Valorization of Residual Biomass from Sargassum filipendula for the Extraction of Phlorotannins and Pigments Using Eutectic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Raw Material
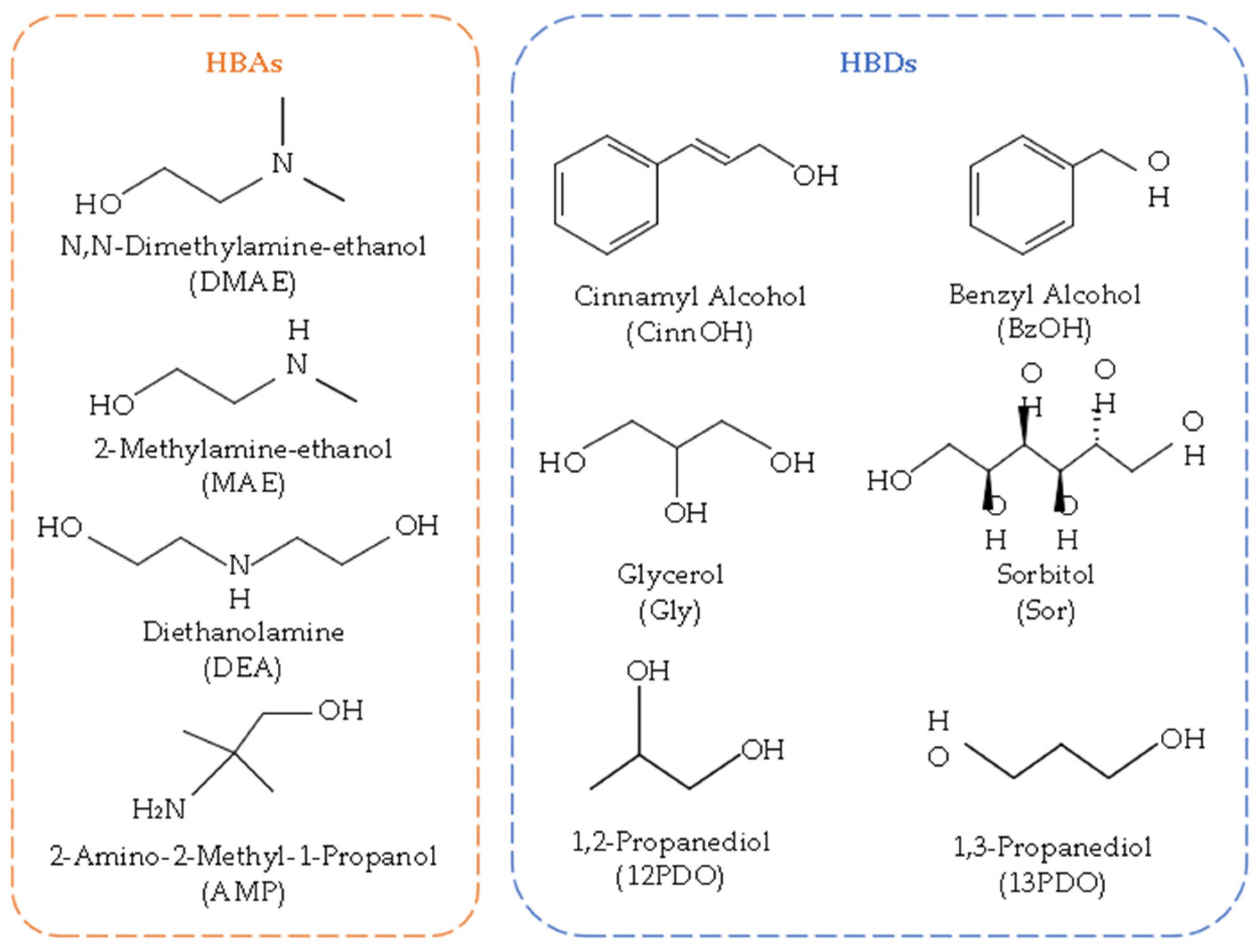
2.3. Preparation of DES
2.3.1. pH Analyzer
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. DSC Analyzer
2.4. Extraction Method
2.5. Total Phenolic Compounds (TPC)
2.6. Total Tannins Compounds (TTC)
2.7. Total Chlorophyll and Pheophytin
2.8. DPPH Free Radical Scavenging
2.9. ABTS Free Radical Scavenging
2.10. FRAP Antioxidant Activity Assay
2.11. COSMO-RS Computational Details
2.12. Experimental Design
2.13. Statistical Test
3. Results and Discussion
3.1. Biomass
3.2. DESs Characterization
3.2.1. pH
3.2.2. Thermogravimetric Analysis (TGA)
3.3. Extraction
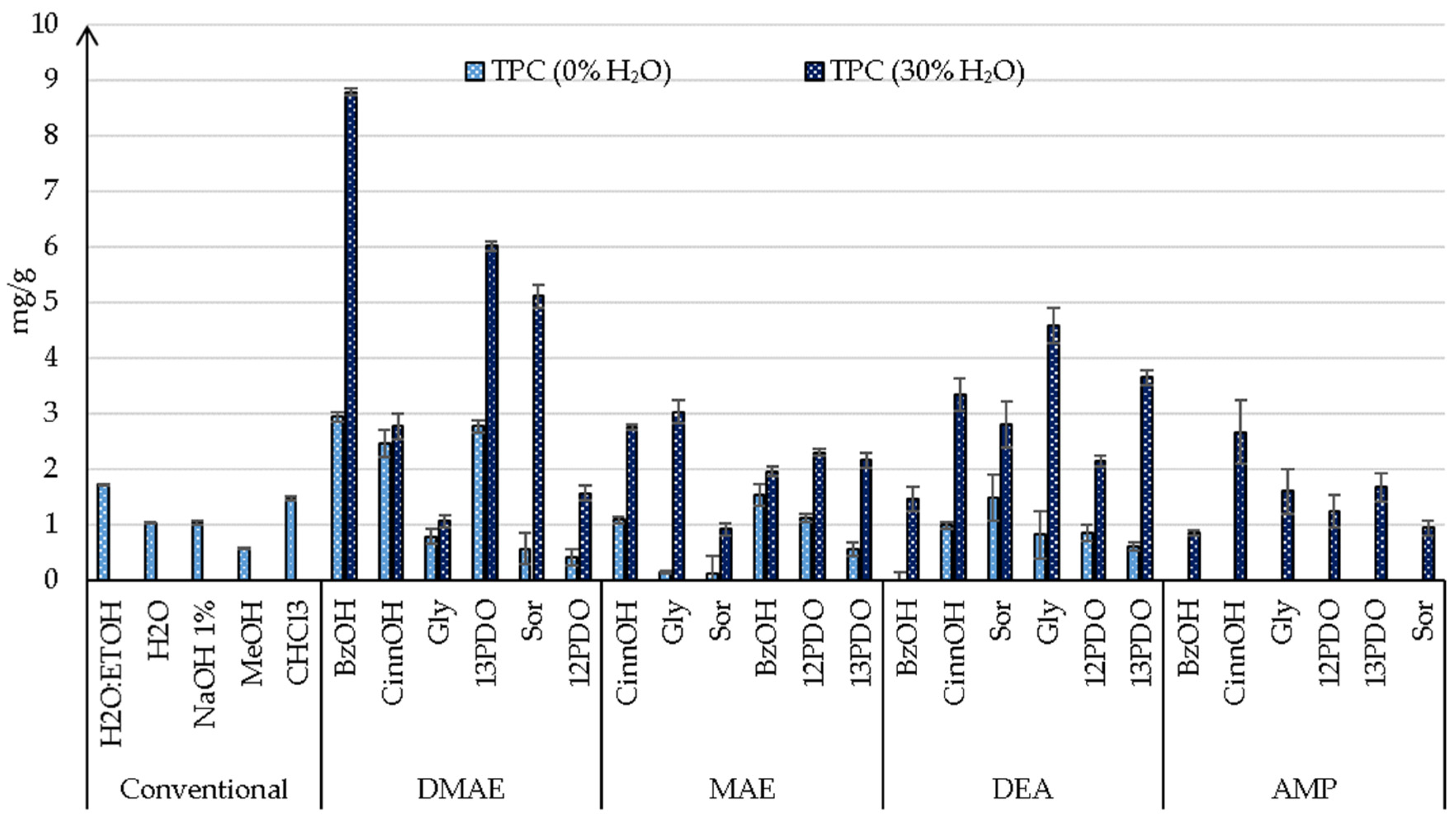
3.3.1. Phenolics Compounds
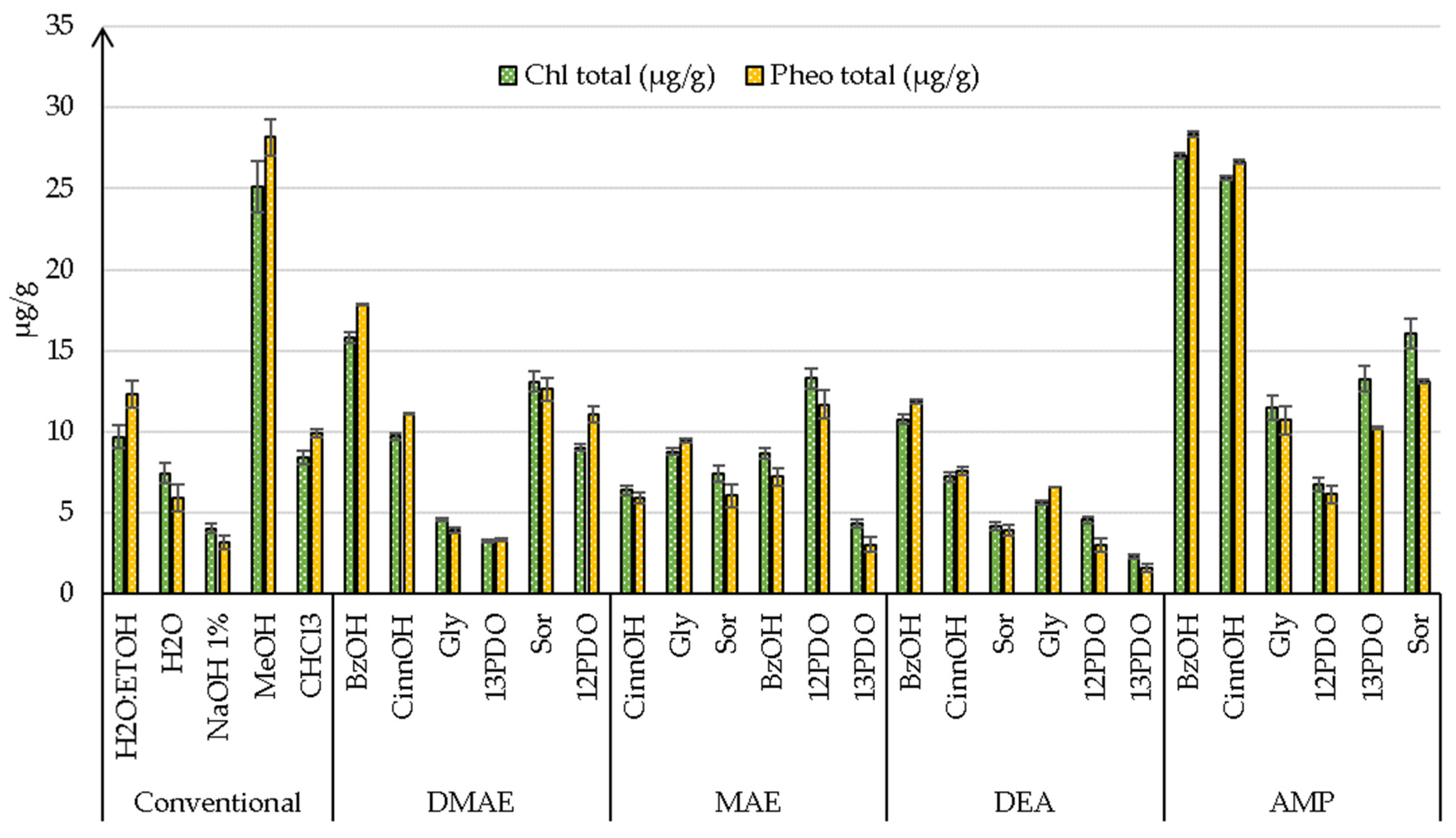
3.3.2. Pigments
3.4. Experimental Optimization
3.4.1. Experimental Design
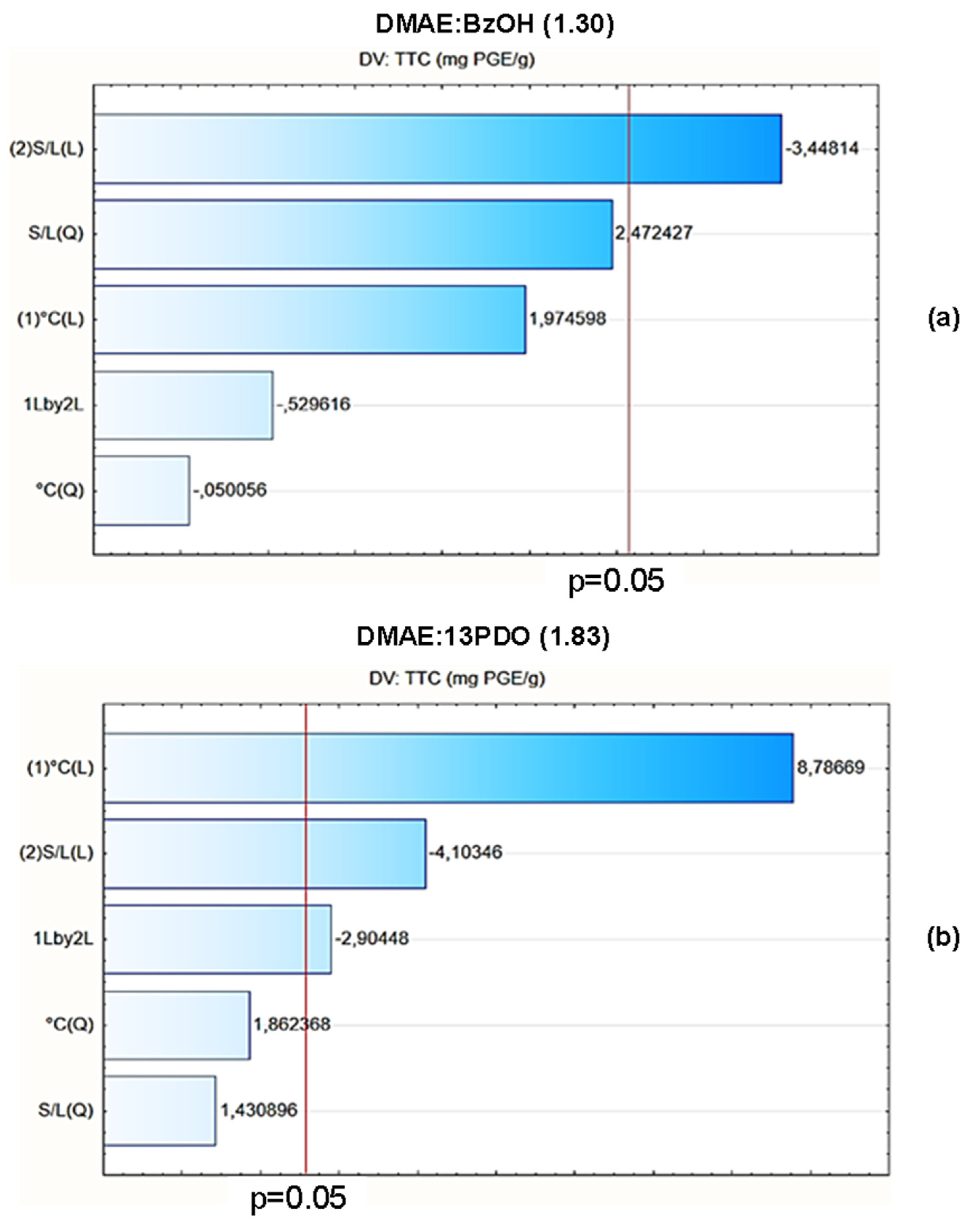
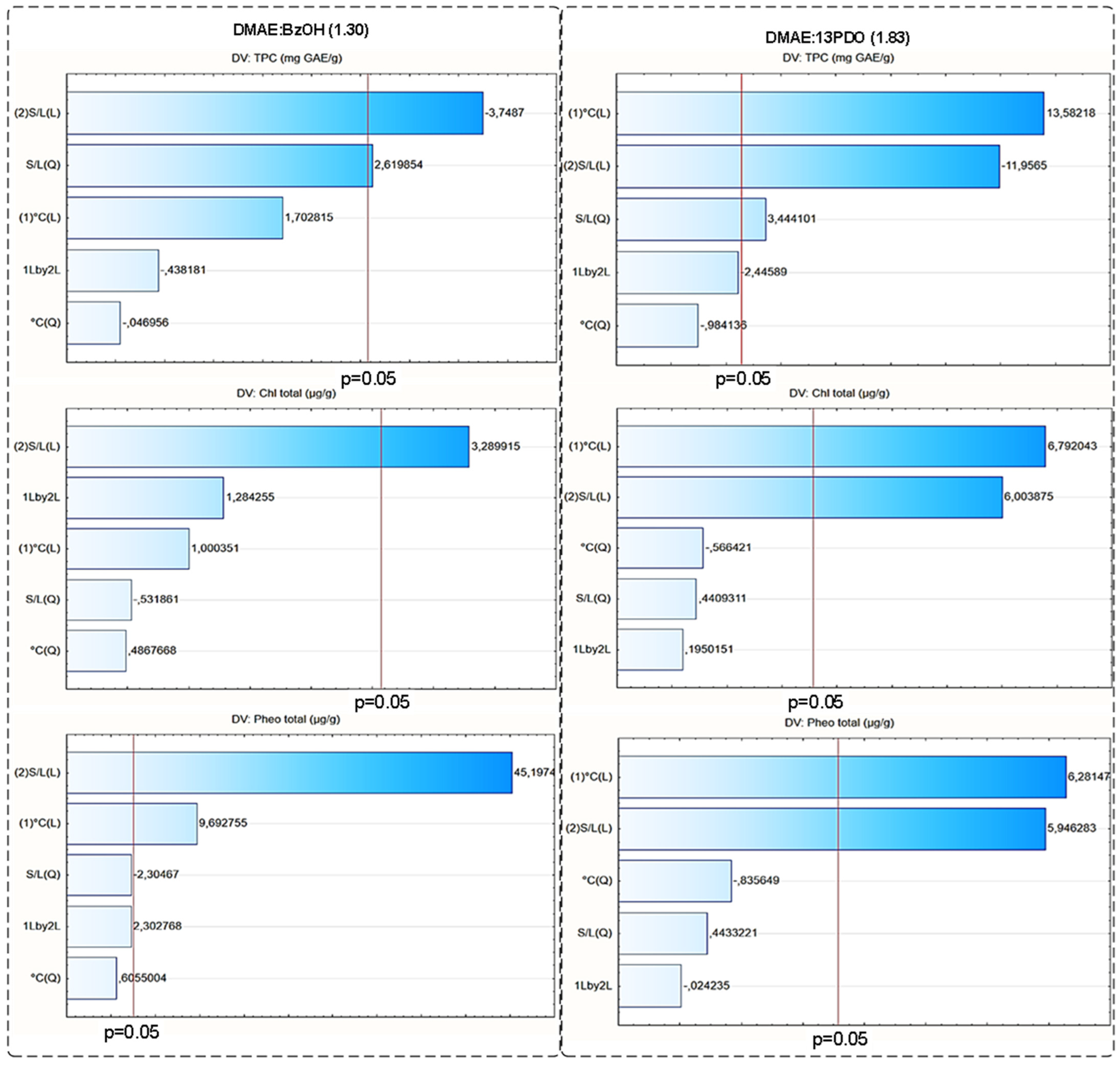
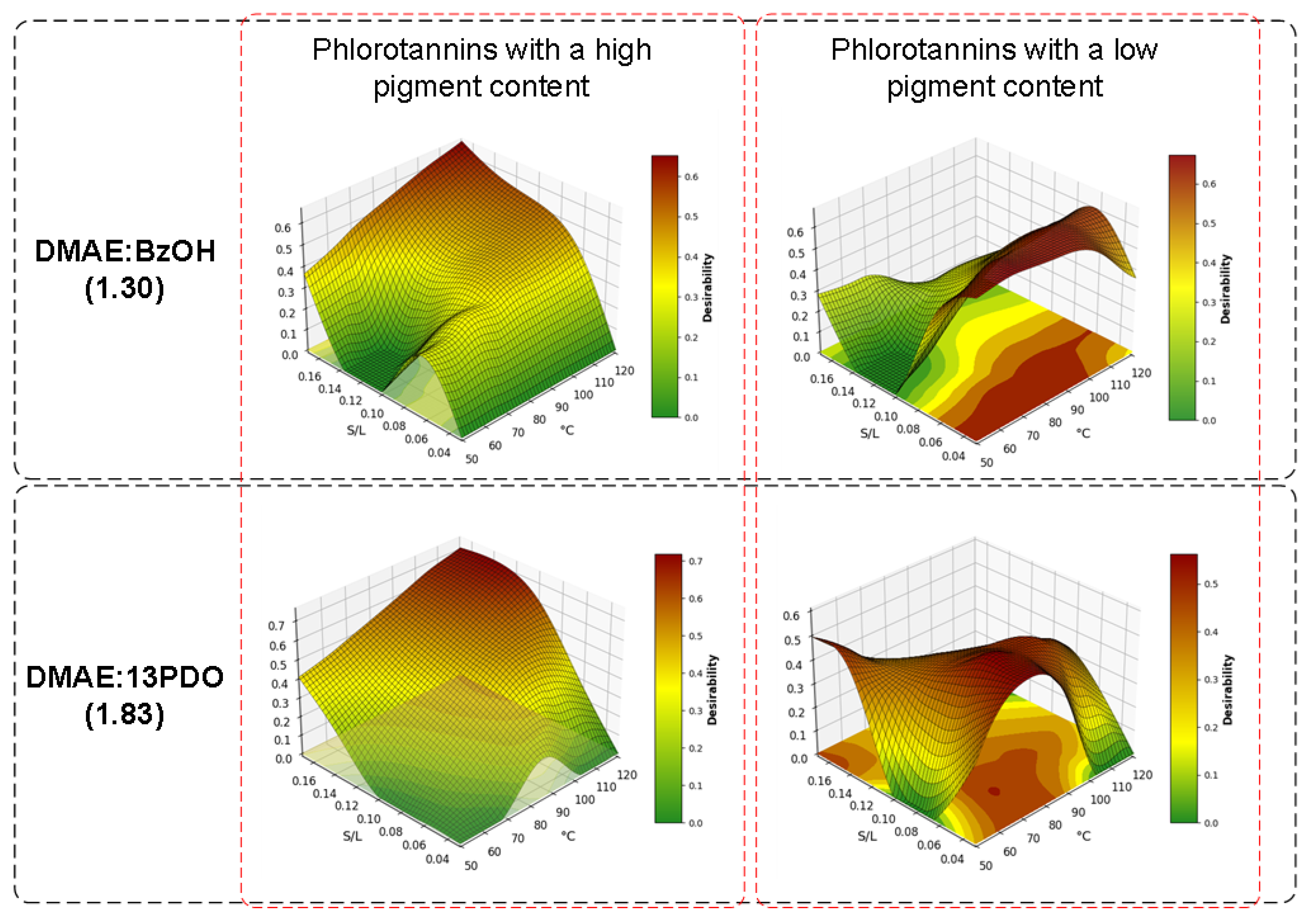
3.4.2. Optimization of Extraction
3.5. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DES | Deep Eutectic Solvent |
| DMAE | N,N-Dimethylaminoethanol |
| MAE | 2-Methylaminoethanol |
| DEA | Diethanolamine |
| AMP | 2-Amino-2-methyl-1-propanol |
| BzOH | Benzyl alcohol |
| CinnOH | Cinnamyl Alcohol |
| GLY | Glycerol |
| SORB | Sorbitol |
| 12PDO | 1,2-Propanediol |
| 13PDO | 1,3-Propanediol |
| TPC | Total phenolic content |
| TTC | Total Tannin Content |
| GAE | Gallic Acid Equivalent |
| PGE | Phloroglucinol Equivalent |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| FRAP | Ferric Reducing Antioxidant Power |
| TE | Trolox equivalent |
| COSMO-RS | Conductor-like Screening Model for Real Solvents |
| DFT | Density Functional Theory |
| CCD | Central Composite Design |
| ANOVA | Analysis of variance |
| S/L | Solid-to-liquid ratio |
| PVPP | Polyvinylpolypyrrolidone |
| LD50 | Lethal Dose 50% |
| FDA | Food and Drug Administration |
| ECHA | European Chemicals Agency |
| CIR | Cosmetic Ingredient Review |
| OSHA | Occupational Safety and Health Administration |
| MWAE | Microwave-Assisted Extraction |
| UAE | Ultrasound-Assisted Extraction |
| SFE | Supercritical fluid extraction |
| DMF | N,N-Dimethylformamide |
| TPTZ | 2,4,6-Tripyridyl-s-triazine |
| Na2CO3 | Sodium Carbonate |
| NaOH | Sodium Hydroxide |
| HCl | Hydrochloric Acid |
| CHCl3 | Chloroform |
| MeOH | Methanol |
