Abstract
In the realm of sustainable and eco-friendly agriculture, current scientific research emphasizes the development of plant-based bioproducts to mitigate the agricultural footprint resulting from excessive fertilizer and pesticide use. This study investigates the cladodes of Opuntia ficus-indica to screen for bioactive compounds and assess their efficacy against fungal pathogens isolated from infected tomato fruits. Quantitative analysis of the methanolic extract revealed substantial concentrations of bioactive compounds: total polyphenols (86.6 mg GAEs/100 g FW), flavonoids (13.4 mg QEs/100 g FW), condensed tannins (8.9 mg TAEs/100 g FW), and carotenoids (0.9 mg β-CEs/100 g FW). Notably, the DPPH assay indicated that the cladode extract exhibited significant antioxidant potential at a concentration of 0.6 mg/mL. Seven fungal pathogens were isolated from infected tomato fruits and identified as belonging to the following genera: Rhizoctonia (EC2), Fusarium (EC1 and EC3), Alternaria (EC4), Mucor (EC5), Aspergillus (EC6), and Penicillium (EC7). At a concentration of 0.02% of the cladode hydroethanolic extract, the antifungal activity results demonstrated mycelial growth inhibition for Alternaria sp. (70.91%), Rhizoctonia solani EC2 (58.49%), Fusarium oxysporum EC3 (57.63%), and Fusarium solani EC1 (53.13%). Conversely, lower inhibitory activities were observed for Mucor sp. EC5 (31.08%), Aspergillus sp. EC6 (35.14%), and Penicillium sp. EC7 (28.38%). At a concentration of 0.04%, all cladode hydroethanolic extracts inhibited mycelial growth by more than 50%. Furthermore, the highest spore inhibition was attained with the 0.04% cladode hydroethanolic extract (exceeding 50%). Inhibition percentages of 83.02%, 85.96%, 87.76%, and 90.20% were recorded for Fusarium oxysporum EC3, Fusarium solani EC1, Rhizoctonia solani EC2, and Alternaria sp. EC4, respectively. Collectively, these findings suggest that Opuntia ficus-indica extract holds significant promise for application as a biopesticide against fungal pathogens affecting tomato fruits.
1. Introduction
The tomato, usually referred to as Lycopersicum esculentum Mill. (syn. Solanum lycopersicum L.), belongs to the Solanaceae family, Solanoideae subfamily, and Solaneae tribe. The correct taxonomic genus is still being debated, although recent studies suggest that Linnaeus was correct in attributing them to Solanum. The Lycopersicum genus includes cultivated tomatoes and a small number of closely related wild species native to Central and South America, from Mexico to Peru [1].
Currently, more than 3000 species of tomato are reported worldwide. Solanum lycopersicum (L.) is the most common species in terms of occurrence and domestication. The tomato can be consumed raw as well as in industrially processed form like pulp and sauces. Tomato is considered as a good source of dietary minerals, vitamins (vitamins C and E), lycopene (80%), folic acid, flavonoids, β-carotene, and potassium ions [2].
Globally, Solanum lycopersicum L. (tomatoes) is the second most widely grown vegetable. This crop is sensitive to over 200 diseases caused by a variety of phytopathogenic microorganisms, specifically, soil-borne fungi. The major fungal pathogen causing diseases in tomatoes are Fusarium oxysporum f. sp. lycopersici, Botrytis cinerea, Verticillium dahliae, Sclerotium rolfsii, Colletotrichum sp., Alternaria sp. Rhizoctonia solani, etc. Even though a wide range of chemical fungicides is now available to combat fungal diseases, the overuse of these chemicals has been shown to leave negative/adverse influences on the texture, yield, and nutritive value of the fruits [3].
Fungi such as Aspergillus, Penicillium, and Fusarium contaminate agricultural products and produce toxic metabolites, causing yield losses of 30% to 50% during epidemics [4]. Chemical pesticides, including fungicides and insecticides, are the primary methods for disease control [5]. Even though a wide range of chemical fungicides is now available to combat fungal diseases, the overuse of these chemicals has been shown to leave negative/adverse influences on the texture, yield, and nutritive value of the fruits [3]. However, the increasing resistance of fungi to these chemicals, combined with associated toxicological and ecological concerns, poses significant challenges [6]. These pesticides also adversely affect beneficial microorganisms essential for soil fertility [7]. Also, numerous studies have shown the emergence of fungal resistance to these chemical substances. These substances cause both toxicological and ecological threats [6]. To address these challenges, research is increasingly focused on natural resources, particularly aromatic and medicinal plants, which offer a rich source of bioactive compounds. Secondary metabolites such as polyphenols, alkaloids, and terpenes exhibit significant biological activities [8].
Among these, Opuntia species, particularly their cladodes, fruits, and flowers, have gained attention for their applications in the food, cosmetic, and pharmaceutical industries [9]. Opuntia ficus-indica, commonly known as prickly pear, is primarily found in the western Mediterranean, including southern Spain, Portugal, and North Africa (Tunisia, Algeria, and Morocco) [10]. As a member of the Cactaceae family, Opuntia comprises approximately 300 species [11]. Native to Mexico, it thrives in arid and semi-arid regions [12], with Opuntia ficus-indica being the most widely studied and consumed species. Its cladodes are notable for their high mucilage content [13], containing polysaccharides, minerals, amino acids, vitamins, phenolic acids, and flavonoids [14]. Furthermore, it exhibits therapeutic potential with antibacterial and antifungal properties. Nevertheless, it has to be said that there is variability in photochemical screening results, which can be attributed to region, seasonality, vegetation stage, agro-edaphic conditions of the plant, age, and genetic variability. Also, these data show that “Opuntia ficus-indica” could be a potential source of biomolecules of interest to medicine, nutrition, and biotechnology [15]. The current study focuses on characterizing the phytochemical composition of Opuntia ficus-indica cladode extracts, evaluating their bioactive substances, and assessing their antifungal activity against specific fungal pathogens isolated from tomato fruits of the species Lycopersicon esculentum L.
2. Materials and Methods
2.1. Sampling of Plant Material
The cladodes of Opuntia ficus-indica L. were collected in Douar Al-Abayatt (Sidi Kadaa: 35°20′51.3″ N, 0°18′46″ E), situated 18 km from the Mascara department (Figure 1). Harvesting took place in February 2023. Seven samples of infected tomato fruits (E1–E7), exhibiting visible symptoms of various diseases, were sourced from local vegetable merchants. The characterization of fungal diseases in tomatoes was based on observable symptoms, including gluiness and the presence of necrotic spots or lesions.
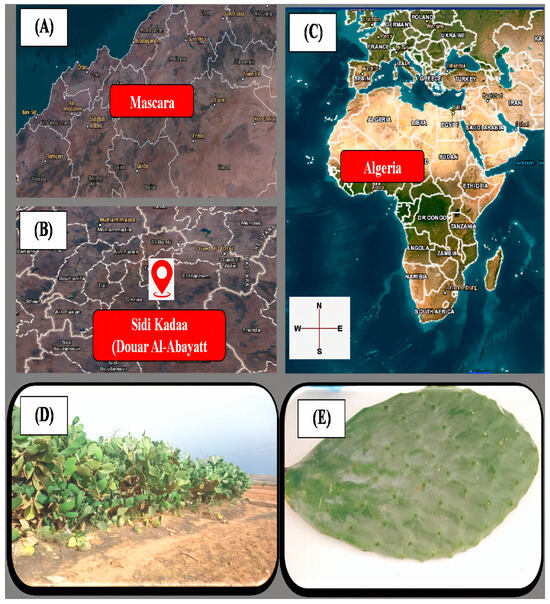
Figure 1.
