Piper aduncum Essential Oil: Toxicity to Sitophilus zeamais and Effects on the Quality of Corn Grains
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Essential Oil
2.3. Initial Characterization of Corn Grain Quality
2.4. Toxicity Bioassays
2.5. Quality Analysis of Grains Sprayed with PAEO
3. Results and Discussion
3.1. PAEO Composition and Toxicity
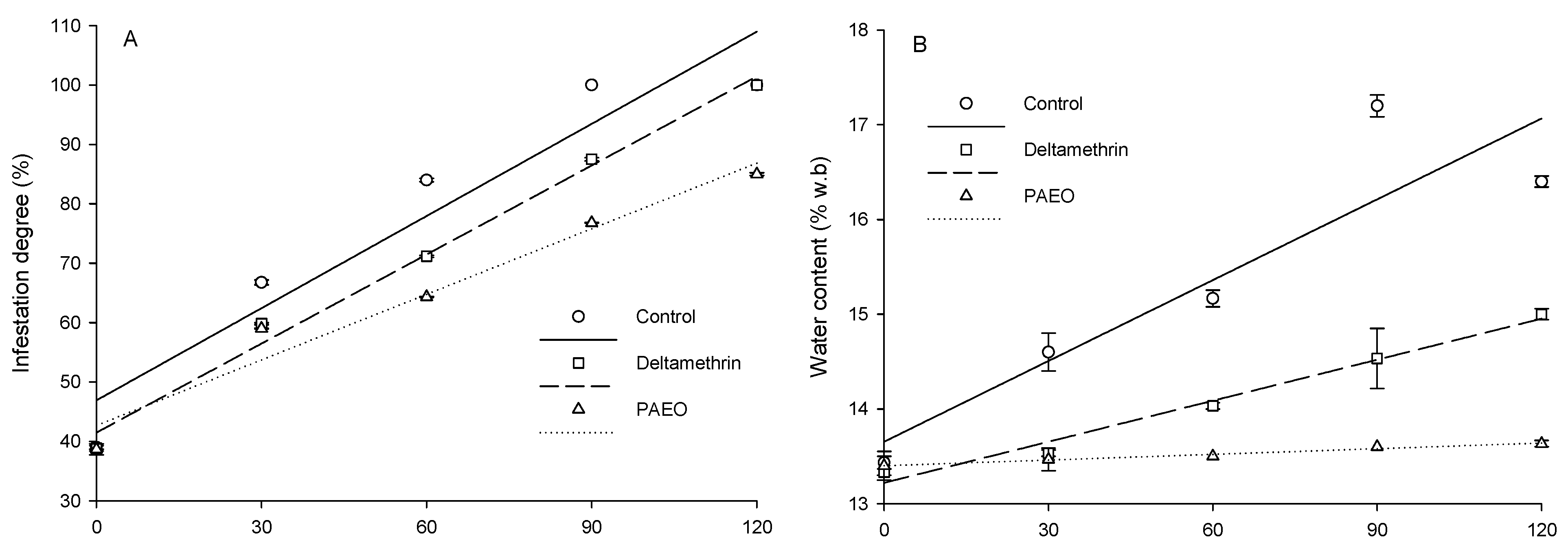
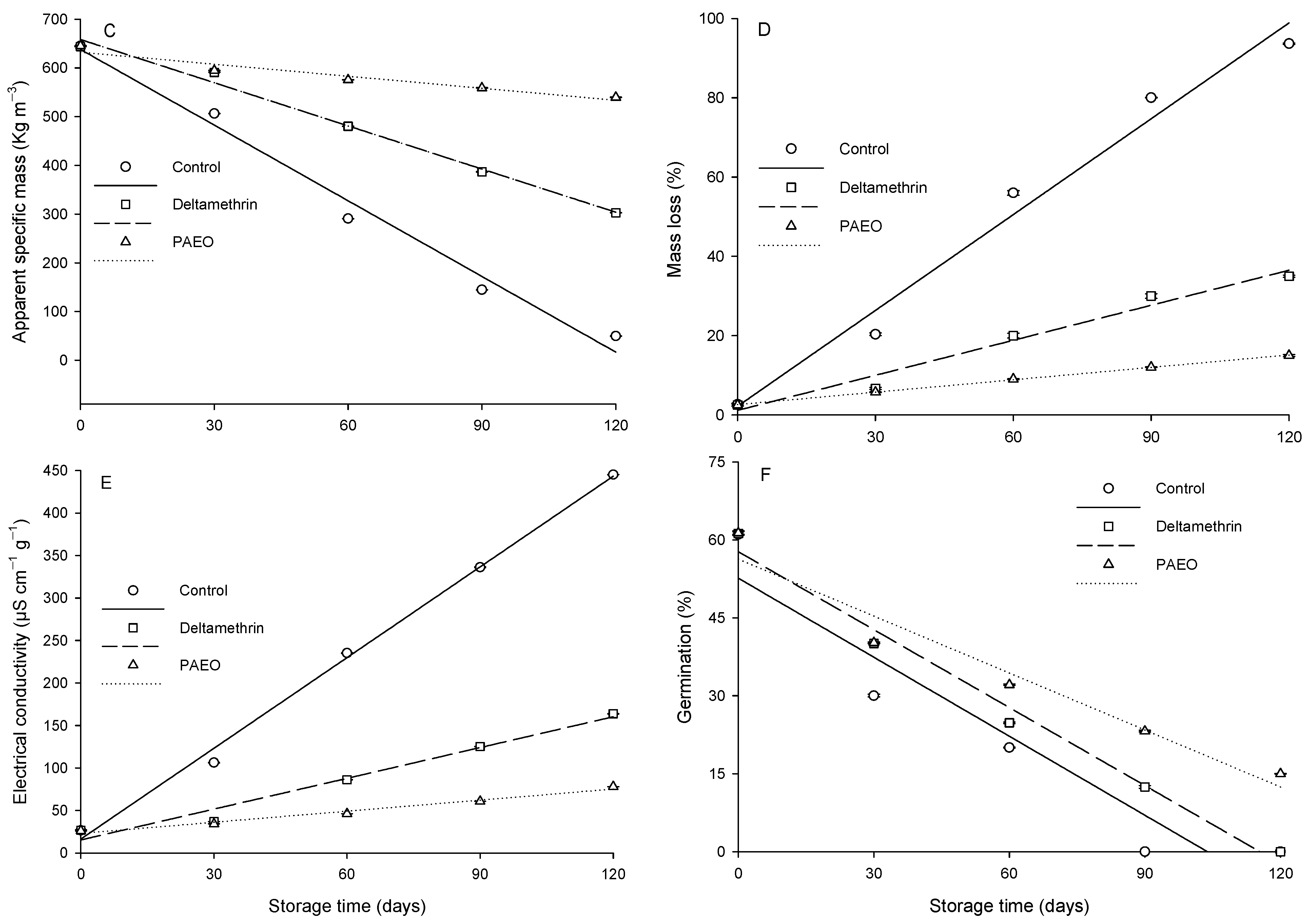
3.2. Quality of Grains Sprayed with PAEO
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Frazão, C.A.V.; Silva, P.R.R.; Almeida, W.A.D.; Pontual, E.V.; Cruz, G.D.S.; Napoleão, T.H.; França, S.M.D. Resistance of maize cultivars to Sitophilus zeamais (Coleoptera: Curculionidae). Arq. Inst. Biol. 2018, 85, e0552017. [Google Scholar] [CrossRef]
- Antunes, L.E.; VIebrantz, P.C.; Gottardi, R.; Dionello, R.G. Características físico-químicas de grãos de milho atacados por Sitophilus zeamais durante o armazenamento. Rev. Bras. Eng. Agric. Ambient. 2011, 15, 615–620. [Google Scholar] [CrossRef]
- Silva, M.G.; Silva, G.N.; Sousa, A.H.; Freitas, R.S.; Silva, M.S.; Abreu, A.O. Hermetic storage as an alternative for controlling Callosobruchus maculatus (Coleoptera: Chrysomelidae) and preserving the quality of cowpeas. J. Stored Prod. Res. 2018, 78, 27–31. [Google Scholar] [CrossRef]
- Freitas, R.S.; Faroni, L.R.A.; Sousa, A.H. Hermetic storage for control of common bean weevil, Acanthoscelides obtectus (Say). J. Stored Prod. Res. 2016, 66, 1–5. [Google Scholar] [CrossRef]
- Ramachandran, R.P. Integrated approach on stored grain quality management with CO2 monitoring—A review. J. Stored Prod. Res. 2022, 96, 101950. [Google Scholar] [CrossRef]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef]
