Extraction, Purification and Characterization of Exopolysaccharide from Lactiplantibacillus plantarum B7 with Potential Antioxidant, Antitumor and Anti-Inflammatory Activities
Abstract
1. Introduction
2. Methodology
2.1. Materials
2.2. Screening, Isolation, Characterization and Identification of EPS-Producing Bacterial Strain from Human Breast Milk
2.2.1. Screening of EPS Producing Isolates
2.2.2. Optimal Growth Temperature of the Isolated Bacterial Strain
2.2.3. NaCl Tolerance Assessment
2.2.4. Acid and Bile Tolerance
2.2.5. Identification of the Chosen Bacterial Isolate Utilizing 16S rRNA
2.3. Probiotic Properties of the Selected Strain
Cell Surface Hydrophobicity
2.4. Antimicrobial Potential of the L. plantarum B7
2.5. Assessment of the Safety Aspects of L. plantarum B7
2.5.1. Hemolytic Activity
2.5.2. Antibiotic Susceptibility Test
2.6. Crude Exopolysaccharide (EPSc) Isolation, Fractionation and Purification
2.6.1. EPSc Isolation
2.6.2. EPSc Fractionation
2.6.3. Fraction Purification
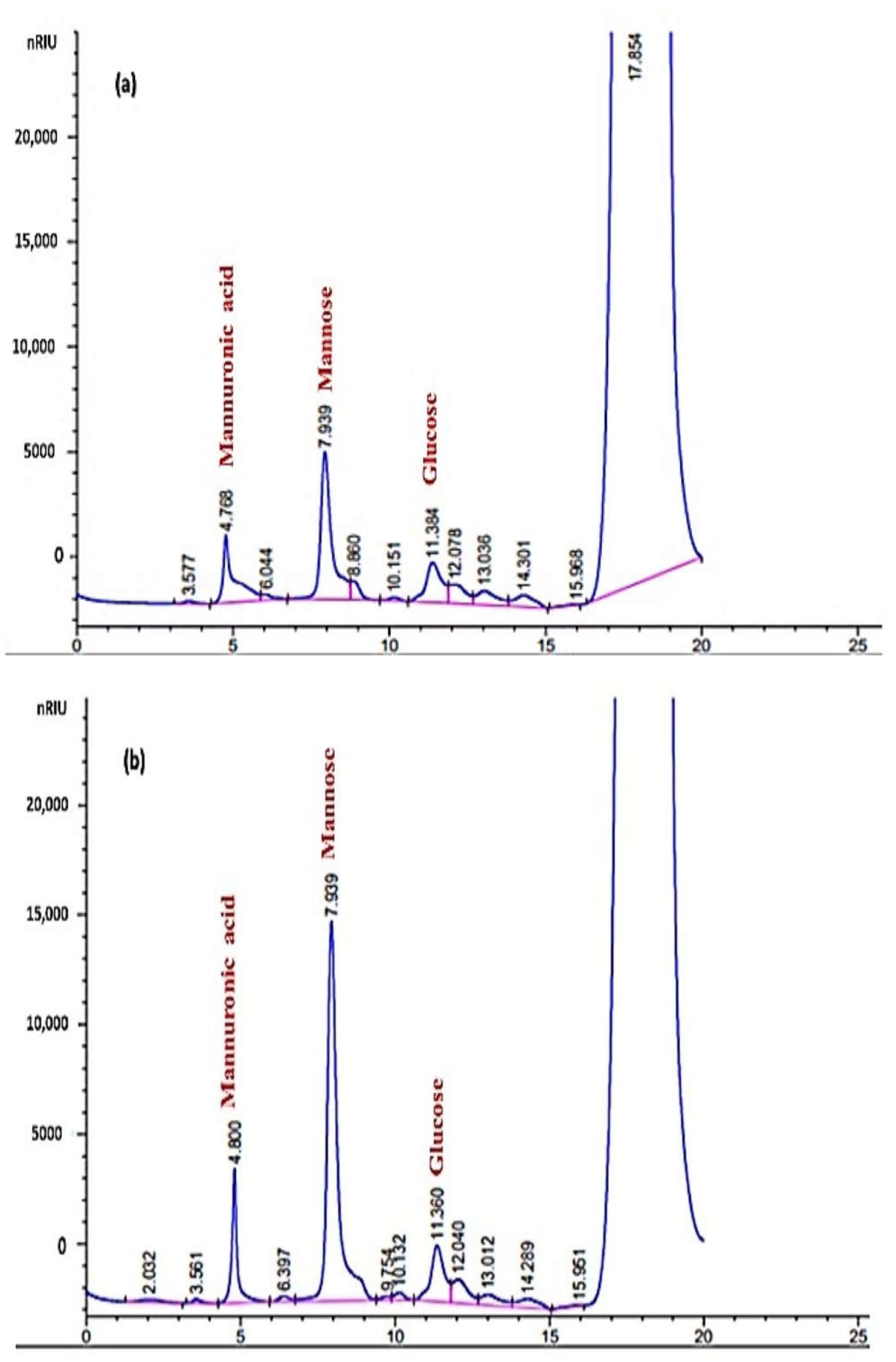
2.7. Determination of Monosaccharide Composition and Molecular Weight (MW) of EPSF1 and EPSF2
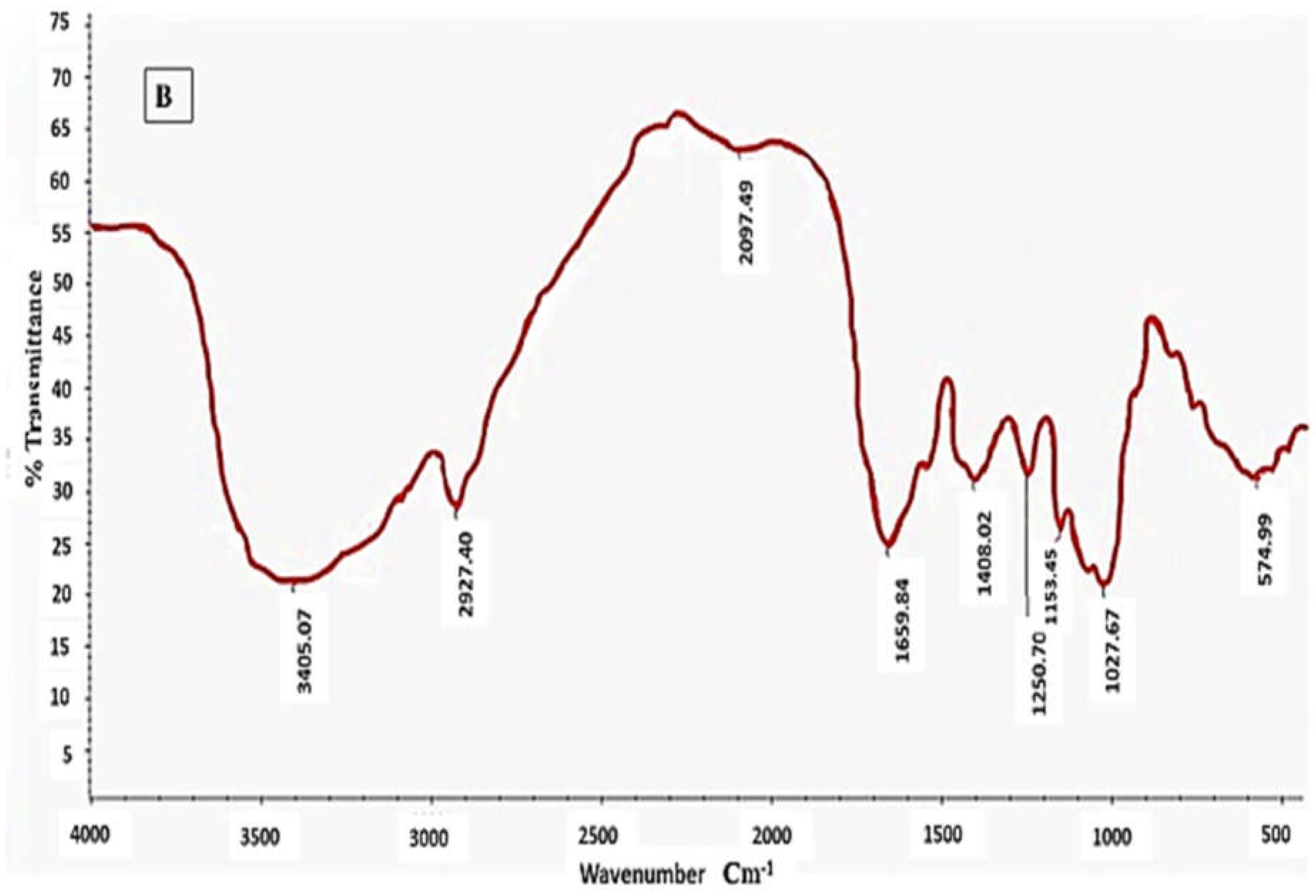
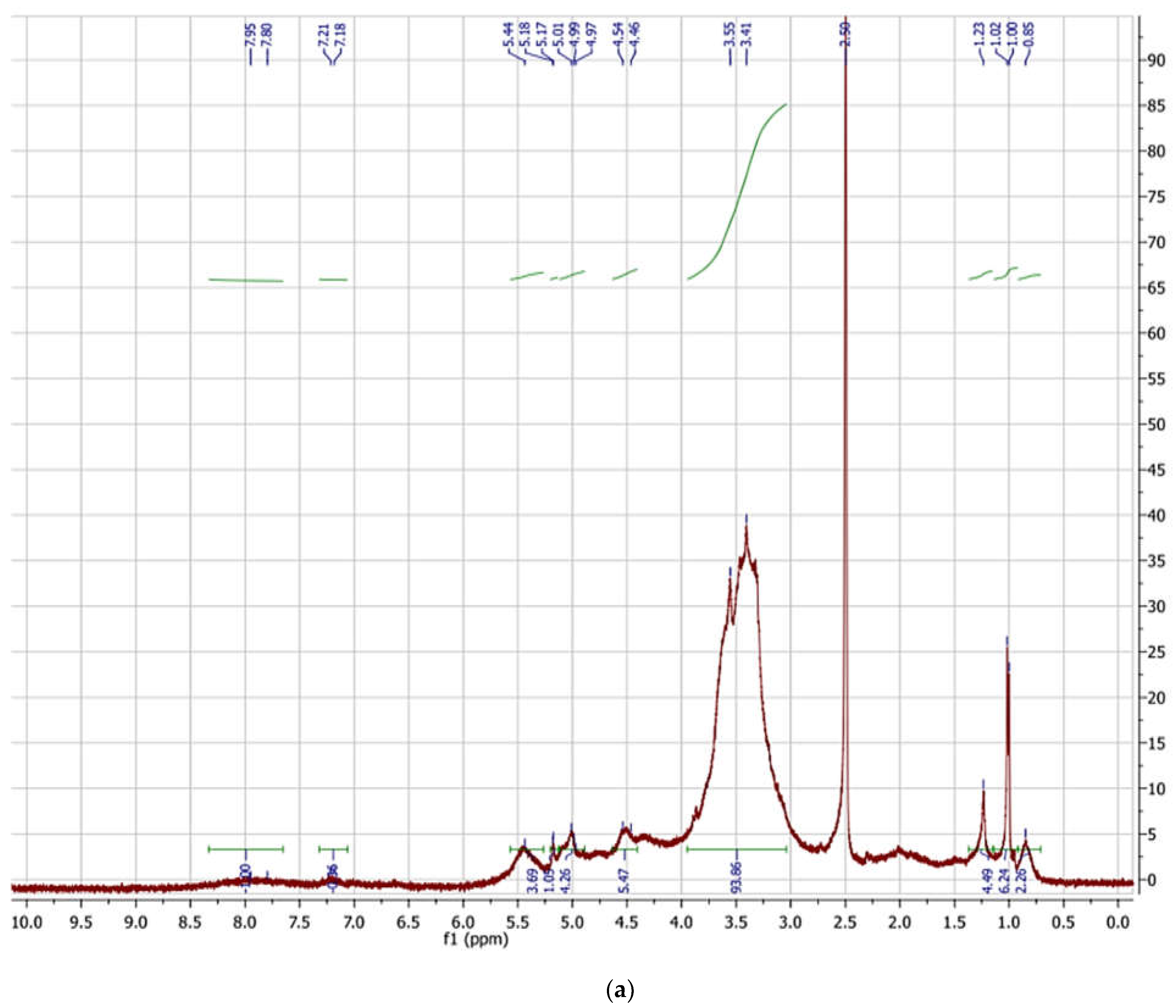
2.8. FT-IR and 1H NMR Spectroscopy
2.9. Antioxidant Capacity of the EPSc, EPSF1 and EPSF2
2.9.1. Radical Scavenging by DPPH
2.9.2. ABTS Assay for Antioxidant Activity Evaluation
2.9.3. Hydroxyl Radical Scavenging (HRS) Activity
2.9.4. Superoxide Scavenging Activity
2.9.5. Antioxidant Power Determination
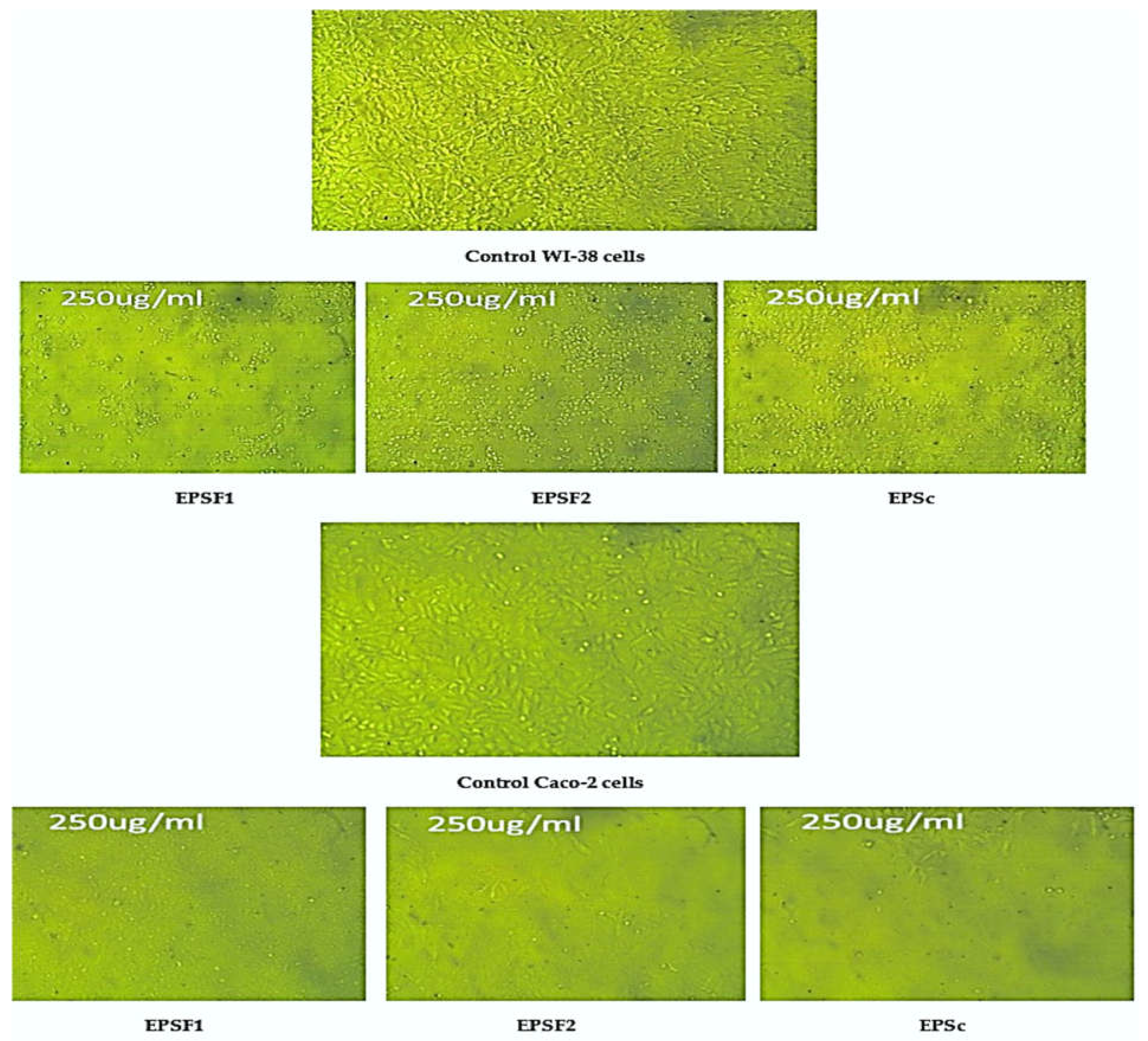
2.10. Cytotoxicity and Anticancer Activity of EPSc, EPSF1 and EPSF2
Determination of IC50 of EPSc, EPSF1 and EPSF2
2.11. In Vitro Anti-Inflammatory Assay (Hypotonicity-Induced Hemolysis)
2.12. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Purification of L. plantarum
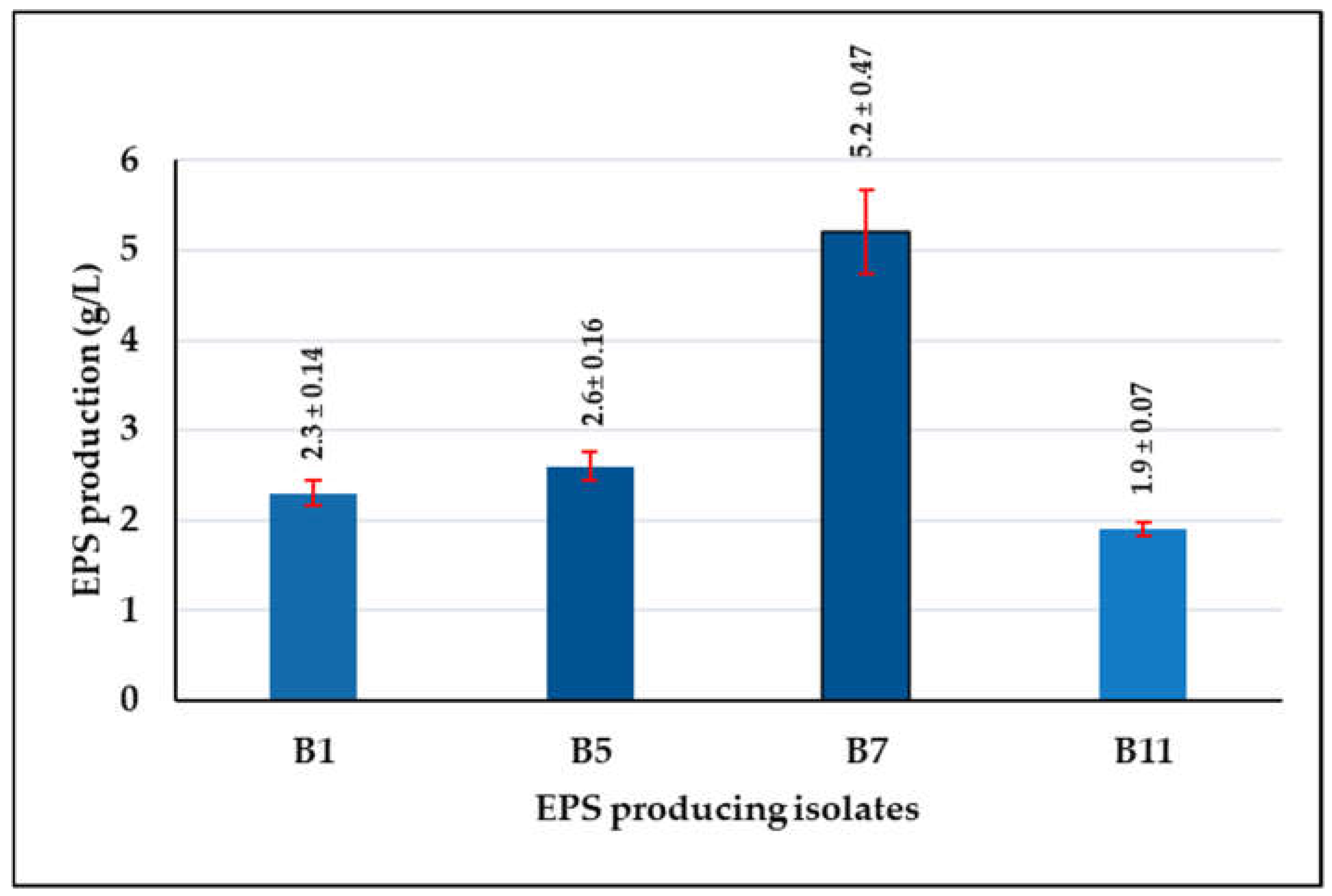
3.1.1. Screening of L. plantarum Isolates for EPS Production
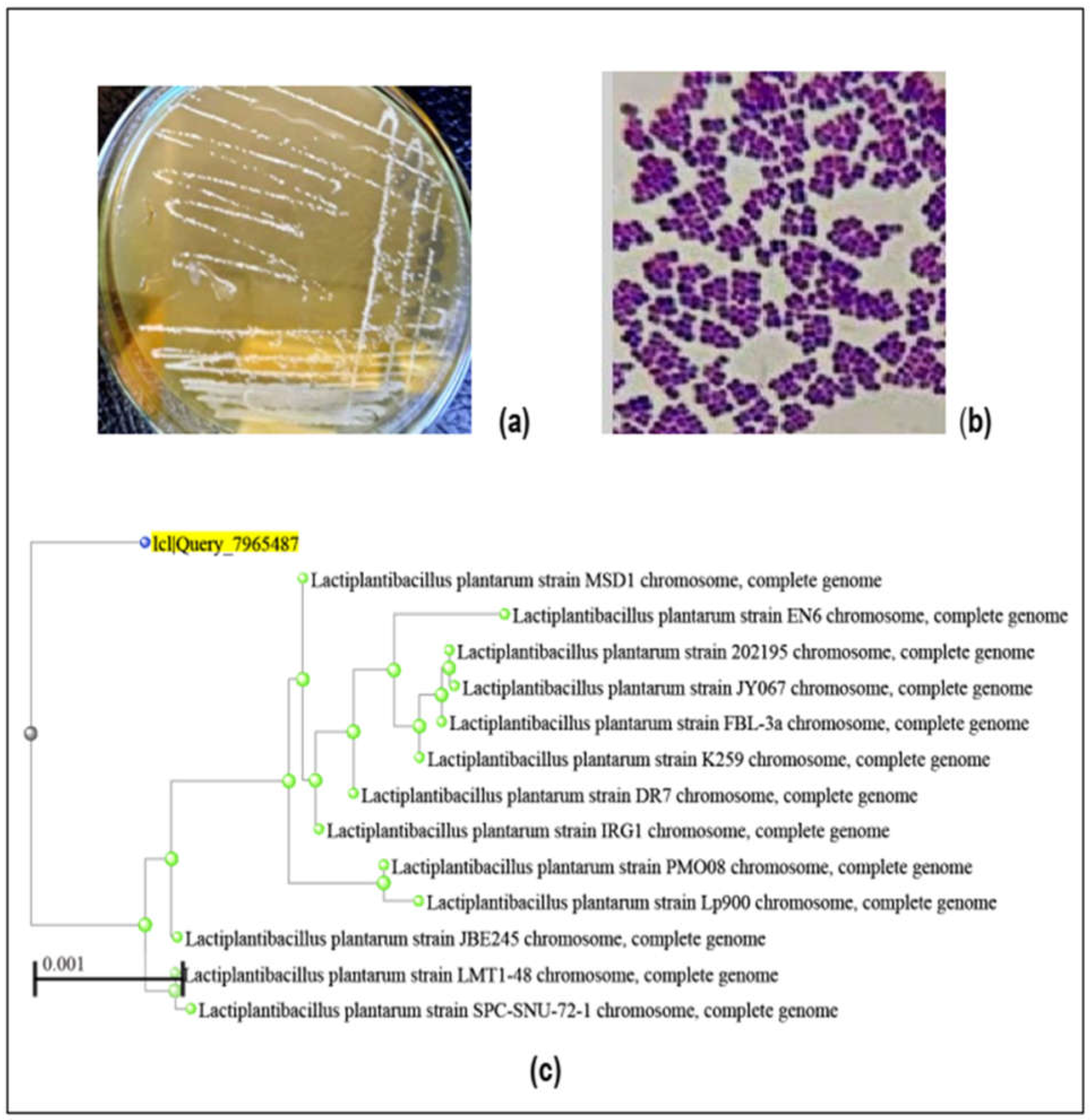
3.1.2. Morphological Characters and Molecular Identification of L. plantarum B7
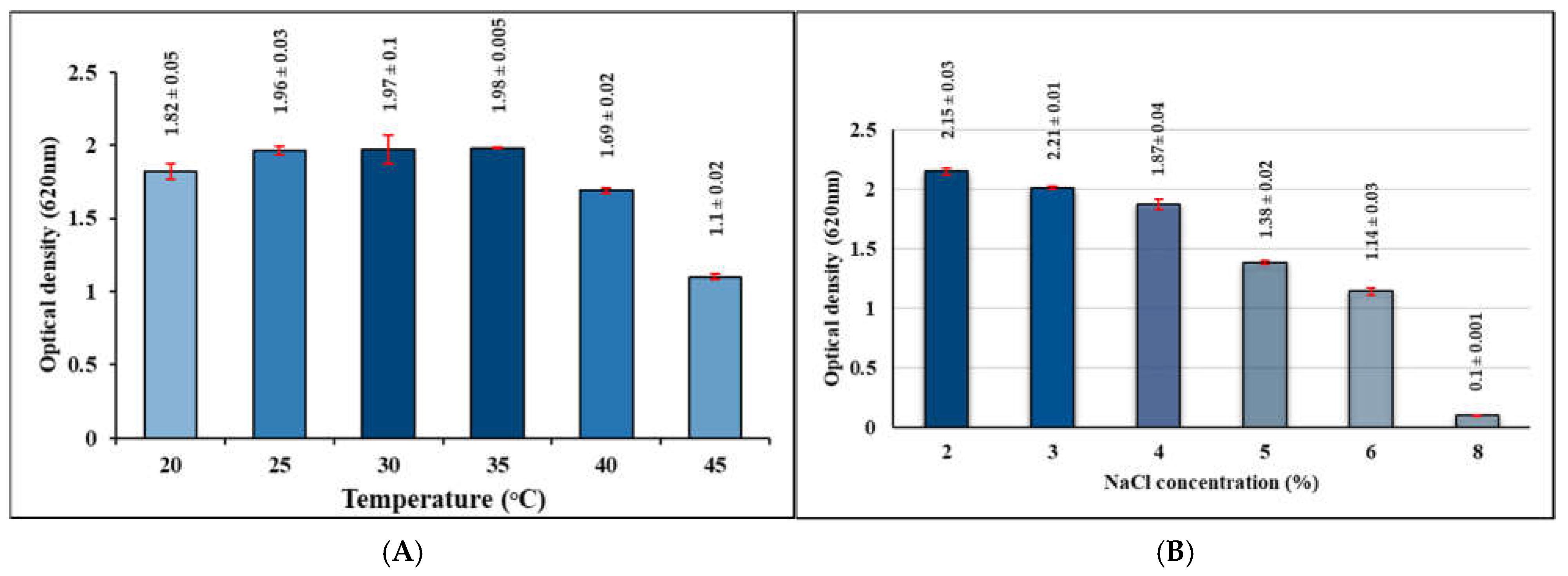
3.1.3. Characteristics of L. plantarum B7
3.2. Probiotic Characteristics of L. plantarum B7
3.2.1. Low pH and High Bile Salt Tolerance
3.2.2. Cell Surface Properties
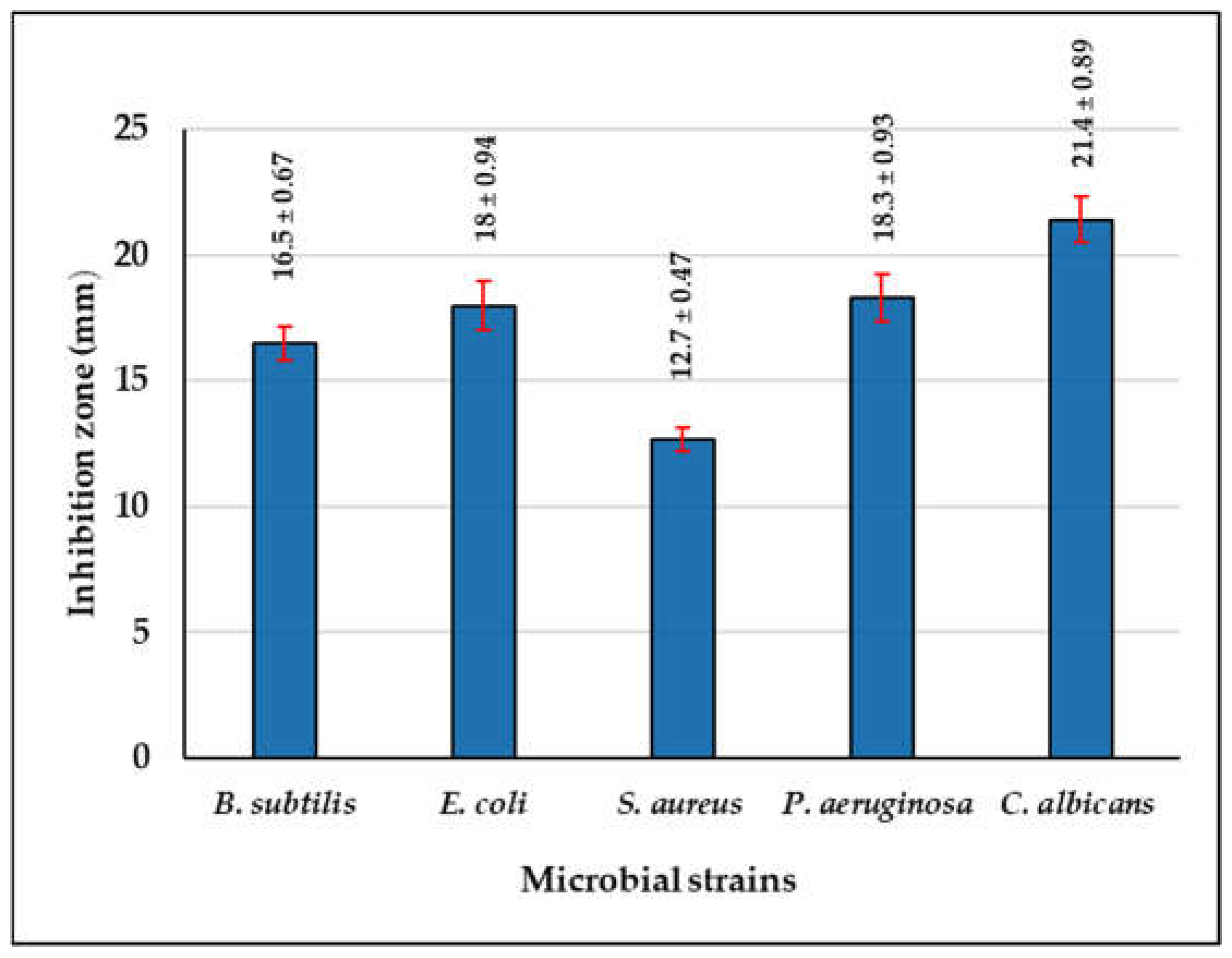
3.3. Antimicrobial Potential of L. plantarum B7
3.4. Safety Aspects of L. plantarum B7
3.4.1. Hemolytic Properties of L. plantarum B7
3.4.2. Antibiotic Susceptibility of L. plantarum B7
3.5. EPS Production and Isolation
3.6. Purification and Characterization of EPSc
3.7. Monosaccharide Composition and MW Determination of EPSF1 and EPSF2
3.8. FT-IR and 1HNMR Spectroscopy of EPSF1 and EPSF2
3.9. Antioxidant Activity of EPSc, EPSF1 and EPSF2
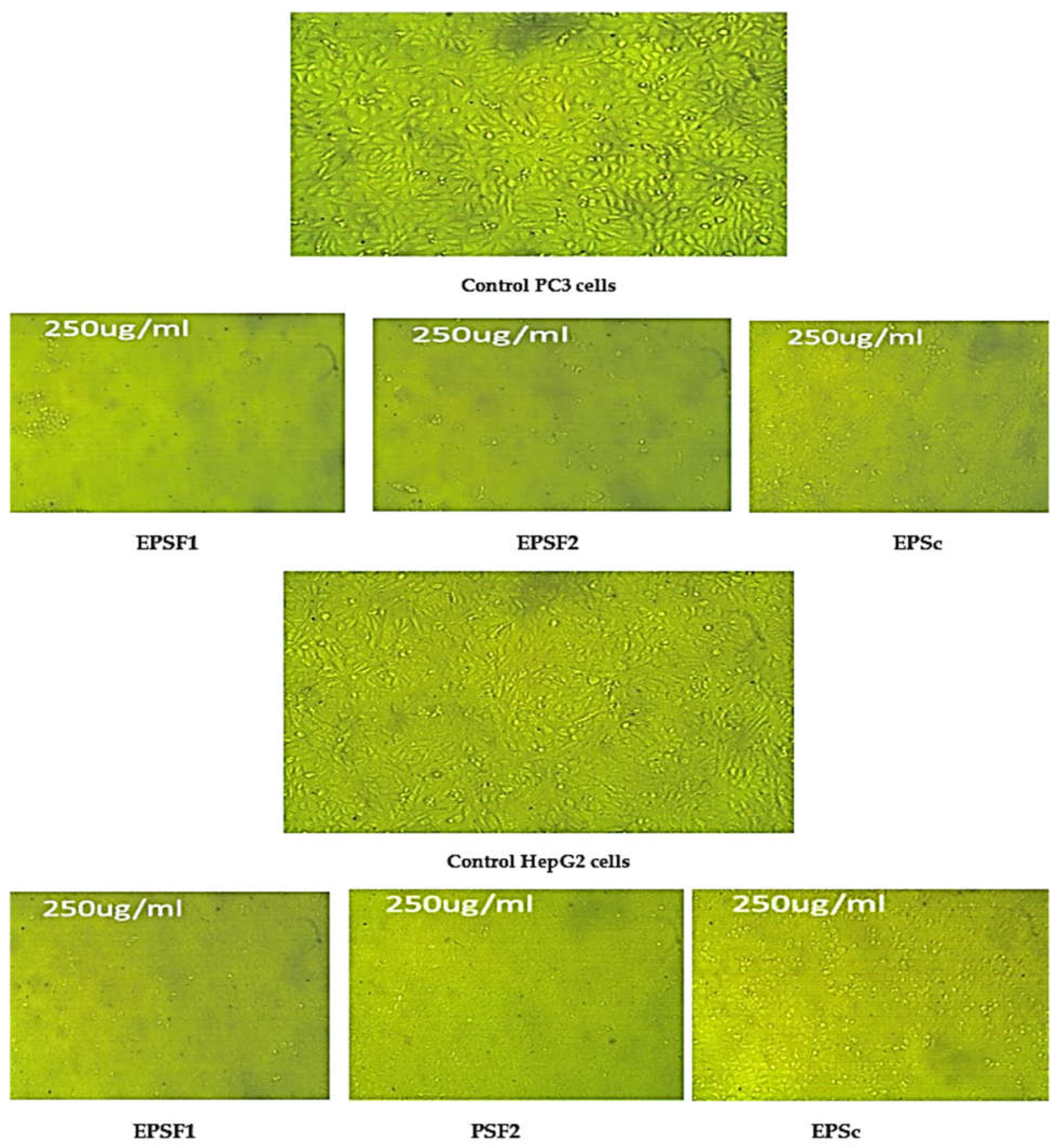
3.10. Cytotoxicity and Antitumor Activity
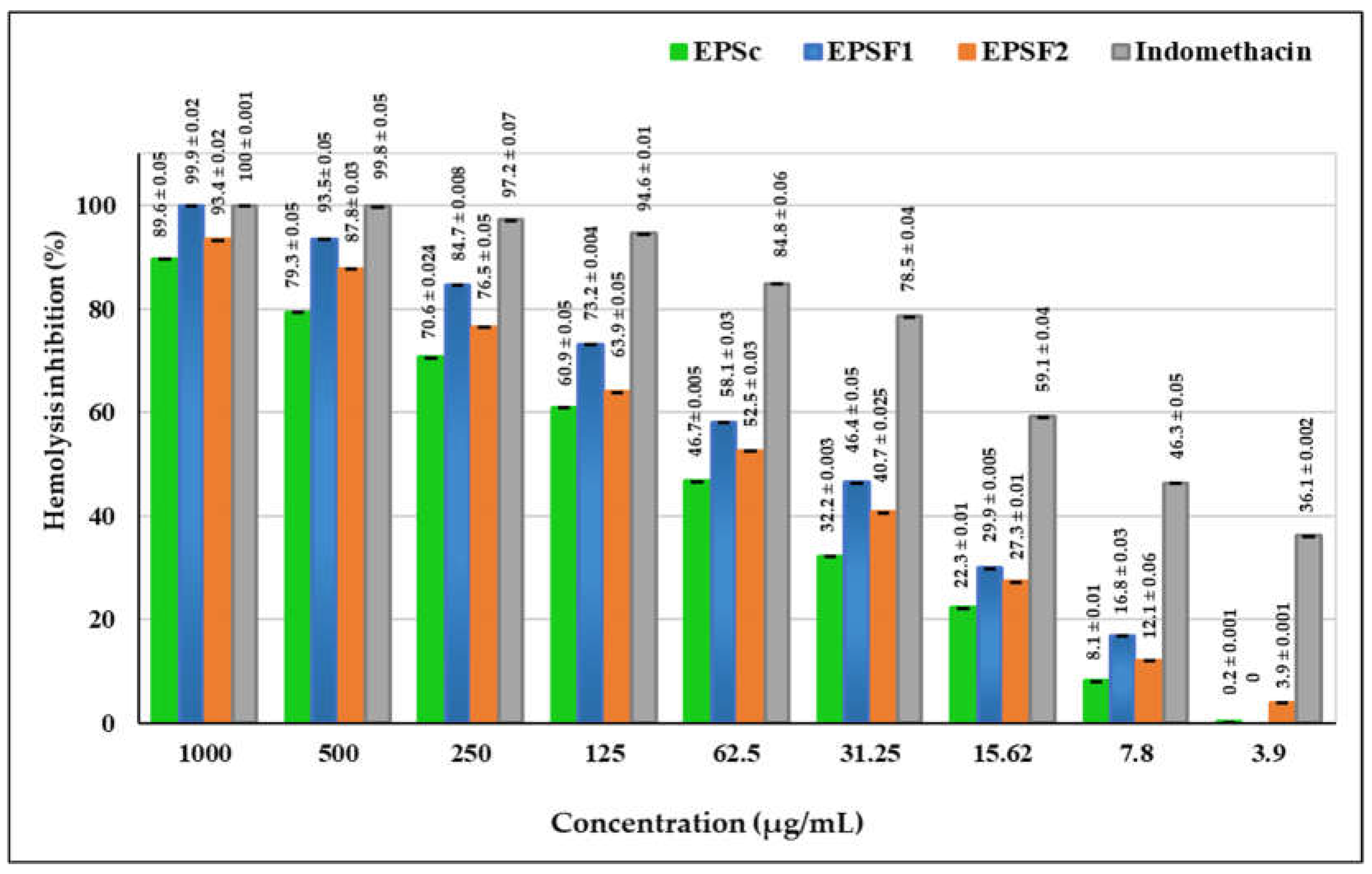
3.11. In Vitro Anti-Inflammatory Activity of EPSc, EPSF1 and EPSF2 (Hemolysis Inhibition)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.S.; Emon, D.D.; Toma, M.A.; Nupur, A.H.; Karmoker, P.; Iqbal, A. Recent advances in probiotication of fruit and vegetable juices. J. Adv. Vet. Anim. Res. 2023, 10, 522–537. [Google Scholar] [PubMed]
- Sharifi-Rad, J.; Rodrigues, C.F.; Stojanović-Radić, Z.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Salehi, B.; Milton Prabu, S.; et al. Probiotics: Versatile Bioactive Components in Promoting Human Health. Medicina 2020, 56, 433. [Google Scholar] [CrossRef] [PubMed]
- Khushboo, K.A.; Malik, T. Characterization and selection of probiotic lactic acid bacteria from different dietary sources for development of functional foods. Front. Microbiol. 2023, 14, 1170725. [Google Scholar]
- Ren, D.; Li, C.; Qin, Y.; Yin, R.; Du, S.; Ye, F.; Liu, C.; Liu, H.; Wang, M.; Li, Y.; et al. In vitro evaluation of the probiotic and functional potential of Lactobacillus strains isolated from fermented food and human intestine. Anaerobe 2014, 30, 1–10. [Google Scholar] [PubMed]
- Saha, S.; Alam, N.N.; Rahman, S.M.N. The Role of Probiotic Supplementation on Insulin Resistance in Obesity-Associated Diabetes: A Mini-Review. Biomedicine 2022, 42, 651–656. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.; Yang, Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 2015, 74, 119–126. [Google Scholar] [CrossRef]