Sampling location of OFI-02-2023 (O. ficus-indica): (A) Mascara, Algeria; (B) Sidi Kada (35.4° N, 0.1° E); (C) national context; (D,E) plant/cladode morphology (bar: 10 cm).
2.2. Isolation and Identification of Fungal Pathogens
2.2.1. Isolation and Purification
Infected tomato fruits (Lycopersicon esculentum L.) were rinsed with 200 mL of sterile distilled water; this was followed by surface sterilization using bleach for 10 min to eliminate external microorganisms. The fruits were then allowed to air-dry (modified from [16]). Eight tissue fragments from the infected fruits were aseptically transferred into individual Petri dishes containing Potato Dextrose Agar (PDA) medium. The dishes were sealed with parafilm and incubated in the dark at 25 °C for seven days [17].
2.2.2. Morphological Identification
Macroscopic characterization
Seven-day-old PDA cultures were examined for colony morphology (diameter, texture, pigmentation) on both the adaxial and abaxial surfaces under standardized lighting conditions [18].
Microscopic characterization
Fungal isolates were microscopically characterized using the Scotch tape technique [19], where adhesive tape impressions of colonies were stained with 0.1% methylene blue and examined at 40× magnification (Olympus CX23, Tokyo, Japan) to evaluate three key diagnostic features: (1) mycelial characteristics including septation patterns and branching architecture, (2) reproductive structures such as conidiophores and sporangia morphology, and (3) conidial morphology encompassing size dimensions, shape profiles, and septation arrangements, with all taxonomic identifications being subsequently verified by the Laboratory of Research on Biological Systems and Geomatics (L.R.S.B.G.).
2.2.3. Methanolic Extract Preparation
Fresh cladodes (10 g FW) of O. ficus-indica (L.) Mill. were homogenized in 95% methanol (1:1 w/v) using a Polytron PT 2500E (Kinematica AG, Malters, Switzerland) at 15,000 rpm for 2 min. The homogenate was stirred (700 rpm, 30 min, 25 ± 2 °C), vacuum-filtered (Whatman No.1, Maidstone, UK), and stored at −20 °C in amber vials [20].
2.2.4. Bioactive Compound Quantification
All spectrophotometric analyses (Shimadzu UV-1800, Kyoto, Japan) were performed in triplicate.
Total phenolics
The total phenolic content of Opuntia ficus-indica cladodes was determined according to a modified Folin–Ciocalteu method [21]. The assay was performed by mixing 100 μL of methanolic extract with 900 μL of 10% (v/v) Folin–Ciocalteu reagent; this was followed by a 5 min incubation at room temperature (25 ± 2 °C). Subsequently, 750 μL of 7% (w/v) sodium carbonate solution was added, and the mixture was vortexed vigorously for 30 s. After 90 min of incubation in darkness, absorbance was measured at 765 nm using a UV-Vis spectrophotometer. Phenolic content was calculated from a gallic acid standard curve (0.006–0.2 mg/mL, R2 > 0.99) and expressed as gallic acid equivalents (GAEs) in mg/100 g fresh weight (mean ± SD, n = 3 independent replicates).
Total flavonoids
Total flavonoid content was determined following the method described by Dehpeur et al. [22]. Briefly, 500 μL of the methanolic extract was mixed with 1500 μL of 95% methanol, 100 μL of 10% (w/v) aluminum chloride (AlCl₃), 100 μL of 1 M sodium acetate, and 2.8 mL of distilled water. The mixture was stirred thoroughly and incubated in the dark at room temperature for 30 min. Absorbance was then measured at 415 nm. A blank sample was prepared by replacing the extract with 95% methanol. The results were expressed as the mean flavonoid content in milligrams of quercetin equivalents (mg QEs ± SD) per 100 g of fresh weight (FW), based on three replicates. Quantification was performed using a standard calibration curve of quercetin ranging from 0.0025 mg/mL to 0.08 mg/mL.
Condensed tannins
The condensed tannin content was determined using a modified colorimetric method [23], based on the reduction of phosphomolybdic–tungstic acid complexes in alkaline medium. In brief, 0.5 mL of methanolic extract was mixed with 2.5 mL of 10% (v/v) Folin–Ciocalteu reagent and 5 mL of 7.5% (w/v) sodium carbonate solution. The reaction mixture was incubated under dark conditions for 30 min at 25 ± 2 °C, followed by 5 min at 55 ± 1 °C in a water bath. After immediate cooling in an ice-water bath for 30 min, absorbance was measured at 760 nm using a UV-Vis spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Tannin concentration was calculated from a tannic acid standard curve (0.02–0.1 mg/mL, R2 > 0.98) and expressed as tannic acid equivalents (TAEs) in mg/100 g fresh weight (mean ± SD, n = 3 analytical replicates).
Carotenoids
Carotenoid content was analyzed using an optimized protocol adapted from Sass-Kiss et al. [24]. Fresh cladode samples (4 g FW) were homogenized with 10 mL of a hexane/acetone/ethanol (1:2:2, v/v/v) solvent mixture using a Polytron homogenizer. The homogenate was agitated at 350 ± 50 rpm for 15 min using an orbital shaker; this was followed by centrifugation at 5500× g for 15 min at 4 °C (Eppendorf 5810R, Hamburg, Germany). The carotenoid-containing hexane phase was carefully collected, and absorbance was measured at 430 nm against a 95% methanol blank using a UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). Quantification was performed using a β-carotene standard curve (0.002–0.01 mg/mL, R2 > 0.99), with results expressed as β-carotene equivalents (β-CEs) in mg/100 g FW (mean ± SD, n = 3 biological replicates).
2.2.5. Antioxidant Activity (DPPH Essay)
The antioxidant capacity was assessed using the 2,2-diphenyl-1-picrylhydrazyl (DPPH•) radical scavenging assay following Aruwa et al. [25], where 50 μL of each extract dilution (1–5 mg/mL) was combined with 5 mL of freshly prepared 0.004% (w/v) DPPH• methanolic solution, vortexed for 30 s, and incubated in darkness for 30 min at 25 ± 2 °C. Absorbance was measured at 517 nm using a UV-1800 spectrophotometer (Shimadzu) with 80% (v/v) methanol as a blank, DPPH• solution as a negative control, and ascorbic acid (0.1–1.0 mg/mL) as a positive control.
DPPH•, a stable nitrogen-centered radical (violet in methanol), is reduced to diphenylpicrylhydrazine (yellow) upon proton donation, with the absorbance decrease at 517 nm (ε = 1.2 × 104 M−1cm−1) [26] being proportional to antioxidant activity.