| H2O | Water |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| UV–Vis | Ultraviolet–Visible Spectroscopy |
| R2 | Coefficient of Determination |
| pKa | Acid Dissociation Constant |
| pH | Potential of Hydrogen |
| ppm | Parts Per Million |
Appendix A

| Solvents | TPC | TPC | TTC (mg GAE/g) | TTC (mg PGE/g) | Chl Total (µg/g) | Pheo Total (µg/g) |
|---|---|---|---|---|---|---|
| (0% H2O) (mg GAE/g) | (30% H2O) (mg GAE/g) | |||||
| H2O:EtOH | 1.72 ± 0.01 b | - | 1.37 ± 0.03 d | 2.77 ± 0.03 d | 9.69 ± 0.69 d | 12.33 ± 0.8 c |
| H2O | 1.03 ± 0.01 d | - | 0.57 ± 0.01 e | 1.15 ± 0.01 e | 7.43 ± 0.62 e | 5.92 ± 0.84 e |
| NaOH 1% | 1.04 ± 0.04 d | - | 0.84 ± 0.02 e | 1.7 ± 0.02 e | 4.05 ± 0.33 f | 3.18 ± 0.45 f |
| MeOH | 0.57 ± 0.01 d | - | 0.45 ± 0.02 f | 0.91 ± 0.02 f | 25.12 ± 1.57 a | 28.18 ± 1.13 a |
| CHCl3 | 1.47 ± 0.04 d | - | 1.18 ± 0.03 d | 2.39 ± 0.03 d | 8.4 ± 0.44 d | 9.89 ± 0.23 d |
| DMAE:BzOH | 2.94 ± 0.6 a | 8.79 ± 0.09 a | 4.57 ± 0.06 a | 9.24 ± 0.06 a | 15.82 ± 0.31 b | 17.86 ± 0.02 b |
| DMAE:CinnOH | 2.47 ± 0.24 b | 2.47 ± 0.24 d | 1.05 ± 0.23 d | 2.12 ± 0.23 d | 9.71 ± 0.22 d | 11.17 ± 0.01 c |
| DMAE:Gly | 0.79 ± 0.14 c | 0.79 ± 0.14 e | 0.33 ± 0.1 f | 0.67 ± 0.1 f | 4.58 ± 0.08 f | 3.96 ± 0.17 f |
| DMAE:13PDO | 2.77 ± 0.6 a | 6.02 ± 0.1 b | 5.63 ± 0.09 a | 11.38 ± 0.09 a | 3.24 ± 0.1 g | 3.32 ± 0.09 f |
| DMAE:Sor | 0.57 ± 0.09 d | 5.12 ± 0.29 b | 3.85 ± 0.21 b | 7.78 ± 0.21 b | 13.1 ± 0.64 c | 12.62 ± 0.7 c |
| DMAE:12PDO | 0.41 ± 0.06 d | 2.21 ± 0.14 e | 1.57 ± 0.17 d | 3.17 ± 0.14 d | 9.02 ± 0.22 d | 11.05 ± 0.51 c |
| MAE:CinnOH | 1.09 ± 0.12 c | 2.75 ± 0.06 d | 1.52 ± 0.05 d | 3.07 ± 0.05 d | 6.39 ± 0.3 e | 5.93 ± 0.36 e |
| MAE:Gly | 0.15 ± 0.05 d | 3.03 ± 0.02 c | 2.57 ± 0.21 c | 5.2 ± 0.21 c | 8.77 ± 0.22 d | 9.43 ± 0.12 d |
| MAE:Sor | 0.12 ± 0.06 d | 0.92 ± 0.32 e | 0.66 ± 0.11 e | 1.33 ± 0.11 e | 7.39 ± 0.49 e | 6.05 ± 0.68 e |
| MAE:BzOH | 1.53 ± 0.08 b | 1.96 ± 0.19 c | 1.41 ± 0.09 d | 2.85 ± 0.09 d | 8.66 ± 0.34 d | 7.24 ± 0.54 e |
| MAE:12PDO | 1.12 ± 0.16 c | 2.3 ± 0.07 d | 2.22 ± 0.06 c | 4.49 ± 0.06 c | 13.31 ± 0.63 c | 11.69 ± 0.86 c |
| MAE:13PDO | 0.55 ± 0.18 c | 2.16 ± 0.12 c | 0.93 ± 0.13 e | 1.88 ± 0.13 e | 4.36 ± 0.24 f | 3.04 ± 0.43 f |
| DEA:BzOH | - | 1.47 ± 0.14 d | 1.06 ± 0.22 d | 2.14 ± 0.22 d | 10.78 ± 0.31 d | 11.88 ± 0.15 c |
| DEA:CinnOH | 0.99 ± 0.07 c | 3.34 ± 0.06 c | 2.34 ± 0.3 c | 4.73 ± 0.3 c | 7.21 ± 0.3 e | 7.59 ± 0.25 e |
| DEA:Sor | 1.49 ± 0.28 b | 2.81 ± 0.42 c | 2.53 ± 0.42 c | 5.11 ± 0.42 c | 4.18 ± 0.27 f | 3.92 ± 0.31 f |