- Bernal, P.; Llamas, M.A. Promising biotechnological applications of antibiofilm exopolysaccharides. Microb. Biotechnol. 2012, 5, 670–673. [Google Scholar]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar]
- El-Deeb, N.M.; Yassin, A.M.; Al-Madboly, L.A.; El-Hawiet, A. A novel purified Lactobacillus acidophilus 20079 exopolysaccharide, LA-EPS-20079, molecularly regulates both apoptotic and NF-ΚB inflammatory pathways in human colon cancer. Microb. Cell Factories 2018, 17, 29. [Google Scholar] [CrossRef]
- Di, W.; Zhang, L.; Yi, H.; Han, X.; Zhang, Y.; Xin, L. Exopolysaccharides produced by Lactobacillus strains suppress HT-29 cell growth via induction of G0/G1 cell cycle arrest and apoptosis. Oncol. Lett. 2018, 16, 3577–3586. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, G.; Zhao, F.; Zhou, L.; Huang, S.; Li, H. The antioxidant activities of six (1→3)-β-d-glucan derivatives prepared from yeast cell wall. Int. J. Biol. Macromol. 2017, 98, 216–221. [Google Scholar] [PubMed]
- Shahid, M.R.R.; Mehwish, M.M.; Kitazawa, H.; Barba, F.J.; Berthelot, L.; Umair, M.; Zhu, Q.; He, Z.; Zhao, L. Techno-functional properties and immunomodulatory potential of exopolysaccharide from Lactiplantibacillus plantarum MM89 isolated from human breast milk. Food Chem. 2022, 377, 131954. [Google Scholar]
- Zou, S.; Zhang, X.; Yao, W.; Niu, Y.; Gao, X. Structure characterization and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Carbohydr. Polym. 2010, 80, 1161–1167. [Google Scholar]
- Abdel-Wahab, B.A.; Abd El-Kareem, H.F.; Al Zamami, A.; Fahmy, C.A.; Elesawy, B.H.; Mahmoud, M.M.; Ghareeb, A.; El Askary, A.; Abo Nahas, H.H.; Attallah, N.G.M.; et al. Novel Exopolysaccharide from Marine Bacillus subtilis with Broad Potential Biological Activities: Insights into Antioxidant, Anti-Inflammatory, Cytotoxicity, and Anti-Alzheimer Activity. Metabolites 2022, 12, 715. [Google Scholar] [CrossRef]
- Tarannum, N.; Ali, F.; Khan, M.S.; Alhumaidan, O.S.; Zawad, A.N.M.; Hossain, T.J. Bioactive exopolysaccharide from Limosilactobacillus fermentum LAB-1: Antioxidant, anti-inflammatory, antibacterial and antibiofilm properties. Bioact. Carbohydr. Diet. Fibre 2024, 31, 100409. [Google Scholar]
- Oleksy, M.; Klewicka, E. Exopolysaccharides produced by Lactobacillus sp.: Biosynthesis and applications. Crit. Rev. Food Sci Nut. 2018, 58, 450–462. [Google Scholar]
- Liu, C.T.; Chu, F.J.; Chou, C.C.; Yu, R.C. Antiproliferative and anticytotoxic effects of cell fractions and exopolysaccharides from Lactobacillus casei 01. Mutat. Res. 2011, 721, 157–162. [Google Scholar]
- Liu, A.; Liu, D.; Zhao, S.; Zheng, J.; Cao, D.; Zhang, H. Up regulation of annexin A2 on murine H22 hepatocarcinoma cells induced by cartilage polysaccharide. Cancer Epidemiol. 2011, 35, 490–496. [Google Scholar]
- Reuben, R.; Roy, P.; Sarkar, S.; Alam, A.R.U.; Jahid, I. Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. J. Dairy Sci. 2020, 103, 1223–1237. [Google Scholar]
- Wang, Y.; Wang, S.X.; Song, R.Z.; Cai, J.J.; Xu, J.J.; Tang, X.Z.; Li, N. Ginger polysaccharides induced cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol. 2018, 123, 81–90. [Google Scholar]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 1, 853–865. [Google Scholar]
- Aziz, T.; Naveed, M.; Jabeen, K.; Shabbir, M.A.; Sarwar, A.; Zhennai, Y.; Alharbi, M.; Alshammari, A.; Alasmari, A.F. Integrated genome-based evaluation of safety and probiotic characteristics of Lactiplantibacillus plantarum YW11 isolated from Tibetan kefir. Front. Microbiol. 2023, 14, 1157615. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Naveed, M.; Sarwar, A.; Makhdoom, S.I.; Mughal, M.S.; Ali, U.; Yang, Z.; Shahzad, M.; Sameeh, M.Y.; Alruways, M.W.; et al. Functional Annotation of Lactiplantibacillus plantarum 13-3 as a Potential Starter Probiotic Involved in the Food Safety of Fermented Products. Molecules 2022, 27, 5399. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Naveed, M.; Shabbir, M.A.; Sarwar, A.; Naseeb, J.; Zhao, L.; Yang, Z.; Cui, H.; Lin, L.; Albekairi, T.H. Unveiling the whole genomic features and potential probiotic characteristics of novel Lactiplantibacillus plantarum HMX2. Front. Microbiol. 2024, 15, 1504625. [Google Scholar]
- Hossain, T.J. Functional genomics of the lactic acid bacterium Limosilactobacillus fermentum LAB-1: Metabolic, probiotic and biotechnological perspectives. Heliyon 2022, 8, e11412. [Google Scholar]