The DPPH inhibition percentage was calculated as
where
I = [(A0 − A1)/A0] × 100
A0 = the absorbance of the negative control (DPPH solution alone);
A1 = the absorbance of the test sample/standard.
The radical scavenging activity was plotted against the extract concentration to determine the EC50 value (effective concentration for 50% inhibition). All assays were performed in triplicate (n = 3), with lower EC50 values indicating greater antioxidant potency.
2.3. Antifungal Activity
2.3.1. Hydroethanolic Extract Preparation
The extraction protocol was adapted from Ghoul et al. [20] with modifications. Fresh Opuntia ficus-indica cladodes (300 g) were homogenized in 70% (v/v) ethanol using a mechanical homogenizer (Kinematica AG, Malters, Switzerland) with constant stirring (500 rpm, 15 min, 25 ± 2 °C). The homogenate was filtered through Whatman No. 4 filter paper (400 × 400 mm; Medilips, Cat# Sp2st 60 f 4040) to remove particulate matter. The filtrate was concentrated using a rotary evaporator (40 °C, 100 mbar) to obtain 6 g of dried extract. For subsequent analyses, aliquots of 2 g and 4 g dried extract were separately reconstituted in 10 mL distilled water (final concentrations: 0.2 g/mL and 0.4 g/mL, respectively). All extracts were sterilized by UV exposure (254 nm) for 24–48 h in a laminar flow hood prior to use.
2.3.2. Preparation of PDA Medium with Different Concentrations of Cladode Hydroethanolic Extract
Under aseptic conditions, 10 mL aliquots of Opuntia ficus-indica cladode hydroethanolic extract (0.2 or 0.4 g/mL) were aseptically combined with 90 mL of Potato Dextrose Agar (PDA) medium in sterile containers. The mixtures were homogenized by continuous shaking (150 rpm) at 50 ± 1 °C using a temperature-controlled orbital shaker, yielding final extract concentrations of 0.02% and 0.04% (w/v) in the culture medium (modified from [27]).
2.3.3. Cladode’s Hydroethanolic Effect on Mycelial Growth
For antifungal testing, 6 mm diameter agar discs were aseptically excised from 7-day-old fungal cultures (grown at 25 ± 1 °C) using a sterile cork borer. These inocula were centrally placed on Potato Dextrose Agar (PDA) plates containing hydroethanolic extract at two concentrations (0.02% and 0.04% w/v) (modified from [27]). All plates were immediately sealed with parafilm M® (Bemis Company, Inc., Neenah, WI, USA) to maintain sterility and prevent desiccation and then incubated under controlled conditions.
The inoculated Petri dishes were incubated at 25 ± 1 °C for 7 days alongside control plates containing extract-free PDA medium. Following incubation, antifungal activity was evaluated by measuring radial inhibition zone diameters (mm) using digital calipers, with all treatments performed in triplicate (n = 3 technical replicates) and independently repeated three times (biological replicates) to ensure result consistency (CV < 5% across repetitions).
The mycelial growth inhibition percentage (MGIP) was determined using the formula MGIP (%) = [(D0 − D1)/D0] × 100 [28], where D0 represents the radial growth diameter (mm) of fungal colonies in control PDA medium and D1 denotes the growth diameter in hydroethanolic extract-amended PDA, with all measurements performed in triplicate using calibrated digital calipers under standardized conditions.
2.3.4. Cladode’s Hydroethanolic Effect on Sporulation
The same Petri dishes used for mycelial growth evaluation were repurposed to analyze sporulation suppression. For each fungal isolate, a 1 cm2 agar explant was aseptically excised from 10-day-old PDA cultures (incubated at 25 ± 1 °C) and transferred to sterile 15 mL conical tubes containing 10 mL of distilled water. The suspension was vortexed vigorously (30 s, 2000 rpm) and filtered through sterile Whatman No. 1 filter paper to remove residual hyphae, yielding a pure spore suspension for quantification. Spore density was determined using a Malassez hemocytometer, where 10 μL of filtered suspension was loaded via Pasteur pipette into the counting chamber and quantified according to standardized protocols. Triplicate measurements across three independent experiments (CV < 5%) yielded reproducible data expressed as spores/mL. Sporulation inhibition percentage (SIP%) was calculated as [(S0 − S1)/S0] × 100 [29], with S0 representing mean control spores (untreated PDA) and S1 denoting treated samples (extract-amended PDA), demonstrating significant antifungal effects at both concentrations tested.
2.4. Statistical Analysis
The antifungal efficacy of Opuntia ficus-indica cladode hydroethanolic extract, as measured through mycelial growth inhibition and sporulation reduction, was statistically evaluated using one-way ANOVA (α = 0.05) followed by post hoc Tukey–Kramer HSD tests for multiple comparisons. All analyses were performed using JMP Pro 17.0 (SAS Institute Inc., Cary, NC, USA), with Pearson correlation tests examining relationships between treatment concentrations and biological effects.
3. Results
3.1. Characterization of Tomato Fungal Diseases
The seven infected tomato fruit samples (E1–E7) exhibited characteristic postharvest fungal infection symptoms, including polymorphic necrotic lesions (black, white, brown, and gray) accompanied by tissue softening and moist maceration zones, with the complete symptom–pathogen–disease correlations documented in Figure 2.
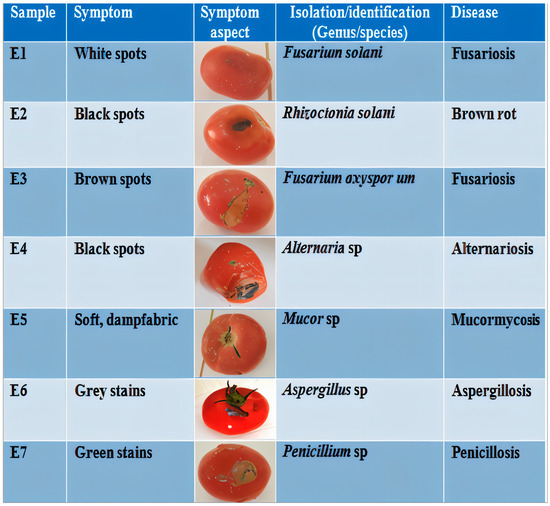
Figure 2.
Diagnostic features of infested tomato samples: symptom presentation, fungal etiology, and corresponding postharvest diseases.
3.2. Identification of Fungal Agents
3.2.1. Macroscopic Identification
The seven fungal isolates obtained from infected tomato fruits were systematically characterized, with their macroscopic morphological features detailed in Table 1.

Table 1.
Diagnostic morphological characteristics of fungal pathogens isolated from tomato samples: macroscopic colony features and microscopic identification.
The seven fungal isolates (EC1–EC7) exhibited distinct colony morphologies (Figure 3): EC1 displayed snow-white cottony mycelium with a beige-white reverse and moderate growth; EC2 showed orange cottony colonies with a dark-orange reverse and slow growth; EC3 presented pinkish-white margins with a beige reverse and dry cottony texture; EC4 featured a brownish-black periphery with a black reverse and thick mat; EC5 demonstrated jet-black granular colonies with a beige reverse and rapid growth; EC6 revealed green powdery colonies with a yellow reverse; while EC7 produced bluish-green powdery colonies with a beige-yellow reverse and exudate droplets.
