Abstract
Aerated accumulation bioreactors represent a promising alternative for the aerobic bioremediation of solid contaminated substrates. However, achieving homogeneous mixing and effective air distribution remains a key design challenge in solid-phase systems. This study presents the design and construction of a novel pilot-scale aerated bioreactor equipped with an angled-paddle agitation system, specifically developed to improve solid mixing and aeration. To evaluate the geometric configuration, a series of simulations were performed using the Discrete Element Method (DEM), with particle dynamics analyzed through the Lacey Mixing Index (). Four paddle angles (0°, 15°, 45°, and 55°) were compared, with the 45° configuration achieving optimal performance, reaching values above 0.95 in less than 15 s and maintaining high homogeneity at a filling volume of 70%. These results confirm that the paddle angle significantly influences mixing efficiency in granular media. While this work focuses on engineering design and DEM-based validation, future studies will include experimental trials to evaluate biodegradation kinetics. The proposed design offers a scalable and adaptable solution for ex situ bioremediation applications. This work reinforces the value of integrating DEM simulations early in the bioreactor development process and opens pathways for further optimization and implementation in real-world environmental remediation scenarios.
1. Introduction
Ongoing global industrialization has led to the release of various pollutants that affect all forms of life [1]. In soils, these contaminants include heavy metals [2], toxic organic compounds such as pesticides [3], biological pathogens, plastic waste, and microplastics [4,5]. The presence of these contaminants is often associated with adverse human health effects, including carcinogenesis and mutagenesis, as well as other toxic impacts [1]. Furthermore, they can disrupt soil ecosystems by affecting soil vitality and reducing the functional diversity of microorganisms responsible for their structural and metabolic stability [6].
Soil contamination alters its physical, chemical, and physicochemical properties, with the magnitude of impact depending on the soil type, its initial condition, the nature of the contaminant, and its concentration [7]. In particular, hydrocarbon contamination creates a film that coats soil particles, causing a color shift to gray or dark brown tones [8]. These chromatic changes affect soil reflectivity and alter its thermal dynamics [9].
To address soil contamination, various remediation strategies have been developed, which are generally classified into physical, chemical, and biological methods [1,10]. Among these, bioremediation stands out as a sustainable alternative that uses microorganisms under aerobic or anaerobic conditions to transform contaminants into less toxic compounds such as CO2, H2O, and biomass through oxidation, degradation, and mineralization processes [11].
This approach employs living organisms, such as bacteria, fungi, algae, and plants, capable of degrading both organic and inorganic pollutants including pesticides, fertilizers, and heavy metals present in soils and water [9,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Microorganisms metabolize contaminants by using them as a source of energy or nutrients, while plants absorb them through their roots and enzymatically convert them into less toxic compounds with simpler chemical structures [19,22,28,31].
Bioremediation offers several advantages, including the preservation of soil texture and structure, and improvements in physicochemical properties such as aeration, pH, water retention capacity, and ion exchange [32]. An efficient bioprocess should ensure the complete removal of pollutants and their intermediate by-products [33]. Compared to physicochemical methods, bioremediation presents a more favorable cost–benefit ratio, lower energy consumption, and reduced generation of toxic residues and sludge [3].
Advances in biotechnology have enabled the development of bioreactors designed to optimize large-scale bioremediation processes [34]. However, compared to submerged biological systems, controlling operational parameters in solid-state bioprocesses is more complex—particularly during scale-up [35]. Since the early 2000s, significant improvements have been made in the design of bioreactors for solid-state fermentation, optimizing key parameters for contaminant biodegradation [36].
Despite these advances, the main limitation to the industrial implementation of solid-state fermentation remains the lack of simple, efficient, and scalable bioreactors capable of adequately managing heat and mass transfer and minimizing process heterogeneity [37]. This challenge arises from the absence of optimized reactor designs, accurate mathematical models to describe transport phenomena and reaction kinetics at both micro- and macroscopic levels, and effective real-time monitoring and control strategies. Although progress has been made in developing more efficient bioreactors, there is still considerable room for improvement regarding their applicability to diverse biotechnological scenarios [37].
In the context of soil bioremediation, the process can be conceptualized as a form of solid-state fermentation, opening the door to adapting technologies used in that domain. A promising approach involves the use of aerated accumulation bioreactors, which function similarly to solid-state fermenters [38,39]. This technique involves excavating a designated area in the soil where the contaminated material is mechanically agitated to maintain aerobic conditions through convective air movement and oxygen diffusion. This setup allows for better control of moisture and temperature—two critical factors driven by microbial activity—which in turn accelerates the kinetics of the bioprocess [38].
Despite these technological developments, significant challenges remain in the design and availability of efficient, scalable bioreactors for soil bioremediation. While some systems have improved control over operational variables such as temperature and humidity, there are still no commercially available solutions adaptable to the specific conditions of contaminated soils, particularly in solid-state systems.
The lack of accessible bioreactors capable of effectively managing heat and mass transfer, preventing thermal accumulation, and minimizing heterogeneity continues to hinder the large-scale implementation of these technologies. Moreover, the absence of robust models to accurately simulate transport phenomena and reaction kinetics at different scales further limits their industrialization.
Stirred bioreactors designed for solid-state fermentation have been explored in various configurations, including horizontal and vertical shaft mixers. For example, Nie et al. [40] employed a laboratory-scale stirred aerobic slurry bioreactor (1 L) to optimize bioremediation conditions for TNT-contaminated soils using nutrient amendments and microbial enhancement strategies. Zhang et al. [41] examined blade geometry in spiral mixers and demonstrated that excessive angular velocity led to energy waste and increased shear stress, which can disrupt microbial consortia. While these studies offer valuable insights into mixing efficiency, they often lack experimental validation or do not advance to practical construction. Moreover, most reported designs are not optimized for handling large volumes of heterogeneous, moist solid matrices like contaminated soils. The current study builds upon these findings by implementing a vertical shaft with angled paddles tailored to promote convective mixing without excessive mechanical stress, thus preserving microbial activity while enhancing mass transfer. The DEM simulations complement this design by enabling a quantitative evaluation of mixing performance over time using the Lacey Index.
While Computational Fluid Dynamics (CFD) has been widely applied to simulate mass and heat transfer in liquid-phase and multiphase bioreactors, its applicability to solid-phase systems such as those used in soil bioremediation remains limited. Recent studies have explored CFD in packed-bed and wave-mixed reactors—such as the work by Pessoa et al. [42], who used CFD to simulate temperature and moisture dynamics in solid-state fermentation, and Wen et al. [43], who applied CFD to investigate oxygen transfer in wave-mixed systems. However, these models focus primarily on fluid behavior or gas–liquid interactions and often neglect particle-scale dynamics critical in solid-phase processes.
This study addresses this gap by presenting the complete design, construction, and DEM-based evaluation of an aerated accumulation bioreactor intended for solid-phase bioremediation. The reactor’s configuration incorporates a vertical shaft paddle mixer designed to enhance the distribution of solids and air within a confined volume. Using DEM simulations, this study evaluates the spatiotemporal evolution of mixing through the Lacey Index, while also validating key geometric design decisions. No previous studies have presented the complete design, engineering construction, and DEM-based validation of an aerated accumulation bioreactor specifically developed for solid-phase bioremediation processes. Unlike previous works limited to simulation or lab-scale devices, this research provides engineering drawings, construction criteria, and operational parameters for a fully functional system. In doing so, it offers both a novel methodological approach and a practical contribution to sustainable waste treatment technologies.