- Araujo, A.; Oliveira, J.V.D.; França, S.M.; Navarro, D.M.; Barbosa, D.R.; Dutra, K.D.A. Toxicity and repellency of essential oils in the management of Sitophilus zeamais. Rev. Bras. Eng. Agric. Ambient. 2019, 23, 372–377. [Google Scholar] [CrossRef]
- Fazolin, M.; Estrela, J.L.V.; Catani, V.; Alécio, M.R.; Lima, M.S.D. Propriedade inseticida dos óleos essenciais de Piper hispidinervum C. DC.; Piper aduncum L. e Tanaecium nocturnum (Barb. Rodr.) Bur. & K. Shum sobre Tenebrio molitor L., 1758. Ciênc. Agrotec. 2007, 31, 113–120. [Google Scholar]
- Santos, T.L.B.; Turchen, L.M.; Dall’oglio, E.L.; Butnariu, A.R.; Pereira, M.J.B. Fitoquímica do óleo essencial de Piper e toxicidade aguda sobre Helicoverpa armigera (Lepidoptera: Noctuidae). Rev. Bras. Cienc. Agrar. 2017, 12, 484–489. [Google Scholar]
- BRASIL; Ministério da Agricultura; Pecuária e Abastecimento (MAPA); Secretaria de Defesa Agropecuária (ACS). Regras para Análise de Sementes (RAS); MAPA: Brasília, Brasil, 2009; p. 399. [Google Scholar]
- Marcondes, E.; Ribeiro, M.A.; Stangerlin, D.M.; Souza, A.P.; de Melo, R.R.; Gatto, D.A. Resistência natural da madeira de duas espécies amazônicas em ensaios de deterioração de campo. Sci. Plena 2013, 9, 067302. [Google Scholar]
- Mendonça, J.F.; Sousa, A.H.; Faroni, L.R.A.; Fernandes, C.C.; Santos, A.C.V.; Lopes, L.M.; Ferraz, M.S.S.; Prates, L.H.F. Yield, composition and toxicity of piperaceae volatiles to pest insects. Rev. Caatinga 2024, 37, e12298. [Google Scholar] [CrossRef]
- Santana, H.T.; Trindade, F.T.T.; Stabeli, R.G.; Silva, A.A.E.; Militão, J.S.T.L.; Facundo, V.A. Essential oils of leaves of Piper species display larvicidal activity against the dengue vector, Aedes aegypti (Diptera: Culicidae). Rev. Bras. Plantas Med. 2015, 17, 105–111. [Google Scholar] [CrossRef]