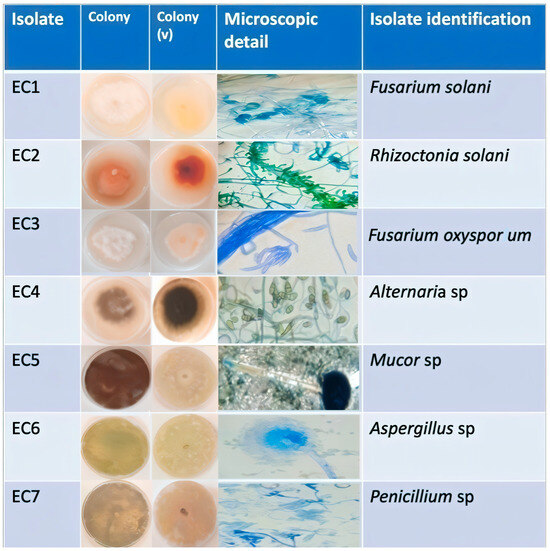
Figure 3.
Morphological characterization of fungal isolates from infected tomato fruits: (A) macroscopic colony features (obverse and reverse [v] views), (B) microscopic structures (40× magnification).
3.2.2. Microscopic Identification
The diagnostic microscopic features of the seven fungal isolates obtained from infected tomato fruits are systematically presented in Table 1.
Microscopic examination of the seven fungal isolates revealed distinct morphological characteristics: EC1 (Fusarium) displayed septate hyphae with cylindrical microconidia, abundant chlamydospores, and branched conidiophores; EC2 (Rhizoctonia) showed septate mycelium with right-angle branching of elongated, slightly swollen hyphae; EC3 (Fusarium) exhibited septate mycelium producing both smooth/rough hyaline chlamydospores and fusiform, multiseptate macroconidia; EC4 (Alternaria) presented septate hyphae generating multicellular conidia with transverse/longitudinal septa; EC5 (Mucor) featured aseptate mycelium with unicellular, globose black conidia; EC6 (Aspergillus) demonstrated septate hyphae bearing conidiophores with terminal globose heads; and EC7 (Penicillium) revealed septate hyaline hyphae with branched conidiophores and phialides, with all isolates successfully classified into six genera (Fusarium, Rhizoctonia, Alternaria, Mucor, Aspergillus, and Penicillium), as illustrated in Figure 2.
3.3. Bioactive Compound Screening
Table 2 summarizes the principal secondary metabolites identified in Opuntia ficus-indica cladode extracts, with the hydroethanolic extract containing 86.63 ± 0.008 mg gallic acid equivalents (GAEs)/100 g FW of total polyphenols, 13.4 ± 0.01 mg quercetin equivalents (QEs)/100 g FW of flavonoids, 8.9 ± 0.11 mg tannic acid equivalents (TAEs)/100 g FW of condensed tannins, and 0.94 ± 0.02 mg β-carotene equivalents (β-CEs)/100 g FW of carotenoids, demonstrating significant phenolic and carotenoid composition.

Table 2.
Phytochemical screening of bioactive molecules in Opuntia ficus-indica cladode extracts.
The DPPH radical scavenging assay revealed significant antioxidant activity, as seen in Opuntia ficus-indica cladode extract (IC50 = 0.64 ± 0.005 mg/mL), while for the control composed of ascorbic acid, IC50 = 0.39 ± 0.003 mg/mL; this demonstrates that the extract has about 61% of the antioxidant activity of ascorbic acid (calculated as 0.39/0.64 × 100%), with both values indicating strong free radical neutralization capacity.
3.4. Antifungal Activity
3.4.1. Cladode’s Hydroethanolic Effect on Mycelial Growth
The inhibitory effect of Opuntia ficus-indica cladode extract on fungal mycelial growth is quantitatively demonstrated, showing dose-dependent suppression of isolate development (Alternaria sp., Rhizoctonia sp., Fusarium sp., Mucor sp., Aspergillus sp., and Penicillium sp.).
The Opuntia ficus-indica cladode hydroethanolic extract exhibited significant, concentration-dependent inhibition of mycelial growth across all tested fungal isolates (Figure 4). At 0.02% concentration, isolate EC4 (Alternaria sp.) showed the highest sensitivity (70.91% inhibition), followed by EC2 (Rhizoctonia sp.; 58.49%), EC3 (Fusarium sp.; 57.63%), and EC1 (Fusarium sp.; 53.13%), while EC5 (Mucor sp.; 35.14%), EC6 (Aspergillus sp.; 31.08%), and EC7 (Penicillium sp.; 28.38%) demonstrated lower susceptibility. Doubling the extract concentration to 0.04% enhanced inhibition significantly (p < 0.05), with EC4 (94.55%), EC3 (92.45%), EC2 (91.53%), and EC1 (90.63%) showing >90% suppression and EC5 (56.76%), EC7 (54.05%), and EC6 (52.70%) exceeding 50% inhibition, establishing a clear positive correlation (R2 = 0.96) between extract concentration and antifungal efficacy.
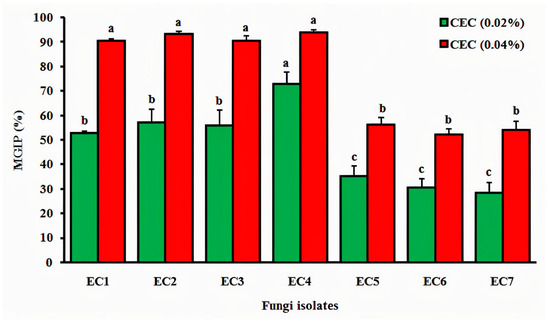
Figure 4.
Antifungal activity of Opuntia ficus-indica cladode hydroethanolic extract against postharvest fungal pathogens from tomato fruits. MGIP (%): mycelial growth inhibition percentage; CEC: cladode extract concentration. Data represent mean ± SD (n = 3). Different lowercase letters indicate significant differences (p < 0.05, one-way ANOVA with Tukey–Kramer HSD post hoc test).
3.4.2. Cladode’s Hydroethanolic Effect on Sporulation
The Opuntia ficus-indica cladode extract significantly inhibited sporulation in all the tested fungal isolates (p < 0.05 vs. control, Figure 5). At 0.02% concentration, the highest inhibition occurred in Alternaria sp. EC4 (68.42%), Rhizoctonia sp. EC2 (64.15%), Fusarium sp. EC3 (61.22%), and Fusarium sp. EC1 (60.78%), while Aspergillus sp. EC6 (49.38%), Mucor sp. EC5 (47.56%), and Penicillium sp. EC7 (47.97%) showed <50% inhibition. Doubling the concentration to 0.04% enhanced efficacy significantly (p < 0.01), with EC4 (90.20%), EC2 (87.76%), EC1 (85.96%), and EC3 (83.02%) achieving >80% suppression and EC6 (70.37%), EC5 (68.29%), and EC7 (60.76%) exceeding 60% inhibition, demonstrating dose-dependent activity (R2 = 0.93).
