Effects of Light Quality Adjustment in Microalgal Cultivation: Flashing Light and Wavelength Shifts in Photobioreactor Design
Abstract
1. Introduction
2. Photosynthesis in Microalgae: Mechanisms and Energy Conversion Pathways
2.1. Properties of Light and Its Role in Photosynthesis

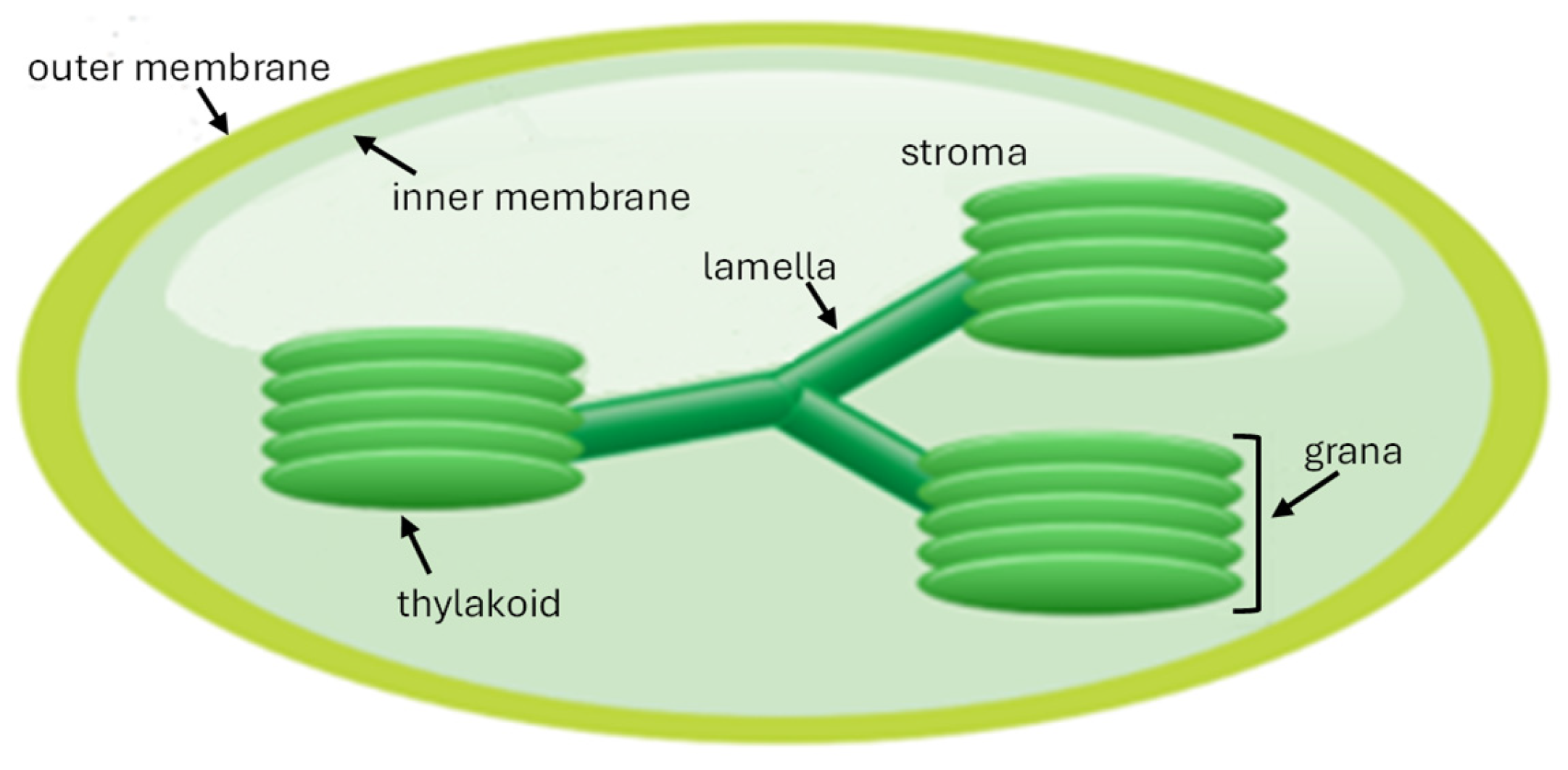
2.2. Mechanism of Photosynthesis in Microalgae
2.2.1. Overview
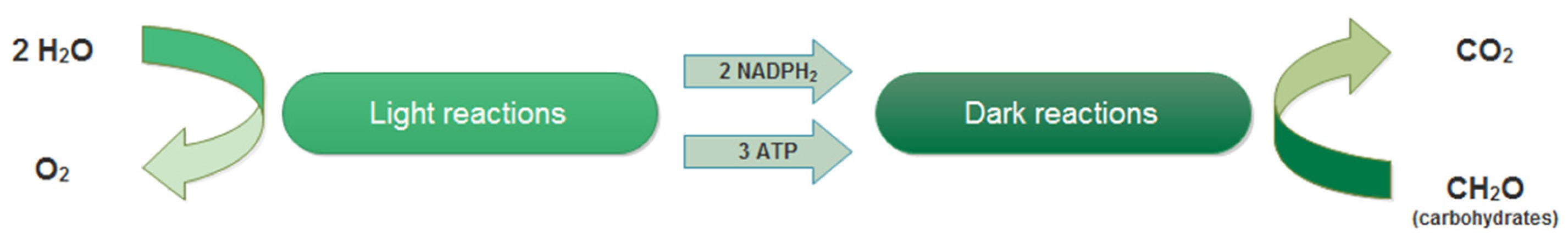
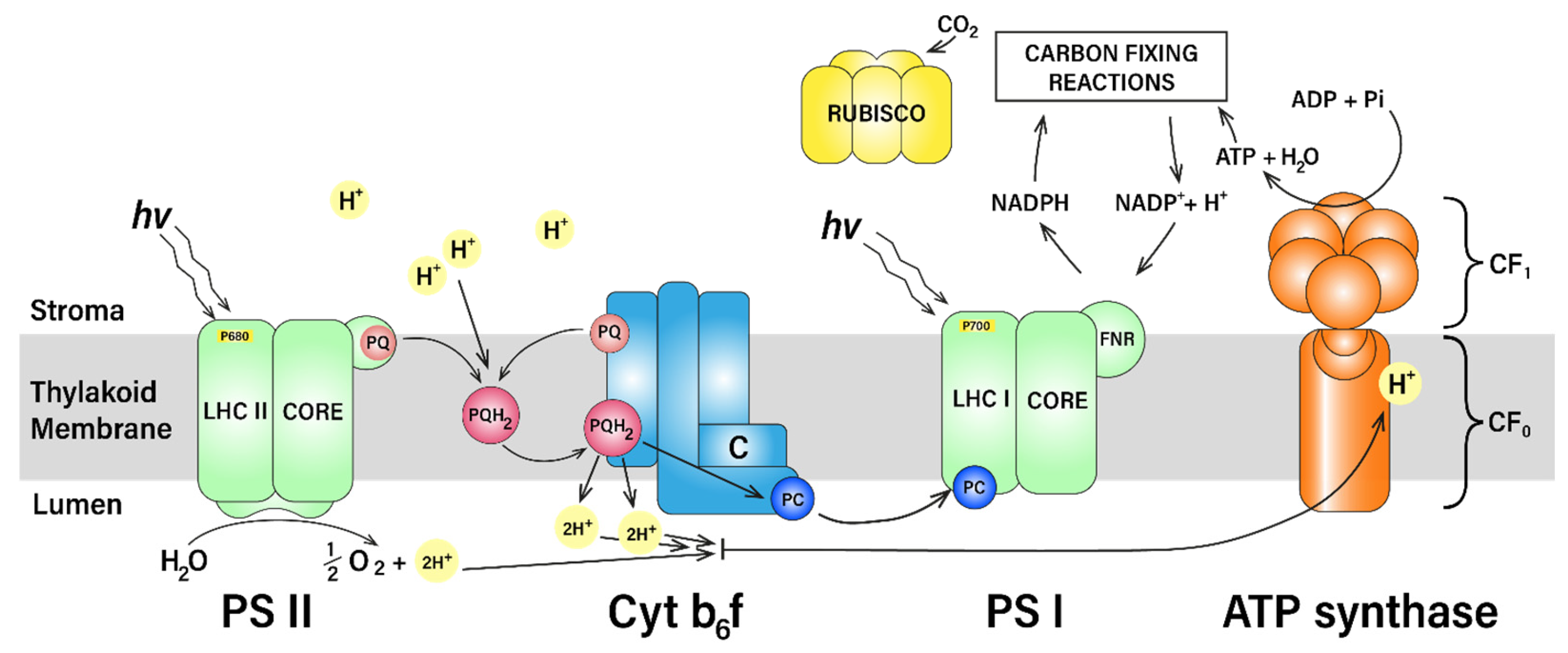
2.2.2. Light Reactions: Photochemical Process
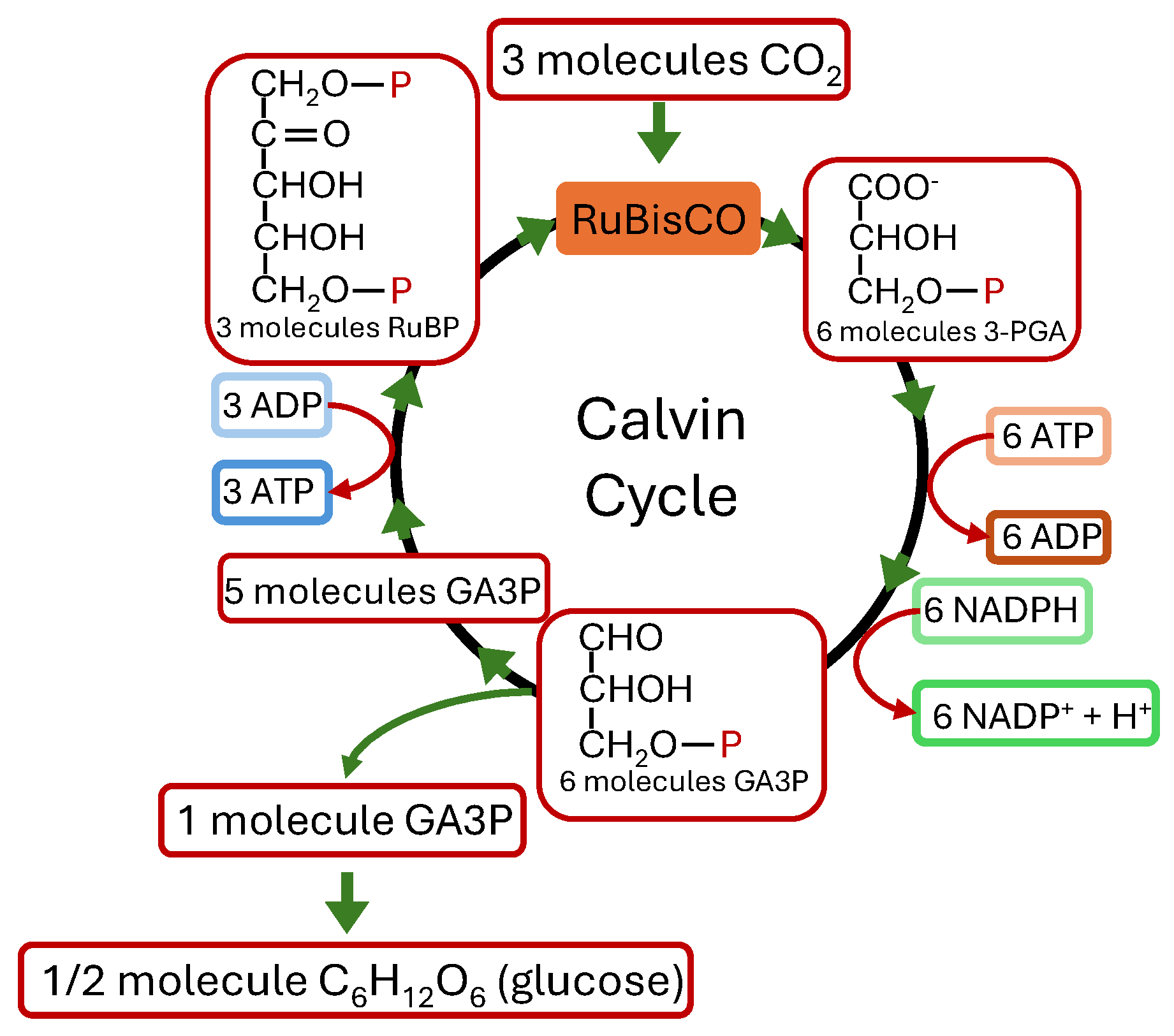
2.2.3. Dark Reactions: Carbon Fixation
- The carboxylation phase, where CO2 is inserted to the 5-carbon sugar ribulose bisphosphate (ribulose-bis-P) to form phosphoglycerate (glycerate-P). This reaction is catalyzed by the enzyme ribulose bisphosphate carboxylase/oxygenase (Rubisco);
- The reduction phase, where the phosphoglycerate is converted to 3-carbon products by its phosphorylation (involving ATP) and the subsequent reduction (involving NADPH2);
- The regeneration phase, where ribulose phosphate is regenerated for further CO2 fixation;
- The production phase, in which carbohydrates but also fatty acids, amino acids and organic acids are produced.
2.2.4. Alternative Sinks for Electrons
2.2.5. Plastoquinone Oxidation State as a Regulation of Photosynthesis
2.2.6. Regulation Through CO2 Availability
3. Light Quality Adjustments for Enhanced Photosynthesis
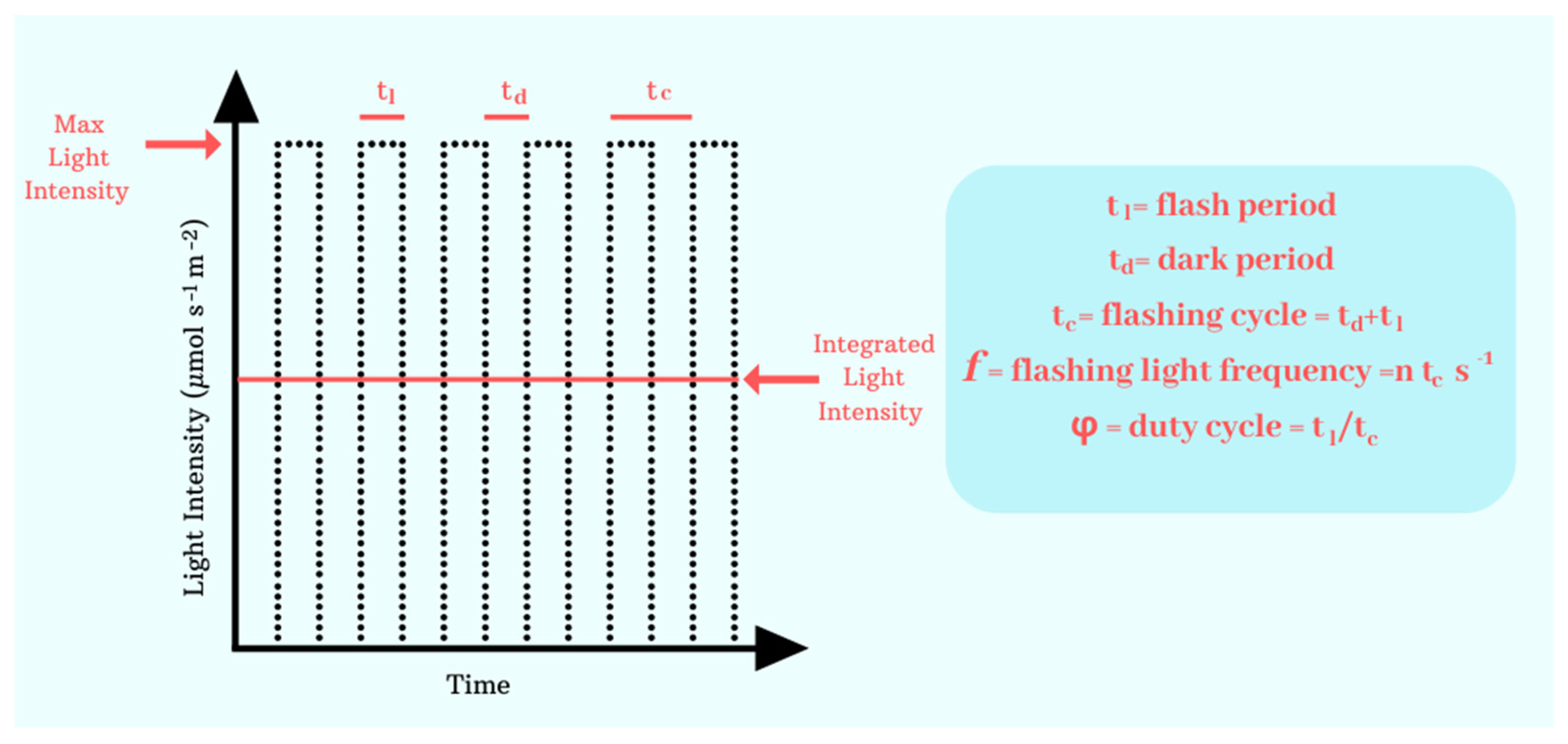
3.1. Application of Flashing Lights
3.2. Tailoring the Wavelength
3.2.1. Green Algae
3.2.2. Diatoms
3.2.3. Red Algae
3.2.4. Cyanobacteria
4. Photobioreactor Design for Optimized Light Utilization
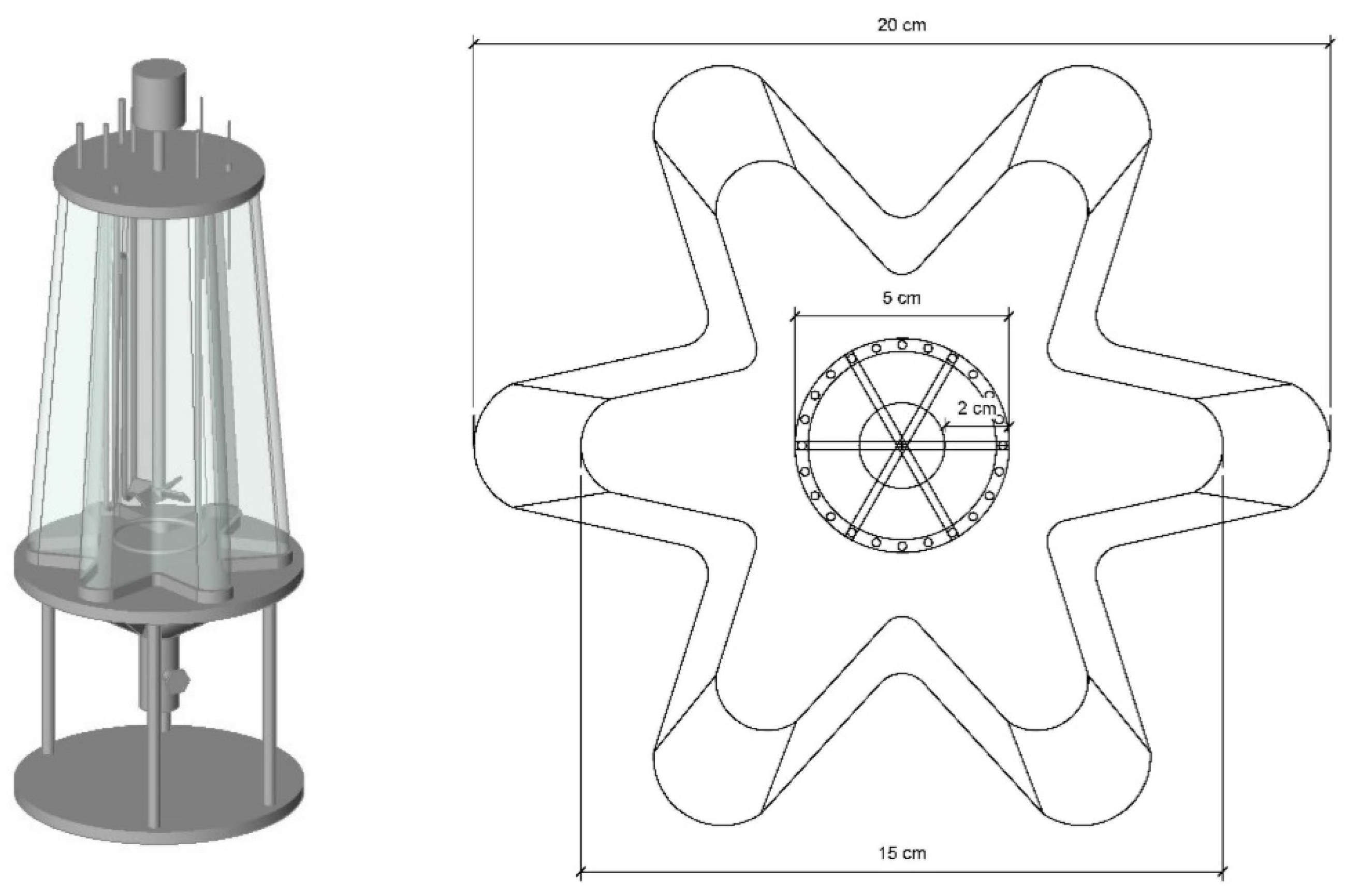
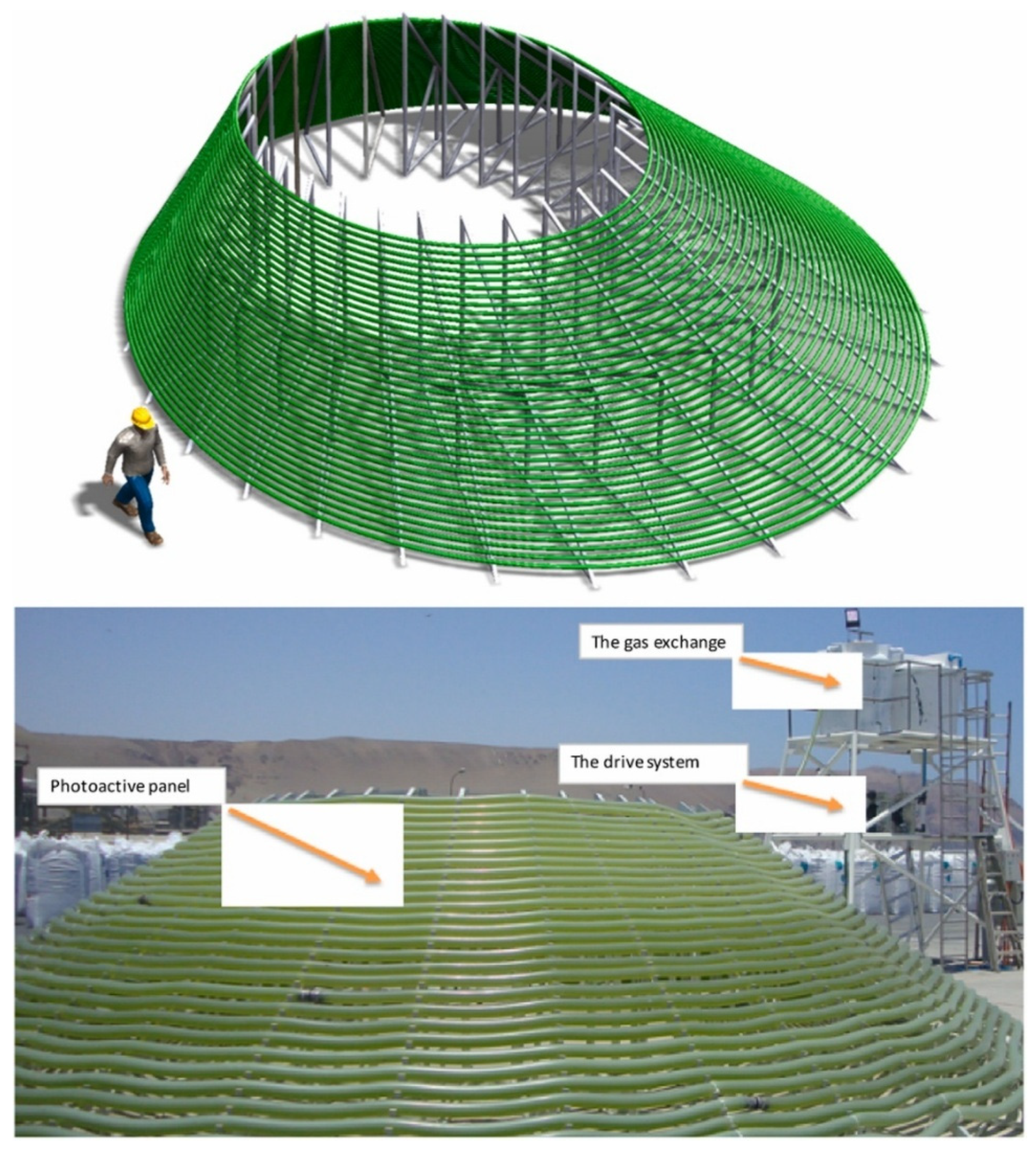
4.1. Strategies to Minimize Light Gradients
4.2. Implementing Effective Dark/Light Cycles
4.3. Optimizing Light Spectrum Through the Application of Filters in Photobioreactors
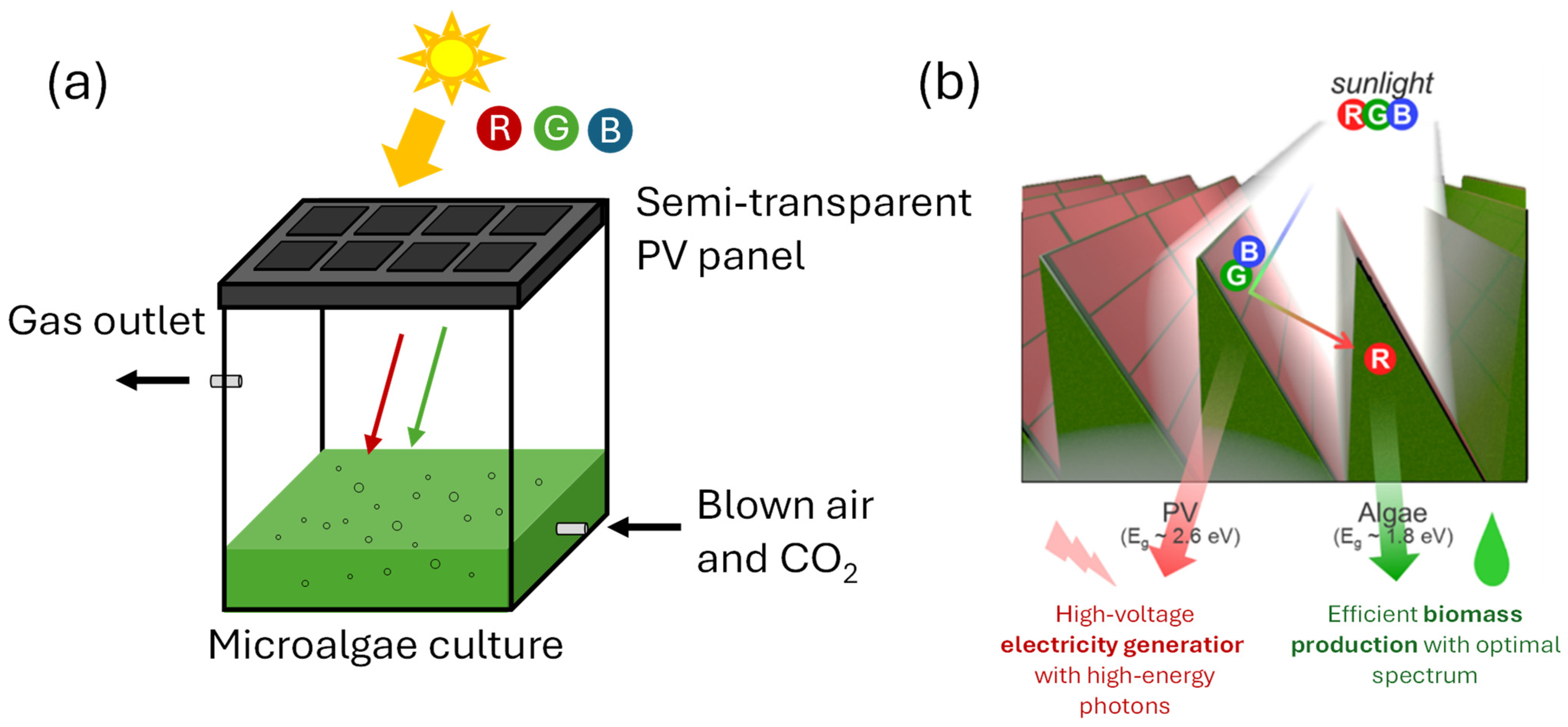
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hemaiswarya, S.; Raja, R.; Ravikumar, R.; Carvalho, I.S. Microalgae Taxonomy and Breeding. In Biofuel Crops: Production, Physiology and Genetics; CABI: Wallingford, UK, 2013; pp. 44–53. [Google Scholar] [CrossRef]
- Pruvost, J.; Cornet, J.-F.; Pilon, L. Large-Scale Production of Algal Biomass: Photobioreactors. In Algae Biotechnology: Products and Processes; Bux, F., Chisti, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 41–66. [Google Scholar]
- Legrand, J.; Artu, A.; Pruvost, J. A Review on Photobioreactor Design and Modelling for Microalgae Production. React. Chem. Eng. 2021, 6, 1134–1151. [Google Scholar] [CrossRef]
- Luzi, G.; McHardy, C. Modeling and Simulation of Photobioreactors with Computational Fluid Dynamics—A Comprehensive Review. Energies 2022, 15, 3966. [Google Scholar] [CrossRef]
- Khan, M.J.; Singh, N.; Mishra, S.; Ahirwar, A.; Bast, F.; Varjani, S.; Schoefs, B.; Marchand, J.; Rajendran, K.; Banu, J.R.; et al. Impact of Light on Microalgal Photosynthetic Microbial Fuel Cells and Removal of Pollutants by Nanoadsorbent Biopolymers: Updates, Challenges and Innovations. Chemosphere 2022, 288, 132589. [Google Scholar] [CrossRef] [PubMed]
- Saccardo, A.; Bezzo, F.; Sforza, E. Microalgae Growth in Ultra-Thin Steady-State Continuous Photobioreactors: Assessing Self-Shading Effects. Front. Bioeng. Biotechnol. 2022, 10, 977429. [Google Scholar] [CrossRef]
- Penloglou, G.; Pavlou, A.; Kiparissides, C. Recent Advancements in Photo-Bioreactors for Microalgae Cultivation: A Brief Overview. Processes 2024, 12, 1104. [Google Scholar] [CrossRef]
- Porto, B.; Silva, T.F.; Gonçalves, A.L.; Esteves, A.F.; de Souza, S.M.; de Souza, A.A.; Pires, J.C.; Vilar, V.J. Tubular Photobioreactors Illuminated with LEDs to Boost Microalgal Biomass Production. Chem. Eng. J. 2022, 435, 134747. [Google Scholar] [CrossRef]
- Lima, S.; Brucato, A.; Caputo, G.; Schembri, L.; Scargiali, F. Modelling Nannochloropsis Gaditana Growth in Reactors with Different Geometries, Determination of Kinetic Parameters and Biochemical Analysis in Response to Light Intensity. Appl. Sci. 2022, 12, 5776. [Google Scholar] [CrossRef]
- Singh, V.; Mishra, V. A Review on the Current Application of Light-Emitting Diodes for Microalgae Cultivation and Its Fiscal Analysis. Crit. Rev. Biotechnol. 2023, 43, 665–679. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Baidya, A.; Akter, T.; Islam, M.R.; Shah, A.K.M.A.; Hossain, M.A.; Salam, M.A.; Paul, S.I. Effect of Different Wavelengths of LED Light on the Growth, Chlorophyll, β-Carotene Content and Proximate Composition of Chlorella ellipsoidea. Heliyon 2021, 7, e08525. [Google Scholar] [CrossRef]
- Borella, L.; Marchese, D.; Trivellin, N.; Sforza, E. Complementary Chromatic Adaptation as a Strategy to Increase Energy Conversion Efficiency of Microalgae-Cyanobacteria Consortia in Continuous LED Photobioreactors. Energy Convers. Manag. 2023, 294, 117549. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light Requirements in Microalgal Photobioreactors: An Overview of Biophotonic Aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Blanken, W.; Cuaresma, M.; Wijffels, R.H.; Janssen, M. Cultivation of Microalgae on Artificial Light Comes at a Cost. Algal Res. 2013, 2, 333–340. [Google Scholar] [CrossRef]
- Kim, Z.H.; Park, Y.S.; Ryu, Y.J.; Lee, C.G. Enhancing Biomass and Fatty Acid Productivity of Tetraselmis sp. in Bubble Column Photobioreactors by Modifying Light Quality Using Light Filters. Biotechnol. Bioprocess. Eng. 2017, 22, 397–404. [Google Scholar] [CrossRef]
