Adsorption of Heavy Metal Pb(II) in Dredged Sediment Using Different Biochar Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus and Materials
2.2. Preparation of Biochar Materials
- (1)
- Pyrolyzed biochar
- (2)
- Hydrochar
2.3. Characterization of Biochar Materials
2.4. Heavy Metal Adsorption Experiment
- (1)
- Adsorption of Pb(II) in water
- (2)
- Adsorption of Pb(II) in sediment
3. Results
3.1. Yield and Characterization of Biochar Materials
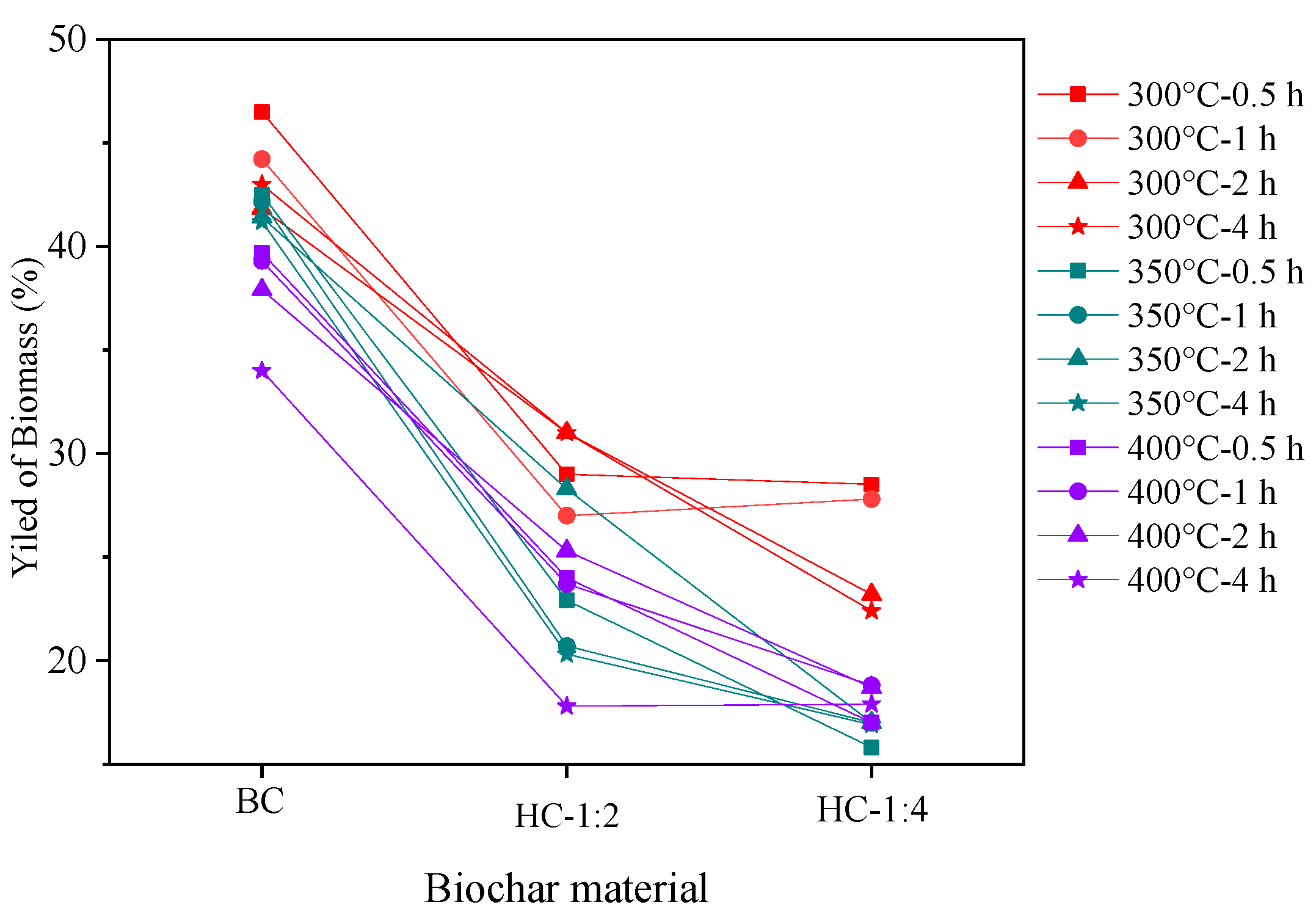
3.1.1. Yield of Biochar Materials
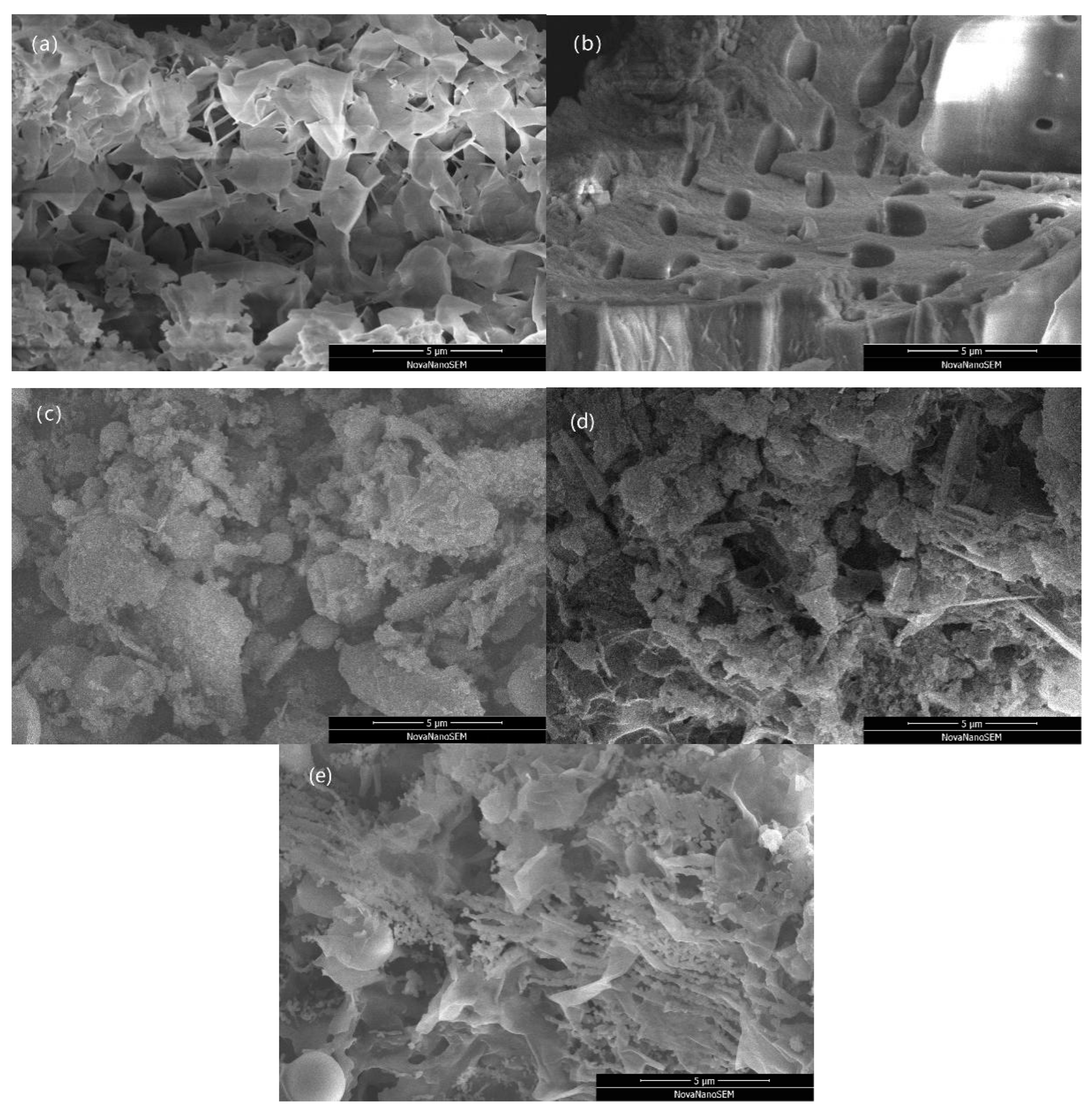
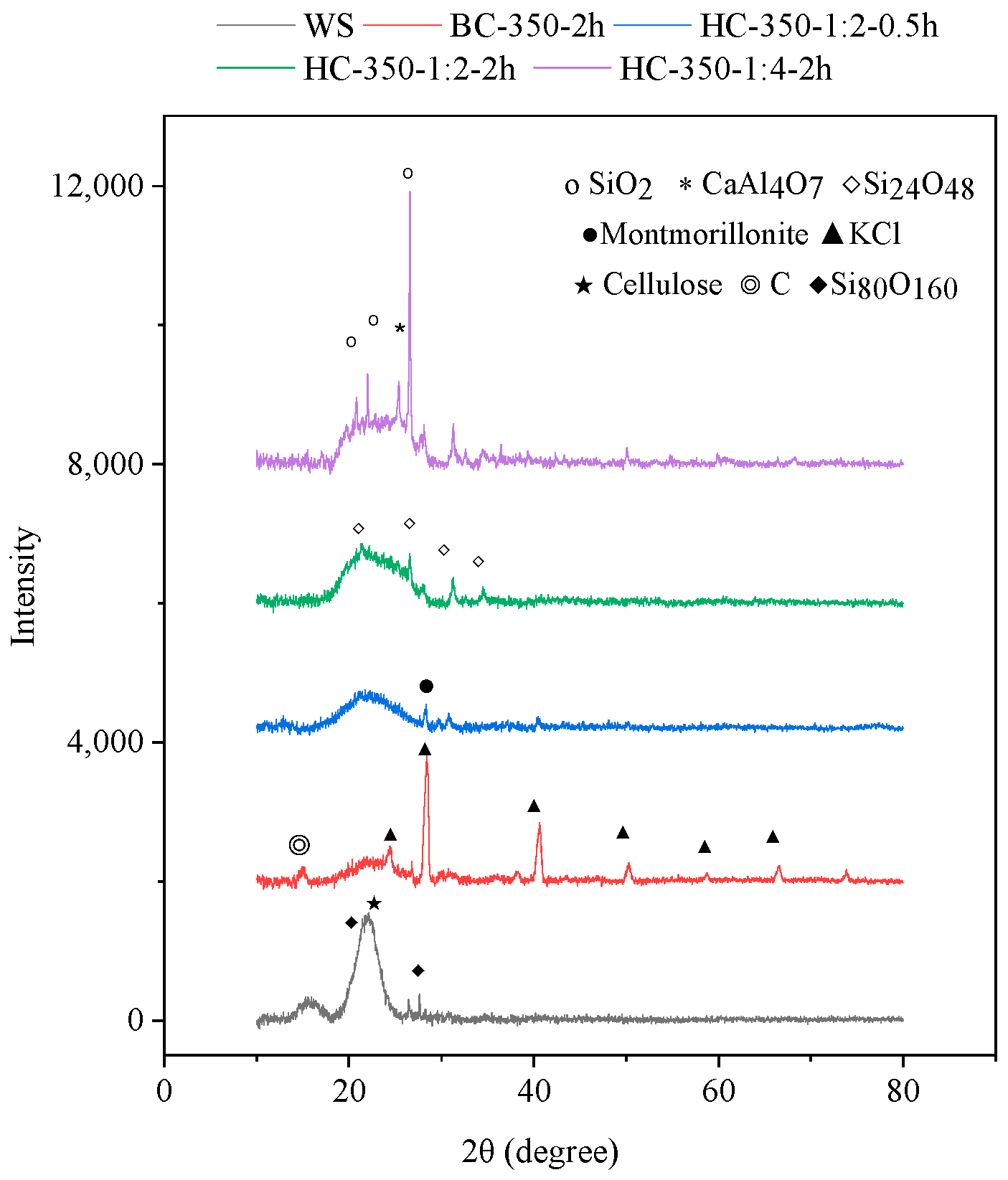
3.1.2. Analysis of Biochar
3.2. Adsorption of Pb(II) in Water by Biochar
3.2.1. Determination of Biochar Dosage
3.2.2. Pb(II) Adsorption with Different Biochar Materials
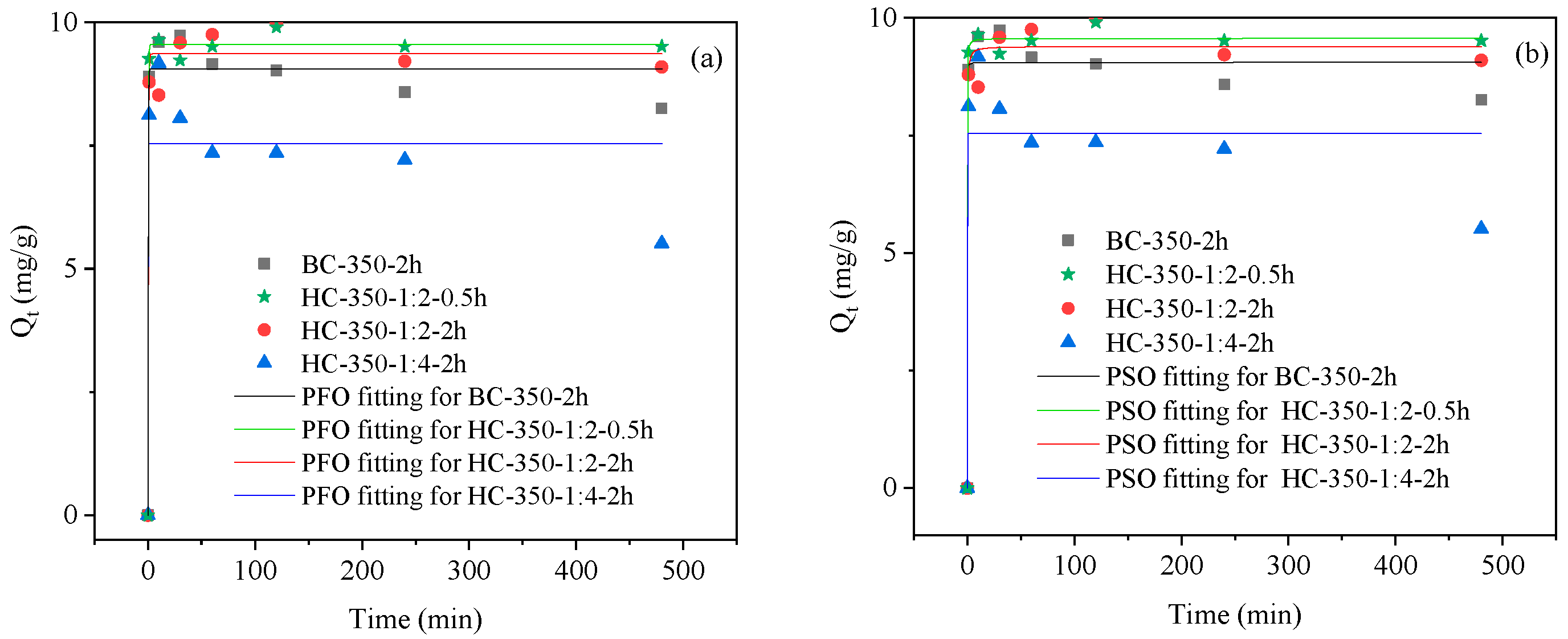