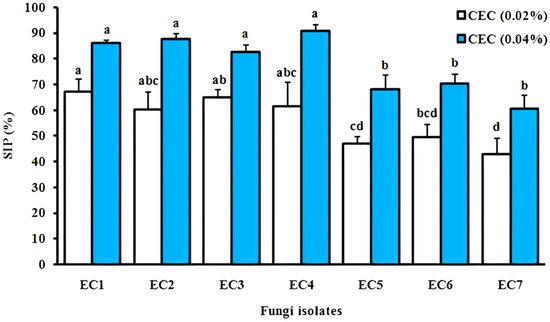
Figure 5.
Dose-dependent inhibition of sporulation in postharvest fungal pathogens by Opuntia ficus-indica cladode hydroethanolic extract. SIP (%): sporulation inhibition percentage; CEC: cladode extract concentration (w/v). Data represent mean ± SD (n = 3 biological replicates). Different lowercase letters indicate statistically significant differences (p < 0.05, one-way ANOVA with Tukey–Kramer HSD post hoc test).
Pearson correlation analysis revealed no statistically significant relationship (p > 0.05) between mycelial growth inhibition and sporulation inhibition by Opuntia ficus-indica cladode extracts across tested fungal isolates. While strong correlation coefficients were observed (r = 0.827 at 0.02% extract concentration; r = 0.949 at 0.04% concentration; Figure 6), these did not reach statistical significance at α = 0.05, suggesting independent mechanisms of action for growth versus sporulation suppression.
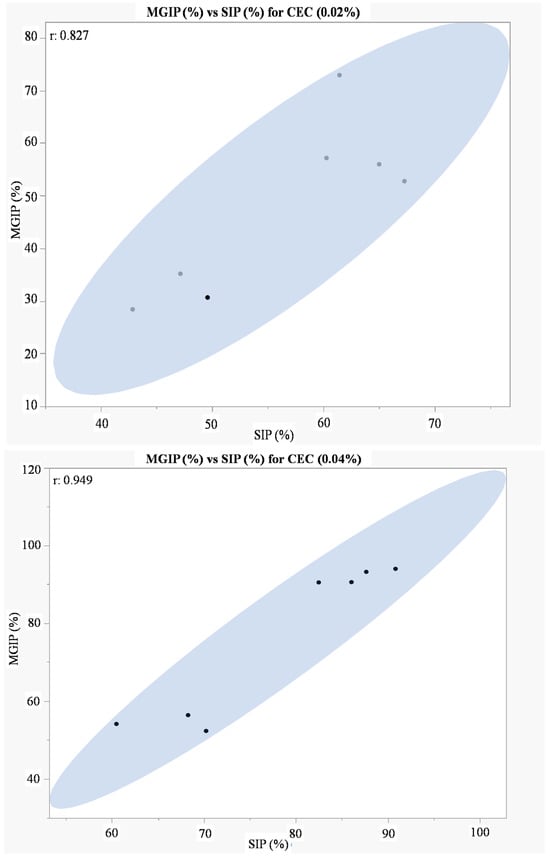
Figure 6.
Correlation analysis between mycelial growth inhibition (MGIP%) and sporulation inhibition (SIP%) for Opuntia ficus-indica cladode extract treatments: 0.02% concentration (r = 0.827, p = 0.062), 0.04% concentration (r = 0.949, p = 0.004). CEC: cladode extract concentration (w/v). Dashed lines indicate 95% confidence intervals.
4. Discussion
Microscopic analysis (40× magnification) revealed distinct morphological features among the fungal pathogens: Fusarium oxysporum [30] exhibited septate, spindle-shaped mycelium producing multiseptate macroconidia; Alternaria sp. [18] displayed septate hyphae with simple or smooth conidiophores and conidia divided by transverse and longitudinal septa; Mucor sp. [19] featured aseptate mycelium with black, unicellular, globose conidia; Aspergillus sp. [31] showed septate hyphae bearing unicellular globose conidia; Penicillium sp. demonstrated septate hyphae with branched conidiophores terminating in phialides; Fusarium solani [30] produced abundant uni- or bicellular micro- and macroconidia; and Rhizoctonia solani [32] formed septate hyphae with 90° branching, basal constrictions, and aging-induced browning of elongated hyphal cells.
The hydroethanolic extract of fresh (<7-day-old) Opuntia ficus-indica cladodes demonstrated promising antifungal activity for tomato crop protection, containing 8–9 mg total polyphenols/100 g fresh weight (FW) [33], compared to 23.4–52.6 mg GAEs/100 mL reported for cladode juice [34,35], though differences in juice density may affect this comparison. Polyphenol levels varied significantly with tissue type (2.3× higher in chlorenchyma than parenchyma), developmental stage (40% greater in young “nopales”), and processing (60% reduction after cooking) [33,36], highlighting their potential as sustainable alternatives to synthetic fungicides.
Phenolic compounds, representing one of the largest classes of plant secondary metabolites with >8000 identified structures [37], are prominently featured in Opuntia ficus-indica. Chromatographic analyses have confirmed the presence of six characteristic phenolic acids: gallic acid, caffeic acid, coumaric acid, ferulic acid, hydroxybenzoic acid, and salicylic acid [33], which collectively contribute to their observed bioactivity.
The total flavonoid content of Opuntia ficus-indica cladodes in our study (13.4 ± 0.01 mg QEs/100 g FW) exceeded previous reports of 1.24 ± 0.01 mg RE/100 mL [35] but was lower than 240 mg QEs/g FW [38], with variations attributable to cultivar differences, extraction methods (e.g., solvent polarity, temperature), and environmental conditions [39]. These water-soluble pigments, characterized by two aromatic rings (A, B) and an oxygenated heterocycle (C ring) [40], fulfill critical ecological functions including UV radiation protection [8] and tissue pigmentation, while demonstrating broad-spectrum antifungal activity against phytopathogens [41]. Notably, O. ficus-indica contains nine bioactive flavonoids: isorhamnetin, kaempferol, quercetin, isoquercitrin, rutin, catechin, epicatechin, nicotiflorin, and narcissin [33], which collectively contribute to its potent antimicrobial properties.
The condensed tannin content in Opuntia ficus-indica shows significant variability, ranging from 6.45–6.93 mg/100 g FW in cladode tissues [34] to 18.23 ± 0.36 mg tannic acid equivalents (TAEs)/100 mL in cladode juice [35], with concentrations influenced by vegetative stage and environmental conditions [42]. Although relatively low in cladodes compared to other plant organs (where they contribute to fruit astringency) [43], tannins (MW: 500–3000 Da) are widely distributed in roots, rhizomes, bark, leaves, flowers, fruits, seeds, and wood. These water-, alcohol-, and acetone-soluble (but ether-insoluble) polyphenols [8] function as crucial biochemical defenses against pathogens (bacteria, fungi, viruses), nematodes, and herbivores [44], with content varying substantially between species and within individuals based on tissue maturity and seasonal factors [42].
