weight components of Opuntia ficus-indica cladodes: Structure and skin repairing properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Malainine, M.E.; Dufresne, A.; Dupeyre, D.; Mahrouz, M.; Vuong, R.; Vignon, M.R. Structure and morphology of cladodes and spines of Opuntia ficus-indica. Cellulose extraction and characterisation. Carbohydr. Polym. 2003, 51, 77–83. [Google Scholar] [CrossRef]
- Goudjil, S.; Naceri, K.; Noura, S. Caractérisation Physicochimique et Effet Antibactérien de la Cladode d’Opuntia ficus-indica Inermis (L) Mill. de la Région de contributtioianret, en Vue D’explorer Son Potentiel Thérapeutique. Ph.D. Thesis, Université Ibn khaldoun, Tiaret, Algeria, 2018. [Google Scholar]
- Meena, M.; Swapnil, P.; Upadhyay, R.S. Isolation, characterization and toxicological potential of Alternaria-mycotoxins (TeA, AOH and AME) in different Alternaria species from various regions of India. Sci. Rep. 2017, 7, 8777. [Google Scholar] [CrossRef] [PubMed]
- Cassagne, H. Milieux de Culture et Leurs Applications; Edition de la Tourelle: Paris, France, 1966. [Google Scholar]
- Bessadat, N. Isolement, Identification et Caractérisation des Alternaria sp. Résponsable de la Détérioration des Plantes Maraichères Par Des Systèmes Enzymatiques et Moléculaires. Ph.D. Thesis, Université d’Oran es-senia, Oran, Algeria, 2014. [Google Scholar]
- Botton, B.; Bretton, A.; Fever, M.; Gautier, S.; Guy, P.; Larpent, J.P.; Reymond, P.; Sanglier, J.; Vayssier, Y.; Veau, P. Moisissures Utiles et Nuisibles. Importance Industriell; Collection Biotechnologie: Paris, France, 1990. [Google Scholar]
- Ghoul, M.; Minet, J.; Bernard, T.; Dupray, E.; Cornier, M. Marine macroalgae as a source for osmoprotection for Escherichia coli. Microb. Ecol. 1995, 30, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Dehpeur, A.A.; Ebrahimzadeh, A.A.; Fazel, N.S.; Nabavi, S.M. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas Aceites 2009, 60, 405–412. [Google Scholar] [CrossRef]
- Joslyn, M. Tannins and related phenolics. J. Cell. Biochem. 1970, 22, 188–919. [Google Scholar]
- Sass-Kiss, A.; Kiss, J.; Milotay, P.; Kerek, M.M.; Toth-Markus, M. Differences in anthocyanin and carotenoid content of fruits and vegetables. Food Res. Int. 2005, 38, 1023–1029. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) plant compounds, biological activities and prospects—A comprehensive review. Food Res. Int. 2018, 112, 328–344. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Int. J. Foods Sci. Tech. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Serhan, A.R. Additional interaction of mint leaf extracts with fungi that have an antagonistic property on certain fungi associated with legume seeds. Arab. J. Plant Prot. 2006, 24, 118–124. [Google Scholar]
- Kismoune, S. L’effet de L’extrait Aqueux de Cyprès Sur la Croissance de Champignon Phythophthora infestans. Master’s Thesis, Université Mouhamed Seddik Benyahia, Jijel, Algeria, 2021. [Google Scholar]
- Leroux, P.; Credet, A. Document Sur L’étude de L’activité des Fongicides; INRA: Versailles, France, 1978. [Google Scholar]
- Chabasse, D.; Bouchara, J.; De Gentile, L.; Brun, S.; Cimon, B.; Penn, P. Les moisissures d’intérêt médical. Cah. Form. Biol. Med. 2002, 25, 46–85. [Google Scholar]
- Guiraud, J.P. Microbiologie Alimentaire; Edition Dunod: Paris, France, 1998. [Google Scholar]
- Blancard, D. 2013. Biologie, Epidémiologie. Available online: http://ephytia.inra.fr/fr/C/5217/Tomate-Biologie-epidemiologie (accessed on 1 November 2023).