- Logan, N.A.; De, V.P.; Genus, I. Bacillus Cohn 1872, 174AL. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; De Vos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K.H., Whitman, W.B., Eds.; Springer: New York, NY, USA, 2009; Volume 3, pp. 21–128. [Google Scholar]
- Kanmani, R.; Kumar, S.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Production and purification of a novel exopolysaccharide from lactic acid bacterium Streptococcus phocae PI80 and its functional characteristics activity in vitro. Bioresour. Technol. 2011, 102, 74827–74833. [Google Scholar]
- Hu, S.M.; Zhou, J.M.; Zhou, Q.Q.; Ping, L.; Yuan-Yuan, X.; Tao, Z.; Qing, G. Purification, characterization and biological activities of exopolysaccharides from Lactobacillus rhamnosus ZFM231 isolated from milk. LWT 2021, 147, 111561. [Google Scholar]
- Zaghloul, E.H.; Ibrahim, M.I.A.N. Production and Characterization of Exopolysaccharide from Newly Isolated Marine Probiotic Lactiplantibacillus plantarum EI6 with in vitro Wound Healing Activity. Front. Microbiol. 2022, 13, 903363. [Google Scholar]
- Chen, Z.; Leng, X.; Zhou, F.; Shen, W.; Zhang, H.; Yu, Q.; Meng, X.; Fan, H.; Qin, M. Screening and identification of probiotic Lactobacilli from the infant gut microbiota to alleviate lead toxicity. Probiot. Antimicrob. Proteins 2022, 21, 9895. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Mulaw, G.; Sisay, T.T.; Muleta, D.; Tesfaye, A. In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian food products. Int. J. Microbiol. 2019, 2019, e7179514. [Google Scholar]
- Mogrovejo, D.C.; Perini, L.; Gostinčar, C.; Sepčić, K.; Turk, M.; Ambrožič-Avguštin, J.; Brill, F.H.H.; Gunde-Cimerman, N. Prevalence of Antimicrobial Resistance and Hemolytic Phenotypes in Culturable Arctic Bacteria. Front. Microbiol. 2020, 3, 570. [Google Scholar]
- Sadeghi, M.; Panahi, B.; Mazlumi, A.; Hejazi, M.A.; Komi, D.E.A.; Nami, Y. Screening of potential probiotic lactic acid bacteria with antimicrobial properties and selection of superior bacteria for application as biocontrol using machine learning models. LWT 2022, 162, 113471. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Sudhamani, S.R.; Tharanathan, R.N.; Prasad, M.S. Isolation and characterization of an extracellular polysaccharide from Pseudomonas caryophylli CFR 1705. Carbohydr. Polym. 2004, 56, 423–427. [Google Scholar]
- Adebayo-Tayo, B.; Fashogbon, R. In vitro antioxidant, antibacterial, in vivo immunomodulatory, antitumor and hematological potential of exopolysaccharide produced by wild type and mutant Lactobacillus delbureckii subsp. bulgaricus. Heliyon 2020, 6, e03268. [Google Scholar]
- Zheng, Z.M.; Huang, Q.L.; Ling, C.Q. Water-soluble yeast β-glucan fractions with different molecular weights: Extraction and separation by acidolysis assisted-size exclusion chromatography and their association with proliferative activity. Int. J. Biol. Macromol. 2019, 123, 269–279. [Google Scholar]
- Kogan, G.; Sandula, J.; Korolenko, T.A.; Falameeva, O.; Poteryaeva, O.; Zhanaeva, S.; Levina, O.; Filatova, T.; Kaledin, V. Increased efficiency of Lewis lung carcinoma chemotherapy with a macrophage stimulator-yeast carboxylmethylglucan. Int. Immunopharmacol. 2002, 2, 775–781. [Google Scholar]
- Wang, J.; Ji, H.F.; Wang, S.X.; Zhang, D.Y.; Liu, H.; Shan, D.C.; Wang, Y.M. Lactobacillus plantarum ZLP001: In vitro Assessment of Antioxidant Capacity and Effect on Growth Performance and Antioxidant Status in Weaning Piglets. Asian-Australas J. Anim. Sci. 2012, 25, 1153–1158. [Google Scholar]
- Zhang, S.; Liu, L.; Su, Y.; Li, H.; Sun, Q.; Liang, X.; Lv, J. Antioxidative activity of lactic acid bacteria in yogurt. Afr. J. Microbiol. Res. 2011, 5, 5194–5201. [Google Scholar]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophyticbacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2009, 78, 275–281. [Google Scholar]
- Abeer, A.A.; Sahera, F.M. New Carrageenan/2-Dimethyl Aminoethyl Methacrylate/Gelatin/ZnO Nanocomposite as a Localized Drug Delivery System with Synergistic Biomedical Applications. Processes 2024, 12, 2702. [Google Scholar] [CrossRef]