Quantitative analyses reveal progressive carotenoid accumulation during cladode development, with concentrations increasing from 0.047 ± 0.05 to 0.077 ± 0.06 mg/100 g FW [34]. Extraction efficiency is predominantly governed by the polarity characteristics of three carotenoid classes—xanthophylls, carotenes, and carotenoid esters—rather than their absolute solvent solubility [45]. These pigments demonstrate potent antioxidant activity through their conjugated polyene chains, which effectively quench reactive oxygen species (ROO•, HO•, O₂•−, and R•) [46]. In Opuntia ficus-indica, the carotenoid profile comprises β-carotene (36%, the predominant form), lutein (44%), and α-cryptoxanthin (20%), collectively totaling 229 μg/g dry mass [47], highlighting their significant contribution to the plant’s photoprotective mechanisms.
This study confirms significant antioxidant activity in Opuntia ficus-indica cladode extracts, consistent with previous reports of 1.45 mg/mL free radical scavenging capacity [9] and 1.78 ± 0.03 μmol Trolox equivalents (TEs)/mL in cladode juice [35]. This activity is primarily attributed to polyphenolic compounds, which show strong correlation with antiradical activity (r > 0.92, p < 0.01) [48]. These bioactive constituents exhibit multifaceted pharmacological potential, including antioxidant, antimicrobial, antiviral, anti-inflammatory, anticancer, antiallergic, and vasodilatory effects [49], while also functioning as the plant’s biochemical defense system against insects and microbial pathogens through their diverse phenolic composition.
The observed antifungal activity against both mycelial growth and sporulation is mediated by high-concentration secondary metabolites in O. ficus-indica cladode extracts, particularly flavonoids (e.g., isorhamnetin and quercetin derivatives), polyphenols (hydroxybenzoic and caffeic acids), and condensed tannins. These compounds exert fungicidal effects through three primary mechanisms: (1) membrane permeabilization via lipid bilayer disruption, (2) intracellular infiltration targeting essential enzymes and proteins, and (3) induction of oxidative stress leading to apoptotic cell death [50]. Notably, flavonoids—which plants biosynthesize as specific antifungal defenses [49]—demonstrate dose-dependent inhibition of fungal virulence factors. These findings support numerous studies documenting O. ficus-indica’s potent broad-spectrum antifungal properties.
Both aqueous and ethanolic extracts of Opuntia ficus-indica demonstrated variable antifungal activity against seven pathogenic fungi: Aspergillus niger (MT628904.1), Curvularia khuzestanica (MH688044.1), Penicillium funiculosum (JX500735.1), Talaromyces funiculosus (KX262973.1), Penicillium minioluteum (JN620402.1), Aspergillus chevalieri (MT487830.1), and A. terreus (MT558939.1). Ethanolic extracts showed superior efficacy, with T. funiculosus exhibiting the largest inhibition zone (0.40 ± 0.10 mm) and P. funiculosum the smallest (0.07 ± 0.06 mm). Aqueous extracts displayed minimal activity (0.13–0.17 mm) [51]. Complementary studies with methanolic extracts revealed 89.57% inhibition of A. fumigatus and 85.40% inhibition of A. flavus at 1000 mg/mL [52], highlighting the potential for developing O. ficus-indica-based biofungicides, particularly for tomato crop protection.
Plants from different genera are a natural source of a wide range of compounds, including secondary metabolites that act as defense agents against environmental aggressions. It is generally accepted that the beneficial effects of herbal remedies can be attributed to active constituents present in either the aerial and subterranean parts or the whole plant, whether in crude or processed form [53]. The antimicrobial activity of Opuntia cladode extracts may be related to their high content of polyphenols, especially isorhamnetin, which has been reported as a substance with antimicrobial activity [54]. Phenolic compounds can act at two different levels: the cell membrane and cell wall of microorganisms [55]. They can interact with membrane proteins, affect membrane permeability, and lead to cell destruction. Furthermore, phenolic compounds are widely known to be synthesized by plants in response to microbial infection [56]. Therefore, it is logical that they can be effective antimicrobial substances in vitro against a wide range of microorganisms [57].
Santana et al. [58] reported that the inhibition of hemagglutinating activity (HA) with glycoproteins indicates that the activity of O. ficus indica cladodes is due to lectin presence. Furthermore, Ochoa et al. [59] indicated that the ethanolic extract of resistant cladodes showed higher levels of total condensed tannins, flavonoids, and polyphenols than those of susceptible genotypes, generating 93% inhibition of P. opuntiae conidial germination in vitro. The total protein in the resistant genotype showed 300% higher β-1,3-glucanase activity than the susceptible genotype. This increased activity was able to inhibit the germination of conidia by 90%, a similar effect to that of the fungicide Captan® (N-trichloromethylthio-4-cyclohexene 1,2-dicarboximide). This study showed, for the first time, that the combined action of cactus polyphenols and β-1,3-glucanase contributes significantly to resistance against P. opuntiae.
Taken together, these findings suggest the need for additional phytochemical analysis, such as characterizing other biomolecules and/or extracting and purifying active substances to test them separately and determine the fraction responsible for the activity. Furthermore, this study can address various gaps, such as variation due to geographic origin, extraction method, or specific plant part influencing the phytochemical screening.
5. Conclusions
This study demonstrates that Opuntia ficus-indica cladode extracts exhibit potent, dose-dependent antifungal activity against seven tomato pathogens: Fusarium oxysporum (90.48%), F. solani (90.55%), Rhizoctonia solani (93.20%), Alternaria sp. (93.98%), Mucor sp. (56.31%), Aspergillus sp. (52.25%), and Penicillium sp. (54.05%). A 0.04% hydroethanolic extract showed superior efficacy in both mycelial growth and sporulation inhibition (>50% for all isolates). The observed bioactivity correlates with the extract’s rich phytochemical profile (flavonoids, polyphenols, carotenoids, and condensed tannins), positioning this CAM species as a promising source for developing natural biofungicides to sustainably manage tomato diseases in agricultural settings.
Nevertheless, it is important to mention that the efficacy of the cladode extract in vivo, for example, on real tomato fruits, may be modified or reduced depending on various parameters such as climatic conditions, the virulence of fungal strains, and the method of application of the cladode extract. Testing the extract in vivo in a greenhouse or on a tomato field is crucial to confirming the practical application of these findings. Thus, future studies should focus on evaluating in vivo efficacy and on the in-depth elucidation of the mechanisms of action of the observed antifungal activity.