- Ievina, B.; Romagnoli, F. Unveiling Underlying Factors for Optimizing Light Spectrum to Enhance Microalgae Growth. Bioresour. Technol. 2025, 418, 131980. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light Emitting Diodes (LEDs) Applied to Microalgal Production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef]
- Aizpuru, A.; González-Sánchez, A. Traditional and New Trend Strategies to Enhance Pigment Contents in Microalgae. World J. Microbiol. Biotechnol. 2024, 40, 272. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Gitelson, A.; Qiuang, H.; Richmond, A. Photic Volume in Photobioreactors Supporting Ultrahigh Population Densities of the Photoautotroph Spirulina platensis. Appl. Environ. Microbiol. 1996, 62, 1570–1573. [Google Scholar] [CrossRef]
- Wu, B.S.; Rufyikiri, A.S.; Orsat, V.; Lefsrud, M.G. Re-Interpreting the Photosynthetically Action Radiation (PAR) Curve in Plants. Plant Sci. 2019, 289, 110272. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Light Matters: Effect of Light Spectra on Cannabinoid Profile and Plant Development of Medical Cannabis (Cannabis sativa L.). Ind. Crops Prod. 2021, 164, 113351. [Google Scholar] [CrossRef]
- Lewicka, K.; Siemion, P.; Kurcok, P. Chemical Modifications of Starch: Microwave Effect. Int. J. Polym. Sci. 2015, 2015, 867697. [Google Scholar] [CrossRef]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; ISBN 9781118567166. [Google Scholar]
- Rochaix, J.-D. Regulation of Photosynthetic Electron Transport. Biochim. Biophys. Acta-Bioenerg. 2011, 1807, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, S.; Finazzi, G.; Wollman, F.-A. The Dynamics of Photosynthesis. Annu. Rev. Genet. 2008, 42, 463–515. [Google Scholar] [CrossRef]
- Drop, B.; Webber-Birungi, M.; Yadav, S.K.N.; Filipowicz-Szymanska, A.; Fusetti, F.; Boekema, E.J.; Croce, R. Light-Harvesting Complex II (LHCII) and Its Supramolecular Organization in Chlamydomonas reinhardtii. Biochim. Biophys. Acta-Bioenerg. 2014, 1837, 63–72. [Google Scholar] [CrossRef]
- Van Amerongen, H.; Croce, R. Light-Harvesting in Photosystem II. Photosynth. Res. 2013, 116, 251–263. [Google Scholar] [CrossRef]
- Nelson, N. Plant Photosystem I–The Most Efficient Nano-Photochemical Machine. J. Nanosci. Nanotechnol. 2009, 9, 1709–1713. [Google Scholar] [CrossRef]
- Fromme, P.; Jordan, P.; Krauß, N. Structure of Photosystem I. Biochim. Biophys. Acta-Bioenerg. 2001, 1507, 5–31. [Google Scholar] [CrossRef]
- Croce, R.; van Amerongen, H. Light-Harvesting in Photosystem I. Photosynth. Res. 2013, 116, 153–166. [Google Scholar] [CrossRef]
- Rumeau, D.; Peltier, G.; Cournac, L. Chlororespiration and Cyclic Electron Flow around PSI during Photosynthesis and Plant Stress Response. Plant Cell Environ. 2007, 30, 1041–1051. [Google Scholar] [CrossRef]
- Shi, X.; Bloom, A. Photorespiration: The Futile Cycle? Plants 2021, 10, 908. [Google Scholar] [CrossRef] [PubMed]
- Badger, M.R.; Andrews, T.J.; Whitney, S.M.; Ludwig, M.; Yellowlees, D.C.; Leggat, W.; Price, G.D. The Diversity and Coevolution of Rubisco, Plastids, Pyrenoids, and Chloroplast-Based CO2-Concentrating Mechanisms in Algae. Can. J. Bot. 1998, 76, 1052–1071. [Google Scholar] [CrossRef]
- Burlacot, A.; Peltier, G. Energy Crosstalk between Photosynthesis and the Algal CO2-Concentrating Mechanisms. Trends Plant Sci. 2023, 28, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.S.C.; Guerra, R.; Pereira, H.; Schüler, L.M.; Varela, J.C.S. Flashing LEDs for Microalgal Production. Trends Biotechnol. 2017, 35, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.N.; Myers, J. Growth Rate of Chlorella in Flashing Light. Plant Physiol. 1953, 29, 152–161. [Google Scholar] [CrossRef]
- Grobbelaar, J.U.; Nedbal, L.; Tichý, V. Influence of High Frequency Light/Dark Fluctuations on Photosynthetic Characteristics of Microalgae Photoacclimated to Different Light Intensities and Implications for Mass Algal Cultivation. J. Appl. Phycol. 1996, 8, 335–343. [Google Scholar] [CrossRef]
- Xue, S.; Su, Z.; Cong, W. Growth of Spirulina Platensis Enhanced under Intermittent Illumination. J. Biotechnol. 2011, 151, 271–277. [Google Scholar] [CrossRef]
- Gordon, J.M.; Polle, J.E.W. Ultrahigh Bioproductivity from Algae. Appl. Microbiol. Biotechnol. 2007, 76, 969–975. [Google Scholar] [CrossRef]
- Luzi, G.; McHardy, C.; Lindenberger, C.; Rauh, C.; Delgado, A. Comparison between Different Strategies for the Realization of Flashing-Light Effects–Pneumatic Mixing and Flashing Illumination. Algal Res. 2019, 38, 101404. [Google Scholar] [CrossRef]
- Kim, D.G.; Choi, Y.-E. Microalgae Cultivation Using LED Light. Korean Chem. Eng. Res. 2014, 52, 8–16. [Google Scholar] [CrossRef]
- Lima, S.; Grisafi, F.; Scargiali, F.; Caputo, G.; Brucato, A. Growing Microalgae in a “Quasi-Isoactinic” Photobioreactor. Chem. Eng. Trans. 2018, 64, 673–678. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, C.G. Effectiveness of Flashing Light for Increasing Photosynthetic Efficiency of Microalgal Cultures over a Critical Cell Density. Biotechnol. Bioprocess Eng. 2001, 6, 189–193. [Google Scholar] [CrossRef]
- Matthijs, H.C.P.; Balke, H.; Van Hes, U.M.; Kroon, B.M.A.; Mur, L.R.; Binot, R.A. Application of Light-Emitting Diodes in Bioreactors: Flashing Light Effects and Energy Economy in Algal Culture (Chlorella pyrenoidosa). Biotechnol. Bioeng. 1996, 50, 98–107. [Google Scholar] [CrossRef]