| DEA:Gly | 0.82 ± 0.19 c | 4.59 ± 0.42 c | 3.49 ± 0.31 b | 7.06 ± 0.31 b | 5.64 ± 0.13 f | 6.57 ± 0 e |
| DEA:12PDO | 0.85 ± 0.31 c | 2.15 ± 0.14 d | 0.93 ± 0.1 e | 1.88 ± 0.1 e | 4.58 ± 0.21 f | 3.02 ± 0.43 f |
| DEA:13PDO | 0.61 ± 0.22 c | 3.65 ± 0.07 c | 2.2 ± 0.13 c | 4.45 ± 0.13 c | 2,29 ± 0.13 g | 1.61 ± 0.23 g |
| AMP:BzOH | - | 0.85 ± 0.33 e | 0.38 ± 0.05 f | 0.77 ± 0.05 f | 27.05 ± 0.16 a | 28.37 ± 0.14 a |
| AMP:CinnOH | - | 2.67 ± 0.33 d | 1.37 ± 0.57 d | 2.77 ± 0.57 d | 25.63 ± 0.13 a | 26.64 ± 0.11 a |
| AMP:Gly | - | 1.6 ± 0.16 e | 1.27 ± 0.41 d | 2.57 ± 0.41 d | 11.5 ± 0.75 d | 10.72 ± 0.86 d |
| AMP:12PDO | - | 1.67 ± 0.61 d | 0.9 ± 0.3 e | 1.82 ± 0.3 e | 6.75 ± 0.42 e | 6.13 ± 0.51 e |
| AMP:13PDO | - | 0.94 ± 0.29 e | 0.63 ± 0.26 e | 1.27 ± 0.26 e | 13.27 ± 0.81 c | 10.24 ± 0.12 d |
| AMP:Sor | - | 1.64 ± 0.13 e | 1.24 ± 0.13 d | 2.51 ± 0.13 d | 16.06 ± 0.9 b | 13.08 ± 0.13 c |
| Extract − Control | Extract + Control | |||||||
|---|---|---|---|---|---|---|---|---|
| Solvents | °C | S/L | DPPH (mg TE/g) | ABTS (mg TE/g) | FRAP (mg TE/g) | DPPH (mg TE/g) | ABTS (mg TE/g) | FRAP (mg TE/g) |
| DMAE:BzOH (1.30) | 74.39 | 0.03 | 3.54 | 18.46 | 3.08 | 13.42 | 786.47 | 66.68 |
| 120.36 | 0.17 | 2.32 | 8.10 | 2.10 | 4.06 | 143.63 | 13.32 | |
| DMAE:13PDO (1.83) | 69.44 | 0.03 | 2.72 | 10.25 | 4.84 | 24.33 | 781.41 | 68.53 |
| 120.36 | 0.14 | 1.59 | 7.78 | 2.35 | 6.22 | 173.03 | 16.00 | |
| Solvents | R$/kg | °C | S/L | $/g PGE | % Cost Reduction |
|---|---|---|---|---|---|
| DMAE:BzOH (1.30) | 60.00 | 0.10 | $ 48.21 | - | |
| R$ 267.25 | 67.32 | 0.03 | $ 12.36 | −74.4% | |
| 120.36 | 0.17 | $ 5.41 | −88.8% | ||
| DMAE:13PDO (1.83) | 60.00 | 0.10 | $ 187.21 | - | |
| R$ 1.278.28 | 67.32 | 0.03 | $ 329.19 | 75.8% | |
| 120.36 | 0.14 | $ 36.03 | −80.8% | ||
| H2O:EtOH | R$ 17.41 | 60.00 | 0.10 | $ 10.48 | - |
| H2O | R$ 10.00 | 60.00 | 0.10 | $ 25.23 | - |
| NaOH 1% | R$ 175.41 | 60.00 | 0.10 | $ 17.07 | - |
| MeOH | R$ 137.81 | 60.00 | 0.10 | $ 31.89 | - |
| CHCl3 | R$ 160.00 | 60.00 | 0.10 | $ 12.14 | - |
References
- FAO. The State of World Fisheries and Aquaculture 2024–Blue Transformation in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024. [Google Scholar]