Author Contributions
Conceptualization, S.M. and B.B.; methodology, S.M. and E.-h.N.; software, K.B.; validation, E.-h.N., K.H., and C.C.; formal analysis, S.M., B.B., F.B., and Z.B.; investigation, S.M. and K.B.; resources, E.-h.N.; data curation, S.M. and E.-h.N.; writing—original draft preparation, S.M. and B.B.; writing—review and editing, S.M., E.-h.N., K.H., and C.C.; visualization, A.D., Z.B., and B.B.; supervision, E.-h.N.; project administration, N.S.B. and M.S.A.; funding acquisition, N.A.I. and N.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data sets analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
This research was conducted through a collaborative effort involving The Centre for Ecology, Evolution and Environmental Changes (cE3c), Universidade de Lisboa, Portugal; The Department of Microbiology, Université de Bejaia, Algeria; and The Department of Agronomy, Université de Mascara, Algeria. We gratefully acknowledge the scientific and technical contributions of all participating institutions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- González, M.; Cid, M.C.; Lobo, M.G. Usage of Tomato (Lycopersicum Esculentum Mill.) Seeds in Health. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Elsevier: San Diego, CA, USA, 2011; pp. 1123–1132. [Google Scholar]
- Willcox, J.K.; Catignani, G.L.; Lazarus, S. Tomatoes and cardiovascular health. Crit. Rev. Food Sci. Nutr. 2003, 43, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Singh, U.B.; Ilyas, T.; Malviya, D.; Vishwakarma, S.K.; Shafi, Z.; Yadav, B.; Singh, H.V. Bacterial inoculants for control of fungal diseases in Solanum lycopersicum L. (tomatoes): A comprehensive overview. In Rhizosphere Microbes. Microorganisms for Sustainability; Singh, U.B., Sahu, P.K., Singh, H.V., Sharma, P.K., Sharma, S.K., Eds.; Springer: Singapore, 2022; pp. 311–339. [Google Scholar]
- Doyle, M.P.; Beuchat, L.R.; Montville, T.J. Food Microbiology: Fundamentals and Frontiers; ASM Press: Washington DC, USA, 1998. [Google Scholar]
- Merghid, M.; Debbache, M.; Foughali, I. Impacts des pesticides utilisés dans la plasticulture sur la santé humaine En Algérie. Etude de cas la wilaya de Constantine. Master’s Thesis, Université des Frères Mentouri, Constantine, Algeria, 2017. [Google Scholar]
- Alleche, N. Activité Antifongique de Quelques Extraits D’une Plante Endémique Sur Des Moisissures du Blé Stocké. Master’s Thesis, Université des Frères Mentouri, Constantine, Algeria, 2017. [Google Scholar]
- Elkady, W.M.; Raafat, M.M.; Abdel-Aziz, M.M.; Al-Huqail, A.A.; Ashour, M.L.; Fathallah, N. Endophytic fungus from Opuntina fiscus-indica: A source of potential bioactive antimicrobial compounds against multidrug-resistant bacteria. Plants 2022, 11, 1070. [Google Scholar] [CrossRef]
- Bruneton, J. Pharmacognosie, Phytochimie, Plantes Médicinales, 3rd ed.; Éditions Tec & Doc Lavoisier: Paris, France, 1999. [Google Scholar]
- Msaddak, L. Propriétés Techno-Fonctionnelles et Substances Bioactives de Deux Ingrédients Alimentaires: Cladodes du Figuier de Barbarie et Feuilles de Vigne. Ph.D. Thesis, Université de Gabès, Tunis, Tunisia, 2018. [Google Scholar]
- Salem, H.B.; Nefzaoui, A.; Salem, L.B. Supplementing spineless cactus (Opuntia ficus-indica f. inermis) based diets with urea-treated straw or old man saltbush (Atriplex nummularia). Effects on intake, digestion and sheep growth. J. Agric. Sci. 2002, 138, 85–92. [Google Scholar] [CrossRef]
- Yahia, E.M.; Sáenz, C. Cactus pear (Opuntia species). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahiya, E.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 290–329. [Google Scholar]
- Felker, P.; Rodriguez, S.; Del, C.; Casoliba, R.M.; Filippini, R.; Medina, D.; Zapata, R. Comparison of Opuntia ficus-indica varieties of Mexican and Argentine origin for fruit yield and quality in Argentina. J. Arid. Environ. 2005, 60, 405–422. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Bonina, F. The polysaccharide and low molecular weight components of Opuntia ficus-indica cladodes: Structure and skin repairing properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Malainine, M.E.; Dufresne, A.; Dupeyre, D.; Mahrouz, M.; Vuong, R.; Vignon, M.R. Structure and morphology of cladodes and spines of Opuntia ficus-indica. Cellulose extraction and characterisation. Carbohydr. Polym. 2003, 51, 77–83. [Google Scholar] [CrossRef]
- Goudjil, S.; Naceri, K.; Noura, S. Caractérisation Physicochimique et Effet Antibactérien de la Cladode d’Opuntia ficus-indica Inermis (L) Mill. de la Région de contributtioianret, en Vue D’explorer Son Potentiel Thérapeutique. Ph.D. Thesis, Université Ibn khaldoun, Tiaret, Algeria, 2018. [Google Scholar]
- Meena, M.; Swapnil, P.; Upadhyay, R.S. Isolation, characterization and toxicological potential of Alternaria-mycotoxins (TeA, AOH and AME) in different Alternaria species from various regions of India. Sci. Rep. 2017, 7, 8777. [Google Scholar] [CrossRef] [PubMed]
- Cassagne, H. Milieux de Culture et Leurs Applications; Edition de la Tourelle: Paris, France, 1966. [Google Scholar]
- Bessadat, N. Isolement, Identification et Caractérisation des Alternaria sp. Résponsable de la Détérioration des Plantes Maraichères Par Des Systèmes Enzymatiques et Moléculaires. Ph.D. Thesis, Université d’Oran es-senia, Oran, Algeria, 2014. [Google Scholar]
- Botton, B.; Bretton, A.; Fever, M.; Gautier, S.; Guy, P.; Larpent, J.P.; Reymond, P.; Sanglier, J.; Vayssier, Y.; Veau, P. Moisissures Utiles et Nuisibles. Importance Industriell; Collection Biotechnologie: Paris, France, 1990. [Google Scholar]
- Ghoul, M.; Minet, J.; Bernard, T.; Dupray, E.; Cornier, M. Marine macroalgae as a source for osmoprotection for Escherichia coli. Microb. Ecol. 1995, 30, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Dehpeur, A.A.; Ebrahimzadeh, A.A.; Fazel, N.S.; Nabavi, S.M. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas Aceites 2009, 60, 405–412. [Google Scholar] [CrossRef]
- Joslyn, M. Tannins and related phenolics. J. Cell. Biochem. 1970, 22, 188–919. [Google Scholar]
- Sass-Kiss, A.; Kiss, J.; Milotay, P.; Kerek, M.M.; Toth-Markus, M. Differences in anthocyanin and carotenoid content of fruits and vegetables. Food Res. Int. 2005, 38, 1023–1029. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) plant compounds, biological activities and prospects—A comprehensive review. Food Res. Int. 2018, 112, 328–344. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Int. J. Foods Sci. Tech. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Serhan, A.R. Additional interaction of mint leaf extracts with fungi that have an antagonistic property on certain fungi associated with legume seeds. Arab. J. Plant Prot. 2006, 24, 118–124. [Google Scholar]
- Kismoune, S. L’effet de L’extrait Aqueux de Cyprès Sur la Croissance de Champignon Phythophthora infestans. Master’s Thesis, Université Mouhamed Seddik Benyahia, Jijel, Algeria, 2021. [Google Scholar]
- Leroux, P.; Credet, A. Document Sur L’étude de L’activité des Fongicides; INRA: Versailles, France, 1978. [Google Scholar]
- Chabasse, D.; Bouchara, J.; De Gentile, L.; Brun, S.; Cimon, B.; Penn, P. Les moisissures d’intérêt médical. Cah. Form. Biol. Med. 2002, 25, 46–85. [Google Scholar]
- Guiraud, J.P. Microbiologie Alimentaire; Edition Dunod: Paris, France, 1998. [Google Scholar]
- Blancard, D. 2013. Biologie, Epidémiologie. Available online: http://ephytia.inra.fr/fr/C/5217/Tomate-Biologie-epidemiologie (accessed on 1 November 2023).