- Combe, C.; Hartmann, P.; Rabouille, S.; Talec, A.; Bernard, O.; Sciandra, A. Long-Term Adaptive Response to High-Frequency Light Signals in the Unicellular Photosynthetic Eukaryote Dunaliella salina. Biotechnol. Bioeng. 2015, 112, 1111–1121. [Google Scholar] [CrossRef]
- Kim, Z.-H.; Kim, S.-H.; Lee, H.-S.; Lee, C.-G. Enhanced Production of Astaxanthin by Flashing Light Using Haematococcus pluvialis. Enzym. Microb. Technol. 2006, 39, 414–419. [Google Scholar] [CrossRef]
- Lima, S.; Schulze, P.S.C.; Schüler, L.M.; Rautenberger, R.; Morales-Sánchez, D.; Santos, T.F.; Pereira, H.; Varela, J.C.S.; Scargiali, F.; Wijffels, R.H.; et al. Flashing Light Emitting Diodes (LEDs) Induce Proteins, Polyunsaturated Fatty Acids and Pigments in Three Microalgae. J. Biotechnol. 2021, 325, 15–24. [Google Scholar] [CrossRef]
- Lima, S.; Lokesh, J.; Schulze, P.S.C.; Wijffels, R.H.; Kiron, V.; Scargiali, F.; Petters, S.; Bernstein, H.C.; Morales-Sánchez, D. Flashing Lights Affect the Photophysiology and Expression of Carotenoid and Lipid Synthesis Genes in Nannochloropsis gaditana. J. Biotechnol. 2022, 360, 171–181. [Google Scholar] [CrossRef]
- Schüler, L.M.; Walter, J.M.; Kato, H.; Suzuki, H.; Hulatt, C.J.; Rautenberger, R.; Navalho, S.; Schmid, B.; Varela, J.; Kiron, V.; et al. High-Value Compound Induction by Flashing Light in Diacronema lutheri and Tetraselmis striata CTP4. Bioresour. Technol. Rep. 2022, 19, 101158. [Google Scholar] [CrossRef]
- Xi, Y.; Bian, J.; Luo, G.; Kong, F.; Chi, Z. Enhanced β-Carotene Production in Dunaliella Salina under Relative High Flashing Light. Algal Res. 2022, 67, 102857. [Google Scholar] [CrossRef]
- Borella, L.; Diotto, D.; Barbera, E.; Fiorimonte, D.; Sforza, E.; Trivellin, N. Application of Flashing Blue-Red LED to Boost Microalgae Biomass Productivity and Energy Efficiency in Continuous Photobioreactors. Energy 2022, 259, 125087. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, R.; Wang, C.; Zhang, G.; Chen, C.; Li, B.; Han, T. Exploration of Flashing Light Interaction Effect on Improving Biomass, Protein, and Pigments Production in Photosynthetic Bacteria Wastewater Treatment. J. Clean. Prod. 2022, 348, 131304. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. Chlorella vulgaris Acclimated Cultivation under Flashing Light: An in-Depth Investigation under Iso-Actinic Conditions. Algal Res. 2023, 70, 102976. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Fixler, D.; Dubinsky, Z.; Solovchenko, A.; Zigman, M.; Yehoshua, Y.; Iluz, D. Flashing Light Enhancement of Photosynthesis and Growth Occurs When Photochemistry and Photoprotection Are Balanced in Dunaliella salina. Eur. J. Phycol. 2015, 50, 469–480. [Google Scholar] [CrossRef]
- Gatamaneni Loganathan, B.; Orsat, V.; Lefsrud, M.; Wu, B. Sen A Comprehensive Study on the Effect of Light Quality Imparted by Light-Emitting Diodes (LEDs) on the Physiological and Biochemical Properties of the Microalgal Consortia of Chlorella variabilis and Scenedesmus obliquus Cultivated in Dairy Wastewater. Bioprocess. Biosyst. Eng. 2020, 43, 1445–1455. [Google Scholar] [CrossRef]
- Ueno, Y.; Aikawa, S.; Kondo, A.; Akimoto, S. Adaptation of Light-Harvesting Functions of Unicellular Green Algae to Different Light Qualities. Photosynth. Res. 2019, 139, 145–154. [Google Scholar] [CrossRef]
- Detweiler, A.M.; Mioni, C.E.; Hellier, K.L.; Allen, J.J.; Carter, S.A.; Bebout, B.M.; Fleming, E.E.; Corrado, C.; Prufert-Bebout, L.E. Evaluation of Wavelength Selective Photovoltaic Panels on Microalgae Growth and Photosynthetic Efficiency. Algal Res. 2015, 9, 170–177. [Google Scholar] [CrossRef]
- Li, X.; Slavens, S.; Crunkleton, D.W.; Johannes, T.W. Interactive Effect of Light Quality and Temperature on Chlamydomonas Reinhardtii Growth Kinetics and Lipid Synthesis. Algal Res. 2021, 53, 102127. [Google Scholar] [CrossRef]
- Li, X.; Huff, J.; Crunkleton, D.W.; Johannes, T.W. Light Intensity and Spectral Quality Modulation for Improved Growth Kinetics and Biochemical Composition of Chlamydomonas reinhardtii. J. Biotechnol. 2023, 375, 28–39. [Google Scholar] [CrossRef]
- de Mooij, T.; de Vries, G.; Latsos, C.; Wijffels, R.H.; Janssen, M. Impact of Light Color on Photobioreactor Productivity. Algal Res. 2016, 15, 32–42. [Google Scholar] [CrossRef]
- Yan, C.; Zhu, L.; Wang, Y. Photosynthetic CO2 Uptake by Microalgae for Biogas Upgrading and Simultaneously Biogas Slurry Decontamination by Using of Microalgae Photobioreactor under Various Light Wavelengths, Light Intensities, and Photoperiods. Appl. Energy 2016, 178, 9–18. [Google Scholar] [CrossRef]
- Parveen, A.; Rawat, J.; Bhatnagar, P.; Gautam, P.; Kumar, S.; Upadhyay, S.; Vlaskin, M.S.; Anna, I.K.; Kumar, V.; Nanda, M. Enhanced Production of High-Value Compounds from Chlorella sorokiniana by Two-Stage Cultivation under Red Light and Salinity Stress. Biocatal. Agric. Biotechnol. 2024, 60, 103315. [Google Scholar] [CrossRef]
- Esteves, A.F.; Pardilhó, S.; Gonçalves, A.L.; Vilar, V.J.P.; Pires, J.C.M. Unravelling the Impact of Light Spectra on Microalgal Growth and Biochemical Composition Using Principal Component Analysis and Artificial Neural Network Models. Algal Res. 2025, 85, 103820. [Google Scholar] [CrossRef]