2. Materials and Methods
2.1. Design of the Aerated Accumulation Bioreactor
The design of the bioreactor was based on principles of solid-state fermentation and soil bioremediation, with criteria focused on optimizing mass and heat transfer, substrate homogeneity, and efficient aeration through mechanical agitation.
The system geometry was defined as a horizontal vessel with a circular bottom, constructed from stainless steel. To achieve aerated accumulation, an impeller consisting of a horizontal shaft with paddles inclined at 45° was incorporated. These paddles were alternately positioned along the shaft to promote a combined radial and axial mixing pattern.
The aeration system was designed as a passive aeration mechanism based on mechanical turning. Additionally, to ensure thermal stability during operation, a convective heating system was implemented. This consisted of a water tank with heating plates and a thermostat, allowing precise temperature control within the bioreactor.
As an initial validation phase, an experimental prototype with a volume of 8.3 × 10−3 m3 was built. It was equipped with thermal control and turning systems to evaluate its scalability and performance under operational conditions.
2.2. Geometric Design Development
The geometric modeling of the bioreactor was performed using the Design Modeler module of Ansys Fluent 2024 R2 (Ansys, Inc., Canonsburg, PA, USA), which enabled the generation of three-dimensional models and verification of the system’s individual components.
The geometric model was constructed based on specified dimensional relationships between the bioreactor body and the impeller.
2.3. Simulation of the Bioreactor Using the Discrete Element Method (DEM)
The mixing dynamics of the aerated accumulation bioreactor were simulated using the Discrete Element Method (DEM) in Rocky DEM 4.5 (Granular Dynamics International, LLC, Louisville, CO, USA). The three-dimensional geometric model of the bioreactor, including the impeller, paddles, and internal surfaces, was created in Autodesk Inventor Professional, version 2021 (Autodesk Inc., San Rafael, CA, USA), and discretized into triangular meshes. A monodisperse distribution of spherical particles of diameter 0.01 m was used to represent the behavior of the solid granular material, with physical properties calibrated from the literature. The simulation input parameters are listed in Table 1.

Table 1.
Simulation input parameters.
The geometry of the bioreactor and its internal components was imported into Rocky DEM in STL format and discretized into triangular surface elements (Figure 1a), which serve as contact boundaries in the DEM environment. No volumetric meshing was required, as particle interactions are governed by contact dynamics with the STL surfaces. The resulting surface mesh consisted of 26,803 triangular elements and 8495 nodes, with a minimum edge length of 1.0 × 10−3 m and an average surface area of 4.43 × 10−3 m2 per element. Adaptive sizing was applied with fine curvature resolution and smooth transitions to accurately capture the reactor contours. Mesh quality was validated using built-in checks, with a target skewness of 0.9 and medium smoothing. This mesh, based solely on surface discretization, was used to define particle–wall interactions in the DEM environment, without volumetric elements or CFD coupling [43].
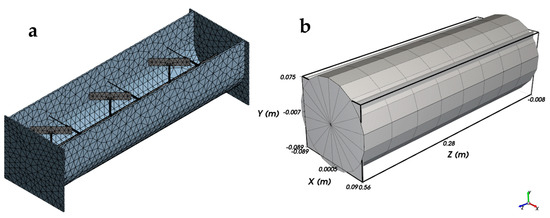
Figure 1.
(a) Triangular surface discretization (STL mesh) of the aerated accumulation bioreactor imported into Rocky DEM. (b) Eulerian grid defined for statistical analysis of particle distribution and mixing, with 1 radial division, 18 tangential divisions, and 8 axial divisions.
Additionally, a Eulerian grid was defined within the domain using 1 radial division, 18 tangential divisions, and 8 axial divisions (Figure 1b), which allowed spatial computation of local particle concentrations. The particle bed was composed of 10,000 spherical particles with a uniform diameter of 5 mm, modeled using the Hertz–Mindlin contact model.
The translational and rotational motion of each particle are tracked by solving Newton’s equations of motion, which are expressed in Equations (1) and (2) [43],
where , , , , denote the mass, linear velocity, angular velocity, and moment of inertia of particle , respectively. The vector represents gravitational acceleration. The terms and correspond to the normal and tangential contact forces between particles (or between particles and walls). The torques and are the contributions from tangential forces and rolling resistance, respectively.
In this work, particle interactions were modeled using the Hertz–Mindlin contact law with rolling friction, which accounts for elastic deformation, damping, and interparticle friction. This model allows an accurate representation of normal-tangential contact behavior and rotational constraints due to surface roughness.
The timestep employed was 9.58 × 10−6 s, determined automatically to maintain contact resolution accuracy. The simulation ran for 60 s of real time, using a time interval of 0.05 s for data output. Solver curves were exported every two data intervals. The simulation was executed on a system with an Intel Core i7-9700 CPU (3.00 GHz), 32 GB RAM (Intel Corporation, Santa Clara, CA, USA), and Windows 11 Enterprise OS (Microsoft Corporation, Redmond, WA, USA), using 4 CPU cores. Total computational time per simulation was approximately 9.5 h. The system did not require volumetric meshing or CFD coupling, and convergence was achieved based on contact overlap stabilization (<3% over 5000+ time steps).
2.4. Evaluation of Mixing Efficiency
The filling volume was set to 85% of the bioreactor’s working capacity, and the impeller rotation speed was fixed at 1 rad/s to simulate operating conditions representative of intermittent aeration. Particle-to-particle and particle-to-wall interactions were modeled using the Hertz–Mindlin contact model with limited sliding. A stability criterion was applied to determine the simulation time step, which was limited to 30% of the critical value estimated from collision analysis. Simulations were run for 60 physical seconds, with positions, velocities, and contact data recorded every 0.05 s.
To quantify the degree of homogeneity achieved during mixing, the Lacey Mixing Index () was used [40]. This statistical metric is based on the spatial variance in species concentration. In the simulation, particles were assigned to two color-coded classes (blue and pink) to represent an initial condition of complete segregation. The evolution was computed based on periodic sampling of local composition across a 3D cubic mesh.
The was calculated as shown in Equation (3):
where is the observed variance in local concentration, and is the maximum expected variance under complete segregation. The data were analyzed over time to identify the stabilization point of the system and define the optimal mixing time. Additionally, direct visualization through snapshots from different angles (front, lateral, and bottom views) was used to validate the quantitative results and observe particle accumulation patterns or dead zones within the bioreactor.
2.5. Assembly and Construction
The main structure of the reactor was manufactured using high-precision laser cutting and TIG (Tungsten Inert Gas) welding. All joints were inspected for airtightness and mechanical integrity. The agitation system was assembled using a set of paddles mounted on a rotating shaft, supported by low-friction bearings.
3. Results and Discussion
The design and construction of the bioreactor were conceived with the goal of optimizing efficiency in bioremediation processes, taking into account key principles of mass transfer, aeration, and thermal control. To evaluate its performance, critical operational conditions were analyzed, with particular emphasis on the application of ex situ remediation techniques, the implementation of bioaugmentation and biostimulation strategies, and the effectiveness of bioventing for contaminant degradation.
This section presents the results obtained, highlighting the effectiveness of the bioreactor under the established operating parameters. The implications of these findings are discussed in the context of bioremediation, as well as the system’s potential scalability for industrial and environmental applications.
