Sustainable Bio-Adsorbent Generated from Coffee Waste for Dual Application in Heavy Metal and Dye Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of Metal-Loaded Biochar Composites (Fe/CB and Cu/CB) via Adsorption and Carbonization
2.3. Characterization of Fe/CB and Cu/CB
2.4. Adsorption Experiments for Organic Dyes
2.5. Desorption and Reusability
3. Results
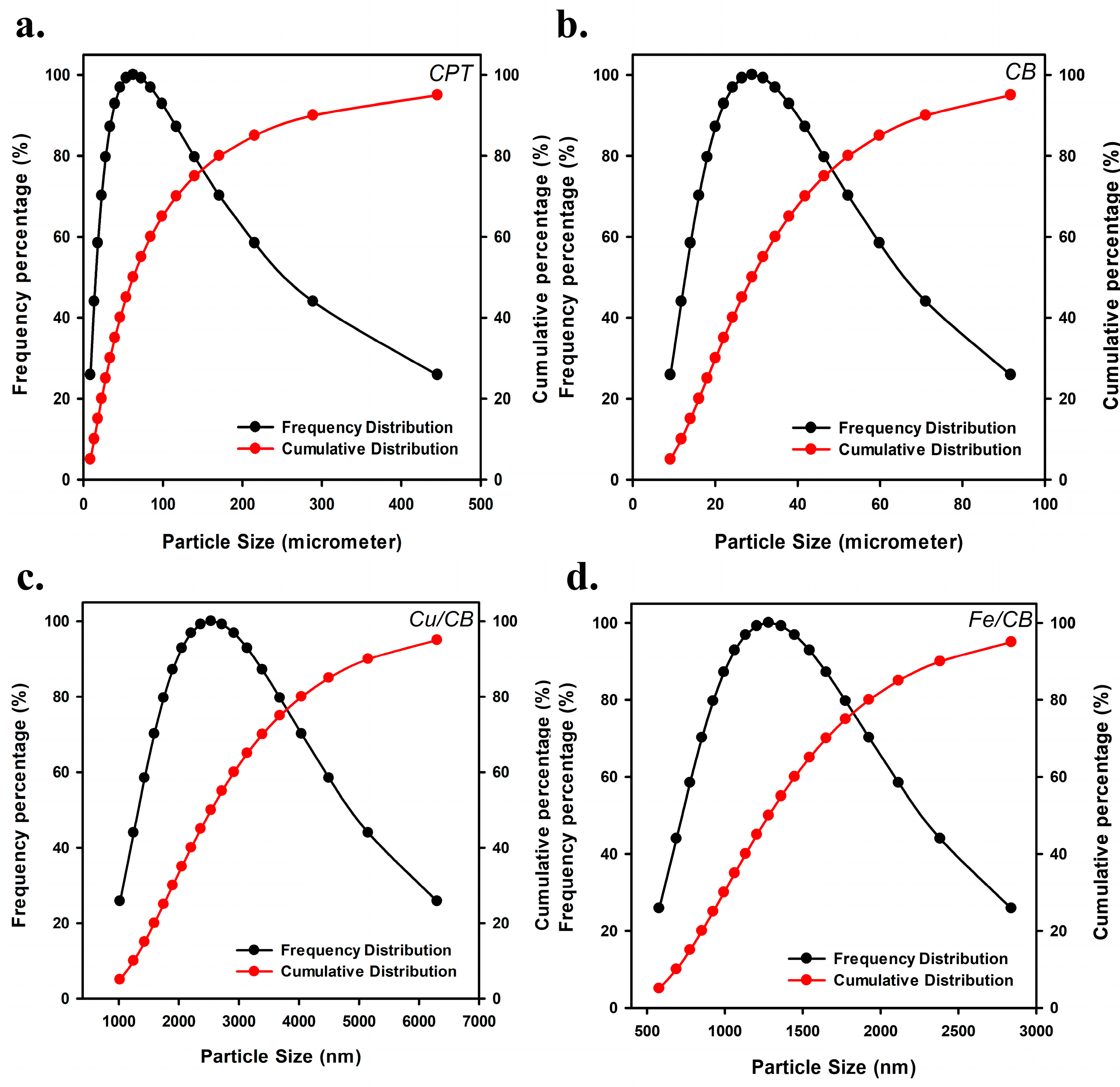
3.1. Characterization of CPT, CB, Cu/CB, and Fe/CB
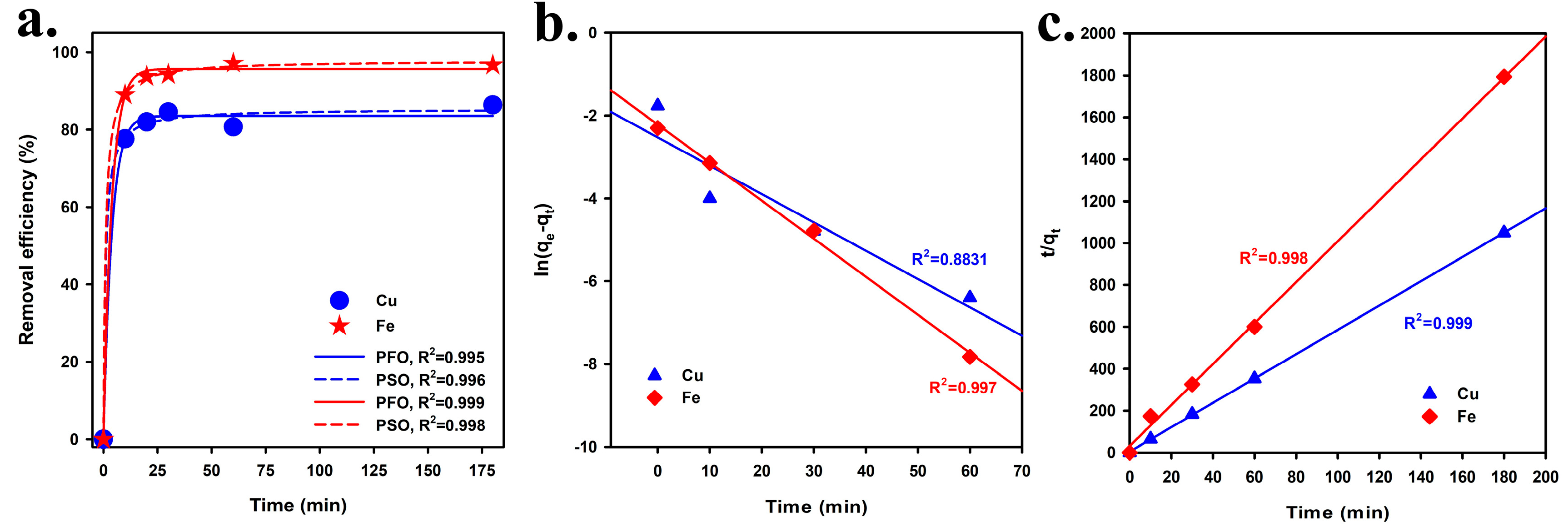
3.2. Effect of Contact Time on Cu2+ and Fe2+ Removal from Aqueous Solutions Using CPT
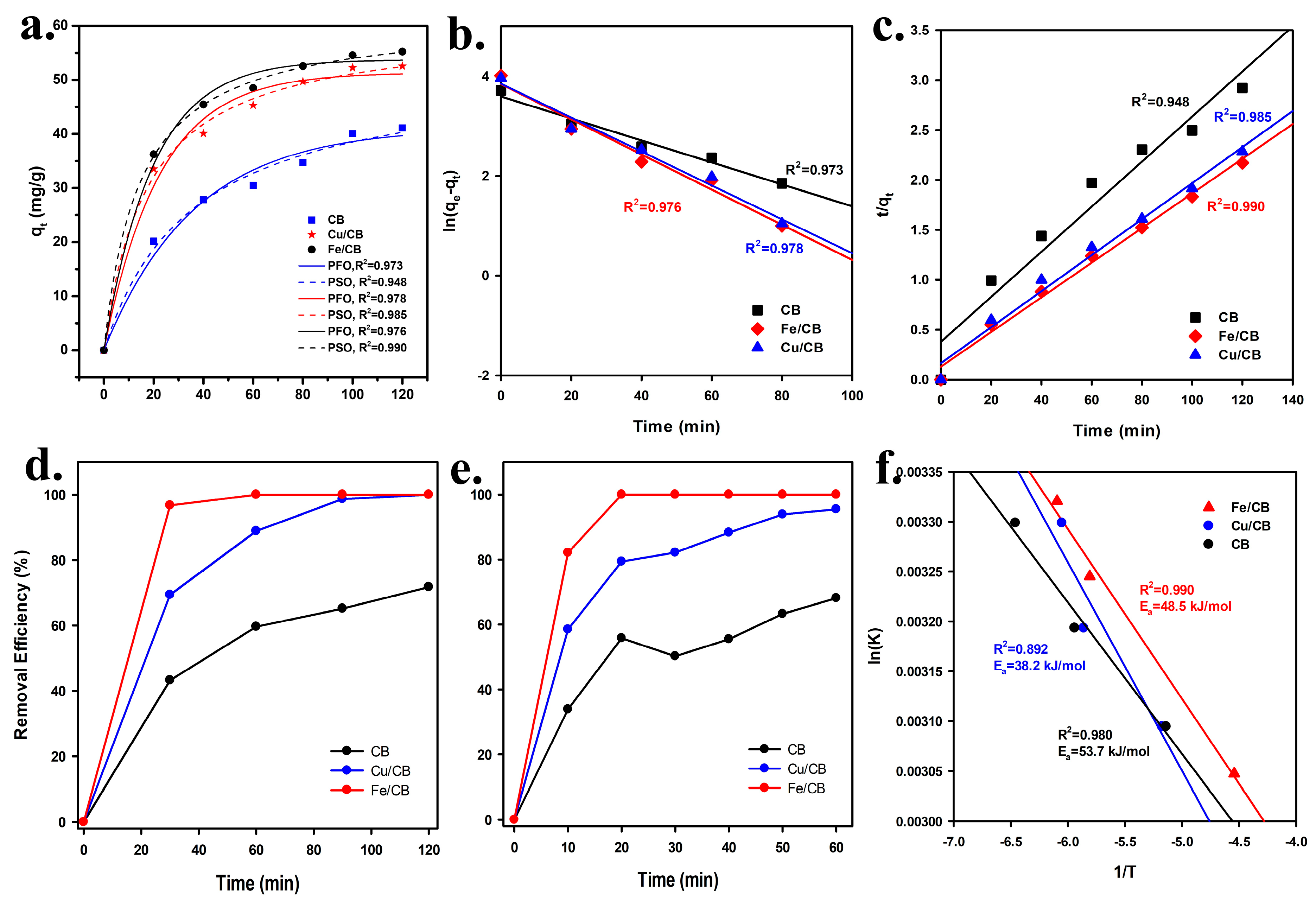
3.3. Effect of Contact Time and Temperature on MB Removal from Aqueous Solutions Using CB, Cu/CB, and Fe/CB
3.4. Influence of Dosage, pH, and Water Bodies on MB Removal from Aqueous Solutions Using Fe/CB
3.5. Recyclability of Fe/CB
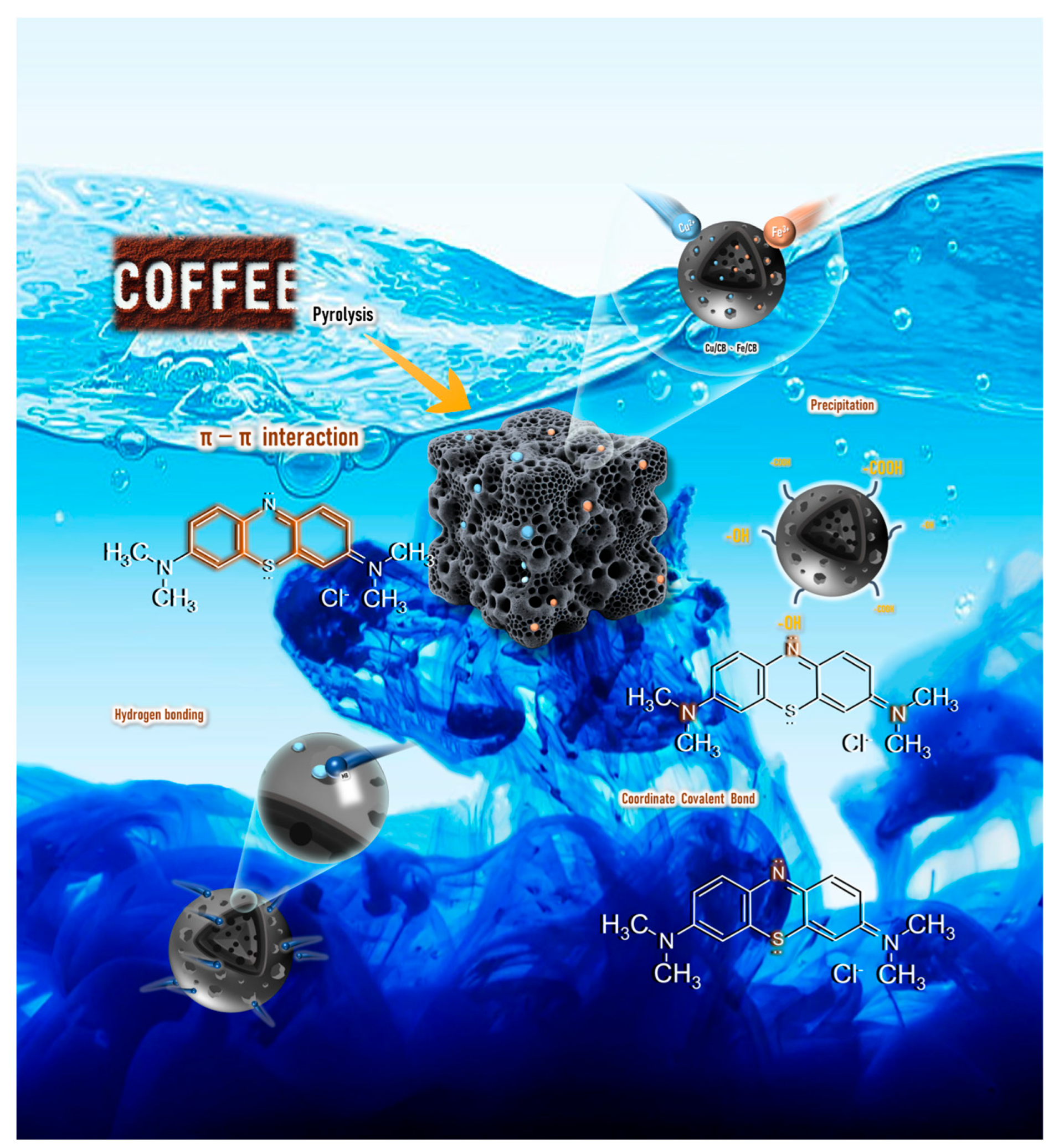
3.6. The Proposed Reaction Mechanism for Metal Ion and MB Removal Using CPT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, X.; Liu, H.; Zhang, L.; Zhang, J.; Fu, C.; Shi, X.; Chen, X.; Mijowska, E.; Chen, M.J.; Wang, D.Y. Large-scale converting waste coffee grounds into functional carbon materials as high-efficient adsorbent for organic dyes. Bioresour. Technol. 2019, 272, 92–98. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Li, G. Polyethyleneimine modified spent coffee grounds as a novel bio-adsorbent for selective adsorption of anionic Congo red and cationic Methylene blue. Desalination Water Treat. 2023, 290, 147–161. [Google Scholar] [CrossRef]