- Kim, S.W.; Kang, J.; Park, I.K. Fumigant toxicity of Apiaceae essential oils and their constituents against Sitophilus oryzae and their acetylcholinesterase inhibitory activity. J. Asia Pac. Entomol. 2013, 16, 443–448. [Google Scholar] [CrossRef]
- Song, H.Y.; Yang, J.Y.; Suh, J.W.; Lee, H.S. Activities of Apiaceae essential oils against armyworm, Pseudaletia unipuncta (Lepidoptera: Noctuidae). J. Agric. Food Chem. 2011, 59, 7759–7764. [Google Scholar] [CrossRef] [PubMed]
- Haddi, K.; Valbon, W.R.; Viteri Jumbo, L.O.; Oliveira, L.O.; Guedes, R.N.C.; Oliveira, E.E. Diversity and convergence of mechanisms involved in pyrethroid resistance in the stored grain weevils, Sitophilus spp. Sci. Rep. 2018, 8, 16361. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.L.V.; Fazolin, M.; Catani, V.; Alécio, M.R.; Lima, M.S.D. Toxicity of essential oils of Piper aduncum and Piper hispidinervum against Sitophilus zeamais. Pesqui. Agropecu. Bras. 2006, 41, 217–222. [Google Scholar] [CrossRef]
- Magalhaes, V.B.; Sousa, A.H. Quality of white Gurgutuba creole beans stored in silo bags and PET bottles. Rev. Agrogeoambiental 2020, 12, 91–104. [Google Scholar] [CrossRef]
- BRASIL, Mistério da Agricultura, Pecuária e Abastecimento (MAPA). Instrução Normativa Nº 60, de 22 de Dezembro de 2011; MAPA: Brasília, Brasil, 2011. [Google Scholar]
- Faroni, L.R.A.; Barbosa, G.D.O.; Sartori, M.A.; Cardoso, F.D.S.; Alencar, E.D. Avaliação qualitativa e quantitativa do milho em diferentes condições de armazenamento. Eng. Agríc. 2005, 13, 193–201. [Google Scholar]
- Paraginski, R.T.; Rockenbach, B.A.; Santos, R.F.D.; Elias, M.C.; Oliveira, M.D. Qualidade de grãos de milho armazenados em diferentes temperaturas. Rev. Bras. Eng. Agric. Ambient. 2015, 19, 358–363. [Google Scholar] [CrossRef]