3.1. Results of the Bioreactor’s Basic Design
The aerated accumulation bioreactor developed in this study was designed according to the concept of a biological solid-phase bioreactor. The system, illustrated in Figure 2, consists of a horizontal vessel with a circumferential bottom and a paddle-type agitator for solid materials. This mixing system is composed of one or more horizontal, vertical, or inclined paddles attached to a horizontal shaft, which rotates axially within the vessel. As a result, the material being mixed is pushed or dragged along a semicircular path following the trajectory of the paddles, moving faster than the material located between them.
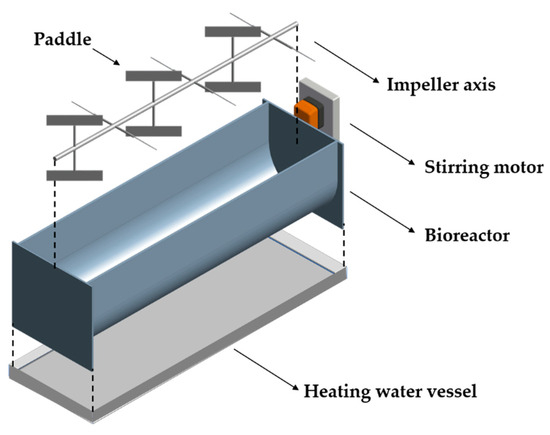
Figure 2.
Isometric view of the aerated accumulation bioreactor, detailing the geometry of the mixing paddles.
The symbolic representation of the dimensions used in the geometry of the aerated accumulation bioreactor is presented in Figure 3.
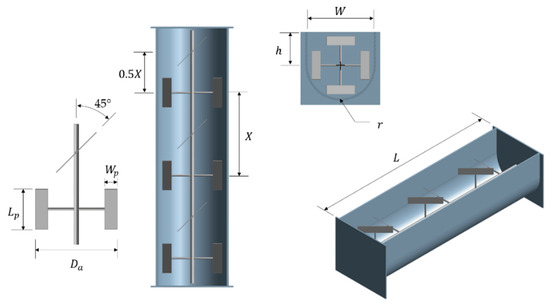
Figure 3.
Symbolic representation of the dimensions used in the geometry of the aerated accumulation bioreactor.
Table 2 shows the geometric relationships and equations for the sizing of the aerated accumulation bioreactor.

Table 2.
Geometric relationships and equations for the sizing of the aerated accumulation bioreactor.
The bioreactor tank was designed with a circular bottom, which—together with the use of close-clearance paddles—helps reduce dead zones during the bioprocess, especially when the target contaminant is in solid form. In contrast, for liquid contaminants, dead zones in bioreactors are typically overcome using diffusers, where key parameters such as diffuser height relative to the total tank height (approximately 5%) and flow velocity play crucial roles [41].
Given that no bioreactor design matching the one proposed in this study was found in the reviewed literature, rotary reactors were consulted as a reference. These systems are closed, horizontally oriented cylindrical vessels, widely used for granular mixing in various industries due to their simple geometry and versatile applications [42]. Particle mixing within these reactors is influenced by several parameters, including static and dynamic angles of repose, particle shape and size, bulk density, Young’s modulus and shear properties, coefficient of restitution, and the fill fraction. The Lacey Mixing Index is one of the most used metrics for quantitatively assessing the degree of granular mixing in rotary drums and has been widely employed to evaluate and compare mixing efficiency [40].
Additionally, paddle-type impellers were used in the bioreactor design, as their rotation within curved vessels has been shown to enhance heat transfer, promote uniform temperature distribution, and improve particle mixing [30].
The aerated accumulation bioreactor can be conceptualized as half of an open rotary drum bioreactor, which facilitates material handling and eliminates the need for complex sealing and agitation systems typically required in closed rotary reactors.
3.2. DEM Simulation Results for the Aerated Accumulation Bioreactor
Figure 4 illustrates the dynamic mixing process within the aerated accumulation bioreactor, simulated using the Discrete Element Method (DEM) over a time span of 60 s. The simulation was conducted with a fill volume of 85% and an impeller rotation speed of 1 rad/s. At the initial time (t = 0 s), a clear segregation is observed between the blue and pink particles, indicating a static system with no phase interaction. As time progresses, the motion induced by the paddles initiates convective flow, gradually dispersing both phases.
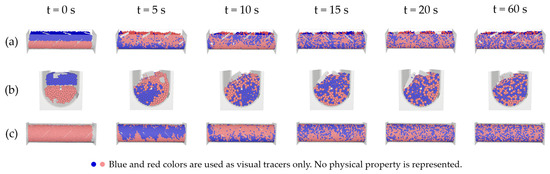
Figure 4.
Mixing evolution in the aerated accumulation bioreactor during the first 60 s of simulation using the Discrete Element Method (DEM). The images show three different views of the system: (a) front view, (b) side view, and (c) bottom view. The two colors represent identical particles initialized with distinct tags to visually evaluate mixing and segregation behavior.
In Figure 4, colors do not represent a physical parameter but indicate identical particles initialized with distinct tracers to assess spatial segregation. Since all particles have identical properties, the distribution may be interpreted equally as mass or volume fraction.
From t = 10 s onward, partial mixing is observed, along with the emergence of zones with greater local heterogeneity—a typical feature of solid-state systems, where granular dynamics are strongly influenced by vessel geometry and impeller design. At t = 20 s and t = 60 s, the system reaches a more homogeneous distribution; however, lateral accumulation patterns persist along the reactor walls, due to wall friction and preferential transport caused by the paddle design.
The front (Figure 4a), side (Figure 4b), and bottom (Figure 4c) views enable the identification of zones with higher or lower mixing efficiency and provide visual confirmation of the system’s dynamic behavior. These findings are critical for understanding how geometric and operational design factors influence mixing quality, which is essential for optimizing the bioremediation process.
To evaluate the impact of paddle inclination on mixing performance, simulations were conducted at four different angles: 0°, 15°, 45°, and 55°. The evolution of the Lacey Mixing Index () as a function of time is presented in Figure 5. This index is a widely used metric for evaluating the progress of granular mixing, where a value of 1 represents a completely random (optimal) mixture, and a value near 0 indicates total segregation.
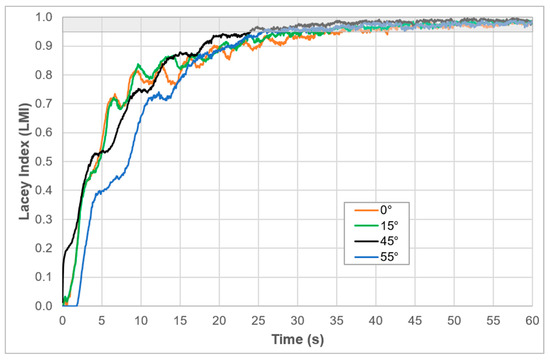
Figure 5.
Evolution of the Lacey Mixing Index () over time for different paddle inclination angles (0°, 15°, 45°, and 55°) in the aerated accumulation bioreactor. The grey-shaded area indicates the mixing threshold above 95% homogeneity ( ≥ 0.95), commonly reported in the literature as the criterion for acceptable mixing performance in particulate systems.