- Guérin, M.; Silva, C.R.; Garcia, C.; Remize, F. Lactic acid bacterial production of exopolysaccharides from fruit and vegetables and associated benefits. Fermentation 2020, 6, 115. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends. Food Sci. Technol. 2021, 110, 375–384. [Google Scholar]
- Sornplang, P.; Piyadeatsoontorn, S. Probiotic isolates from unconventional sources: A review. J. Anim. Sci. Technol. 2016, 58, 26. [Google Scholar]
- Khalil, E.S.; Abd Manap, M.Y.; Mustafa, S.; Alhelli, A.M.; Shokryazdan, P. Probiotic Properties of Exopolysaccharide-Producing Lactobacillus Strains Isolated from Tempoyak. Molecules 2018, 23, 398. [Google Scholar] [CrossRef]
- Aarti, C.; Khusro, A. Functional and technological properties of exopolysaccharide producing autochthonous Lactobacillus plantarum strain AAS3 from dry fish based fermented food. LWT 2019, 114, 108387. [Google Scholar]
- Tarique, M.; Abdalla, A.; Masad, R.; Al Sbiei, A.; Kizhakkayi, J.; Osaili, T.; Olaimat, A.; Liu, S.Q.; Cabezudo, F.M.; al-Ramadi, B.; et al. Potential probiotics and postbiotic characteristics including immunomodulatory effects of lactic acid bacteria isolated from traditional yogurt-like products. LWT 2022, 159, 113207. [Google Scholar]
- Ayyash, M.; Abushelaibi, A.; AlMahadin, S.; Enan, M.; El-Tarabily, K.; Shah, N. In-vitro investigation into probiotic characterisation of Streptococcus and Enterococcus isolated from camel milk. LWT 2018, 87, 478–487. [Google Scholar]
- Yamada, T.; Shimizu, K.; Ogura, H.; Asahara, T.; Nomoto, K.; Yamakawa, K.; Hamasaki, T.; Nakahori, Y.; Ohnishi, M.; Kuwagata, Y.; et al. Rapid and Sustained Long-Term Decrease of Fecal Short-Chain Fatty Acids in Critically Ill Patients with Systemic Inflammatory Response Syndrome. JPEN J. Parenter. Enter. Nutr. 2015, 39, 569–577. [Google Scholar]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An overview of the functionalityof exopolysaccharides produced by lactic acid bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar]
- Čuljak, N.; Bellich, B.; Pedroni, A.; Butorac, K.; Pavunc, A.L.; Novak, J.; Banić, M.; Šušković, J.; Cescutti, P.; Kos, B. Limosilactobacillus fermentum strains MC1 and D12: Functional properties and exopolysaccharides characterization. Int. J. Biol. Macromol. 2024, 273, 133215. [Google Scholar]
- Dos, S.L.E.; Ginani, V.C.; de Alencar, E.R.; Pereira, O.G.; Rose, E.C.P.; do Vale, H.M.M.; Pratesi, R.; Hecht, M.M.; Cavalcanti, M.H.; Tavares, C.S.O. Isolation, identification, and screening of lactic acid bacteria with probiotic potential in silage of different species of forage plants, cocoa beans, and artisanal salami. Probiotics Antimicrob. Proteins 2021, 13, 173–186. [Google Scholar]
- De Souza, B.M.S.; Borgonovi, T.F.; Casarotti, S.N.; Todorov, S.D.; Penna, A.L.B. Lactobacillus casei and Lactobacillus fermentum strains isolated from mozzarella cheese: Probiotic potential, safety, acidifying kinetic parameters and viability under gastrointestinal tract conditions. Probiotics Antimicrob. Proteins 2019, 11, 382–396. [Google Scholar]
- Dertli, E.; Mayer, M.J.; Narbad, A. Impact of the exopolysaccharide layer on biofilms, adhesion and resistance to stress in Lactobacillus johnsonii FI9785. BMC Microbiol. 2015, 15, 8. [Google Scholar]
- Sivasankar, P.; Seedevi, P.; Poongodi, S.; Sivakumar, M.; Murugan, T.; Sivakumar, L.; Balasubramanian, T. Characterization, antimicrobial and antioxidant property of exopolysaccharide mediated silver nanoparticles synthesized by Streptomyces violaceus MM72. Carbohydr. Polym. 2018, 181, 752–759. [Google Scholar]
- Salachna, P.; Mizieliñska, M.; Soból, M. Exopolysaccharide gellan gum and derived oligo-gellan enhance growth and antimicrobial activity in Eucomis plants. Polymers 2018, 10, 42. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Wang, Y.; Bandara, H.; Mayer, M.P.A.; Samaranayake, L.P. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl. Microbiol. Biotechnol. 2016, 100, 6415–6426. [Google Scholar]
- Asadi, A.; Lohrasbi, V.; Abdi, M.; Mirkalantari, S.; Esghaei, M.; Kashanian, M. The probiotic properties and potential of vaginal Lactobacillus spp. isolated from healthy women against some vaginal pathogens. Lett. Appl. Microbiol. 2022, 74, 752–764. [Google Scholar]
- Onyibe, J.E.; Oluwole, O.B.; Ogunbanwo, S.T.; Sanni, A.I. Antibiotic Susceptibility Profile and Survival of Bifidobacterium adolescentis and Bifidobacterium catenulatum of Human and Avian Origin in Stored Yoghurt. Niger. Food 2013, 31, 73–83. [Google Scholar] [CrossRef]