- Kim, S.H.; Sunwoo, I.Y.; Hong, H.J.; Awah, C.C.; Jeong, G.T.; Kim, S.K. Lipid and Unsaturated Fatty Acid Productions from Three Microalgae Using Nitrate and Light-Emitting Diodes with Complementary LED Wavelength in a Two-Phase Culture System. Bioprocess. Biosyst. Eng. 2019, 42, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Mohsenpour, S.F.; Richards, B.; Willoughby, N. Spectral Conversion of Light for Enhanced Microalgae Growth Rates and Photosynthetic Pigment Production. Bioresour. Technol. 2012, 125, 75–81. [Google Scholar] [CrossRef]
- Hashmi, Z.; Zaini, J.; Abdullah, R.; Abu Bakar, M.S.; Bilad, M.R. Effects of Light Color on Growth, Nutrient Uptake, and Harvesting of the Indigenous Strain of Chlorococcum sp. Bioresour. Technol. Rep. 2024, 28, 101966. [Google Scholar] [CrossRef]
- Nie, C.; Jiang, L.; Yu, Z.; Yang, Z.; Hou, Q.; Pei, H. Campus Sewage Treatment by Golenkinia SDEC-16 and Biofuel Production under Monochromic Light. J. Chem. 2020, 2020, 5029535. [Google Scholar] [CrossRef]
- Pereira, S.; Otero, A. Haematococcus pluvialis Bioprocess Optimization: Effect of Light Quality, Temperature and Irradiance on Growth, Pigment Content and Photosynthetic Response. Algal Res. 2020, 51, 102027. [Google Scholar] [CrossRef]
- Teo, C.L.; Atta, M.; Bukhari, A.; Taisir, M.; Yusuf, A.M.; Idris, A. Enhancing Growth and Lipid Production of Marine Microalgae for Biodiesel Production via the Use of Different LED Wavelengths. Bioresour. Technol. 2014, 162, 38–44. [Google Scholar] [CrossRef]
- Abomohra, A.E.F.; Shang, H.; El-Sheekh, M.; Eladel, H.; Ebaid, R.; Wang, S.; Wang, Q. Night Illumination Using Monochromatic Light-Emitting Diodes for Enhanced Microalgal Growth and Biodiesel Production. Bioresour. Technol. 2019, 288, 121514. [Google Scholar] [CrossRef]
- Diaz-MacAdoo, D.; Nagai, S.; Mata, M.T.; Minei, R.; Ogura, A.; Riquelme, C. Comparative Analysis of Carotenoid Synthesis and Transcriptome of a Microalga Chlorophyta MCH-35, Potential Lutein Producer, in Response to Different Quality Light. Algal Res. 2023, 74, 103206. [Google Scholar] [CrossRef]
- Niizawa, I.; Heinrich, J.M.; Irazoqui, H.A. Modeling of the Influence of Light Quality on the Growth of Microalgae in a Laboratory Scale Photo-Bio-Reactor Irradiated by Arrangements of Blue and Red LEDs. Biochem. Eng. J. 2014, 90, 214–223. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Qi, Y.; Wei, Q.; Yang, G.; Ma, X. A Modified Cultivation Strategy to Enhance Biomass Production and Lipid Accumulation of Tetradesmus Obliquus FACHB-14 with Copper Stress and Light Quality Induction. Bioresour. Technol. 2024, 400, 130677. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Woolschot, S.; Cabanelas, I.T.D.; Wijffels, R.H.; Barbosa, M.J. Light Spectra as Triggers for Sorting Improved Strains of Tisochrysis lutea. Bioresour. Technol. 2021, 321, 124434. [Google Scholar] [CrossRef] [PubMed]
- Costa Shellenberger, B.; Jungandreas, A.; Jakob, T.; Weisheit, W.; Mittag, M.; Wilhelm, C. Blue Light Is Essential for High Light Acclimation and Photoprotection in the Diatom Phaeodactylum tricornutum. J. Exp. Bot. 2012, 63, 695–709. [Google Scholar] [CrossRef]
- Xu, L.; Pan, W.; Yang, G.; Tang, X.; Martin, R.M.; Liu, G.; Zhong, C. Impact of Light Quality on Freshwater Phytoplankton Community in Outdoor Mesocosms. Environ. Sci. Pollut. Res. 2021, 28, 58536–58548. [Google Scholar] [CrossRef]
- Baer, S.; Heining, M.; Schwerna, P.; Buchholz, R.; Hübner, H. Optimization of Spectral Light Quality for Growth and Product Formation in Different Microalgae Using a Continuous Photobioreactor. Algal Res. 2016, 14, 109–115. [Google Scholar] [CrossRef]
- Bahman, M.; Aghanoori, M.; Jalili, H.; Bozorg, A.; Danaee, S.; Bidhendi, M.E.; Amrane, A. Effect of Light Intensity and Wavelength on Nitrogen and Phosphate Removal from Municipal Wastewater by Microalgae under Semi-Batch Cultivation. Environ. Technol. 2022, 43, 1352–1358. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781439867334. [Google Scholar]
- Fu, J.; Huang, Y.; Liao, Q.; Xia, A.; Fu, Q.; Zhu, X. Photo-Bioreactor Design for Microalgae: A Review from the Aspect of CO2 Transfer and Conversion. Bioresour. Technol. 2019, 292, 121947. [Google Scholar] [CrossRef]
- Venancio, H.C.; Cella, H.; Lopes, R.G.; Derner, R.B. Surface-to-Volume Ratio Influence on the Growth of Scenedesmus Obliquus in a Thin-Layer Cascade System. J. Appl. Phycol. 2020, 32, 821–829. [Google Scholar] [CrossRef]
- Sivakaminathan, S.; Wolf, J.; Yarnold, J.; Roles, J.; Ross, I.L.; Stephens, E.; Henderson, G.; Hankamer, B. Light Guide Systems Enhance Microalgae Production Efficiency in Outdoor High Rate Ponds. Algal Res. 2020, 47, 101846. [Google Scholar] [CrossRef]
- Štěrbová, K.; Manoel, J.C.; Lakatos, G.E.; Grivalský, T.; Masojídek, J. Microalgae as an Aquaculture Feed Produced in a Short Light-Path Annular Column Photobioreactor. J. Appl. Phycol. 2023, 35, 603–611. [Google Scholar] [CrossRef]
- González-Camejo, J.; Aparicio, S.; Jiménez-Benítez, A.; Pachés, M.; Ruano, M.V.; Borrás, L.; Barat, R.; Seco, A. Improving Membrane Photobioreactor Performance by Reducing Light Path: Operating Conditions and Key Performance Indicators. Water Res. 2020, 172, 115518. [Google Scholar] [CrossRef] [PubMed]
- Chuka-ogwude, D.; Ogbonna, J.C.; Moheimani, N.R. Depth Optimization of Inclined Thin Layer Photobioreactor for Efficient Microalgae Cultivation in High Turbidity Digestate. Algal Res. 2021, 60, 102509. [Google Scholar] [CrossRef]