The results reveal significant differences in mixing kinetics across the evaluated angles. The paddle inclined at 45° (black line) consistently outperformed the other configurations, achieving an above 0.95 in less than 15 s, and reaching nearly complete homogeneity well before the 30 s mark. This rapid convergence toward ideal mixing conditions demonstrates that a 45° inclination facilitates efficient radial and axial movement of particles, enhancing dispersion and minimizing segregation.
The 15° and 0° configurations also exhibited satisfactory performance, although their trajectories fluctuated more and required longer periods (~25–30 s) to stabilize within the 95% homogeneity zone. In contrast, the 55° paddle angle (blue line) showed the slowest mixing response, with a delayed rise in the and longer residence in the suboptimal mixing range, particularly in the first 20 s. This behavior may be attributed to reduced radial displacement and an increased tendency for layered particle flow at higher inclinations.
These results highlight the sensitivity of solid-phase mixing to agitator geometry, particularly paddle angle, and validate the use of DEM simulations to optimize mechanical design choices. Among all tested configurations, the 45° design offers the best compromise between speed and mixing quality, supporting its selection for the construction of the pilot-scale bioreactor. This finding is also consistent with previous studies that emphasize the importance of paddle geometry in enhancing convective mixing in confined volumes of granular media.
In addition to the visual validation of mixing efficiency through the time-lapse views and the quantitative assessment via the Lacey Index, the results are further reinforced when compared with previous studies on horizontal paddle mixers. For instance, Wen et al. [43] reported that in monodisperse systems, mixing performance is highly dependent on loading configuration and fill level, with residual values above 10% even after 60 s of operation at 40 RPM. In contrast, the present study achieved an value greater than 0.98 at 60 s with an 85% fill level—demonstrating higher efficiency despite the increased occupied volume, which typically poses a challenge due to limited particle mobility.
Moreover, Füvesi et al. [44] noted that continuous mixing conditions require equivalent residence times close to 180 s to approach random mixing in polydisperse systems. This highlights the effectiveness of the intermittent mixing strategy proposed here for batch or semi-continuous operations. The optimized impeller design, which combines angular inclination and alternating blade arrangement, appears to play a key role in inducing unpredictable particle trajectories that accelerate the breakdown of segregated fronts [45]. This behavior was not observed in mixers with more conventional geometries, as reported by Emmerink et al. [46] and Wen et al. [43], where mixing is more dependent on the number of rotations than on the local flow efficiency.
The following subsections are intended to contextualize the engineering design presented in this study within its practical application to soil bioremediation. While Section 3.1 outlines the geometric characteristics of the proposed aerated accumulation bioreactor and Section 3.2 describes the internal mixing dynamics simulated via DEM, the subsequent Section 3.3, Section 3.4 and Section 3.5 focus on the operational and biological principles that underpin the reactor’s functionality. These include the rationale for selecting ex situ remediation as a guiding strategy, as well as the integration of bioaugmentation, biostimulation, and bioventing protocols tailored to solid-phase treatment. Additionally, environmental variables such as nutrient availability, pH, moisture, and aeration are examined as critical factors for ensuring the effectiveness of the bioprocess. These sections serve to bridge the reactor’s structural design with its intended use in field applications, highlighting its viability as a scalable and adaptable solution.
3.3. Ex Situ Technique in the Bioreactor Design
The bioreactor was designed to operate under the ex situ remediation approach, which involves the removal of contaminated media to a separate treatment area [1]. This technique is used to clean polluted sites by relocating contaminants without disturbing the original location [10,19,28,47,48,49]. It is especially suitable when the contaminants have not reached the groundwater table or do not significantly impact subsurface layers [34].
The efficiency of this technique depends on various factors, including degradation and transport costs, environmental regulations, social impact, the type of pollutant, and site-specific characteristics [1,19,28,48].
Ex situ solid-phase bioremediation involves treating contaminated soil above ground using containment systems to prevent the release of pollutants into the environment [22,49]. Parameters such as moisture content, oxygen supply, nutrient concentration, and heat are carefully managed to facilitate the degradation process [28]. This technique is applicable to organic waste such as leaves, manure, and agricultural residues, as well as problematic waste streams like household and industrial waste and sewage sludge or municipal solid waste [22].
Ex situ methods are often preferred for safe, rapid, and effective remediation, especially in soils contaminated with persistent and hazardous substances. These methods help minimize secondary contamination in surrounding environments and reduce ecotoxic risks. They are particularly advantageous in soils with low hydraulic conductivity, low permeability, and high organic matter content, as well as in cold climates or adverse environmental conditions that hinder in situ bioremediation [12]. Ex situ bioremediation can be implemented through systems such as land farming, composting, biopiles, aerated static piles, and slurry-phase bioreactors [46,50]. These approaches are generally more effective than alternative techniques in removing a wide variety of contaminants, in shorter times and with better operational control, resulting in higher biodegradation rates [46]. This technique is commonly used to treat pesticides and toxins [49].
Ex situ bioremediation methods offer several advantages over in situ approaches in terms of environmental control, leading to faster biodegradation rates. This technique is increasingly used due to its cost-effectiveness and the ability to homogenize contaminated soil, making the process more uniform and time-efficient [1]. The ex situ method also allows for the regulation of nutrients, pH, and the use of exogenous microorganisms [12,14], enhancing mass transfer and improving biodegradation kinetics [46]. Several studies have demonstrated the successful application of ex situ strategies to treat petroleum-contaminated soils in cold environments [48].
Bioreactors provide ideal conditions for microbial growth, enabling the ex situ bioremediation of contaminants [14,50], and can operate in batch, semi-continuous, or continuous modes [31,48].
Biological reactions can be triggered and controlled within the bioreactor system [14]. Bioreactor designs can maximize microbial degradation while minimizing abiotic losses due to their operational flexibility [14]. Bioreactors are widely considered to be among the most effective methods for treating contaminated soils, owing to their ability to control operational parameters and enhance microbial biodegradation activity [1]. Furthermore, bioreactors support normal cellular processes by simulating and maintaining a natural environment that fosters optimal microbial growth conditions [31].
3.4. Bioremediation Operations
To stimulate and enhance microbial activity—and, consequently, the bioremediation process—microorganisms (bioaugmentation), nutrients (biostimulation), air (bioventing), and organic substrates or other electron donors/acceptors can be added to the system [21,26,51].
3.4.1. Bioaugmentation
Bioaugmentation is a method used in bioremediation to accelerate the decomposition or degradation of compounds in contaminated environments [31,47,50,52]. It is typically applied when the native microbial content in the material is low (less than 105 microorganisms/g of dry material) or when the present microorganisms lack the ability to degrade the target contaminants [51].
Bioaugmentation involves the addition of microbial strains with high catalytic activity to enhance contaminant degradation [27,31,47,48,52,53,54,55,56,57]. These strains may consist of microbial consortia, genetically modified microorganisms, or vectors containing degradation genes that supplement the existing microflora [47,48,55,56,58,59]. The introduction of microbial biomass into contaminated areas can significantly improve biodegradation performance [60].
The inoculated microorganisms can be either endogenous or exogenous [22,47,55]. Endogenous microorganisms are naturally present in the contaminated soil and may already possess the ability to use the contaminant as a carbon and energy source, albeit inefficiently or at low concentrations. These microbes can be isolated and cultivated in the lab to enhance their growth [55,58], and later reintroduced into the contaminated site at higher concentrations. This reinoculation can significantly increase the degradation of specific contaminants [55,58].