- FAO. Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.; Reddy, C.R. Seaweed biorefinery: A sustainable process for valorizing the biomass of brown seaweed. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Yun, J.-H.; Archer, S.D.; Price, N.N. Valorization of waste materials from seaweed industry: An industry survey-based biorefinery approach. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Narayanan, M. Promising biorefinery products from marine macro and microalgal biomass: A review. Renew. Sustain. Energy Rev. 2024, 190 Pt B, 114081. [Google Scholar] [CrossRef]
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins–bioactivity and extraction perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef]
- Bertagnolli, C.; Espíndola, A.; Kleinübing, S.; Tasić, L.; Silva, M. Sargassum filipendula alginate from Brazil: Seasonal influence and characteristics. Carbohydr. Polym. 2014, 111, 619–623. [Google Scholar] [CrossRef]
- National Confederation of Family Farmers and Rural Family Entrepreneurs (CONAFER – BR). Agriculture in the Amazon ‘Feeds’ the Formation of a Gigantic Patch of Brown Algae. 2018. Available online: https://conafer.org.br/en/Agriculture-in-the-Amazon-fuels-the-formation-of-a-giant-patch-of-brown-algae (accessed on 10 April 2025).
- Pérez, Y.; López, S.G.; Tejada, E.L.; Giraldo, J.C.; Mejía, M.Á. Sargassum filipendula, a source of bioactive compounds with antioxidant and matrix metalloproteinase inhibition activities in vitro with potential dermocosmetic application. Antioxidants 2023, 12, 876. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Conde, E.; Moure, A.; Domínguez, H. Supercritical CO2 extraction of fatty acids, phenolics, and fucoxanthin from freeze-dried Sargassum muticum. J. Appl. Phycol. 2014, 27, 957–964. [Google Scholar] [CrossRef]
- Cui, X.; Liao, J.; Liu, H.; Tang, W.; Tie, C.; Tian, S.; Li, Y. Adsorption of phenols from aqueous solution with a pH-sensitive surfactant-modified bentonite. Separations 2023, 10, 523. [Google Scholar] [CrossRef]
- Jesus, B.C.; Sáenz de Miera, B.; Santiago, R.; Martins, A.; Pedrosa, R.; González-Miquel, M.; Marrucho, I.M. Valorisation of Sargassum muticum through the extraction of phenolic compounds using eutectic solvents and intensification techniques. RSC Sustain. 2023, 1, 14. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 22nd ed.; Association of Official Analytical Chemistry International: New York, NY, USA, 2005. [Google Scholar]
- Dische, Z. A new specific color reaction of hexuronic acids. J. Biol. Chem. 2021, 167, 189–198. [Google Scholar] [CrossRef]