- Huys, G.; D’Haene, K.; Swings, J. Genetic basis of tetracycline and minocycline resistance in potentially probiotic Lactobacillus plantarum strain CCUG 43738. Antimicrob. Agents Chemother. 2006, 50, 1550–1551. [Google Scholar]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The Spread of Antibiotic Resistance Genes In Vivo Model. Can. J. Infect. Dis. Med. Microbiol. 2022, 18, 3348695. [Google Scholar]
- Fraqueza, M.J. Antibiotic resistance of lactic acid bacteria isolated from dry-fermented sausages. Int. J. Food Microbiol. 2015, 212, 76–88. [Google Scholar] [CrossRef]
- Federic, S.; Ciarrocchi, F.; Campana, R.; Ciandrini, E.; Blasi, G.; Baffone, W. Identification and functional traits of lactic acid bacteria isolated from Ciauscolo salami produced in Central Italy. Meat Sci. 2014, 98, 575–584. [Google Scholar]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.M.; Mitra, S.; Emran, T.B. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar]
- Lerner, A.; Benzvi, C.; Vojdani, A. The Potential Harmful Effects of Genetically Engineered Microorganisms (GEMs) on the Intestinal Microbiome and Public Health. Microorganisms 2024, 12, 238. [Google Scholar] [CrossRef]
- Tarannum, N.; Hossain, T.J.; Ali, F.; Das, T.; Dhar, K.; Nafiz, I.H. Antioxidant, antimicrobial and emulsification properties of exopolysaccharides from lactic acid bacteria of bovine milk: Insights from biochemical and genomic analysis. LWT 2023, 186, 115263. [Google Scholar]
- Dwivedi, M. Exopolysaccharide (EPS) producing isolates from sugarcane field soil and antibacterial activity of extracted EPSs. Acta Sci. Microbiol. 2018, 1, 6–13. [Google Scholar]
- Santschi, P.H.; Xu, S.C.; Lin, K.A.; Sun, P.; Chin, L.; Kamalanathan, W.C.; Bacosa, M.P.; Quigg, A. Can the protein/ carbohydrate (P/C) ratio of exopolymeric substances (EPS) be used as a proxy for their ‘stickiness’ and aggregation propensity? Mar. Chem. 2020, 218, 103734. [Google Scholar]
- Li, W.; Ji, J.; Chen, X.; Jiang, M.; Rui, X.; Dong, M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014, 102, 351–359. [Google Scholar]
- Rajoka, M.S.R.; Mehwish, H.M.; Fang, H.; Padhiar, A.A.; Zeng, X.; Khurshid, M.; He, Z.; Zhao, L. Characterization and anti-tumor activity of exopolysaccharide produced by Lactobacillus kefiri isolated from Chinese kefir grains. J. Funct. Foods 2019, 63, 103588. [Google Scholar]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and ioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy Sci. 2017, 100, 6895–6905. [Google Scholar]
- Bamigbade, G.; Ali, A.H.; Subhash, A.; Tamiello-Rosa, C.; Al Qudsi, F.R.; Esposito, G.; Hamed, F.; Liu, S.Q.; Gan, R.Y.; Abu-Jdayil, B.; et al. Structural characterization, biofunctionality, and environmental factors impacting rheological properties of exopolysaccharide produced by probiotic Lactococcus lactis C15. Sci. Rep. 2023, 13, 17888. [Google Scholar] [CrossRef]
- Tallon, R.; Bressollier, P.; Urdaci, M.C. Isolation and characterization of two exopolysaccharides produced by Lactobacillus plantarum EP56. Res. Microbiol. 2003, 154, 705–712. [Google Scholar]
- Zhou, K.; Zeng, Y.; Yang, M.; Chen, S.; He, L.; Ao, X.; Zou, L.; Liu, S. Production, purification and structural study of an exopolysaccharide from Lactobacillus plantarum BC-25. Carbohydr. Polym. 2016, 144, 205–214. [Google Scholar]
- Ziadi, M.; Bouzaiene, T.; Sana, M.H.; Kaouther, Z.; Mokhtar, F.; Hamdi, M.; Boisset-Helbert, C. Evaluation of the Efficiency of Ethanol Precipitation and Ultrafiltration on the Purification and Characteristics of Exopolysaccharides Produced by Three Lactic Acid Bacteria. BioMed Res. Int. 2018, 2018, e1896240. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X.; Yang, Z.; Li, S. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Zhang, L.; Wang, Z.; Li, L. Structural characteristics and antioxidant properties of exopolysaccharides isolated from soybean protein gel induced by lactic acid bacteria. LWT 2021, 150, 111811. [Google Scholar]
- Feng, X.; Zhag, H.; Lai, P.F.; Xiong, Z.; Ai, L. Structure characterization of a pyruvated exopolysaccharide from Lactobacillus plantarum AR307. Int. J. Biol. Macromol. 2021, 178, 113–120. [Google Scholar]