- Ventura-Aguilar, R.I.; Bosquez-Molina, E.; Bautista-Baños, S.; Rivera-Cabrera, F. Cactus stem (Opuntia ficus-indica Mill): Anatomy, physiology and chemical composition with emphasis on its biofunctional properties. J. Sci. Food Agric. 2017, 97, 5065–5073. [Google Scholar] [CrossRef] [PubMed]
- Hadj Sadok, T.; Aid, F.; Bellal, M.; Abdul Hussain, M.S. Composition Chimique Des Jeunes Cladodes d’Opuntia ficus-indica et Possibilité de Valorisation Alimentaire. Ph.D. Thesis, Ecole Nationale Supérieur Agronomique, Alger, Algeria, 2010. [Google Scholar]
- Boutakiout, A. Etude physico-chimique, biochimique et stabilité d’un nouveau produit: Jus de cladode du figuier de Barbarie marocain (Opuntia ficus-indica et Opuntia megacantha). Ph.D. Thesis, Université d’Angers, Angers, France, 2015. [Google Scholar]
- Aganga, A.A.; Mosase, K.W. Tannins content, nutritive value and dry matter digestibility of Lonchocarpuscapassa, Ziziphus mucronata, Sclerocarya birrea, Kirkia acuminata and Rhus lancea seeds. Anim. Feed Sci. Technol. 2001, 91, 107–113. [Google Scholar] [CrossRef]
- Bruneton, J. Pharmacognosie, Phytochimie, Plantes Médicinales, 5th ed.; Éditions Tec & Doc Lavoisier: Paris, France, 2015. [Google Scholar]
- Boukhalfa, S.; Hamdi, S. Évaluation phytochimique et étude des activités biologiques des extraits bruts des plantes médicinales locales: Opuntia ficus-indica et Thymus lanceolatus. Master’s Thesis, Université des Frères Mentouri, Constantine, Algeria, 2016. [Google Scholar]
- Maataoui, B.S.; Hmyene, A.; Hilali, S. Activites anti-radicalaires d’extraits de jus de fruits du figuier de barbarie (Opuntia ficus-indica). Leban. Sci. J. 2006, 7, 3–8. [Google Scholar]
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef]
- Al Aboody, M.S.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Skadhauge, B.; Gruber, M.Y.; Thomsen, K.K.; Von Wettstein, D. Leucocyanidin reductase activity and accumulation of proanthocyanidins in developing legume tissues. Am. J. Bot. 1997, 84, 494–503. [Google Scholar] [CrossRef]
- Tirilly, Y.; Bourgeois, C.M. Technologie des Légumes; Editions Tec & Doc Lavoisier: Paris, France, 1999. [Google Scholar]
- Collingborn, F.M.; Gowen, S.R.; Mueller-Harvey, I. Investigations into the biochemical basis for nematode resistance in roots of three musa cultivars in response to radopholussimilis infection. J. Agric. Food Chem. 2000, 48, 5297–5301. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.G.; Camões, M.F.G.; Oliveira, L. Carotenoids in traditional Portuguese fruits and vegetables. Food Chem. 2009, 113, 808–815. [Google Scholar] [CrossRef]
- López-Lázaro, M.; Martín-Cordero, C.; Ayuso, M.J. Two new flavonol glycosides as DNA topoisomerase I poisons. Z. Naturforsch C 2000, 55, 898–902. [Google Scholar] [CrossRef]
- Jaramillo-Flores, M.E.; González-Cruz, L.; Cornejo-Mazon, M.; Dorantes-Alvarez, L.; Gutierrez-Lopez, G.F.; Hernandez-Sanchez, H. Effect of thermal treatment on the antioxidant activity and content of carotenoids and phenolic compounds of cactus pear cladodes (Opuntia ficus-indica). Food Sci. Technol. Int. 2003, 9, 271–278. [Google Scholar] [CrossRef]
- Mariod, A.A.; Ibrahim, R.M.; Ismail, M.; Ismail, N. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 2009, 116, 306–312. [Google Scholar] [CrossRef]
- Benhamama, L. Contribution à l’étude phytochimique et évaluation de l’activité Antioxydante de la plante médicinale Crataegus monogyna. Master’s Thesis, Université des Frères Mentouri, Constantine, Algeria, 2015. [Google Scholar]
- Cristani, M.; D’arrigo, M.; Giuseppina, M.; Castelli, F.; Sarpietro, M.; Micieli, M.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implicationsfor their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6301–6305. [Google Scholar] [CrossRef]
- Alghamdi, A.; Alshehri, W.; Sajer, B.; Ashkan, M.; Ashy, R.; Gashgari, R.; Hakmi, H. Biological Activities and GC-MS Analysis of Aloe vera and Opuntia ficus-indica Extracts. J. Chem. 2023, 2023, 6504505. [Google Scholar] [CrossRef]
- Hajar, N.; Nawal, A.; Amjad, D. A study to determine total phenolic content of Opuntia ficus-indica extracts and their activity against some pathogenic fungi. World J. Pharm. Pharm. Sci. 2018, 8, 98–109. [Google Scholar]
- Riyazzudin, M.D.; Sharma, M.E.; Kaur, G.U.; Chaudhry, N.A. A review on therapeutic potential of plant derived natural compounds. Bio-Chem. Acta 2016, 1, 30–34. [Google Scholar]