- Alhadid, A.; Mokrushina, L.; Minceva, M. Formation of glassy phases and polymorphism in deep eutectic solvents. J. Mol. Liq. 2020, 314, 113667. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Marques, C.; Igarashi-Mafra, L.; Coutinho, J.A.; Mafra, M.R. Extraction of phenolic compounds from rosemary using choline chloride–based Deep Eutectic Solvents. Sep. Purif. Technol. 2020, 247, 117975. [Google Scholar] [CrossRef]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents, and aqueous glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef]
- Malta, L.; Liu, R. Analyses of Total Phenolics, Total Flavonoids, and Total Antioxidant Activities in Foods and Dietary Supplements. Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 305–314. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Coelho, M.A.Z.; Barreto, D.W. Obtaining caffeine-rich guarana extracts by enzymatic process and tannin adsorption. Braz. J. Food Technol. 2012, 15, 261–270. [Google Scholar] [CrossRef]
- Toth, G.B.; Pavia, H. Removal of dissolved brown algal phlorotannins using insoluble polyvinylpolypyrrolidone (PVPP). J. Chem. Ecol. 2001, 27, 1899–1910. [Google Scholar] [CrossRef]
- Targett, N.M.; Arnold, T.M. Minireview—Predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J. Phycol. 1998, 34, 195–205. [Google Scholar] [CrossRef]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef]
- Vernon, L.P. Spectrophotometric determination of chlorophylls and pheophytins in plant extracts. Anal. Chem. 1960, 32, 1144–1150. [Google Scholar] [CrossRef]
- Hill, R. Chlorophyll. In Comprehensive Biochemistry; Florkin, M., Stotz, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1963; Volume 9, p. 73. [Google Scholar]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.C.; Kassuia, Y.S.; Domahovski, R.C.; Lazzarotto, M. Método de DPPH Adaptado: Uma Ferramenta para Analisar Atividade Antioxidante de Polpa de Frutos da Erva-Mate de Forma Rápida e Reprodutível; Embrapa Florestas. Comunicado técnico, 421; Embrapa Florestas: Colombo, Brazil, 2018; Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1101294/metodo-de-dpph-adaptado-uma-ferramenta-para-analisar-atividade-antioxidante-de-polpa-de-frutos-da-erva-mate-de-forma-rapida-e-reprodutivel (accessed on 22 March 2025).
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Jimenes-Perez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas pelo Método de Redução do Ferro (FRAP); Comunicado Técnico; Embrapa Agroindústria Tropical: Fortaleza, Brazil, 2006; Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/664098/metodologia-cientifica-determinacao-da-atividade-antioxidante-total-em-frutas-pelo-metodo-de-reducao-do-ferro-frap (accessed on 15 March 2025).