- Rahim, A.R.A.; Iswarya; Johari, K.; Shehzad, N.; Saman, N.; Mat, H. Conversion of coconut waste into cost effective adsorbent for Cu(II) and Ni(II) removal from aqueous solutions. Environ. Eng. Res. 2020, 26, 200250. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; He, Z.; Li, X.; Jiang, Z.; Xuan, F.; Tang, B.; Bian, X. Behavior and toxicity assessment of copper nanoparticles in aquatic environment: A case study on red swamp crayfish. J. Environ. Manag. 2022, 313, 114986. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Jung, K.W.; Choi, B.H.; Hwang, M.J.; Jeong, T.U.; Ahn, K.H. Fabrication of granular activated carbons derived from spent coffee grounds by entrapment in calcium alginate beads for adsorption of acid orange 7 and methylene blue. Bioresour. Technol. 2016, 219, 185–195. [Google Scholar] [CrossRef]
- R Ananthashankar, A.E.G. Production, Characterization and Treatment of Textile Effluents: A Critical Review. J. Chem. Eng. Process Technol. 2013, 5, 182. [Google Scholar] [CrossRef]
- Sutar, S.; Patil, P.; Jadhav, J. Recent advances in biochar technology for textile dyes wastewater remediation: A review. Environ. Res. 2022, 209, 112841. [Google Scholar] [CrossRef]
- Shin, J.; Bae, S.; Chon, K. Fenton oxidation of synthetic food dyes by Fe-embedded coffee biochar catalysts prepared at different pyrolysis temperatures: A mechanism study. Chem. Eng. J. 2021, 421, 129943. [Google Scholar] [CrossRef]