- Naď, M.; Brummer, V.; Lošák, P.; Máša, V.; Sukačová, K.; Tatarová, D.; Pernica, M.; Procházková, M. Waste-to-Energy Plants Flue Gas CO2 Mitigation Using a Novel Tubular Photobioreactor While Producing Chlorella algae. J. Clean. Prod. 2023, 385, 135721. [Google Scholar] [CrossRef]
- Hijazi, R.M.; Mounsef, J.R.; Kanaan, H.Y. Comparison of Light Intensity Effect on Microalgal Growth in Cactus-like and Cylindrical Photo Bioreactors. Processes 2024, 12, 1664. [Google Scholar] [CrossRef]
- Díaz, J.P.; Inostroza, C.; Acién, F.G. Yield and Production Cost of Chlorella Sp. Culture in a Fibonacci-Type Photobioreactor. Process Biochem. 2023, 129, 209–220. [Google Scholar] [CrossRef]
- Díaz, J.P.; Inostroza, C.; Acién Fernández, F.G. Fibonacci-Type Tubular Photobioreactor for the Production of Microalgae. Process Biochem. 2019, 86, 1–8. [Google Scholar] [CrossRef]
- Chin-On, R.C.; Barbosa, M.J.; Wijffels, R.H.; Janssen, M. A Novel V-Shaped Photobioreactor Design for Microalgae Cultivation at Low Latitudes: Modelling Biomass Productivities of Chlorella sorokiniana on Bonaire. Chem. Eng. J. 2022, 449, 137793. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, K.; Huang, Y.; Xia, A.; Zhu, X.; Zhu, X.; Liao, Q. Three-Dimensional Porous Biofilm Photobioreactor with Light-Conducting Frameworks for High-Efficiency Microalgal Growth. Algal Res. 2023, 69, 102942. [Google Scholar] [CrossRef]
- Ahangar, A.K.; Yaqoubnejad, P.; Divsalar, K.; Mousavi, S.; Taghavijeloudar, M. Design a Novel Internally Illuminated Mirror Photobioreactor to Improve Microalgae Production through Homogeneous Light Distribution. Bioresour. Technol. 2023, 387, 129577. [Google Scholar] [CrossRef]
- Deprá, M.C.; Mérida, L.G.R.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. A New Hybrid Photobioreactor Design for Microalgae Culture. Chem. Eng. Res. Des. 2019, 144, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Zhao, R.; Tao, Y.; Ying, K.Z.; Mao, X.Z. Novel Flat-Plate Photobioreactor with Inclined Baffles and Internal Structure Optimization to Improve Light Regime Performance. ACS Sustain. Chem. Eng. 2021, 9, 1550–1558. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, G.; Xiao, G.; Duan, Z.; Dai, C.; Hu, J.; Wang, Y.; Yang, Y.; Jiang, X. Enhancing CO2 Photo-Biochemical Conversion in a Newly-Designed Attached Photobioreactor Characterized by Stacked Horizontal Planar Waveguide Modules. Sci. Total Environ. 2021, 760, 144041. [Google Scholar] [CrossRef] [PubMed]
- Nwoba, E.G.; Parlevliet, D.A.; Laird, D.W.; Alameh, K.; Moheimani, N.R. Light Management Technologies for Increasing Algal Photobioreactor Efficiency. Algal Res. 2019, 39, 101433. [Google Scholar] [CrossRef]
- Brzychczyk, B.; Hebda, T.; Fitas, J.; Giełżecki, J. The Follow-up Photobioreactor Illumination System for the Cultivation of Photosynthetic Microorganisms. Energies 2020, 13, 1143. [Google Scholar] [CrossRef]
- Zhang, Q.; Xue, S.; Yan, C.; Wu, X.; Wen, S.; Cong, W. Installation of Flow Deflectors and Wing Baffles to Reduce Dead Zone and Enhance Flashing Light Effect in an Open Raceway Pond. Bioresour. Technol. 2015, 198, 150–156. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, Z.; Ye, Q.; Zhou, J.; Cen, K. Enhanced Flashing Light Effect with Up-down Chute Baffles to Improve Microalgal Growth in a Raceway Pond. Bioresour. Technol. 2015, 190, 29–35. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, J.; Xu, X.; Zhou, J.; Cen, K. Enhanced Solution Velocity between Dark and Light Areas with Horizontal Tubes and Triangular Prism Baffles to Improve Microalgal Growth in a Flat-Panel Photo-Bioreactor. Bioresour. Technol. 2016, 211, 519–526. [Google Scholar] [CrossRef]
- Xue, S.; Zhang, Q.; Wu, X.; Yan, C.; Cong, W. A Novel Photobioreactor Structure Using Optical Fibers as Inner Light Source to Fulfill Flashing Light Effects of Microalgae. Bioresour. Technol. 2013, 138, 141–147. [Google Scholar] [CrossRef]
- Sero, E.T.; Siziba, N.; Bunhu, T.; Shoko, R. Light Filtration Technology for Sustainable Microalgal Biomass Production. Biotechnol. Biotechnol. Equip. 2022, 36, 914–924. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Parlevliet, D.A.; Laird, D.W.; Vadiveloo, A.; Alameh, K.; Moheimani, N.R. Can Solar Control Infrared Blocking Films Be Used to Replace Evaporative Cooling for Growth of Nannochloropsis sp. in Plate Photobioreactors? Algal Res. 2019, 39, 101441. [Google Scholar] [CrossRef]
- Cho, C.; Nam, K.; Kim, G.-Y.; Seo, Y.H.; Hwang, T.G.; Seo, J.-W.; Kim, J.P.; Han, J.-I.; Lee, J.-Y. Multi-Bandgap Solar Energy Conversion via Combination of Microalgal Photosynthesis and Spectrally Selective Photovoltaic Cell. Sci. Rep. 2019, 9, 18999. [Google Scholar] [CrossRef] [PubMed]
- Balbuena-Ortega, A.; Flores-Bahena, P.D.; Villa-Calderón, A.; del Río, J.A.; Arias, D.M. Impact of Light Spectrum on Outdoors Tubular Photobioreactors Used for Microalgae-Based Wastewater Treatment. J. Environ. Chem. Eng. 2024, 12, 114884. [Google Scholar] [CrossRef]
- Sero, E.T.; Siziba, N.; Bunhu, T.; Shoko, R.; Jonathan, E. Biophotonics for Improving Algal Photobioreactor Performance: A Review. Int. J. Energy Res. 2020, 44, 5071–5092. [Google Scholar] [CrossRef]
- Saeli, M.; Piccirillo, C.; Warwick, M.E.; Binions, R. Thermochromic Thin Films: Synthesis, Properties and Energy Consumption Modelling; Formatex Research Center: Badajoz, Spain, 2013. [Google Scholar]
- Kalyani, V.L.; Sharma, V. Different Types of Optical Filters and Their Realistic Application. J. Manag. Eng. Inf. Technol. 2016, 3, 2394–8124. [Google Scholar]