In cases where the soil lacks native microorganisms capable of degrading specific contaminants, exogenous microbes must be introduced. These can be isolated from other contaminated environments and applied to the affected sites [57]. The success of these interventions depends on the ability of the introduced microorganisms to compete with indigenous species and survive under abiotic conditions [58].
Genetically modified microorganisms can also be used, engineered with genes encoding enzymes for specific contaminant degradation [57,61]. However, the instability of their genetic material presents a challenge during application [48].
Using microbial consortia or mixed cultures for bioaugmentation is considered more advantageous than relying on single strains. Consortia offer broader enzymatic and catabolic capabilities, enabling more complete degradation of contaminants [54]. These organisms can adapt to a variety of environmental conditions—including fluctuations in temperature and pH—and utilize accessible nutrients such as carbon, nitrogen, phosphorus, and oxygen, facilitating the remediation process [31].
Numerous studies have shown that microbial consortia outperform single strains in contaminant removal. In addition, strains isolated from contaminated sites have been found to be more effective in bioremediation [58]. For example, Rendón-Castrillón et al. [62] used sewage sludge from wastewater treatment plants as a microbial source for bioaugmentation, while Jabbar et al. [31] employed mixed fungal strains isolated from petroleum-contaminated soils.
The effectiveness of bioaugmentation depends on several factors, including soil type, moisture content, aeration, temperature, pH, and organic matter content [63]. These abiotic parameters must be considered when designing a bioaugmentation strategy [64]. Extreme pH values and high temperatures can negatively affect bacterial and fungal viability. Additionally, organic matter levels influence contaminant bioavailability and microbial activity. One study showed that aromatic carbon in humic acids, along with total organic matter, is essential for the activity and survival of Achromobacter xylosoxidans during PCB degradation. Intermediate levels of organic and aromatic carbon were found to yield better results. Nevertheless, some contaminant characteristics can limit microbial activity and hinder mass transfer during bioaugmentation [58].
Bioaugmentation success also hinges on biotic factors, such as competition between native and introduced microorganisms for limited carbon sources, antagonistic interactions, and predation by protozoa or bacteriophages. It is important to note that not all individuals of a given genus are equally adapted to every task; some may thrive across a wide range of environments, while others are specialized for narrow ecological niches [58].
Actinobacteria are frequently used for bioaugmentation due to their ability to degrade pollutants and adapt to various soil types [52]. Research has primarily focused on microbial species such as Flavobacterium, Pseudomonas, Sphingomonas, Lysinibacillus sp., Alcaligenes, Rhodococcus, Achromobacter, Bacillus, and Mycobacterium. Microbial consortia involving fungi and bacteria have also been explored, including fungal genera such as Acremonium, Absidia, Aspergillus, and Penicillium, and bacterial genera like Alcaligenes, Bacillus, Flavobacterium, Mycobacterium, Pseudomonas, and Sphingobium [31].
Bioaugmentation has proven especially effective for organic contaminants [65], chlorinated soils [10], and has been successfully applied to cases involving benzene, toluene, ethylbenzene, xylene (BTEX), and metallurgical effluents [61].
3.4.2. Biostimulation
Biostimulation is the process of biologically stimulating the native microbial activity through the addition of nutrients to the soil—such as nitrogen, carbon, and phosphorus [1,11,47,59,66]—which promotes the development of diverse microbial species already present in the soil but previously inhibited by the contaminants [59]. Some stimulating agents are typically applied underground through injection wells [1].
Biostimulation involves the addition of electron donors, electron acceptors, nutrients, and water [16,22,48,50,52,58], all essential for microbial survival by providing energy, enzymes, and microbial populations capable of degrading pollutants [59].
Various organic residues from agricultural and agro-industrial activities are used as biostimulants, including wood chips, straw, sugarcane filter cake, and orange peel, due to their high nutritional content and their ability to improve soil properties [52]. Additionally, manure, urea, compost, and inorganic fertilizers can also be used as nutrient sources for biostimulation [31].
Biostimulation is considered an effective solution due to its ease of implementation and management, as it utilizes well-adapted indigenous microorganisms already distributed in the environment [1,16,31]. It allows for the optimization of site-specific conditions such as aeration, pH, and temperature, or the addition of organic or inorganic nutrients to enhance contaminant degradation [27]. It is an affordable process that increases the bioavailability of microbial communities by improving the soil’s nutrient content [31].
In bioremediation, bioaugmentation can also be combined with biostimulation to enhance the capabilities of the existing microbiota and improve the overall efficiency of the bioprocess [50].
Both bioaugmentation and biostimulation require effective mixing, which significantly influences the efficiency of biochemical reactions [62]. However, optimizing the performance of a mixer is a critical task for industries involved in particle processing. Achieving higher product homogeneity in a shorter time is often the main goal when optimizing mixer performance [67].
Efficient mixing is essential for achieving complete homogenization of process substances within a reactor, ensuring that all reactive components can interact uniformly to produce the desired product. Furthermore, the level of homogeneity in a system significantly affects heat transfer. An ideal system can maintain a constant temperature throughout the reactor volume and respond rapidly to changes [68].
In solid-state bioprocesses, mixing is so critical that some authors focus specifically on analyzing the parameters of various mixer configurations, including blade spacing, wall clearance, and blade width and angle [67].
Therefore, the aerated accumulation bioreactor in this study was designed with a paddle system constructed from stainless steel. The paddle system consists of six pairs of longitudinal plow-like blades perpendicular to the shaft, each rake inclined at 45° and arranged in alternating positions, as shown in Figure 2.
Additionally, the paddles were designed with narrow clearance, which is advantageous among the various types of impellers commonly used in industrial applications [68].
The paddle system is driven by a geared motor consisting of a shaded-pole induction motor rated at 30 watts and 110 volts AC, coupled with gear mechanisms that reduce the speed from 150 rpm to 5 rpm. The central paddle shaft is mounted using a bearing system.
Emmerink et al. employed a twin-shaft, batch-type paddle mixer. The shafts are horizontally aligned, each equipped with 14 paddles uniformly distributed in 4 radial quadrants. The paddles are configured to direct the granular material radially toward the center of the mixing chamber and axially to move in a circular pattern throughout the chamber [46].
To prevent buildup in the corners of the mixing chamber, the last two paddles are positioned in the opposite direction with a 15° inclination and function as “transfer paddles”. To avoid collisions between the paddles on the two shafts, a 45° phase angle is applied [45].
The presence of baffles can guide the fluid to enhance mixing effectiveness by increasing the turbulence and directional flow. Properly designed baffles allow precise control of mixing intensity to meet specific application requirements, reduce flow inhomogeneities, and ensure more uniform blending [62].
3.4.3. Bioventing
Bioventing is a process used to stimulate the bioremediation of soil contaminants by supplying air or oxygen to native soil microorganisms, either through the injection of a controlled airflow or by turning the material to enhance microbial activity [14,22,24,28,48,69].
Conventional bioventing involves the injection of low-flow air using blowers to promote the microbial degradation of organic compounds while minimizing the volatilization and release of contaminants into the atmosphere [22,24,48,69,70]. Bioventing is sometimes combined with biostimulation through the addition of nutrients such as phosphorus or nitrogen to support microbial growth [10,31].
Bioventing can be carried out using either active or passive aeration. In the active method, air is injected into the soil using a blower. Conversely, the passive method relies on gas exchange through ventilation wells driven by atmospheric pressure. The duration of bioventing-based bioremediation depends on factors such as contaminant type and concentration, biodegradation rates, and soil characteristics, including permeability and moisture content [59].