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Palomar, J.; Torrecilla, J.S.; Lemus, J.; Ferro, V.R.; Rodríguez, F. A COSMO-RS based guide to analyze/quantify the polarity of ionic liquids and their mixtures with organic cosolvents. Phys. Chem. Chem. Phys. 2010, 12, 1863–1870. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Zhang, J.; Li, Q.; Yuan, M.; Kang, Z. Ionic liquid screening for lignocellulosic biomass fractionation: COSMO–RS prediction and experimental verification. J. Mol. Liq. 2024, 407, 125214. [Google Scholar] [CrossRef]
- Bhattacharya, S. Central Composite Design for Response Surface Methodology and Its Application in Pharmacy. Response Surface Methodology in Engineering Science; IntechOpen: London, UK, 2021; pp. 1–19. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Hernández-Carmona, G.; McHugh, D.J.; Arvizu-Higuera, D.L. Pilot plant scale extraction of alginates from Macrocystis pyrifera. J. Appl. Phycol. 1999, 11, 493–502. [Google Scholar] [CrossRef]
- Torres, M.R.; Sousa, A.P.A.; Silva Filho, E.A.T.; Melo, D.F.; Feitosa, J.P.A.; de Paula, R.C.M.; Lima, M.G.S. Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydr. Res. 2007, 342, 2067–2074. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Ale, M.T.; Ajalloueian, F.; Meyer, A.S. Characterization of alginates from Ghanaian brown seaweeds: Sargassum spp. and Padina spp. Food Hydrocoll. 2017, 71, 236–244. [Google Scholar] [CrossRef]
- Lee, J.-E.; Xu, X.; Jeong, S.-M.; Kang, W.-S.; Ryu, S.-H.; Kim, H.-H.; Kim, S.-R.; Lee, G.-H.; Kim, M.-J.; Ahn, D.-H. Properties and anti-inflammatory effects of Sargassum muticum enzymatic extracts decomposed using crude enzyme from Shewanella oneidensis. Food Sci. Biotechnol. 2022, 31, 1299–1307. [Google Scholar] [CrossRef]
- Garcia-Ríos, V.; Ríos-Leal, E.; Robledo, D.; Freile-Pelegrin, Y. Polysaccharides composition from tropical brown seaweeds. Phycol. Res. 2012, 60, 305–315. [Google Scholar] [CrossRef]
- Dia Filippo-Herrera, D.A.; Hernández-Carmona, G.; Muñoz-Ochoa, M.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E. Monthly variation in the chemical composition and biological activity of Sargassum horridum. Bot. Mar. 2018, 61, 1–12. [Google Scholar] [CrossRef]
- Sari, R.I.; Suryati, I.R.A.; Taslihan, A. Laminarin crude extract characterization of Sargassum sp. originated from Jepara-Indonesia with the laminarin acid extraction method using an acetic acid solvent. J. Kim. Dan Pendidik. Kim. (JKPK) 2023, 8, 60–74. [Google Scholar] [CrossRef]
- Paredes-Camacho, R.M.; González-Morales, S.; González-Fuentes, J.A.; Rodríguez-Jasso, R.M.; Benavides-Mendoza, A.; Charles-Rodríguez, A.V.; Robledo-Olivo, A. Characterization of Sargassum spp. from the Mexican Caribbean and its valorization through fermentation process. Processes 2023, 11, 685. [Google Scholar] [CrossRef]
- Wardani, A.K.; Herrani, R. Bioethanol from Sargassum sp. using acid hydrolysis and fermentation method using microbial association. J. Phys. Conf. Ser. 2019, 1241, 012008. [Google Scholar] [CrossRef]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soulé, F.; Bhaw-Luximon, A.; Meilhac, O.; D’Hellencourt, C.L.; Jhurry, D.; Couprie, J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef]
- Holme, H.K.; Lindmo, K.; Kristiansen, A.; Smidsrød, O. Thermal depolymerization of alginate in the solid state. Carbohydr. Polym. 2003, 54, 431–438. [Google Scholar] [CrossRef]
- Vauchel, P.; Arhaliass, A.; Legrand, J.; Kaas, R.; Baron, R. Decrease in dynamic viscosity and average molecular weight of alginate from Laminaria digitata during alkaline extraction. J. Phycol. 2008, 44, 515–517. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M.; Hifney, A.F.; Abdel-Gawad, K.M. Optimization of alginate alkaline extraction technology from Sargassum latifolium and its potential antioxidant and emulsifying properties. Carbohydr. Polym. 2017, 157, 1903–1912. [Google Scholar] [CrossRef]
- Mohammed, A.; Bissoon, R.; Bajnath, E.; Mohammed, K.; Lee, T.; Bissram, M.; John, N.; Jalsa, N.K.; Lee, K.-Y.; Ward, K. Multistage extraction and purification of waste Sargassum natans to produce sodium alginate: An optimization approach. Carbohydr. Polym. 2018, 198, 109–118. [Google Scholar] [CrossRef]
- Mohammed, A.; Rivers, A.; Stuckey, D.C.; Ward, K. Alginate extraction from Sargassum seaweed in the Caribbean region: Optimization using response surface methodology. Carbohydr. Polym. 2020, 245, 116419. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Gama-Arachchige, N.S.; Merah, O.; Madhujith, T. Seaweeds as a Source of Functional Proteins. Phycology 2022, 2, 216–243. [Google Scholar] [CrossRef]
- Diniz, G.S.; Barbarino, E.; Oiano-Neto, J.; Pacheco, S.; Lourenço, S.O. Gross Chemical Profile and Calculation of Nitrogen-to-Protein Conversion Factors for Five Tropical Seaweeds. Am. J. Plant Sci. 2011, 2, 287–296. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Salvador-Reyes, R.; Pinto, C.C.; Souza, S.M.; Pallone, J.A.L.; Bezerra, J.A.; Mar, J.M.; Sanches, E.A.; Clerici, M.T.P.S. Nutritional and techno-functional properties of the brown seaweed Sargassum filipendula. Food Res. Int. 2024, 191, 114728. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Cian, R.E.; Martínez-Augustin, O.; Drago, S.R. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Bioactivity of sulfated polysaccharides from the edible red seaweed Mastocarpus stellatus. Bioact. Carbohydr. Diet. Fibre 2014, 3, 29–40. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Mišurcová, L.; Ambrožová, J.V.; Samek, D. Seaweed lipids as nutraceuticals. Adv. Food Nutr. Res. 2012, 64, 339–355. [Google Scholar] [CrossRef]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Laeliocattleya, R.A.; Yunianta, Y. Fucoidan content from brown seaweed (Sargassum filipendula) and its potential as radical scavenger. J. Phys. Conf. Ser. 2020, 1430, 012023. [Google Scholar] [CrossRef]
- Hardouin, K.; Burllot, A.-S.; Umami, A.; Tanniou, A.; Stiger-Pouvreau, V.; Widowati, I.; Bedoux, G.; Bourgougnon, N. Biochemical and antiviral activities of enzymatic hydrolysates from different invasive French seaweeds. Int. J. Mol. Sci. 2021, 22, 3263. [Google Scholar] [CrossRef]
- Davis, T.; Volesky, B.; Vieira, R. Sargassum seaweed as biosorbent for heavy metals. Water Res. 2000, 34, 4270–4278. [Google Scholar] [CrossRef]
- Adeyemi, I.; Abu-Zahra, M.R.M.; Alnashef, I.M. Physicochemical properties of alkanolamine-choline chloride deep eutectic solvents: Measurements, group contribution and artificial intelligence prediction techniques. J. Mol. Liq. 2018, 256, 581–590. [Google Scholar] [CrossRef]
- Ishaq, M.; Gilani, M.A.; Bilad, M.R.; Faizan, A.; Raja, A.A.; Afzal, Z.M.; Khan, A.L. Exploring the potential of highly selective alkanolamine containing deep eutectic solvents based supported liquid membranes for CO2 capture. J. Mol. Liq. 2021, 341, 117274. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shevyrin, V.A.; Kovaleva, E.G.; Flisyuk, E.V.; Shikov, A.N. Optimization of Extraction of Phlorotannins from the Arctic Fucus vesiculosus Using Natural Deep Eutectic Solvents and Their HPLC Profiling with Tandem High-Resolution Mass Spectrometry. Mar. Drugs 2023, 21, 263. [Google Scholar] [CrossRef]
- Sushil, S.; Sakpal, S.H. Transition of a deep eutectic solution to aqueous solution: A dynamical perspective of the dissolved solute. J. Phys. Chem. Lett. 2021, 12, 8784–8789. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, L.; Feng, Y.; Li, X.-S.; Guo, Q.-X. Effects of hydrogen bonding to amines on the phenol/phenoxyl radical oxidation. J. Phys. Chem. A 2002, 106, 4669–4678. [Google Scholar] [CrossRef]
- Xiong, D.; Zhang, Q.; Ma, W.; Wang, Y.; Wan, W.; Shi, Y.; Wang, J. Temperature-switchable deep eutectic solvents for selective separation of aromatic amino acids in water. Sep. Purif. Technol. 2021, 264, 118479. [Google Scholar] [CrossRef]
- Thakuria, R.; Nath, N.K.; Saha, B.K. The nature and applications of π–π interactions: A perspective. Cryst. Growth Des. 2019, 19, 791–805. [Google Scholar] [CrossRef]
- Yao, X.-H.; Zhang, D.-Y.; Duan, M.-H.; Cui, Q.; Xu, W.-J.; Luo, M.; Li, C.-Y.; Zu, Y.-G.; Fu, Y.-J. Preparation and determination of phenolic compounds from Pyrola incarnata Fisch. with a green polyols-based deep eutectic solvent. Sep. Purif. Technol. 2015, 149, 116–123. [Google Scholar] [CrossRef]
- Sardari, R.R.R.; Prothmann, J.; Gregersen, O.; Turner, C.; Karlsson, E.N. Identification of phlorotannins in the brown algae, Saccharina latissima and Ascophyllum nodosum by ultra-high-performance liquid chromatography coupled to high-resolution tandem mass spectrometry. Molecules 2021, 26, 43. [Google Scholar] [CrossRef]
- Taniguchi, M.; LaRocca, C.A.; Bernat, J.D.; Lindsey, J.S. Digital database of absorption spectra of diverse flavonoids enables structural comparisons and quantitative evaluations. J. Nat. Prod. 2023, 86, 1087–1119. [Google Scholar] [CrossRef]
- Ishiyama, T.; Tahara, T.; Morita, A. Why the photochemical reaction of phenol becomes ultrafast at the air–water interface: The effect of surface hydration. J. Am. Chem. Soc. 2022, 144, 6321–6325. [Google Scholar] [CrossRef]
- Costa, J.I. Síntese de Compostos Multiporfirínicos Covalentes. Tese de doutorado em Química. Departamento de Química, Universidade de Aveiro, Aveiro, Portugal. 2015. Available online: http://hdl.handle.net/10773/16049 (accessed on 26 March 2025).