- Huang, W.; Chen, J.; Zhang, J. Adsorption characteristics of methylene blue by biochar prepared using sheep, rabbit and pig manure. Environ. Sci. Pollut. Res. Int. 2018, 25, 29256–29266. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Yousaf, B.; Fu, B.; Ahmed, R.; Abbas, Q.; Munir, M.A.M.; Ruijia, L. One-step synthesis of N-doped metal/biochar composite using NH3-ambiance pyrolysis for efficient degradation and mineralization of Methylene Blue. J. Environ. Sci. (China) 2019, 78, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, T.E.; Oyedemi, M.; Emetere, M.E.; Agboola, O.; Adeoye, J.B.; Odunlami, O.A. Review on the impact of heavy metals from industrial wastewater effluent and removal technologies. Heliyon 2024, 10, e40370. [Google Scholar] [CrossRef]
- Wołowicz, A.; Wawrzkiewicz, M. Screening of Ion Exchange Resins for Hazardous Ni(II) Removal from Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method. Processes 2021, 9, 285. [Google Scholar] [CrossRef]
- Gwanya, H.; Laifa, J. Copper Concentrations Found from Drinking Water, Soils, and Vegetables. Am. J. Environ. Prot. 2023, 12, 18–22. [Google Scholar] [CrossRef]
- Shahin, K.; Elsayed, A.; Zeineldin, A.; Ismail, S. Sustainable treatment for high iron concentration in groundwater for irrigation purposes. In Proceedings of the 2019 ASABE Annual International Meeting, Boston, MA, USA, 7–10 July 2019; ASABE: St. Joseph, MI, USA, 2019; p. 1. [Google Scholar]