Additionally, bioventing often involves the injection of humidified air to maintain adequate moisture levels in the treated soil, since soil moisture and water retention are key limiting factors in bioprocesses. Excessive moisture reduces air permeability and impedes gas transmission, while insufficient moisture inhibits microbial metabolism and activity [69].
Bioventing has demonstrated efficacy in the bioremediation of soils contaminated with petroleum-derived products [1], the degradation of organic compounds in agricultural fields [70], and the treatment of hazardous waste in vadose zone soils [71]. It is also effective for simple hydrocarbons [31], and BTEX compounds (benzene, toluene, ethylbenzene, and xylene). Furthermore, bioventing can be combined with soil vapor extraction (SVE), which uses higher airflow rates [48].
In the design of the aerated accumulation bioreactor, aeration is provided by turning the material using backhoes, shovels, or paddle systems, depending on the size of the bioreactor [72].
Agitation or mixing plays a role equivalent to aeration. Agitation is a viable alternative for addressing heterogeneity in solid systems, potentially improving homogeneity and mitigating gradients. Another benefit of agitation is the more uniform distribution of airflow, which enhances conditions for microbial growth throughout the treatment bed [73].
In these systems, where agitation is used as a means of aeration, low agitation speeds are essential, since high speeds can negatively affect mycelial development and shear forces may cause cellular damage [73].
The aerated accumulation bioreactor was designed with sequential control, implemented via a PLC system (LOGO! 230RC, Siemens AG, Munich, Germany), responsible for managing the mixing time for each bioreactor. The mixing schedule consisted of intermittent turning cycles, initially programmed for 10 min intervals during laboratory-scale bioremediation trials.
Two aerated accumulation bioreactors were designed and connected to the same control system to allow for duplicate trials, as illustrated in Figure 6.
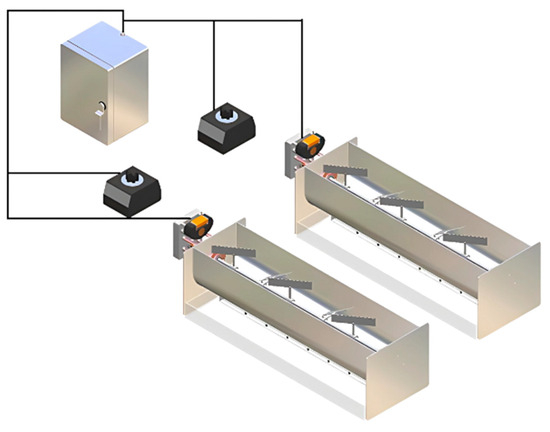
Figure 6.
Complete system of the aerated accumulation bioreactor and its basic components.
The bioreactor was configured to operate with intermittent agitation. When such agitation is applied at appropriate intervals, it does not hinder mycelial development or soil particle mixing. Any forming agglomerates are rapidly broken apart [74].
Studies on solid-state agitation recommend initiating mixing early in the bioprocess to break down agglomerates before they harden and to ensure proper distribution of inoculum, nutrients, and moisture uniformity [74].
This on/off control strategy ensures effective mixing with improved energy efficiency and eliminates the need for complex operational procedures or parameter tuning during the process.
In bioremediation processes, appropriate bioreactor selection and the adjustment of operational conditions are critical to success, as detailed in the following section.
3.5. Operational Conditions
For effective contaminant degradation, microorganisms involved in the bioremediation process must come into contact with compounds that provide the energy and nutrients necessary for their growth [75,76,77,78]. Several factors influence bioremediation, including physical, chemical, and biological parameters, the type of soil, sources of carbon and nitrogen, and the nature of the microorganisms—whether individual strains or microbial consortia [14]. These factors must be properly adjusted to meet the operational requirements of the bioremediation process [25], including nutrients, pH, temperature, moisture, intrinsic permeability, contaminant concentration, inoculum, and additives.
3.5.1. Nutrients
Nutrients are classified into macronutrients, micronutrients, and trace elements, depending on the quality and requirements of the microorganisms for bioremediation [79]. Microbial cells require three main macronutrients: carbon, phosphorus, and nitrogen, which represent about 14% of their dry weight and are essential for microbial growth and metabolic activity [79,80]. Therefore, a sufficient supply of nutrients is needed to support microbial proliferation and function. In some cases, the performance of bioremediation systems can be enhanced by being supplementing with specific nutrients or organic amendments [80]. A minimum organic matter content of 40% and a C:N ratio below 50 are recommended to ensure rapid biodegradation [81]. The suggested C:N:P ratio for bioremediation ranges from 100:10:1 to 100:5:1, which corresponds to the typical nutrient requirements of microorganisms [82,83].
3.5.2. pH
Soil pH variations can significantly affect the bioremediation process by influencing microbial efficiency. Microorganisms operate within an optimal pH range, and deviations from this range can reduce their effectiveness [73]. Therefore, a pH range between 6 and 8 is recommended [79], with a value close to neutral being the most desirable [31,81]. The degradability of compounds depends on enzymes, which are themselves affected by pH. For example, fungi may degrade hydrocarbons more effectively at pH 7 than bacteria, which may only be active at pH 5 [79].
3.5.3. Temperature
Temperature is a key factor in the efficiency of bioremediation [80] because microorganisms generate metabolic heat during the bioprocess. Solid media usually have low thermal conductivity, which can lead to heat buildup and harmful thermal gradients [84]. Each microbial species has specific temperature requirements for growth and metabolic activity [80]. Generally, higher temperatures accelerate biodegradation rates, while lower temperatures slow them down [79].
Microorganisms are most efficient within a certain temperature range. For instance, filamentous fungi and yeasts thrive at temperatures between 25 °C and 30 °C [84]. A temperature increase of just 5 °C can significantly enhance the biodegradation rate of petroleum hydrocarbons. Elevating temperatures into the mesophilic and thermophilic range (20–60 °C) can notably improve bioremediation outcomes [85].
On the other hand, excessive temperatures can cause irreversible damage to microorganisms due to heat stress. Temperature fluctuations can also impact the adsorption and desorption of pesticides and heavy metals in soil, alter microbial activity, and change the physicochemical properties of contaminants. For example, higher temperatures can increase the solubility of hydrophobic contaminants, enhancing their bioavailability, whereas low temperatures may inhibit the transition of contaminants from the solid to the liquid phase [73].
For the proposed aerated accumulation bioreactor, a dedicated heating system was designed to ensure adequate thermal regulation during the bioremediation process. This system includes a reservoir tank constructed from acrylic (ethyl polymethacrylate), which serves as a water container that facilitates evaporation through direct heating. The core of the heating mechanism comprises two stainless steel plates, each measuring 540 mm by 192 mm with a height of 39.2 mm, strategically positioned 4 mm below the base of the mixing chamber. This narrow separation allows for the generation of steam via electrical conduction through the water, as current preferentially travels through the liquid medium rather than the metal plates. Electrical energy is thus, transformed into heat, producing vapor as the current passes between electrodes. To maintain proper conductivity, and therefore consistent heating performance, salts may be added to the water, or the system may be purged depending on its initial conditions.