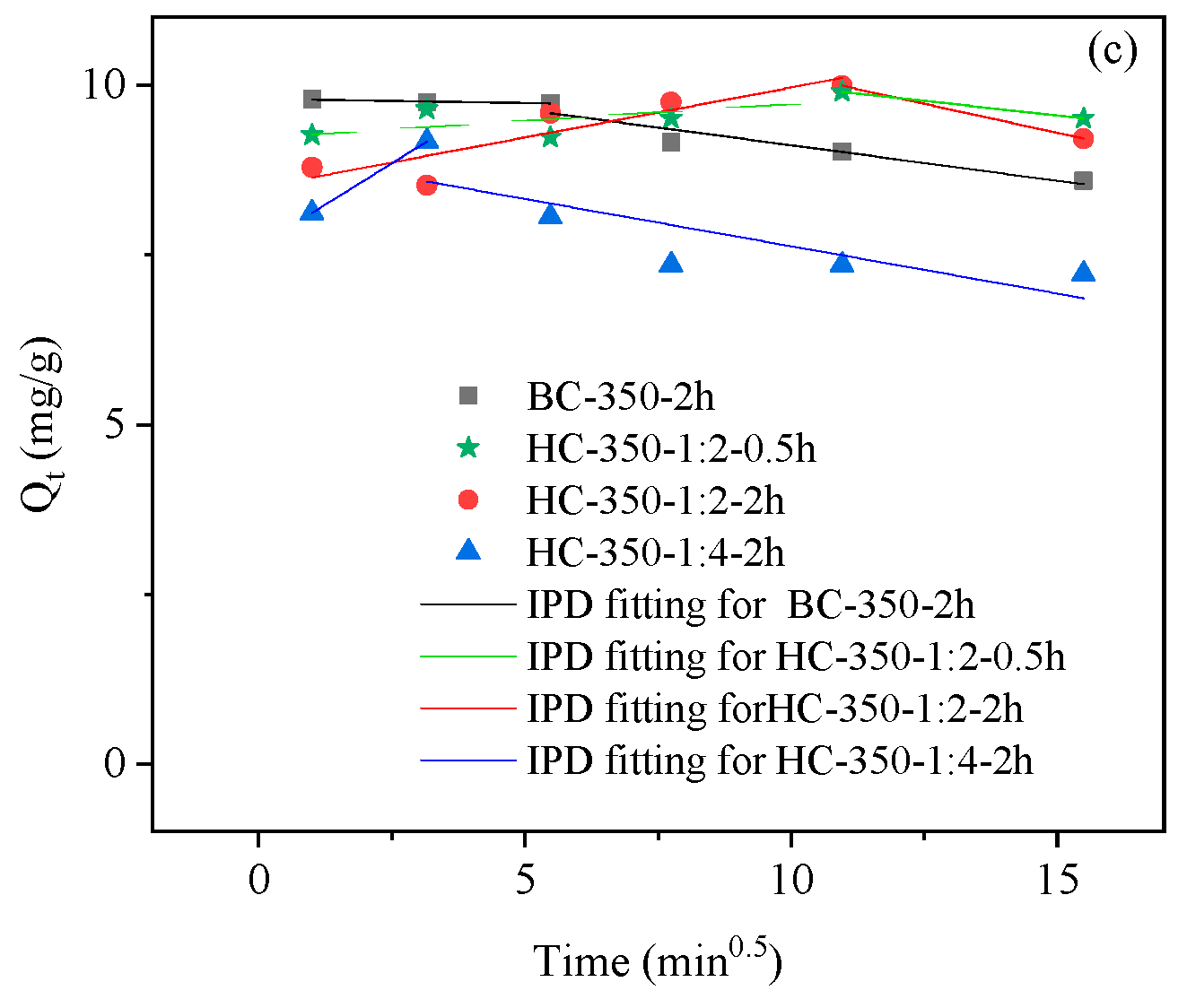
3.2.3. Adsorption Kinetics of Biochar Materials
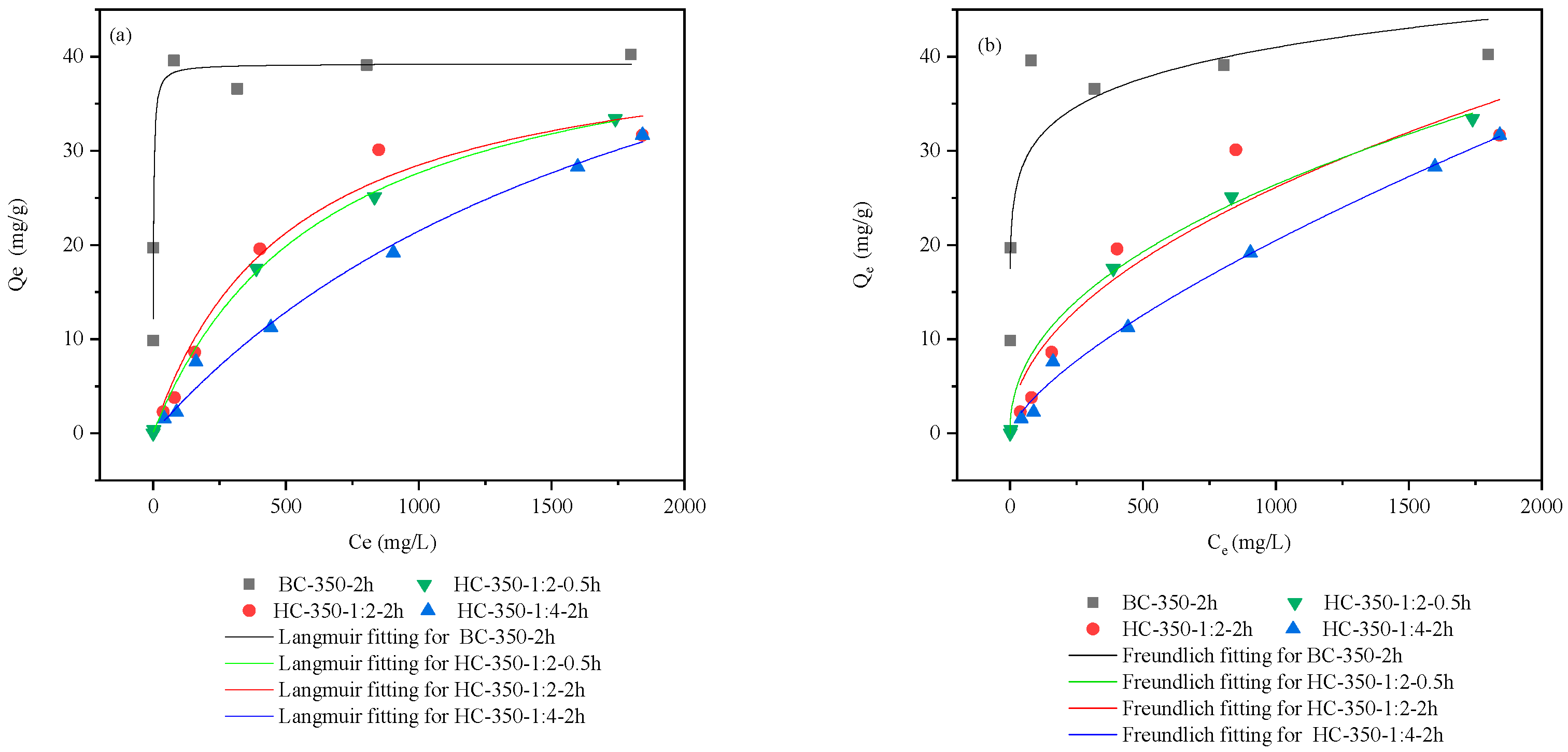
3.2.4. Isothermal Adsorption Modeling
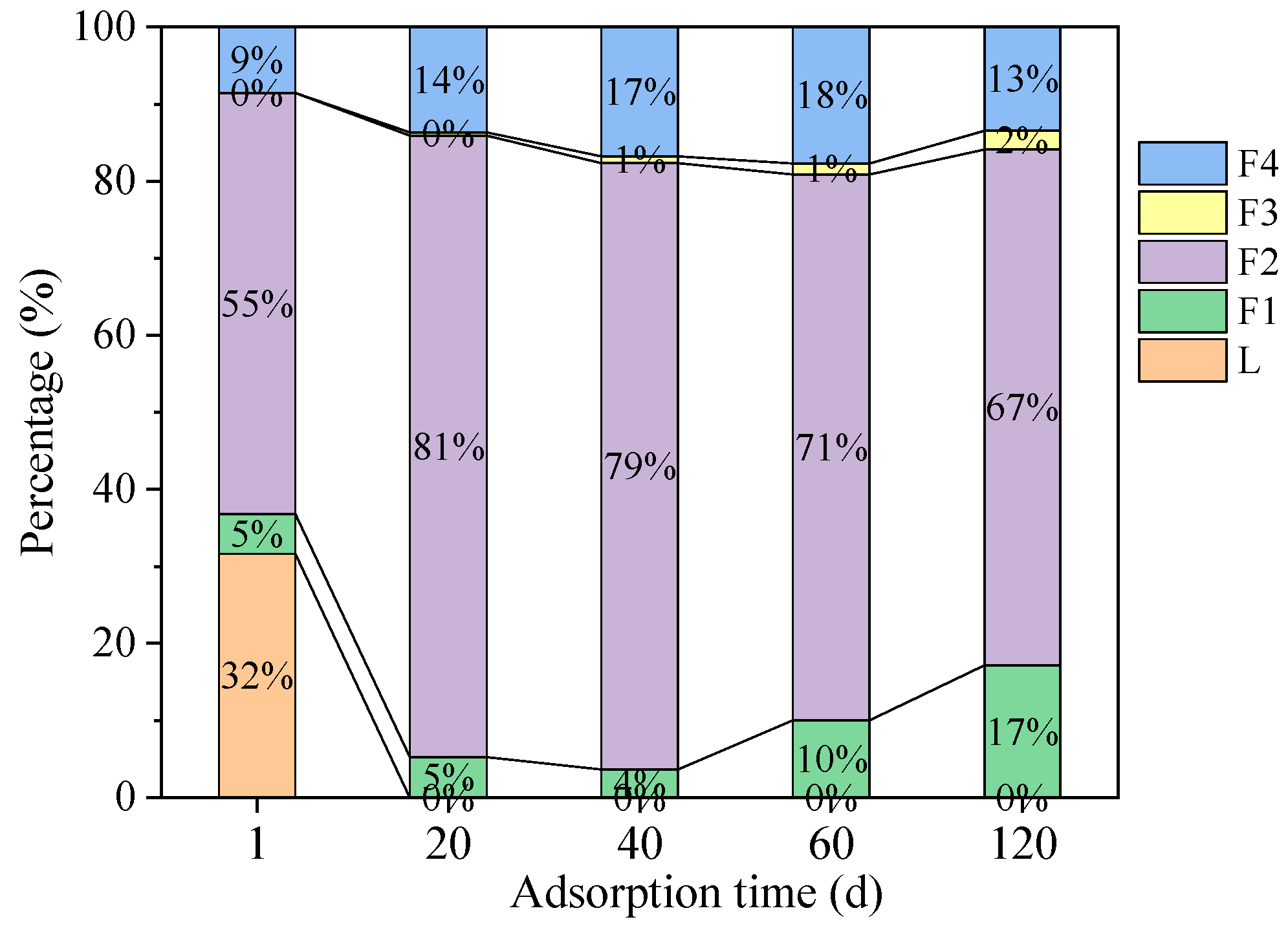
3.3. Adsorption of Pb(II)-Contaminated Sediment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Wang, Z.W.; Yang, W.C.; Jia, H.L.; You, Z.J. Characterization, pollution, and beneficial utilization assessment of dredged sediments from coastal ports in China. Mar. Pollut. Bull. 2025, 211, 117389. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Wu, S.Q.; Sui, X.Q.; Cheng, S.P. Phytoremediation of contaminated sediment combined with biochar: Feasibility, challenges and perspectives. J. Hazard. Mater. 2024, 465, 133135. [Google Scholar] [CrossRef]