- Ediagbonya, T.F.; Francis, S.D.; Omotayo-Tomo, M.S.; Oziegbe, F.E.; Awojobi, O.A. Risk assessment and quantification of elemental concentrations in river and stream in Nigeria. Discov. Toxicol. 2024, 1, 6. [Google Scholar] [CrossRef]
- Ahmed, H.; Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Toward Circular Economy: Potentials of Spent Coffee Grounds in Bioproducts and Chemical Production. Biomass 2024, 4, 286–312. [Google Scholar] [CrossRef]
- Cardarelli, A.; Pinzi, S.; Barbanera, M. Effect of torrefaction temperature on spent coffee grounds thermal behaviour and kinetics. Renew. Energy 2022, 185, 704–716. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, J.-G. Adsorption Characteristics of Spent Coffee Grounds as an Alternative Adsorbent for Cadmium in Solution. Environments 2020, 7, 24. [Google Scholar] [CrossRef]
- Edathil, A.A.; Shittu, I.; Hisham Zain, J.; Banat, F.; Haija, M.A. Novel magnetic coffee waste nanocomposite as effective bioadsorbent for Pb(II) removal from aqueous solutions. J. Environ. Chem. Eng. 2018, 6, 2390–2400. [Google Scholar] [CrossRef]
- Utomo, H.D.; Hunter, K.A. Adsorption of heavy metals by exhausted coffee grounds as a potential treatment method for waste waters. e-J. Surf. Sci. Nanotechnol. 2006, 4, 504–506. [Google Scholar] [CrossRef]