- Kamalisarvestani, M.; Saidur, R.; Mekhilef, S.; Javadi, F.S. Performance, Materials and Coating Technologies of Thermochromic Thin Films on Smart Windows. Renew. Sustain. Energy Rev. 2013, 26, 353–364. [Google Scholar] [CrossRef]
- Louveau, J.; Titica, M.; Moheimani, N.R.; Pruvost, J. Coupling Semi-Transparent PV PANELS with Thin-Film PBRs for Sustainable Microalgal Biomass Production. Chem. Eng. Res. Des. 2024, 205, 722–732. [Google Scholar] [CrossRef]
- Morales, M.; Hélias, A.; Bernard, O. Optimal Integration of Microalgae Production with Photovoltaic Panels: Environmental Impacts and Energy Balance. Biotechnol. Biofuels 2019, 12, 239. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Parlevliet, D.A.; Laird, D.W.; Alameh, K.; Moheimani, N.R. Pilot-Scale Self-Cooling Microalgal Closed Photobioreactor for Biomass Production and Electricity Generation. Algal Res. 2020, 45, 101731. [Google Scholar] [CrossRef]
- Damergi, E.; Qin, P.; Sharma, S.; Nazeeruddin, M.K.; Ludwig, C. Enhancing Algae Biomass Production by Using Dye-Sensitized Solar Cells as Filters. ACS Sustain. Chem. Eng. 2021, 9, 14353–14364. [Google Scholar] [CrossRef]
- Rodrigues Dias, R.; Costa Deprá, M.; Basso Sartori, R.; Terezinha Schneider, A.; Queiroz Zepka, L.; Jacob-Lopes, E. Integrating Solar Cell Technologies in Microalgae Facilities: Environmental Sustainability Metrics and Indicators. Sustain. Energy Technol. Assess. 2023, 58, 103362. [Google Scholar] [CrossRef]
- Barbera, E.; Sforza, E.; Guidobaldi, A.; Di Carlo, A.; Bertucco, A. Integration of Dye-Sensitized Solar Cells (DSC) on Photobioreactors for Improved Photoconversion Efficiency in Microalgal Cultivation. Renew. Energy 2017, 109, 13–21. [Google Scholar] [CrossRef]

| Groups of Microalgae (Phylum) | Microalgae | Cultivation Conditions | Type of Lighting | Wavelength | Growth Rate and Biomass Production | Biochemical Composition | Ref. |
|---|---|---|---|---|---|---|---|
| Green Algae | Botryococcus sudeticus (UTEX 2629) | Conical flask (250 mL) ATCC BG11 + medium Aerated agitation Tav = 18.5 °C | LSC panels | White light Red light (600–730 nm) | Red light slightly promoted growth rate compared to white light | Red light promoted pigment production | [59] |
| Chlamydomonas reinhardtii WT CC-125 | Batch flask (250 mL) TAP medium 6 days Mechanical agitation T = 24–32 °C 24:0 h light/dark cycle 105 ± 3 μmol/m2 s | LED | Blue light White-Yellow light Red-Orange light | Red-orange light was the most effective in promoting biomass production at 24 °C Blue light was the most effective in promoting biomass production at 32 °C | Red light promoted lipid production | [60] | |
| Chlamydomonas reinhardtii WT CC-125 | Batch flask (250 mL) TAP medium 7 days Mechanical agitation T = 28 ± 0.3 °C 45–305 μmol/m2 s | LED | Blue light White-Yellow light Red-Orange light | Blue light was the most effective in promoting biomass production at 35 μmol/m2 s Red-orange and white-yellow light were the most effective in promoting biomass production at 305 μmol/m2 s | Red-orange light promoted lipid and carbohydrate production Blue light and white-yellow light promoted protein production | [61] | |
| Chlamydomonas reinhardtii CC-1690 | Flat-panel photobioreactors Sueoka high salt medium Aerated agitation T = 25 °C pH = 7 1500 μmol/m2 s | LED | White light Blue light Yellow light Orange-Red light Red light | Red light was the most effective in promoting growth rate Yellow light was the most effective in promoting biomass production Blue light was the least effective for growth rate and biomass production | [62] | ||
| Chlamydomonas reinhardtii (CC 1690) | Conical flask (250 mL) P49 medium Aerated agitation Tav = 18.5 °C | LSC panels | White light Red light (600–730 nm) | Red light slightly promoted growth rate compared to white light | No difference was found between the use of red light and white light in pigment production | [59] | |
| Chlorella sp. | Transparent polyethylene bag Biogas slurry 6 days Manual agitation T = 25 ± 0.5 °C 12:12 light/dark cycle 600 μmol/m2 s | LED | White light Blue light (460 nm) Red light (660 nm) Mixed red–blue (2:8; 5:5; 8:2) | Red–blue light ratio (5:5) was the most effective in promoting growth rate | [63] | ||
| Chlorella sorokiniana (UUIND6) | Conical flask (250 mL) BBM medium 7 days (stationary phase) T = 25 °C 18:6 h light/dark cycle 300 μmol/m2 s | LED | White light Blue light Green light Red light | Red light was the most effective in promoting growth rate but the least effective in biomass production White light was the most effective in promoting biomass production Green light was the least effective for growth rate | Red light promoted lipid, pigment and carbohydrate production White light promoted protein production Green light was the least effective for protein and carbohydrate production | [64] | |
| Chlorella vulgaris | Batch flask (1 L) BG-11 medium 17 days (stationary growth phase) Aerated agitation (+0.04% CO2) T = 25 ± 2 °C 24:0 h light/dark cycle 71 ± 2 μmol/m2 s | LED | White light Blue light (420–450 nm) Green light (495–570 nm) Orange light (590–625 nm) Red light (620–700 nm) | Red light was the most effective in promoting growth rate Blue light was the least effective for growth rate | White light promoted lipid and pigment production Blue light promoted protein production Red light promoted carbohydrate production | [65] | |