- Deng, X.; Chen, G.; Zhang, C.; Gao, X.; Sun, B.; Shan, B. Manganese-modified biochar for sediment remediation: Effect, microbial community response, and mechanism. Environ. Pollut. 2024, 363, 125175. [Google Scholar] [CrossRef]
- Liu, H.; Bai, J.; Zhang, K.; Wang, C.; Liang, J.; Zhang, L.; Wang, Y.; Xiao, R. Heavy metal pollution and ecological risk assessment of surface sediments covered by emerged and submerged plants in a shallow lake. Ecohydrol. Hydrobiol. 2024, 24, 849–856. [Google Scholar] [CrossRef]
- Niu, Y.; Jiang, X.; Wang, K.; Xia, J.; Jiao, W.; Niu, Y.; Yu, H. Meta analysis of heavy metal pollution and sources in surface sediments of Lake Taihu, China. Sci. Total Environ. 2020, 700, 134509. [Google Scholar] [CrossRef]
- Negi, M.; Thankachan, V.; Rajeev, A.; Vairamuthu, M.; Arundhathi, S.; Nidheesh, P.V. Clean and Green Bamboo Magic: Recent Advances in Heavy Metal Removal from Water by Bamboo Adsorbents. Water 2025, 17, 454. [Google Scholar] [CrossRef]