- Młynarczykowska, A.; Orlof-Naturalna, M. Biosorption of Copper (II) Ions Using Coffee Grounds—A Case Study. Sustainability 2024, 16, 7693. [Google Scholar] [CrossRef]
- Alorabi, A.Q.; Shamshi Hassan, M.; Azizi, M. Fe3O4-CuO-activated carbon composite as an efficient adsorbent for bromophenol blue dye removal from aqueous solutions. Arab. J. Chem. 2020, 13, 8080–8091. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, X.; Zhang, X.; Wang, X.; Zhang, J.; Liu, Y.; Tan, X.; Zhao, Y.; Kang, D.; Guo, W.; et al. New easily recycled carrier based polyurethane foam by loading Al-MOF and biochar for selective removal of fluoride ion from aqueous solutions. Sci. Total Environ. 2023, 901, 166312. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, E. Comparison between ancient and fresh biochar samples, a study on the recalcitrance of carbonaceous structures during soil incubation. Int. J. New Technol. Res. 2017, 3, 39–46. [Google Scholar]
- Yang, H.; Shang, L.; Zhang, Q.; Shi, R.; Waterhouse, G.I.N.; Gu, L.; Zhang, T. A universal ligand mediated method for large scale synthesis of transition metal single atom catalysts. Nat. Commun. 2019, 10, 4585. [Google Scholar] [CrossRef] [PubMed]
- Wei-Lung, C. Investigation of indium ions removal from aqueous solutions using spent coffee grounds. Int. J. Phys. Sci. 2012, 7, 2445–2454. [Google Scholar] [CrossRef]