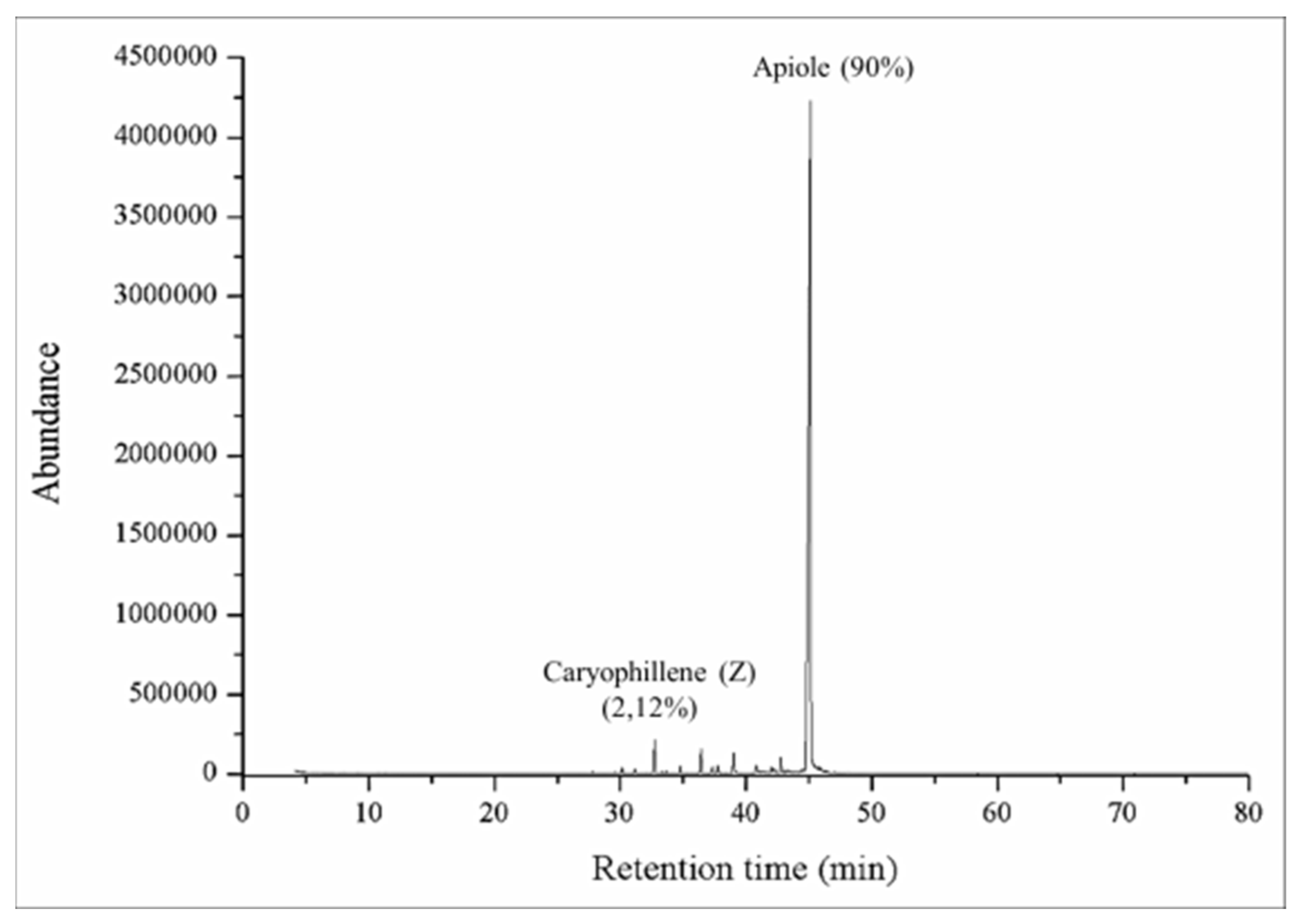
| Grain Characteristics | Average | S.E.M 1 |
|---|---|---|
| Degree of infestation by pest insects (%) | 0 | 0.0 |
| Germination percentage (%) | 98.5 | 0.9 |
| Moisture content on a wet basis (%) | 13.0 | 0.0 |
| Electrical conductivity (µS cm−1 g−1) | 15.7 | 0.6 |
| Apparent specific mass (kg m−3) | 685.3 | 2.7 |
| Insecticide * | Inclination (±S.E.M.) | LC50 (95% FI) (µL kg−1 of Grains) | TR50 (CI 95%) | LC95 (95% FI) (µL kg−1 of Grains) | TR95 (CI 95%) | χ2 | p |
|---|---|---|---|---|---|---|---|
| Deltamethrin | 4.94 ± 0.52 | 0.42 (0.37–0.46) | 1.00 | 0.90 (0.79–1.09) | 1.00 | 2.02 | 0.57 |
| PAEO | 5.62 ± 0.61 | 298.50 (279.80–316.45) | 710.71 | 585.20 (519.88–697.11) | 650.00 | 7.32 | 0.29 |
| Variable | Treatments | Adjusted Equations | G.L. Error | F | p | R2 |
|---|---|---|---|---|---|---|
| Infestation degree | PAEO | ŷ = 0.3682x + 43.4020 | 10 | 65.19 | 0.004 | 0.96 |
| Deltamethrin | ŷ = 0.5003x + 42.0400 | 10 | 248.94 | 0.0006 | 0.99 | |
| Control | ŷ = 0.5200x + 46.600 | 10 | 31.04 | 0.0114 | 0.91 | |
| Water content | PAEO | ŷ = 0.0017x + 13.4200 | 10 | 25.00 | 0.0154 | 0.89 |
| Deltamethrin | ŷ = 0.0147x + 13.1800 | 10 | 161.33 | 0.0011 | 0.98 | |
| Control | ŷ = 0.0287x + 13.6400 | 10 | 14.64 | 0.0315 | 0.83 | |
| Apparent specific mass | PAEO | ŷ = −0.8220x + 632.1600 | 10 | 44.14 | 0.0069 | 0.94 |
| Deltamethrin | ŷ = −2.9533x + 658.0600 | 10 | 341.64 | 0.0003 | 0.99 | |
| Control | ŷ = −5.1780x + 638.0200 | 10 | 189.96 | 0.0008 | 0.98 | |
| Mass loss | PAEO | ŷ = 0.1043x + 2.5800 | 10 | 6835.05 | <0.0001 | 0.99 |
| Deltamethrin | ŷ = 0.2943x + 1.1800 | 10 | 107.68 | 0.0019 | 0.97 | |