- Ye, S.Y.; Jiang, X.Y.; Yang, Y.R.; Xu, X.M.; Zhao, C.H.; Ma, J.Z.; Yang, W.; Liu, L.F. Removal of Pb2+ from Aqueous Media by Solidago canadensis L.-Derived and Crab Shell-Derived Biochar: Adsorption Behavior and Optimization of Adsorption Conditions. Water Air Soil. Pollut. 2025, 236, 87. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R. Conversion of crop, weed and tree biomass into biochar for heavy metal removal and wastewater treatment. Biomass Convers. Biorefinery 2023, 13, 4901–4914. [Google Scholar] [CrossRef]
- Lebrun, M.; Macri, C.; Miard, F.; Hattab-Hambli, N.; Motelica-Heino, M.; Morabito, D.; Bourgerie, S. Effect of biochar amendments on As and Pb mobility and phytoavailability in contaminated mine technosols phytoremediated by Salix. J. Geochem. Explor. 2017, 182, 149–156. [Google Scholar] [CrossRef]
- Kiran, B.R.; Prasad, M.N.V. Biochar and rice husk ash assisted phytoremediation potentials of Ricinus communis L. for lead-spiked soils. Ecotoxicol. Environ. Saf. 2019, 183, 109574. [Google Scholar] [CrossRef]
- Dong, Y.C.; Yu, B.; Jia, Y.F.; Xu, X.K.; Zhou, P.; Yu, M.D.; Liu, J.G. Influence of sewage sludge compost on heavy metals in abandoned mine land reclamation: A large-scale field study for three years. J. Hazard. Mater. 2025, 486, 137098. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Ren, L.S.; Wang, D.Y.; Cai, Z.S.; Xia, X.F.; Ding, A.Z. Assessing the capacity of biochar to stabilize copper and lead in contaminated sediments using chemical and extraction methods. J. Environ. Sci. 2019, 79, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, W.; Liu, C.; Liu, G.; Li, Y.; Ding, B.; Zhang, P.; Zhang, X.; Zhang, Z. Remediation of Pb and Cd contaminated sediments by wheat straw biochar and microbial community analysis. Environ. Technol. Innov. 2024, 36, 103849. [Google Scholar] [CrossRef]
- Xu, Z.R.; Zhu, W.; Gong, M.; Zhang, H.W. Direct gasification of dewatered sewage sludge in supercritical water. Part 1: Effects of alkali salts. Int. J. Hydrogen Energy 2013, 38, 3963–3972. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, W.; Gong, M.; Zhou, H.; Fan, Y.; Amuzu-Sefordzi, B. Interaction between sewage sludge components lignin (phenol) and proteins (alanine) in supercritical water gasification. Int. J. Hydrogen Energy 2015, 40, 9125–9136. [Google Scholar] [CrossRef]
- GB/T 15224.1-2018; Classification for Quality of Coal. Part 1: Ash. Standards Press of China: Beijing, China, 2018.