- Ventura-Aguilar, R.I.; Bosquez-Molina, E.; Bautista-Baños, S.; Rivera-Cabrera, F. Cactus stem (Opuntia ficus-indica Mill): Anatomy, physiology and chemical composition with emphasis on its biofunctional properties. J. Sci. Food Agric. 2017, 97, 5065–5073. [Google Scholar] [CrossRef] [PubMed]
- Hadj Sadok, T.; Aid, F.; Bellal, M.; Abdul Hussain, M.S. Composition Chimique Des Jeunes Cladodes d’Opuntia ficus-indica et Possibilité de Valorisation Alimentaire. Ph.D. Thesis, Ecole Nationale Supérieur Agronomique, Alger, Algeria, 2010. [Google Scholar]
- Boutakiout, A. Etude physico-chimique, biochimique et stabilité d’un nouveau produit: Jus de cladode du figuier de Barbarie marocain (Opuntia ficus-indica et Opuntia megacantha). Ph.D. Thesis, Université d’Angers, Angers, France, 2015. [Google Scholar]
- Aganga, A.A.; Mosase, K.W. Tannins content, nutritive value and dry matter digestibility of Lonchocarpuscapassa, Ziziphus mucronata, Sclerocarya birrea, Kirkia acuminata and Rhus lancea seeds. Anim. Feed Sci. Technol. 2001, 91, 107–113. [Google Scholar] [CrossRef]
- Bruneton, J. Pharmacognosie, Phytochimie, Plantes Médicinales, 5th ed.; Éditions Tec & Doc Lavoisier: Paris, France, 2015. [Google Scholar]
- Boukhalfa, S.; Hamdi, S. Évaluation phytochimique et étude des activités biologiques des extraits bruts des plantes médicinales locales: Opuntia ficus-indica et Thymus lanceolatus. Master’s Thesis, Université des Frères Mentouri, Constantine, Algeria, 2016. [Google Scholar]
- Maataoui, B.S.; Hmyene, A.; Hilali, S. Activites anti-radicalaires d’extraits de jus de fruits du figuier de barbarie (Opuntia ficus-indica). Leban. Sci. J. 2006, 7, 3–8. [Google Scholar]
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef]
- Al Aboody, M.S.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Skadhauge, B.; Gruber, M.Y.; Thomsen, K.K.; Von Wettstein, D. Leucocyanidin reductase activity and accumulation of proanthocyanidins in developing legume tissues. Am. J. Bot. 1997, 84, 494–503. [Google Scholar] [CrossRef]
- Tirilly, Y.; Bourgeois, C.M. Technologie des Légumes; Editions Tec & Doc Lavoisier: Paris, France, 1999. [Google Scholar]
- Collingborn, F.M.; Gowen, S.R.; Mueller-Harvey, I. Investigations into the biochemical basis for nematode resistance in roots of three musa cultivars in response to radopholussimilis infection. J. Agric. Food Chem. 2000, 48, 5297–5301. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.G.; Camões, M.F.G.; Oliveira, L. Carotenoids in traditional Portuguese fruits and vegetables. Food Chem. 2009, 113, 808–815. [Google Scholar] [CrossRef]
- López-Lázaro, M.; Martín-Cordero, C.; Ayuso, M.J. Two new flavonol glycosides as DNA topoisomerase I poisons. Z. Naturforsch C 2000, 55, 898–902. [Google Scholar] [CrossRef]
- Jaramillo-Flores, M.E.; González-Cruz, L.; Cornejo-Mazon, M.; Dorantes-Alvarez, L.; Gutierrez-Lopez, G.F.; Hernandez-Sanchez, H. Effect of thermal treatment on the antioxidant activity and content of carotenoids and phenolic compounds of cactus pear cladodes (Opuntia ficus-indica). Food Sci. Technol. Int. 2003, 9, 271–278. [Google Scholar] [CrossRef]
- Mariod, A.A.; Ibrahim, R.M.; Ismail, M.; Ismail, N. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 2009, 116, 306–312. [Google Scholar] [CrossRef]
- Benhamama, L. Contribution à l’étude phytochimique et évaluation de l’activité Antioxydante de la plante médicinale Crataegus monogyna. Master’s Thesis, Université des Frères Mentouri, Constantine, Algeria, 2015. [Google Scholar]
- Cristani, M.; D’arrigo, M.; Giuseppina, M.; Castelli, F.; Sarpietro, M.; Micieli, M.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implicationsfor their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6301–6305. [Google Scholar] [CrossRef]
- Alghamdi, A.; Alshehri, W.; Sajer, B.; Ashkan, M.; Ashy, R.; Gashgari, R.; Hakmi, H. Biological Activities and GC-MS Analysis of Aloe vera and Opuntia ficus-indica Extracts. J. Chem. 2023, 2023, 6504505. [Google Scholar] [CrossRef]
- Hajar, N.; Nawal, A.; Amjad, D. A study to determine total phenolic content of Opuntia ficus-indica extracts and their activity against some pathogenic fungi. World J. Pharm. Pharm. Sci. 2018, 8, 98–109. [Google Scholar]
- Riyazzudin, M.D.; Sharma, M.E.; Kaur, G.U.; Chaudhry, N.A. A review on therapeutic potential of plant derived natural compounds. Bio-Chem. Acta 2016, 1, 30–34. [Google Scholar]
- Bhattacharya, D.; Koley, H. Antibacterial activity of polyphenolic fraction ofkombucha against enteric bacterial pathogens. Curr. Microbiol. 2016, 73, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial Spectrum of Plant Polyphenols and Extracts Depending upon Hydroxyphenyl Structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Sengul, M.; Yildiz, H.; Gungor, N.; Cetin, B.; Eser, Z.; Ercisli, S. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak. J. Pharm. Sci. 2009, 22, 102–106. [Google Scholar]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Santana, G.M.S.; Albuquerque, L.P.; Simoes, D.A.; Gusmao, N.B.; Coelho, L.C.B.B.; Paiva, P.M.G. Isolation of a lectin from Opuntia ficus indica cladodes. Acta Hortic. 2009, 811, 281–286. [Google Scholar] [CrossRef]
- Ochoa, M.J.; González-Flores, L.M.; Cruz-Rubio, J.M.; Rivera-López, L.A.; Rodríguez, S.; Nazareno, M.A.; Gómez-Leyva, J.F. Resistance of cactus pear (Opuntia ficus-indica) against Pseudocercospora opuntiae through β-1, 3-glucanase activity and polyphenolic compounds in cladodes. In IX International Congress on Cactus Pear and Cochineal: CAM Crops for a Hotter and Drier World 1247; International Society for Horticultural Science: Leuven, Belgium, 2017; pp. 183–190. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).