| Chlorella vulgaris | Flask (1 L) Modified f/2 medium 14 days Air bubble agitation T = 20 °C 12:12 light/dark cycle 100 μmol/m2 s | LED/Fluorescence tubes | White light Purple light (400 nm) Blue light (465 nm) Green light (520 nm) Yellow light (590 nm) Red light (625 nm) | Red light was the most effective in promoting biomass production | Green light promoted lipid production | [66] | |
| Chlorella vulgaris (ATCC-29498) | Conical flask (250 mL) ATCC 847 medium Aerated agitation Tav = 18.5 °C | LSC panels | White light Red light (600–730 nm) | Red light slightly promoted growth rate compared to white light | Red light promoted pigment production | [59] | |
| Chlorella vulgaris (CCAP 211/79) | Rectangular chambers 3n-BBM + V medium 14 days T = 23 ± 2 °C 250 μmol/m2 s | Light filters | Violet light Green light Orange light Red light | Orange light was the most effective in promoting growth rate Violet light was the most effective in promoting biomass production Red light was the least effective for growth rate | Red light promoted pigment production | [67] | |
| Chlorococcum sp. | Batch flask (250 mL) 15 days Aerated agitation T = 23 ± 2 °C 24:0 light/dark cycle 104 μmol/m2 s (red and blue light) 150 μmol/m2 s (white light) 48 μmol/m2 s (violet light) | LED | White light (450 nm) Blue light (388 nm) Violet light (368 nm) Red light (638 nm) | Red light was the most effective in promoting growth rate Violet light was the least effective for growth rate | [68] | ||
| Dunaliella salina (CCAO 19/18) | Raceway (50 L) Instant Ocean Aquarium medium 18 days Mechanical agitation Tav = 18.5 °C | LSC panels | White light Red light (600–730 nm) | Red light slightly promoted growth rate compared to white light | No significant difference was found between the effects of red and white light on pigment production | [59] | |
| Golenkinia SDEC-16 | Batch flask (1 L) Wastewater 7 days Aerated agitation Tav = 25 ± 2 °C 24:0 light/dark cycle | LED | Blue light (460 nm) Green light (520 nm) Red light (660 nm) | Red light and blue light, respectively, promoted biomass production Green light was the least effective for growth rate | Green light was the most effective in promoting lipid production Blue light was the least effective for lipid production | [69] | |
| Haematococcus pluvialis | Cylindrical photobioreactors (1 L) OHM medium 4 days (late exponential phase) Aerated agitation (+10% CO2) T = 21 °C pH = 7/7.5 12:12 h light/dark cycle 100 μmol/m2 s | LED | Blue light (450 nm) Red light (660 nm) Mixed red–blue ≈ 1:1; 1:4; 4:1 | Red light was the most effective in promoting growth rate Blue light was the least effective for growth rate | Mixed red–blue promoted pigment production | [70] | |
| Nannochloropsis sp. | Batch flask (1 L) Walne’s medium 14 days (late exponential phase) T = 23 ± 0.5 °C pH = 8 ± 0.2 24:0 h light/dark cycle 100 μmol/m2 s | LED/Fluorescence lamp | White fluorescence lamp Blue light (457 nm) Red light (660 nm) Mix red–blue | Blue light was the most effective in promoting growth rate | Blue light promoted lipid production White light was the least effective in promoting lipid production | [71] | |
| Scenedesmus obliquus (SAG276-10) | Bubble photobioreactors column Wastewater 14 days (exponential phase) Air bubble agitation (+3% CO2 at aeration rate of 0.2 vvm) 25.0 ± 1.5 °C | LED | White light (400–700 nm) Blue light (460 nm) Green light (540 nm) Red light (650 nm) | Blue light and red light, respectively, promoted biomass production [g/L], with blue light having a moderately greater effect | Blue light and red light promoted lipids and carbohydrate production White and green light promoted protein production | [72] | |
| Scenedesmus rubescens | Multi-Cultivator MC 1000-Mix equipment (100 mL) UMA-5 medium 7 days Air bubble agitation 24:0 light/dark cycle 80 μmol/m2 s | LED | White light (404–789 nm) Blue light (453 nm) Red light (633 nm) | Blue light and red light, respectively, promoted growth rate, with blue light having a moderately greater effect White light promoted biomass production Blue light was the least effective for biomass production | Blue light and white light promoted pigment production Red light inhibited pigment production | [73] | |
| Scenedesmus quadricauda 276/21 | Cylindrical PBR (2 L) BBM medium 9 days Aerated agitation T = 25–28 °C | LED | Blue light (470 nm) Red light (630 nm) | No effect of wavelength on biomass production was observed | Blue light promoted pigment production | [74] | |
| Tetraselmis sp. | Batch flask (1 L) Walne’s medium 14 days (late exponential phase) T = 23 ± 0.5 °C pH = 8 ± 0.2 24:0 h light/dark cycle 100 μmol/m2 s | LED/Fluorescence lamp | White fluorescence lamp Blue light (457 nm) Red light (660 nm) Mix red–blue | Blue light was the most effective in promoting growth rate | Blue light promoted lipid production White light was the least effective for lipid production | [71] | |
| Tetradesmus obliquus FACHB-14 | Batch flask (1 L) BG11 medium 9 days Aerated agitation T = 25 ± 1 °C pH = 7.1 ± 0.1 68 μmol/m2 s | White light Blue light Green light Red light (660 nm) | Red light and blue light, respectively, promoted growth rate and biomass production Green light is the lowest promoter for growth rate and biomass production | Red light promoted lipid production | [75] | ||