- Khan, B.M.; Qiu, H.M.; Wang, X.F.; Liu, Z.Y.; Zhang, J.Y.; Guo, Y.J.; Chen, W.Z.; Liu, Y.; Cheong, K.L. Physicochemical characterization of Gracilaria chouae sulfated polysaccharides and their antioxidant potential. Int. J. Biol. Macromol. 2019, 134, 255–261. [Google Scholar]
- Cui, L.; Butler, H.J.; Martin-Hirsch, P.L.; Martin, F.L. Aluminium foil as a potential substrate for ATR-FTIR, transflection FTIR or Raman spectrochemical analysis of biological specimens. Anal. Methods 2016, 8, 463–690. [Google Scholar]
- Li, J.; Niu, D.; Zhang, Y.; Zeng, X.A. Physicochemical properties, antioxidant and antiproliferative activities of polysaccharides from Morinda citrifolia L. (Noni) based on different extraction methods. Int. J. Biol. Macromol. 2020, 150, 114–121. [Google Scholar]
- Min, W.H.; Fang, X.B.; Wu, T.; Fang, L.; Liu, C.L.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar]
- Amiri, S.; Mokarram, R.R.; Khiabani, M.S.; Bari, M.R.; Khaledabad, M.A. Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of fermentation variables and characterization of structure and bioactivities. Int. J. Biol. Macromol. 2019, 12, 3752–3765. [Google Scholar]
- Yuan, Q.; Zhang, J.; Xiao, C.; Harqin, C.; Ma, M.; Long, T.; Li, Z.; Yang, Y.; Liu, J.; Zhao, L. Structural Characterization of a Low-Molecular-Weight Polysaccharide from Angelica pubescens Maxim. F. Biserrata Shan Et Yuan Root and Evaluation of Its Antioxidant Activity. Carbohydr. Polym. 2020, 236, 116047. [Google Scholar]
- Yao, H.Y.Y.; Wang, J.Q.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. A Review of NMR Analysis in Polysaccharide Structure and Conformation: Progress, Challenge and Perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar]
- Du, R.; Yu, L.; Yu, N.; Ping, W.; Song, G.; Ge, J. Characterization of exopolysaccharide produced by Levilactobacillus brevis HDE-9 and evaluation of its potential use in dairy products. Int. J. Biol. Macromol. 2022, 217, 303–311. [Google Scholar]
- Du, R.; Yu, L.; Sun, M.; Ye, G.; Yang, Y.; Zhou, B.; Qian, Z.; Ling, H.; Ge, J. Characterization of Dextran Biosynthesized by Glucansucrase from Leuconostoc pseudomesenteroides and Their Potential Biotechnological Applications. Antioxidants 2023, 12, 275. [Google Scholar] [CrossRef]
- Do, T.B.T.; Tran, B.K.; Tran, T.V.T.; Le, T.H.; Cnockaert, M.; Vandamme, P.; Nguyen, T.H.C.; Nguyen, C.C.; Hong, S.H.; Kim, S.Y.; et al. Decoding the capability of Lactobacillus plantarum W1 isolated from soybean whey in producing an exopolysaccharide. ACS Omega 2020, 5, 33387–33394. [Google Scholar]
- Andrew, M.G. Jayaraman Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carboh. Res. 2020, 487, 107881. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar]
- Yang, H.; Deng, J.; Yuan, Y.; Fan, D.; Zhang, Y.; Zhang, R.; Han, B. Two novel exopolysaccharides from bacillus amyloliquefaciens C-1: Antioxidation and effect on oxidative stress. Curr. Microbiol. 2015, 70, 298–306. [Google Scholar]
- Lobo, R.E.; Gómez, M.I.; de Valdez, G.F.; Torino, M.I. Physicochemical and antioxidant properties of a gastro protective exopolysaccharide produced by Streptococcus thermophilus CRL1190. Food Hydrocoll. 2019, 96, 625–633. [Google Scholar]
- Sharma, P.; Sharma, A.; Lee, H.J. Antioxidant potential of exopolysaccharides from lactic acid bacteria: A comprehensive review. Int. J. Biol. Macromol. 2024, 281, 135536. [Google Scholar]
- Dai, Y.; Guo, Y.; Tang, W.; Chen, D.; Xue, L.; Chen, Y.; Guo, Y.; Wei, S.; Wu, M.; Dai, J.; et al. Reactive oxygen species-scavenging nanomaterials for the prevention and treatment of age-related diseases. J. Nanobiotechnol. 2024, 22, 252. [Google Scholar]
- Ali, A.H.; Bamigbade, G.; Tarique, M.; Esposito, G.; Obaid, R.; Abu-Jday, B.; Ayyash, M. Physicochemical, rheological, and bioactive properties of exopolysaccharide produced by a potential probiotic Enterococcus faecalis 84B. Int. J. Biol. Macromol. 2023, 240, 124425. [Google Scholar]
- Trabelsi, I.; Ktari, N.; Ben, S.S.; Triki, M.; Bardaa, S.; Mnif, H.; Ben, S.R. Evaluation of dermal wound healing activity and in vitro antibacterial and antioxidant activities of a new exopolysaccharide produced by Lactobacillus sp. Ca6. Int. J. Biol. Macromol. 2017, 103, 194–201. [Google Scholar]
- Tan, X.; Ma, B.; Wang, X.; Cui, F.; Li, X.; Li, J. Characterization of Exopolysaccharides from Lactiplantibacillus plantarum PC715 and Their Antibiofilm Activity Against Hafnia alvei. Microorganisms 2024, 12, 2229. [Google Scholar] [CrossRef]