- Yin, H.; Zhu, J. In situ remediation of metal contaminated lake sediment using naturally occurring, calcium-rich clay mineral-based low-cost amendment. Chem. Eng. J. 2016, 285, 112–120. [Google Scholar] [CrossRef]
- Tan, L.; Nie, Y.; Chang, H.; Zhu, L.; Guo, K.; Ran, X.; Zhong, N.; Zhong, D.; Xu, Y.; Ho, S.H. Adsorption performance of Ni(II) by KOH-modified biochar derived from different microalgae species. Bioresour. Technol. 2024, 394, 130287. [Google Scholar] [CrossRef]
- Park, G.; Phule, A.D.; Elkaee, S.; Kim, S.Y.; Zaman, M.W.U.; Yang, J.H.; Jeon, S.-C. Conversion of PET bottles into carbonaceous adsorbents for Pb(II) removal from aqueous solutions via KOH activation. J. Water Process Eng. 2024, 66, 106092. [Google Scholar] [CrossRef]
- Yun, X.; Ma, Y.; Zheng, H.; Zhang, Y.; Cui, B.; Xing, B.J.B. Pb(II) adsorption by biochar from co-pyrolysis of corn stalks and alkali-fused fly ash. Biochar 2022, 4, 66. [Google Scholar] [CrossRef]
- Uchimiya, M.; Ohno, T.; He, Z.Q. Pyrolysis temperature-dependent release of dissolved organic carbon from plant, manure, and biorefinery wastes. J. Anal. Appl. Pyrolysis 2013, 104, 84–94. [Google Scholar] [CrossRef]
- Qu, X.L.; Fu, H.Y.; Mao, J.D.; Ran, Y.; Zhang, D.N.; Zhu, D.Q. Chemical and structural properties of dissolved black carbon released from biochars. Carbon 2016, 96, 759–767. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, S.A.; Yu, K.; Guo, J.Z.; Wang, Y.X.; Li, B. Adsorption of lead ions and methylene blue on acrylate-modified hydrochars. Bioresour. Technol. 2023, 379, 129067. [Google Scholar] [CrossRef]
- Bai, J.; Li, L.; Chen, Z.; Chang, C.; Pang, S.; Li, P. Study on the optimization of hydrothermal liquefaction performance of tobacco stem and the high value utilization of catalytic products. Energy 2023, 281, 128283. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Su, Y.; Liao, Q.; Xia, S.; Yang, Y.; Sun, Y.; Wang, W. Adsorption of Pb(II) by hydrochar derived from wheat straw and swine manure: Focus on mineral constituents. Energy Sources Part A-Recovery Util. Environ. Eff. 2024, 46, 14977–14988. [Google Scholar] [CrossRef]
- Hong, C.; Wang, Z.; Si, Y.; Xing, Y.; Yang, J.; Feng, L.; Wang, Y.; Hu, J.; Li, Z.; Li, Y. Catalytic Hydrothermal Liquefaction of Penicillin Residue for the Production of Bio-Oil over Different Homogeneous/Heterogeneous Catalysts. Catalysts 2021, 11, 849. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Naeth, M.A.; Chang, S.X. Lead(II) adsorption on microwave-pyrolyzed biochars and hydrochars depends on feedstock type and production temperature. J. Hazard. Mater. 2021, 412, 125255. [Google Scholar] [CrossRef]
- Skodras, G.; Diamantopoulou, I.; Pantoleontos, G.; Sakellaropoulos, G.P. Kinetic studies of elemental mercury adsorption in activated carbon fixed bed reactor. J. Hazard. Mater. 2008, 158, 1–13. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mittal, A.; Malviya, A.; Mittal, J. Adsorption of carmoisine A from wastewater using waste materials—Bottom ash and deoiled soya. J. Colloid. Interface Sci. 2009, 335, 24–33. [Google Scholar]
- Raji, C.; Anirudhan, T.S. Batch Cr(VI) removal by polyacrylamide-grafted sawdust: Kinetics and thermodynamics. Water Res. 1998, 32, 3772–3780. [Google Scholar]