| Control | ŷ = 0.8069x + 2.0560 | 10 | 140.22 | 0.0013 | 0.98 | |
| Electrical conductivity | PAEO | ŷ = 0.4347x + 22.9600 | 10 | 161.04 | 0.0011 | 0.98 |
| Deltamethrin | ŷ = 1.2080x + 15.2800 | 10 | 109.37 | 0.0019 | 0.97 | |
| Control | ŷ = 3.5587x + 16.3600 | 10 | 811.78 | <0.0001 | 0.99 | |
| Germination | PAEO | ŷ = −0.3653x + 56.2800 | 10 | 57.09 | 0.0048 | 0.95 |
| Deltamethrin | ŷ = −0.5010x + 57.8200 | 10 | 205.84 | 0.0007 | 0.99 | |
| Control | ŷ = −0.5067x + 52.6000 | 10 | 28.13 | 0.0131 | 0.90 |
| Product | Storage Period (Days) | ||||
| 0 | 30 | 60 | 90 | 120 | |
| Infestation degree (%) 1 | |||||
| Control | 39.0 ± 0.3 a | 66.0 ± 0.4 a | 84.0 ± 0.3 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| Deltamethrin | 38.8 ± 0.4 a | 59.8 ± 0.2 b | 71.2 ± 0.2 b | 91.2 ± 0.3 b | 100.0 ± 0.0 a |
| PAEO | 38.7 ± 0.9 a | 59.0 ± 0.1 b | 64.3 ± 0.1 c | 72.3 ± 0.1 c | 85.0 ± 0.2 b |
| Water content (% w.b.) 1 | |||||
| Control | 13.5 ± 0.1 a | 14.6 ± 0.2 a | 15.2 ± 0.1 a | 17.2 ± 0.1 a | 16.4 ± 0.1 a |
| Deltamethrin | 13.3 ± 0.0 a | 13.5 ± 0.0 b | 14.0 ± 0.0 b | 14.5 ± 0.3 b | 15.0 ± 0.1 b |
| PAEO | 13.4 ± 0.2 a | 13.5 ± 0.1 b | 13.5 ± 0.0 c | 13.6 ± 0.0 c | 13.9 ± 0.0 c |
| Apparent specific mass (kg m−3) 1 | |||||
| Control | 645.3 ± 0.3 a | 506.3 ± 0.3 b | 290.7 ± 0.3 c | 144.7 ± 0.3 c | 49.7 ± 0.7 c |
| Deltamethrin | 643.7 ± 0.3 a | 590.7 ± 0.3 a | 480.3 ± 0.3 b | 386.7 ± 0.3 b | 302.7 ± 0.3 b |
| PAEO | 645.0 ± 0.6 a | 594.7 ± 0.3 a | 575.7 ± 0.3 a | 559.0 ± 0.6 a | 539.7 ± 0.3 a |
| Mass loss (%) 1 | |||||
| Control | 2.7 ± 0.3 a | 20.3 ± 0.3 a | 47.0 ± 0.6 a | 69.0 ± 0.1 a | 93.8 ± 0.2 a |
| Deltamethrin | 2.5 ± 0.3 a | 9.0 ± 0.3 b | 21.0 ± 0.6 b | 30.0 ± 0.6 b | 45.0 ± 0.3 b |