- Tan, X.; Wang, X.; Cui, F.; Zeshan, A.; Wang, D.; Li, X.; Li, J. Effect of Enterococcus hirae GS22 Fermentation-Assisted Extraction on the Physicochemical and Bioactivities of Sea Cucumber Intestinal Polysaccharides. Molecules 2024, 29, 5800. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Nguyen, H.T. Antioxidant property and structural characteristics of exopolysaccharides derived from Lactiplantibacillus plantarum VAL6. Discov. Bact. 2024, 1, 6. [Google Scholar] [CrossRef]
- Wu, B.; Cui, J.; Zhang, C.; Li, Z. A polysaccharide from Agaricus blazei inhibits proliferation and promotes apoptosis of osteosarcoma cells. Int. J. Biol. Macromol. 2012, 50, 1116–1120. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Ye, L.; Wang, C. The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro: A review. Carbohydr. Polym. 2021, 253, 117308. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Khomeiri, M.; Saeidi, M. Anticancer potential of fermented milk with autochthonous lactic acid bacteria. J. Appl. Microbiol. 2023, 134, lxad041. [Google Scholar] [CrossRef]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci. Rep. 2019, 9, 8268. [Google Scholar] [CrossRef]
- Deepak, V.; Ramachandran, S.; Balahmar, R.M.; Pandian, S.R.; Sivasubramaniam, S.D.; Nellaiah, H.; Sundar, K. In vitro evaluation of anticancer properties of exopolysaccharides from Lactobacillus acidophilus in colon cancer cell lines. Vitr. Cell Dev. Biol. Anim. 2016, 52, 163–173. [Google Scholar] [CrossRef]
- Kawanabe-Matsuda, H.; Takeda, K.; Nakamura, M. Dietary Lactobacillus-derived exopolysaccharide enhances immune-checkpoint blockade therapy. Cancer Discov. 2022, 12, 1336–1355. [Google Scholar] [CrossRef]
- Tiwari, S.; Kavitake, D.; Devi, P.B.; Halady, S.P. Bacterial exopolysaccharides for improvement of technological, functional and rheological properties of yoghurt. Int. J. Biol. Macromol. 2021, 183, 1585–1595. [Google Scholar] [CrossRef]
- Zaidman, B.Z.; Yassin, M.; Mahajna, J.; Wasser, S.P. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl. Microbiol. Biotechnol. 2005, 67, 453–468. [Google Scholar] [CrossRef]
- Yousef, R.H.; Baothman, A.S.; Abdulaal, W.H.; Golayel, K.A.; Darwish, A.A.; Moselhy, S.S.; Ahmed, Y.M.; Hakeem, K.R. Potential antitumor activity of exopolysaccharide produced from date seed powder as a carbon source for Bacillus Subtilis. J. Microbiol. Methods 2020, 170, 105853. [Google Scholar] [CrossRef]
- Alshawwa, S.Z.; Alshallash, K.S.; Ghareeb, A.; Elazzazy, A.M.; Sharaf, M.; Alharthi, A.; Abdelgawad, F.E.; El-Hossary, D.; Jaremko, M.; Emwas, A.H. Assessment of Pharmacological Potential of Novel Exopolysaccharide Isolated from Marine Kocuria sp. Strain AG5: Broad-Spectrum Biological Investigations. Life 2022, 12, 1387. [Google Scholar] [CrossRef] [PubMed]
- Vinothkanna, A.; Sathiyanarayanan, G.; Rai, A.K.; Mathivanan, K.; Saravanan, K.; Sudharsan, K.; Kalimuthu, P.; Ma, Y.; Sekar, S. Exopolysaccharide Produced by Probiotic Bacillus albus DM-15 Isolated from Ayurvedic Fermented Dasamoolarishta: Characterization, Antioxidant, and Anticancer Activities. Front. Microbiol. 2022, 13, 832109. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ye, M.; Jing, L.; Du, Z.; Surhio, M.M.; Xu, H.; Li, J. Purification, characterization and promoting effect on wound healing of an exopolysaccharide from Lachnum YM405. Carbohydr. Polym. 2014, 105, 169–176. [Google Scholar] [PubMed]
- Tahmourespour, A.; Ahmadi, A.; Fesharaki, M. The anti-tumor activity of exopolysaccharides from Pseudomonas strains against HT-29 colorectal cancer cell line. Int. J. Biol. Macromol. 2020, 149, 1072–1076. [Google Scholar]
- Slïzewska, K.; Markowiak-Kopeć, P.; Ślïzewska, W. The Role of Probiotics in Cancer Prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar]
- Li, S.; Shah, N.P. Characterization, Anti-Inflammatory and Antiproliferative Activities of Natural and Sulfonated Exopolysaccharides from Streptococcus thermophilus ASCC 1275. J. Food Sci. 2016, 81, 1167–1176. [Google Scholar]
- Anosike, C.A.; Obidoa, O.; Ezeanyika, L.U. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). DARU J. Pharm. Sci. 2012, 20, 76. [Google Scholar]
- Fehervari, Z.; Philips, J.M.; Dunne, D.W.; Cooke, A. Parasitic worms and inflammatory disease. Parasite Immunol. 2005, 28, 515–523. [Google Scholar]