- Tang, J.; Li, X.; Luo, Y.; Li, G.; Khan, S. Spectroscopic characterization of dissolved organic matter derived from different biochars and their polycylic aromatic hydrocarbons (PAHs) binding affinity. Chemosphere 2016, 152, 399–406. [Google Scholar] [CrossRef] [PubMed]




| Adsorption Experiment | Biochar Mass (g) | Pb(II) Concentration (mg/L) | Adsorption Time (min) |
|---|---|---|---|
| Determination of biochar dosage | 0.2–0.8 | 50 | 10 |
| Adsorption of lead by different carbon materials | 0.2 | 50 | 10 |
| Adsorption kinetics experiment | 0.2 | 50 | 10–240 |
| Isothermal adsorption experiment | 0.2 | 50–1800 | 10 |
| Biochar Materials | Ash (%) | pH | pHpzc | Bet Surface Area (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|---|---|
| BC-350-2h | 24.85 | 8.06 | 9.45 | 4.083 | 0.03424 |
| HC-350-1:2-0.5h | 25.36 | 6.78 | 6.48 | 8.126 | 0.07032 |
| HC-350-1:2-2h | 24.88 | 7.52 | 5.97 | 4.457 | 0.04020 |
| HC-350-1:4-2h | 24.92 | 7.67 | 6.01 | 4.113 | 0.02841 |
| Biochar Materials | Experimental Data | PFO | PSO | ||||
|---|---|---|---|---|---|---|---|
| Qe,exp | Qe,cal | k1 | R2 | Qe,cal | k2 | R2 | |
| mg·g−1 | mg·g−1 | min−1 | mg·g−1 | g·mg−1·min−1 | |||
| BC-350-2h | 9.74 | 9.06 | 4.06 | 0.97 | 9.05 | 15.04 | 0.98 |
| HC-350-1:2-0.5h | 9.90 | 9.55 | 3.50 | 0.99 | 9.56 | 3.03 | 0.99 |
| HC-350-1:2-2h | 9.75 | 9.36 | 2.79 | 0.98 | 9.39 | 1.37 | 0.98 |
| HC-350-1:4-2h | 9.17 | 7.53 | 131.65 | 0.86 | 7.54 | 3.52E44 | 0.85 |
| Biochar materials | IPD | ||||||
| k3-1 | C1 | R2 | k3-2 | C2 | R2 | ||
| g·mg−1·min−1/2 | mg·g−1 | g·mg−1·min−1/2 | mg·g−1 | ||||
| BC-350-2h | −0.012 | 9.80 | 0.87 | −0.10 | 10.16 | 0.91 | |
| HC-350-1:2-0.5h | 0.049 | 9.23 | 0.46 | −0.087 | 10.85 | 1 | |
| HC-350-1:2-2h | 0.15 | 8.49 | 0.8 | −0.17 | 11.87 | 1 | |
| HC-350-1:4-2h | 0.48 | 7.63 | 1 | −0.14 | 9.03 | 0.67 | |
| Biochar Materials | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qmax | K L | R2 | K F | n | R2 | |
| mg·g−1 | L·mg−1 | mg1−1/n·g−1·L1/n | ||||
| BC-350-2h | 39.22 | 1.89 | 0.98 | 17.84 | 0.12 | 0.80 |
| HC-350-1:2-0.5h | 45.45 | 645.16 | 0.99 | 1.13 | 0.46 | 0.99 |
| HC-350-1:2-2h | 43.06 | 512.82 | 0.98 | 0.83 | 0.50 | 0.91 |
| HC-350-1:4-2h | 64.97 | 2026.02 | 0.99 | 0.16 | 0.71 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Liao, Q.; Xia, S.; Shen, X.; Zhu, J.; Liao, Y.; Wang, W.; Fang, Z.; Liu, D. Adsorption of Heavy Metal Pb(II) in Dredged Sediment Using Different Biochar Materials. Processes 2025, 13, 957. https://doi.org/10.3390/pr13040957
Su Y, Liao Q, Xia S, Shen X, Zhu J, Liao Y, Wang W, Fang Z, Liu D. Adsorption of Heavy Metal Pb(II) in Dredged Sediment Using Different Biochar Materials. Processes. 2025; 13(4):957. https://doi.org/10.3390/pr13040957
Chicago/Turabian StyleSu, Ying, Qianyi Liao, Shuhan Xia, Xu Shen, Jiang Zhu, Yubing Liao, Wenhao Wang, Zhou Fang, and Debin Liu. 2025. "Adsorption of Heavy Metal Pb(II) in Dredged Sediment Using Different Biochar Materials" Processes 13, no. 4: 957. https://doi.org/10.3390/pr13040957
APA StyleSu, Y., Liao, Q., Xia, S., Shen, X., Zhu, J., Liao, Y., Wang, W., Fang, Z., & Liu, D. (2025). Adsorption of Heavy Metal Pb(II) in Dredged Sediment Using Different Biochar Materials. Processes, 13(4), 957. https://doi.org/10.3390/pr13040957