- Malagón, D.; Torres-Velasquez, A.; Tinoco, K.; Arrubla-Vélez, J.P. Pyrolysis of Colombian spent coffee grounds (SCGs), characterization of bio-oil, and study of its antioxidant properties. Int. J. Sustain. Energy 2023, 42, 811–829. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Wang, Y.; Yu, J.; Li, N.; Wang, S. Cu/CuO-Decorated Peanut-Shell-Derived Biochar for the Efficient Degradation of Tetracycline via Peroxymonosulfate Activation. Catalysts 2023, 13, 1246. [Google Scholar] [CrossRef]
- Devrajani, S.K.; Ahmed, Z.; Qambrani, N.A.; Kanwal, S.; Sundaram, U.M.; Mubarak, N.M. Mechanism of arsenic removal using brown seaweed derived impregnated with iron oxide biochar for batch and column studies. Sci. Rep. 2024, 14, 18102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, X.; Zhou, J.; Peng, Z.; Shen, L.; Li, W. Insights of the adsorption mechanism of methylene blue on biochar from phytoextraction residues of Citrus aurantium L.: Adsorption model and DFT calculations. J. Environ. Chem. Eng. 2023, 11, 110496. [Google Scholar] [CrossRef]
- Liyanaarachchi, H.; Thambiliyagodage, C.; Lokuge, H.; Vigneswaran, S. Kinetics and Thermodynamics Study of Methylene Blue Adsorption to Sucrose- and Urea-Derived Nitrogen-Enriched, Hierarchically Porous Carbon Activated by KOH and H3PO4. ACS Omega 2023, 8, 16158–16173. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, J.; Ma, R.; Xue, Y.; Ma, Q.; Yuan, S.; Teng, W. Enhanced removal of heavy metals by α-FeOOH incorporated carboxylated cellulose nanocrystal: Synergistic effect and removal mechanism. Environ. Sci. Pollut. Res. Int. 2023, 30, 19427–19438. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, B.; Zhang, H.; Zhang, W.; Wang, Z. Activated municipal wasted sludge biochar supported by nanoscale Fe/Cu composites for tetracycline removal from water. Chem. Eng. Res. Des. 2019, 149, 209–219. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, G.; Wei, D.; Yan, T.; Xue, X.; Shi, S.; Wei, Q. Preparation and utilization of anaerobic granular sludge-based biochar for the adsorption of methylene blue from aqueous solutions. J. Mol. Liq. 2014, 198, 334–340. [Google Scholar] [CrossRef]

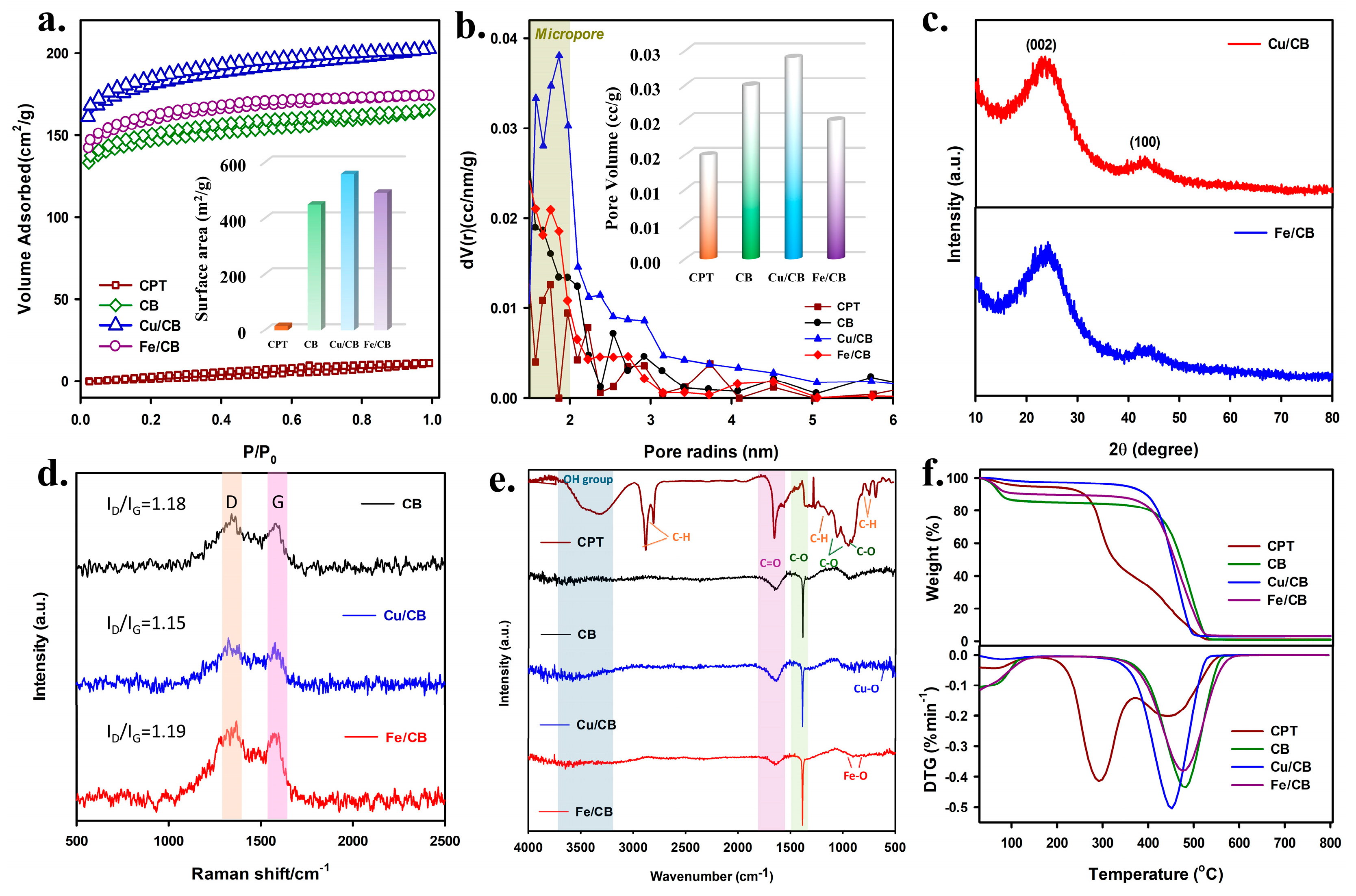

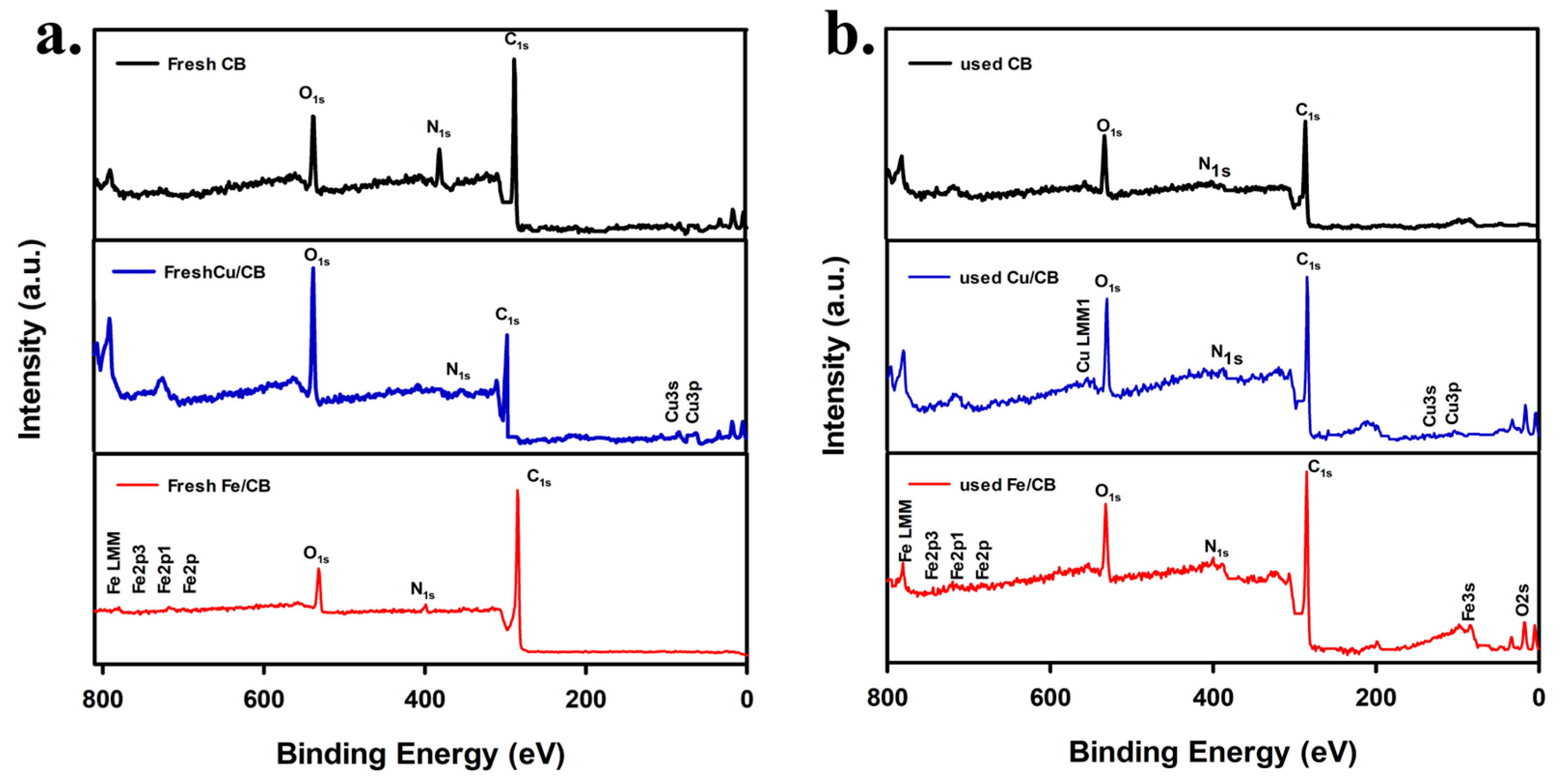
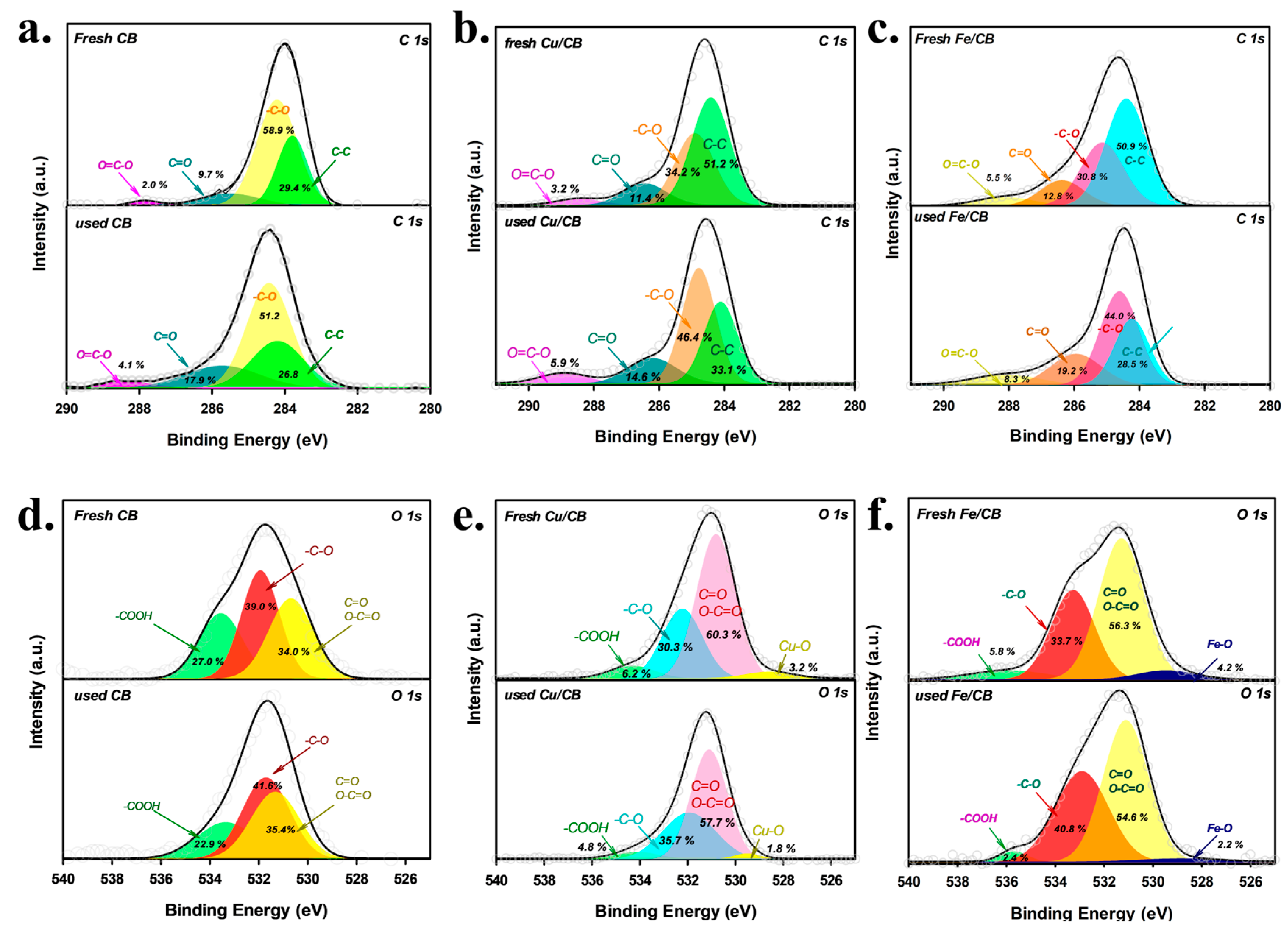

| Sample | SBET (m2 g−1) | Vpore (cc g−1) | ID/IG |
|---|---|---|---|
| CPT | 15.230 | 0.015 | - |
| CB | 450.493 | 0.025 | 1.18 |
| Cu/CB | 559.213 | 0.029 | 1.15 |
| Fe/CB | 492.097 | 0.020 | 1.19 |
| Sample | Experiment qe (mg/g) | Pseudo-First Order Kinetics | Pseudo-Second Order Kinetics | ||||
|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (min−1) | R2 | qe (mg/g) | k2 (g/mg·min) | R2 | ||
| CB | 41.086 | 47.050 | −0.032 | 0.973 | 44.316 | 0.002 | 0.948 |
| Cu/CB | 52.520 | 64.846 | −0.046 | 0.978 | 55.431 | 0.002 | 0.985 |
| Fe/CB | 55.242 | 46.610 | −0.035 | 0.976 | 57.607 | 0.003 | 0.990 |
| Materials | k (min−1) at Room Temperature | qe (mg/g) | Ea (kJ/mol) | Reference |