Temperature control is achieved through an integrated system composed of a thermistor and a controller. The thermistor functions as a temperature-sensitive resistive element that detects thermal variations and transmits resistance-based signals to the controller. This controller, positioned at the lower portion of the tank, executes three sequential tasks: it receives the signal from the thermistor, compares the measured value against a user-defined temperature setpoint, and activates or deactivates the power supply to the heating unit via a breaker based on the comparison outcome. This setup allows for precise and automated regulation of both temperature and humidity within the reactor.
Previous studies involving large-scale applications of aerated accumulation bioreactors for the treatment of hydrocarbon-contaminated soils have demonstrated that additional water input was unnecessary during the bioprocess. The internal temperature of the system increased spontaneously and remained consistently 10 to 15 °C above ambient conditions. Moreover, these thermally controlled environments supported significantly higher concentrations of aerobic microorganisms when compared to conventional landfarming treatments, highlighting the relevance of this design approach for effective bioremediation [86].
3.5.4. Moisture
Moisture is a critical factor in the bioremediation process, particularly in solid-phase biotreatment systems, where the water content of the medium influences the rate of microbial growth and propagation in soils—ultimately determining the performance of the bioprocess [84]. Excessive moisture can reduce soil air permeability and hinder gas transfer, while insufficient moisture may inhibit biomass metabolism and microbial activity [73]. A moisture range between 30 and 50% is recommended [81], although the optimal level depends on the specific soil. Furthermore, high moisture content can reduce the porosity of the substrate, which in turn limits the surface area available for microbial attachment. On the other hand, low moisture levels are associated with decreased enzymatic activity and total protein content, leading to reduced bioremediation efficiency [87].
Filamentous fungi that produce biosurfactants, such as A. fumigatus and T. versicolor, and yeasts such as S. bombicola, require an optimal moisture content between 44.7% and 50%. Bacteria generally demand higher moisture levels than fungi and yeasts, with water activity requirements ranging from 0.8 to 0.9 [84].
The design of the aerated accumulation bioreactor promotes moisture retention, heat conservation, and limited oxygenation. Consequently, it leads to an increase in system temperature, accelerated microbial growth rates, higher concentrations of active microbial biomass, and enhanced overall biodegradation kinetics. Additionally, this system minimizes water loss due to evaporation.
3.5.5. Contaminant Concentration
The presence of contaminants in soil can significantly affect biodegradation efficiency. Both excessively low and high concentrations can negatively impact the microorganisms responsible for biodegradation. The rate of biodegradation is influenced by the chemical complexity and molecular weight of the contaminants; simpler and lighter compounds tend to degrade faster [73].
When contaminant concentrations are particularly high, the adoption of a fed-batch (FB) reactor strategy is recommended [88].
Fed-batch mode combines batch and continuous processing, allowing for the subsequent addition of substrates—which, in this context, refers to the contaminant itself and/or microorganisms (re-inoculation) [89]. Feeding strategies can be classified into three categories [89].
Feeding strategies in solid-state bioprocesses can be classified based on the type of nutrient input, the timing of additions, and the quantity supplied. In terms of nutrient type, a distinction is made between single and combined feeding strategies. Single feeding involves the addition of a single component—typically a nitrogen source—whereas combined feeding strategies incorporate multiple inputs, such as enzymes and/or microbial consortia, along with the primary substrate to enhance biodegradation efficiency. Regarding timing, feeding may occur at variable or constant intervals. Variable, or pulse, feeding introduces solid supplements at irregular stages throughout the process, allowing for adaptive responses to microbial activity. In contrast, constant interval feeding involves the systematic addition of nutrients, such as nitrogen, at predefined and regular time points. Finally, the feeding quantity can either remain constant or vary across the process. In equal quantity feeding strategies, a uniform amount of nutrient is added at each interval. Alternatively, unequal quantity strategies adjust the amount of nitrogen or other nutrients based on the specific needs or metabolic stages of the process. Importantly, these three dimensions, timing, and quantity—are not mutually exclusive and can be combined simultaneously to tailor the bioprocessing environment for optimal microbial performance.
3.5.6. Inoculum
The effectiveness of the bioremediation process is largely determined by the presence of a suitable microbial population [81]. A higher concentration of viable inoculated cells results in a higher remediation rate and increased productivity. Therefore, it is recommended that the maximum feasible inoculum concentration be used. However, in practice, extremely high inoculum levels are not feasible due to the resulting increase in medium viscosity and the emergence of diffusion limitations. These effects can reduce productivity, as predicted by the bioprocess theory [90]. Typically, the inoculum volume for bioremediation processes is around 20% (v/v) [62,91,92].
3.5.7. Amendments
Amendments are used to improve soil porosity and, depending on the material selected, may also serve to enhance microbial flora, provide nitrogen, or add organic matter [93]. For example, wheat bran is considered one of the most effective agricultural residues for keratin degradation and increasing enzyme production. Additionally, a wheat bran bed enhances air circulation and oxygen transfer, thereby improving heat and mass transfer [87].
A study by Ercoli et al. [38] reported full-scale bioremediation processes using more than 8000 m3 of soil in aerated accumulation bioreactors for treating hydrocarbon-contaminated soils. Compared to landfarming, the bioreactor reduced the time required to lower contaminant concentrations to three months with 58% efficiency, while landfarming required six months to achieve only 25% efficiency.
To guide the effective operation of the proposed bioreactor under real conditions, several key parameters must be quantitatively considered due to their critical influence on microbial metabolism and contaminant degradation kinetics. Optimal moisture content is typically reported between 60 and 70%, which ensures adequate water availability while maintaining porosity for oxygen diffusion [9,31]. Similarly, temperatures in the range of 30–35 °C have been associated with enhanced enzymatic activity and biodegradation rates, particularly for hydrocarbon-contaminated soils [28]. The pH should be maintained within 6.5–8.0 to preserve microbial viability and enzymatic stability [22,32]. In terms of nutrient balance, C/N ratios between 20:1 and 30:1 are considered ideal for promoting aerobic microbial growth without inducing nitrogen limitation [19]. Deviations from these ranges have been shown to reduce biodegradation efficiency by 30–50% in comparable systems [9,19]. These values serve as operational targets in future validation experiments using the constructed reactor.
4. Conclusions
This study presents the design, construction, and DEM-based evaluation of a novel aerated accumulation bioreactor intended for the solid-phase bioremediation of contaminated soils. The reactor’s geometric configuration, particularly the paddle inclination angle, was critically analyzed through discrete element method simulations using the Lacey Mixing Index () as a quantitative performance metric. Among the four configurations tested (0°, 15°, 45°, and 55°), the 45° paddle angle achieved the most efficient mixing, reaching values above 0.95 in under 15 s at 70% reactor filling, indicating excellent homogenization. These results support the selection of this configuration for scale-up. While no biodegradation assays were conducted at this stage, a set of optimal operational parameters is proposed based on the established literature: moisture content between 60 and 70%, temperature around 30–35 °C, pH between 6.5 and 8.0, and C/N ratios from 20:1 to 30:1. These conditions are expected to support microbial viability and enhance contaminant degradation kinetics. The proposed bioreactor offers a scalable alternative for ex situ bioremediation, particularly in scenarios where conventional systems are limited by poor heat and mass transfer. Future work will focus on experimental validation of microbial degradation performance and long-term operational stability under various environmental conditions, further bridging the gap between computational design and field application.