- Berlanga-Del Pozo, M.; Gallardo-Guerrero, L.; Gandul-Rojas, B. Influence of alkaline treatment on structural modifications of chlorophyll pigments in NaOH—Treated table olives preserved without fermentation. Foods 2020, 9, 701. [Google Scholar] [CrossRef]
- Gandul-Rojas, B.; Roca, M.; Gallardo-Guerrero, L. Chlorophylls and carotenoids in food products from olive tree. In Olive and Olive Oil Bioactive Constituents; InTech Open Limited: London, UK, 2016. [Google Scholar] [CrossRef]
- Kang, Y.-R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, characterization, and functional properties of chlorophylls, pheophytins, and Zn-pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids, volume 1A, isolation and analysis; volume 1B, spectroscopy: Edited by G. Britton, S. Liaaen-Jensen and H. Pfander, Birkhauser-Verlag, Basel, 1995. 1st Vol, 328 pp. 2nd Vol, 360 pp. £74.00 ISBN 3-7643-2908-4 & 3-7643-2909-2. Phytochemistry 1995, 45, 1095. [Google Scholar] [CrossRef]
- Horta, A.; Duarte, A.M.; Barroso, S.; Pinto, F.R.; Mendes, S.; Lima, V.; Saraiva, J.A.; Gil, M.M. Extraction of antioxidants from brown macroalgae Fucus spiralis. Molecules 2024, 29, 2271. [Google Scholar] [CrossRef]
- Subbiah, V.; Ebrahimi, F.; Agar, O.T.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A.R. Comparative study on the effect of phenolics and their antioxidant potential of freeze-dried Australian beach-cast seaweed species upon different extraction methodologies. Pharmaceuticals 2023, 16, 773. [Google Scholar] [CrossRef]
- García-Vaquero, M.; Ravindran, R.; Walsh, O.; O’Doherty, J. Evaluation of ultrasound, microwave, ultrasound–microwave, hydrothermal and high pressure assisted extraction technologies for the recovery of phytochemicals and antioxidants from brown macroalgae. Mar. Drugs 2021, 19, 309. [Google Scholar] [CrossRef]
- Dinh, T.V.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]
- Toan, T.Q.; Phong, T.D.; Tien, D.D.; Linh, N.M.; Anh, N.T.M.; Minh, P.T.H.; Duy, L.X.; Nghi, D.H.; Thi, H.H.P.; Nhut, P.T.; et al. Optimization of Microwave-Assisted Extraction of Phlorotannin From Sargassum swartzii (Turn.) C. Ag. With Ethanol/Water. Nat. Prod. Commun. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Garcia-Perez, P.; Carreira-Casais, A.; Silva, A.; Simal-Gandara, J.; Prieto, M. A HPLC-DAD method for identifying and estimating the content of fucoxanthin, β-carotene and chlorophyll a in brown algal extracts. Food Chem. Adv. 2022, 1, 100095. [Google Scholar] [CrossRef]
- Sanger, G.; Wonggo, D.; Montolalu, L.A.D.Y.; Dotulong, V. Pigments constituents, phenolic content and antioxidant activity of brown seaweed Sargassum sp. IOP Conf. Ser. Earth Environ. Sci. 2022, 1033, 012057. [Google Scholar] [CrossRef]
- Usman, H.; Farouq, A.; Baki, A.; Abdulkadir, N.; Gani, M. Production and characterization of orange pigment produced by Halophilic bacterium Salinococcus roseus isolated from Abattoir soil. J. Microbiol. Exp. 2018, 6, 6. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- EU. Implementing Regulation (EU) No 371/2011 Regarding the Maximum Limit of Dimethylaminoethanol (DMAE); European Union: Brussels, Belgium, 2018; Available online: https://eur-lex.europa.eu/legal-content/pt/TXT/?uri=CELEX%3A32018R1936 (accessed on 30 March 2025).
- ECHA. Search for Chemicals. 2-Dimethylaminoethanol (CAS 108-01-0); European Chemicals Agency: Helsinki, Finland, 2024; Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.003.221 (accessed on 30 March 2025).
- Lethesh, K.C.; Parmentier, D.; Dehaen, W.; Binnemans, K. Phenolate platform for anion exchange in ionic liquids. RSC Adv. 2012, 2, 11936–11943. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Yao, L.; Qian, M.; Li, Z.; Han, Y.; Bai, S.; Lee, M. pH-responsive switchable deep eutectic solvents to mediate pretreatment method for trace analysis of triazole fungicides in peel wastes. Food Chem. 2023, 417, 135486. [Google Scholar] [CrossRef]
- Yu, X.; Li, M.; Yagoub, A.E.A.; Chen, L.; Zhou, C.; Yan, D. Switchable (pH driven) aqueous two-phase systems formed by deep eutectic solvents as integrated platforms for production-separation 5-HMF. J. Mol. Liq. 2020, 310, 115158. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Bai, Y.; Xu, Z.; Shi, Y.; Sun, Y. Phlorotannins from Undaria pinnatifida sporophyll: Extraction, antioxidant, and anti-inflammatory activities. Mar. Drugs 2019, 17, 434. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Park, Y.-S.; Roy, V.C.; Park, J.-S.; Zhang, W.; Chun, B.-S. Optimization of subcritical water extraction parameters of phlorotannins from brown alga (Ecklonia stolonifera): Bipotentialities and possible applications. J. Supercrit. Fluids 2025, 218, 106502. [Google Scholar] [CrossRef]
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-assisted extraction of phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef] [PubMed]
- Erge, H.S.; Karadeniz, F.; Koca, N.; Soyer, Y. Effect of heat treatment on chlorophyll degradation and color loss in green peas. GIDA 2008, 33, 225–233. [Google Scholar]
- Sukumaran, S.T.; Sugathan, S.; Abdulhameed, S. (Eds.) Plant Metabolites: Methods, Applications and Prospects: Springer Nature: Berlin/Heidelberg, Germany, 2020. [CrossRef]
- Kardile, N.B.; Nanda, V.; Thakre, S. Thermal degradation kinetics of total carotenoid and colour of mixed juice. Agric. Res. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Predescu, N.C.; Papuc, C.; Nicorescu, V.; Gajaila, I.; Goran, G.V.; Petcu, C.D.; Stefan, G. The influence of solid-to-solvent ratio and extraction method on total phenolic content, flavonoid content and antioxidant properties of some ethanolic plant extracts. Rev. Chim. 2016, 67, 1921–1926. [Google Scholar]
- Boi, V.N.; Cuong, D.X.; Vinh, P.T.K. Effects of extraction conditions over the phlorotannin content and antioxidant activity of extract from brown algae Sargassum serratum. Free. Radic. Antioxid. 2001, 7, 1. [Google Scholar] [CrossRef]