|---|---|---|---|---|
| CB | 0.032 | 41.086 | 53.7 | This study |
| Cu/CB | 0.046 | 52.520 | 35.6 | This study |
| Fe/CB | 0.035 | 55.242 | 48.4 | This study |
| FRB/O71-H89—N4 | 0.02 | 280.61 | 54.21 | [33] |
| SU-KOH | 0.039 | 384 | 92.4 | [34] |
| AC-GS | 0.027 | 208.29 | 94.65 | |
| SS+TW biochar | 0.008 | 5.8871 | 86.3 | [35] |
| Sludge-derived biochar | 0.057 | 1.083 | 72.93 | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-Y.; Chang, P.-T.; Shi, J.-R.; Liu, F.-C.; Wang, C.-Y.; Tsao, N.-W. Sustainable Bio-Adsorbent Generated from Coffee Waste for Dual Application in Heavy Metal and Dye Removal. Processes 2025, 13, 1364. https://doi.org/10.3390/pr13051364
Lin J-Y, Chang P-T, Shi J-R, Liu F-C, Wang C-Y, Tsao N-W. Sustainable Bio-Adsorbent Generated from Coffee Waste for Dual Application in Heavy Metal and Dye Removal. Processes. 2025; 13(5):1364. https://doi.org/10.3390/pr13051364
Chicago/Turabian StyleLin, Jia-Yin, Pei-Tzu Chang, Jun-Ren Shi, Fu-Chen Liu, Chih-Ying Wang, and Nai-Wen Tsao. 2025. "Sustainable Bio-Adsorbent Generated from Coffee Waste for Dual Application in Heavy Metal and Dye Removal" Processes 13, no. 5: 1364. https://doi.org/10.3390/pr13051364
APA StyleLin, J.-Y., Chang, P.-T., Shi, J.-R., Liu, F.-C., Wang, C.-Y., & Tsao, N.-W. (2025). Sustainable Bio-Adsorbent Generated from Coffee Waste for Dual Application in Heavy Metal and Dye Removal. Processes, 13(5), 1364. https://doi.org/10.3390/pr13051364