- Bhattacharya, D.; Koley, H. Antibacterial activity of polyphenolic fraction ofkombucha against enteric bacterial pathogens. Curr. Microbiol. 2016, 73, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial Spectrum of Plant Polyphenols and Extracts Depending upon Hydroxyphenyl Structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Sengul, M.; Yildiz, H.; Gungor, N.; Cetin, B.; Eser, Z.; Ercisli, S. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak. J. Pharm. Sci. 2009, 22, 102–106. [Google Scholar]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Santana, G.M.S.; Albuquerque, L.P.; Simoes, D.A.; Gusmao, N.B.; Coelho, L.C.B.B.; Paiva, P.M.G. Isolation of a lectin from Opuntia ficus indica cladodes. Acta Hortic. 2009, 811, 281–286. [Google Scholar] [CrossRef]
- Ochoa, M.J.; González-Flores, L.M.; Cruz-Rubio, J.M.; Rivera-López, L.A.; Rodríguez, S.; Nazareno, M.A.; Gómez-Leyva, J.F. Resistance of cactus pear (Opuntia ficus-indica) against Pseudocercospora opuntiae through β-1, 3-glucanase activity and polyphenolic compounds in cladodes. In IX International Congress on Cactus Pear and Cochineal: CAM Crops for a Hotter and Drier World 1247; International Society for Horticultural Science: Leuven, Belgium, 2017; pp. 183–190. [Google Scholar]


| Isolate Codes | Macroscopic Characteristics | Microscopic Characteristics |
|---|---|---|
| EC1 | Snow-white colony color Beige-white verso side color Cottony surface texture Medium growth speed | Septate mycelia Cylindrical microconidia Frequent chlamydospores Branched conidiophores |
| EC2 | Orange colony color Dark-orange verso side color Cottony surface texture Slow growth speed | Septate mycelia Elongate and often slightly swollen hyphae Branched hyphae at the right angles Dolipore septa with formless transverse walls |
| EC3 | Pink-white colony color at the tips Beige verso side color Cottony and dry surface texture Slow growth speed | Septate mycelia Hyaline, smooth or rough, and globose chlamydospores Fusiform and multiseptate macroconidia produced by phialides on branched conidiophores |
| EC4 | Brown-black colony color at the tips Black verso side color Cottony surface texture Slow growth speed | Septate mycelia bearing numerous chains of conidia Multi-celled and asexual conidia divided transversally and/or longitudinally |
| EC5 | Black colony color Beige verso side color Granular and dry surface texture Fast growth speed | Nonseptate mycelia Unicellular, black, and globose conidia |
| EC6 | Green colony color Yellow verso side color Granular and powdery surface texture Fast growth speed | Septate mycelia Conidiophores terminated by an erect globose head Unicellular and globose conidia |
| EC7 | Blue-green colony color Yellow-beige verso side color High powdery surface texture forming droplets Fast growth speed | Hyaline and septate mycelia Numerous, isolated, and branched conidiophores Penicillates made up of branched phialides |
| Component | Content | |
|---|---|---|
| Total polyphenols | 86.63 ± 0.008 mg GAEs/100 g FW | |
| Flavonoids | 13.40 ± 0.01 mg QEs/100 g FW | |
| Condensed tannins | 08.90 ± 0.11 mg TAEs/100 g FW | |
| Carotenoids | 0.94 ± 0.02 mg β-CEs/100 g FW | |
| Antioxidant activity (DPPH test) | IC 50 (%) | |
| Cladode extract | Ascorbic acid | |
| 0.64 ± 0.005 mg/mL | 0.39 ± 0.003 mg/mL | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokrani, S.; Ibrahim, N.A.; Benaricha, B.; Houali, K.; Cruz, C.; Boungab, K.; Bousedra, F.; Bensekrane, Z.; Aleissa, M.S.; Basher, N.S.; et al. Phytochemical Characterization and Antifungal Potential of Opuntia ficus-indica Cladode Extracts Against Tomato Pathogens. Processes 2025, 13, 1412. https://doi.org/10.3390/pr13051412
Mokrani S, Ibrahim NA, Benaricha B, Houali K, Cruz C, Boungab K, Bousedra F, Bensekrane Z, Aleissa MS, Basher NS, et al. Phytochemical Characterization and Antifungal Potential of Opuntia ficus-indica Cladode Extracts Against Tomato Pathogens. Processes. 2025; 13(5):1412. https://doi.org/10.3390/pr13051412
Chicago/Turabian StyleMokrani, Slimane, Nasir A. Ibrahim, Boumediene Benaricha, Karim Houali, Cristina Cruz, Karima Boungab, Fatma Bousedra, Zakia Bensekrane, Mohammed Saad Aleissa, Nosiba S. Basher, and et al. 2025. "Phytochemical Characterization and Antifungal Potential of Opuntia ficus-indica Cladode Extracts Against Tomato Pathogens" Processes 13, no. 5: 1412. https://doi.org/10.3390/pr13051412
APA StyleMokrani, S., Ibrahim, N. A., Benaricha, B., Houali, K., Cruz, C., Boungab, K., Bousedra, F., Bensekrane, Z., Aleissa, M. S., Basher, N. S., Derguini, A., & Nabti, E.-h. (2025). Phytochemical Characterization and Antifungal Potential of Opuntia ficus-indica Cladode Extracts Against Tomato Pathogens. Processes, 13(5), 1412. https://doi.org/10.3390/pr13051412