- Cunha, T.M.; Verri, W.A.; Schivo, I.R.; Napimoga, M.H.; Parada, C.A.; Poole, S.; Teixeira, M.M.; Ferreira, S.H.; Cunha, F.Q. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J. Leukocyte Biol. 2008, 83, 824–832. [Google Scholar]
- Cunha, T.M.; Verri, W.A.; Poole, S.; Parada, C.A.; Cunha, F.Q.; Ferreira, S.H. Pain Facilitation by Proinflammatory Cytokine Actions at Peripheral Nerve Terminals; Immune and Glial Regulation of Pain; Sorkin, L., DeLeo, J., Watkins, L.R., Eds.; IASP: Seattle, WA, USA, 2007; pp. 67–83. [Google Scholar]
- Mounnissamy, V.M.; Kavimani, S.; Balu, V.; Drlin, Q.S. Evaluation of anti-inflammatory and membrane stabilizing properties of ethanol extract of Canjera rehedi. Iranian J. Pharmacol. Therapeut. 2008, 6, 235–237. [Google Scholar]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Oweyele, B.; Oloriegbe, Y.Y.; Balaogun, E.A.; Soladoye, A.O. Analgesis and anti-inflammatory properties of Nelsonia Canescens leaf extract. J. Ethnopharmacol. 2005, 99, 153–156. [Google Scholar]
- Metowogo, K.; Agbonon, A.; Eklu-Gadegbeku, K.; Aklikokou, A.K.; Gbeassor, M. Anti-ulcer and anti-inflammtory effects of Hydro-alcohol extract of Aloe buettneri A. Berger (Lilliaceae). Trop. J. Pharmaceut. Res. 2008, 7, 907–912. [Google Scholar]
- Ng, T.B.; Liu, F.; Wang, Z. Antioxidative activity of natural products from plants. Life Sci. 2000, 66, 709–723. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Holt, R.R.; Lazarus, S.A.; Orozco, T.J.; Keen, C.L. Inhibitory effects of cocoa flavanols and procyanidin oligomers on free radical-induced erythrocyte hemolysis. Exp. Biol. Med. 2002, 227, 321–329. [Google Scholar]
- Saengkhae, C.; Arunnopparat, W.; Sungkhajorn, P. Antioxidative activity of the leaf of Nelumbo nucifera Gaertn. on oxidative stress-induced erythrocyte hemolysis in hypertensive and normotensive rats. Thai. J. Physiol. Sci. 2007, 20, 70–78. [Google Scholar]
- Ajila, C.M.; Prasada, U. Protection against hydrogen peroxide induced oxidative damage in rat erythrocytes by Mangifera indica L. peel extract. Food Chem. Toxicol. 2008, 46, 303–309. [Google Scholar]





| Time (h) | pH (3) | Bile Salt (0.3%) |
|---|---|---|
| Survival rate (%) | ||
| 1 | 98.56 ± 2.65 | 99.25 ± 3.15 |
| 2 | 97.83 ± 1.76 | 98.11 ± 2.35 |
| 3 | 95.82 ± 1.37 | 97.66 ± 1.85 |
| 4 | 79.12 ± 1.71 | 97.23 ± 1.78 |
| 5 | 47.36 ± 1.63 | 81.35 ± 1.65 |
| Antibiotic | Concentration (µg) | Inhibition Zone (mm) | Sensitivity |
|---|---|---|---|
| Streptomycin | 10 | 0.0 | R |
| Fusidic acid | 10 | 10.8 ± 0.65 | L |
| Tetracycline | 30 | 8.3 ± 0.37 | L |
| Chloramphenicol | 30 | 18.6 ± 0.74 | H |
| Penicillin | 10 | 23.5 ± 1.33 | H |
| Aztreonam | 30 | 0.0 | R |
| Imipenem | 10 | 25.7 ± 1.65 | H |
| Ciprofloxacin | 5 | 9.7 ± 0.35 | L |
| Norfloxacin | 10 | 0.0 | R |
| Ofloxacin | 5 | 8.4 ± 0.41 | L |
| Clindamycin | 2 | 6.7 ± 0.47 | L |
| EPS | Monosaccharide Composition | Molar Ratio | MW (Da) | Mn (Da) | PI (Mw/Mn) |
|---|---|---|---|---|---|
| EPSF1 | manuronic acid/mannose/glucose | 2.76:2.15:1.00 | 4.36 × 104 | 3.51 × 104 | 1.24 |
| EPSF2 | manuronic acid/mannose/glucose | 3.92:2.65:1.00 | 5.27 × 105 | 3.18 × 105 | 1.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ageeli, A.A.; Mohamed, S.F. Extraction, Purification and Characterization of Exopolysaccharide from Lactiplantibacillus plantarum B7 with Potential Antioxidant, Antitumor and Anti-Inflammatory Activities. Processes 2025, 13, 935. https://doi.org/10.3390/pr13040935
Ageeli AA, Mohamed SF. Extraction, Purification and Characterization of Exopolysaccharide from Lactiplantibacillus plantarum B7 with Potential Antioxidant, Antitumor and Anti-Inflammatory Activities. Processes. 2025; 13(4):935. https://doi.org/10.3390/pr13040935
Chicago/Turabian StyleAgeeli, Abeer A., and Sahera F. Mohamed. 2025. "Extraction, Purification and Characterization of Exopolysaccharide from Lactiplantibacillus plantarum B7 with Potential Antioxidant, Antitumor and Anti-Inflammatory Activities" Processes 13, no. 4: 935. https://doi.org/10.3390/pr13040935
APA StyleAgeeli, A. A., & Mohamed, S. F. (2025). Extraction, Purification and Characterization of Exopolysaccharide from Lactiplantibacillus plantarum B7 with Potential Antioxidant, Antitumor and Anti-Inflammatory Activities. Processes, 13(4), 935. https://doi.org/10.3390/pr13040935