| PAEO | 2.5 ± 0.3 a | 8.3 ± 0.1 b | 10.7 ± 0.1 c | 12.8 ± 0.1 c | 15.2 ± 0.3 c |
| Electrical conductivity (µS cm−1 g−1) 1 | |||||
| Control | 26.5 ± 0.8 a | 106.2 ± 0.4 a | 235.2 ± 0.5 a | 336.2 ± 0.5 a | 445.3 ± 0.2 a |
| Deltamethrin | 26.4 ± 0.7 a | 37.2 ± 0.1 b | 86.2 ± 0.2 b | 125.3 ± 0.2 b | 163.7 ± 0.2 b |
| PAEO | 26.1 ± 0.4 a | 34.2 ± 0.2 b | 46.1 ± 0.1 c | 60.8 ± 0.1 c | 78.0 ± 0.1 c |
| Germination (%) 1 | |||||
| Control | 61.0 ± 0.1 a | 34.0 ± 0.3 b | 14.0 ± 0.1 c | 0.0 ± 0.0 c | 0.0 ± 0.0 b |
| Deltamethrin | 61.2 ± 0.4 a | 40.0 ± 0.1 a | 24.8 ± 0.2 b | 8.5 ± 0.3 b | 0.0 ± 0.0 b |
| PAEO | 61.3 ± 0.4 a | 40.2 ± 0.2 a | 32.1 ± 0.1 a | 23.2 ± 0.2 a | 15.0 ± 0.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, W.P.; Lopes, L.M.; Silva, G.N.; Carvalho, M.S.; de Sousa, A.H. Piper aduncum Essential Oil: Toxicity to Sitophilus zeamais and Effects on the Quality of Corn Grains. Processes 2025, 13, 1363. https://doi.org/10.3390/pr13051363
Santos WP, Lopes LM, Silva GN, Carvalho MS, de Sousa AH. Piper aduncum Essential Oil: Toxicity to Sitophilus zeamais and Effects on the Quality of Corn Grains. Processes. 2025; 13(5):1363. https://doi.org/10.3390/pr13051363
Chicago/Turabian StyleSantos, Weverton Peroni, Lucas Martins Lopes, Gutierres Nelson Silva, Marcela Silva Carvalho, and Adalberto Hipólito de Sousa. 2025. "Piper aduncum Essential Oil: Toxicity to Sitophilus zeamais and Effects on the Quality of Corn Grains" Processes 13, no. 5: 1363. https://doi.org/10.3390/pr13051363
APA StyleSantos, W. P., Lopes, L. M., Silva, G. N., Carvalho, M. S., & de Sousa, A. H. (2025). Piper aduncum Essential Oil: Toxicity to Sitophilus zeamais and Effects on the Quality of Corn Grains. Processes, 13(5), 1363. https://doi.org/10.3390/pr13051363