- Isci, A.; Kaltschmitt, M. Recovery and recycling of deep eutectic solvents in biomass conversions: A review. Biomass Convers. Biorefinery 2022, 12, 197–226. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Yuan, F.; Li, Y.; Yu, D.; Liang, X. Quantitative recovery and regeneration of basic deep eutectic solvent for biomass treatment with industrialized membrane-based strategy. Ind. Crops Prod. 2024, 221, 119419. [Google Scholar] [CrossRef]
- Della, S.P.; Gallo, V.; Gentili, A.; Fanali, C. Strategies for the recovery of bioactive molecules from deep eutectic solvents extracts. TrAC Trends Anal. Chem. 2022, 157, 116798. [Google Scholar] [CrossRef]
- Silva, C.N.; Silva, R.M.; Lemes, A.C.; Ribeiro, B.D. Recovery of phenolic compounds by deep eutectic solvents in orange by-products and spent coffee grounds. Sustainability 2024, 16, 7403. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18 Pt B, 820–897. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef]
- Barbosa, M.; Fernandes, F.; Carlos, M.J.; Valentão, P.; Andrade, P.B. Adding value to marine invaders by exploring the potential of Sargassum muticum (Yendo) Fensholt phlorotannin extract on targets underlying metabolic changes in diabetes. Algal Res. 2021, 59, 102455. [Google Scholar] [CrossRef]
- Farvin, K.H.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]

| HBA | HBD | HBA/ HBD | Abbreviation | pH (0–30% H2O) | Stage 1 Degradation | Stage 2 Degradation | −50% wt |
|---|---|---|---|---|---|---|---|
| DMAE | BzOH | 1.30 | DMAE:BzOH (1.30) | 11.18–10.63 | 102.57 | 135.33 | 105.14 |
| CinnOH | 3.25 | DMAE:CinnOH (3.25) | 10.32–10.05 | 79.02 | 161.22 | 78.14 | |
| GLY | 2.57 | DMAE:GLY (2.57) | 11.40–10.91 | 81.78 | 209.27 | 86.65 | |
| 13PDO | 1.83 | DMAE:13PDO (1.83) | 11.47–10.78 | 94.98 | 159.21 | 105.27 | |
| Sor | 5.36 | DMAE:Sor (5.36) | 11.50–10.91 | 82.49 | 326.34 | 75.85 | |
| 12PDO | 0.86 | DMAE:12PDO (0.86) | 11.45–10.90 | 92.74 | 129.44 | 96.83 | |
| MAE | CinnOH | 1.83 | 2-MAE:CinnOH (1.83) | 12.49–11.86 | 107.33 | 166.00 | 112.07 |
| GLY | 1.44 | 2-MAE:GLY (1.44) | 12.50–12.06 | 114.84 | 216.19 | 132.11 | |
| Sor | 4.2 | 2-MAE:Sor (4.2) | 12.90–12.25 | 109.92 | 317.10 | 106.03 | |
| BzOH | 0.82 | 2-MAE:BzOH (0.82) | 12.52–11.65 | 146.66 | - | 126.86 | |
| 12PDO | 0.44 | 2-MAE:12PDO (0.44) | 12.22–11.66 | 147.22 | - | 129.70 | |
| 13PDO | 0.63 | 2-MAE:13PDO (0.63) | 12.59–11.93 | 155.53 | - | 135.03 | |
| DEA | BzOH | 0.37 | DEA:BzOH (0.37) | 11.47–11.93 | 155.61 | 193.94 | 152.20 |
| CinnOH | 0.90 | DEA:CinnOH (0.9) | 11.40–10.77 | 196.99 | - | 186.09 | |
| Sor | 2.32 | DEA:Sor (2.32) | 11.60–10.96 | 200.99 | 327.20 | 202.63 | |
| GLY | 0.65 | DEA:GLY (0.65) | 11.60–10.84 | 224.61 | - | 208.71 | |
| 12PDO | 0.15 | DEA:12PDO (0.15) | 11.85–10.63 | 150.20 | - | 133.26 | |
| 13PDO | 0.19 | DEA:13PDO (0.19) | 12.00–10.77 | 179.12 | - | 167.59 | |
| AMP | BzOH | 0.85 | AMP:BzOH (0.85) | 11.03–10.65 | 121.85 | - | 118.26 |
| CinnOH | 1.67 | AMP:CinnOH (1.67) | 11.60–10.02 | 110.54 | 163.03 | 118.69 | |
| GLY | 1.35 | AMP:GLY (1.35) | 11.66–11.13 | 119.59 | 203.72 | 130.42 | |
| 12PDO | 0.53 | AMP:12PDO (0.53) | 11.52–10.97 | 129.14 | - | 125.16 | |
| 13PDO | 0.63 | AMP:13PDO (0.63) | 11.70–11.21 | 150.22 | - | 140.19 | |
| Sor | 3.97 | AMP:Sor (3.97) | 12.12–11.67 | 102.15 | 310.33 | 107.05 |
| Products | Species | Operational Condictions | Content | Reference |
|---|---|---|---|---|
| Polyphenols | Fucus vesiculosus | UAE; ChCl:LA 1:3 + 30% H2O; 22.80 min | 137.30 mg GAE/g | [66] |
| Fucus spiralis | HPAE; 300 s; 50 g/100 mL; 25% EtOH; 600 Mpa | 141.92 mg GAE/g | [81] | |
| Sargassum muticum | MAE; 1:10; 100 °C; 3 min; Pro:1,2-But (1:4) | 24.00 mg GAE/g | [14] | |
| SLE; 1:20 (w/v); 16 h; 10 °C; 120 rpm; EtOH 96% | 3.20 mg GAE/g | [82] | ||
| Phlorotannins | Pelvetia canaliculate | MAE; 1:10 (w/v); 250 W; 10 min; EtOH 50% | 40.85 mg PGE/g | [83] |
| Sargassum japonica | SLE; 1:32 (w/v); 24 h; RT; 500 rpm; 0.5 M [C4C1im][BF4] | 2.10 mg PGE/g | [84] | |
| Sargassum swartzii | MAE:H2O:EtOH (70:30); (35 v/m); 60 min; 560 W | 5.28 mg PGE/g | [85] | |
| Chlorophyll | Ulva Pinnatifida | UAE; 500 W; 55 min; EtOH (30 g/L)/Centrifugation (8400 rpm, 7 min)/filtration (0.22 μm nylon) | 0.30 mg/g dw | [86] |
| Saccharina latissimi | 0.07 mg/g dw | |||
| Sargassum sp. | SLE; EtOH (1:10 m/v); 50 °C; 3 days | 0.62 mg/g dw | [87] |
| Solvent | Sample | Temperature (°C) | Solid-Liquid S/L | DPPH (mg TE/mL) | ABTS (mg TE/mL) | FRAP (mg TE/mL) |
|---|---|---|---|---|---|---|
| DMAE:BzOH (1.30) | Control | - | - | 0.30 c | 23.04 c | 1.91 c |
| Extract 1 | 120.4 | 0.17 | 0.69 a | 24.42 a | 2.26 a | |
| Extract 2 | 74.4 | 0.03 | 0.40 b | 23.59 b | 2.00 b | |
| DMAE:13PDO (1.83) | Control | - | - | 0.65 b | 23.13 c | 1.91 b |
| Extract 1 | 120.4 | 0.14 | 0.87 a | 24.22 a | 2.24 a | |
| Extract 2 | 68.4 | 0.03 | 0.73 b | 23.44 b | 2.06 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mello, P.A.V.P.; da Silva, C.N.; Ribeiro, B.D. Valorization of Residual Biomass from Sargassum filipendula for the Extraction of Phlorotannins and Pigments Using Eutectic Solvents. Processes 2025, 13, 1345. https://doi.org/10.3390/pr13051345
Mello PAVP, da Silva CN, Ribeiro BD. Valorization of Residual Biomass from Sargassum filipendula for the Extraction of Phlorotannins and Pigments Using Eutectic Solvents. Processes. 2025; 13(5):1345. https://doi.org/10.3390/pr13051345
Chicago/Turabian StyleMello, Pedro Afonso Vasconcelos Paes, Cristiane Nunes da Silva, and Bernardo Dias Ribeiro. 2025. "Valorization of Residual Biomass from Sargassum filipendula for the Extraction of Phlorotannins and Pigments Using Eutectic Solvents" Processes 13, no. 5: 1345. https://doi.org/10.3390/pr13051345
APA StyleMello, P. A. V. P., da Silva, C. N., & Ribeiro, B. D. (2025). Valorization of Residual Biomass from Sargassum filipendula for the Extraction of Phlorotannins and Pigments Using Eutectic Solvents. Processes, 13(5), 1345. https://doi.org/10.3390/pr13051345