| Tetraselmis lutua | Flat panel photobioreactor (400 mL) NutriBloom Plus medium 16 days Air bubble agitation pH = 8 18:6 light/dark cycle 50 μmol/m2 s | LED | Blue light Green light Red light Red–reen–blue ≈ 1.6:1:1.3 Blue–red ≈ 1:5 Blue–green ≈ 6:5 | Red–green–lue and blue light promoted biomass production Red light was the least effective for biomass production | Blue–green light promoted pigment production No effect of wavelength on lipid production was observed | [76] | |
| Diatoms | Pavlova lutheri | Flask (1 L) Modified f/2 medium 14 days Air bubble agitation T = 20 °C 12:12 light/dark cycle 100 μmol/m2 s | LED/Fluorescence tubes | White light Purple light (400 nm) Blue light (465 nm) Green light (520 nm) Yellow light (590 nm) Red light (625 nm) | Blue light was the most effective in promoting biomass production | Yellow light promoted lipid production | [66] |
| Phaeodactylum tricornutum (UTEX 646) | Air-lifted rectangular bioreactor f/2 medium 7 days T = 20 °C 14:10 light/dark cycle 120 μmol/m2 s (white light) 72 μmol/m2 s (blue light) 123 μmol/m2 s (red light) | LED/Fluorescence tubes | White light Blue light (469 ± 10 nm) Red light (659 ± 11 nm) | Blue light was the most effective in promoting growth rate and biomass production | Blue light promoted pigment production, but, in particular, red light promoted violaxanthin production | [77] | |
| Synedra | Cylindrical plastic buckets (100 L) Lake water medium (Meiliang Bay) Mechanical agitation 25 days | Light filters | White light (400–700 nm) Blue light (444 nm) Green light (543 nm) Red light (700 nm) | Green light was the most effective in promoting growth rate Blue light was the least effective for growth rate | [78] | ||
| Red Algae | Galdieria sulphuraria | Continuos bubble column photobioreactors (700 mL) Gross and Schnarrenberger medium Air bubble agitation (+3% CO2 at aeration) 100 μmol/m2 s | LED | Blue light (490 nm) Green light (525 m) Red light (625 nm) | Red light was the most effective in promoting biomass production Green light was the least effective for biomass production | Red light promoted phycobiliprotein production Green light was the least effective in promoting phycobiliprotein production | [79] |
| Porphyridium cruentum | Flask (1 L) Modified f/2 medium 14 days Air bubble agitation T = 20 °C 12:12 light/dark cycle 100 μmol/m2 s | LED/Fluorescence tubes | White light Purple light (400 nm) Blue light (465 nm) Green light (520 nm) Yellow light (590 nm) Red light (625 nm) | Green light was the most effective in promoting biomass production | Red light promoted lipid production | [66] | |
| Porphyridium purpureum | Continuous bubble column photobioreactors (700 mL) Artificial seawater medium Air bubble agitation (+3% CO2 at aeration) 100 μmol/m2 s 16 days | LED | Blue light (490 nm) Green light (525 m) Red light (625 nm) | Green light was the most effective in promoting biomass production Blue light had a negative effect on biomass production | Red light promoted phycobiliprotein production Blue light had a negative effect on phycobiliprotein production | [79] | |
| Cyanobacteria | Gloeothece membranacea | Rectangular chambers 3n-BBM + V medium 14 days T = 23 ± 2 °C 150 μmol/m2 s | Light filters | Violet light Green light Orange light Red light | Violet light was the most effective in promoting growth rate | Green light promoted pigment production | [67] |
| Microcystis | Cylindrical plastic buckets (100 L) Lake water medium (Meiliang Bay) Mechanical agitation 25 days | Light filters | White light (400–700 nm) Blue light (444 nm) Green light (543 nm) Red light (700 nm) | White and red light promoted growth rate Green light was the least effective for growth rate | [78] | ||
| Spirulina platensis | Open raceway pond (4 L) Artificial wastewater 8 days Mechanical agitation pH = 10/11 12:12 light/dark cycle 90 μmol/m2 s | Light filters | White light Blue light (470 nm) Purple light (415 nm) Red light (685 nm) | Blue light was the most effective in promoting biomass production White light was the least effective for biomass production | Blue light promoted protein production | [80] | |
| Spirulina platensis (ATCC 2940) | Conical flask (250 mL) Spirulina medium Aerated agitation Tav = 18.5 °C | LSC panels | White light Red light (600–730 nm) | Red light slightly promoted growth rate compared to white light | No significant difference was found between the effects of red and white light on pigment production | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchese, A.; Lima, S.; Cosenza, A.; Giambalvo, F.; Scargiali, F. Effects of Light Quality Adjustment in Microalgal Cultivation: Flashing Light and Wavelength Shifts in Photobioreactor Design. Processes 2025, 13, 1159. https://doi.org/10.3390/pr13041159
Marchese A, Lima S, Cosenza A, Giambalvo F, Scargiali F. Effects of Light Quality Adjustment in Microalgal Cultivation: Flashing Light and Wavelength Shifts in Photobioreactor Design. Processes. 2025; 13(4):1159. https://doi.org/10.3390/pr13041159
Chicago/Turabian StyleMarchese, Arima, Serena Lima, Alessandro Cosenza, Francesco Giambalvo, and Francesca Scargiali. 2025. "Effects of Light Quality Adjustment in Microalgal Cultivation: Flashing Light and Wavelength Shifts in Photobioreactor Design" Processes 13, no. 4: 1159. https://doi.org/10.3390/pr13041159
APA StyleMarchese, A., Lima, S., Cosenza, A., Giambalvo, F., & Scargiali, F. (2025). Effects of Light Quality Adjustment in Microalgal Cultivation: Flashing Light and Wavelength Shifts in Photobioreactor Design. Processes, 13(4), 1159. https://doi.org/10.3390/pr13041159