Author Contributions
Conceptualization, M.R.-C., L.R.-C. and C.O.-L.; methodology, M.R.-C., L.R.-C. and C.O.-L.; validation, M.R.-C., L.R.-C. and C.O.-L.; formal analysis, M.R.-C., L.R.-C. and C.O.-L.; investigation, M.R.-C., L.R.-C., V.Á.-F. and C.O.-L.; data curation, M.R.-C., L.R.-C. and C.O.-L.; writing—original draft preparation, M.R.-C., L.R.-C., V.Á.-F. and C.O.-L.; writing—review and editing, M.R.-C., L.R.-C. and C.O.-L.; visualization, M.R.-C., L.R.-C. and C.O.-L.; supervision, M.R.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by the Universidad Pontificia Bolivariana.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DEM | Discrete Element Method |
| BTEX | Benzene, Toluene, Ethylbenzene, and Xylenes |
| PLC | Programmable Logic Controller |
References
- Sales da Silva, I.G.; Gomes de Almeida, F.C.; da Rocha e Silva, N.M.P.; Casazza, A.A.; Converti, A.; Asfora Sarubbo, L. Soil Bioremediation: Overview of Technologies and Trends. Energy 2020, 13, 4664. [Google Scholar] [CrossRef]
- Chernysh, Y.; Chubur, V.; Ablieieva, I.; Skvortsova, P.; Yakhnenko, O.; Skydanenko, M.; Plyatsuk, L.; Roubík, H. Soil Contamination by Heavy Metals and Radionuclides and Related Bioremediation Techniques: A Review. Soil. Syst. 2024, 8, 36. [Google Scholar] [CrossRef]
- Dar, M.A.; Kaushik, G. Biodegradation of Malathion in Amended Soil by Indigenous Novel Bacterial Consortia and Analysis of Degradation Pathway. Soil. Syst. 2023, 7, 81. [Google Scholar] [CrossRef]
- Münzel, T.; Hahad, O.; Daiber, A.; Landrigan, P.J. Soil and Water Pollution and Human Health: What Should Cardiologists Worry About? Cardiovasc. Res. 2023, 119, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Santini, G.; Zizolfi, M.; Santorufo, L.; Memoli, V.; D’Ascoli, R.; Maisto, G. Soil Microbial Biomass and Microarthropod Community Responses to Conventional and Biodegradable Plastics. Soil. Syst. 2024, 8, 92. [Google Scholar] [CrossRef]
- Androudi, M.; Liava, V.; Tsaliki, E.; Ipsilantis, I.; Golia, E.E. Use of Cannabis Sativa L. for Improving Cadmium-Contaminated Mediterranean Soils—Effect of Mycorrhizal Colonization on Phytoremediation Capacity. Soil. Syst. 2024, 8, 100. [Google Scholar] [CrossRef]
- Biswal, T.; Malik, J.A. Effect of Pollution on Physical and Chemical Properties of Soil. In Handbook of Research on Microbial Remediation and Microbial Biotechnology for Sustainable Soil; IGI Global: Hershey, PA, USA, 2021; pp. 1–37. [Google Scholar]
- Kvashuk, A.V. The Effect of Sandy Soil Contamination with Petroleum Products on the Soil’s Physical Properties. Bull. Civ. Eng. 2023, 20, 57–66. [Google Scholar] [CrossRef]
- Stepanova, A.Y.; Gladkov, E.A.; Osipova, E.S.; Gladkova, O.V.; Tereshonok, D.V. Bioremediation of Soil from Petroleum Contamination. Processes 2022, 10, 1224. [Google Scholar] [CrossRef]
- Praveen, R.; Nagalakshmi, R. Review on Bioremediation and Phytoremediation Techniques of Heavy Metals in Contaminated Soil from Dump Site. Mater. Today Proc. 2022, 68, 1562–1567. [Google Scholar] [CrossRef]
- Hernández-Adame, N.M.; López-Miranda, J.; Martínez-Prado, M.A.; Cisneros-de la Cueva, S.; Rojas-Contreras, J.A.; Medrano-Roldán, H. Increase in Total Petroleum Hydrocarbons Removal Rate in Contaminated Mining Soil Through Bioaugmentation with Autochthonous Fungi During the Slow Bioremediation Stage. Water Air Soil. Pollut. 2021, 232, 95. [Google Scholar] [CrossRef]
- Balseiro-Romero, M.; Monterroso, C.; Kidd, P.S.; Lu-Chau, T.A.; Gkorezis, P.; Vangronsveld, J.; Casares, J.J. Modelling the Ex Situ Bioremediation of Diesel-Contaminated Soil in a Slurry Bioreactor Using a Hydrocarbon-Degrading Inoculant. J. Environ. Manag. 2019, 246, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Rosatelli, A.; Prasad, S.; Gomez, F.H.; Sbaffoni, S.; Franzetti, A.; Vaccari, M. Ex-Situ Bioremediation of Petroleum Hydrocarbon Contaminated Soil Using Mixed Stimulants: Response and Dynamics of Bacterial Community and Phytotoxicity. J. Environ. Chem. Eng. 2022, 10, 108814. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Cara, I.G.; Țopa, D.; Puiu, I.; Jităreanu, G. Biochar a Promising Strategy for Pesticide-Contaminated Soils. Agriculture 2022, 12, 1579. [Google Scholar] [CrossRef]
- Castro Rodríguez, D.J.; Gutiérrez Benítez, O.; Casals Pérez, E.; Demichela, M.; Godio, A.; Chiampo, F. Bioremediation of Hydrocarbon-Polluted Soil: Evaluation of Different Operative Parameters. Appl. Sci. 2022, 12, 2012. [Google Scholar] [CrossRef]
- Celin, S.M.; Sahai, S.; Kalsi, A.; Bhanot, P. Environmental Monitoring Approaches Used during Bioremediation of Soils Contaminated with Hazardous Explosive Chemicals. Trends Environ. Anal. Chem. 2020, 26, e00088. [Google Scholar] [CrossRef]
- Crecca, V.d.M.T.; da Silva, J.M.; de Souza, P.A.R. Technological Prospecting: Patent Mapping of Bioremediation of Soil Contaminated with Agrochemicals Using Fungi. World Pat. Inf. 2023, 73, 102196. [Google Scholar] [CrossRef]
- Haque, S.; Srivastava, N.; Pal, D.B.; Alkhanani, M.F.; Almalki, A.H.; Areeshi, M.Y.; Naidu, R.; Gupta, V.K. Functional Microbiome Strategies for the Bioremediation of Petroleum-Hydrocarbon and Heavy Metal Contaminated Soils: A Review. Sci. Total Environ. 2022, 833, 155222. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, V.; Yildirim, N.; Erguven, G.O.; Durmus, B.; Nuhoglu, Y. The Bioremediation of Glyphosate in Soil Media by Some Newly Isolated Bacteria: The COD, TOC Removal Efficiency and Mortality Assessment for Daphnia Magna. Environ. Technol. Innov. 2021, 22, 101535. [Google Scholar] [CrossRef]
- Malla, M.A.; Dubey, A.; Kumar, A.; Yadav, S.; Kumari, S. Modeling and Optimization of Chlorpyrifos and Glyphosate Biodegradation Using RSM and ANN: Elucidating Their Degradation Pathways by GC-MS Based Metabolomics. Ecotoxicol. Environ. Saf. 2023, 252, 114628. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Kumar, C. Biotechnological Advances in Bioremediation of Heavy Metals Contaminated Ecosystems: An Overview with Special Reference to Phytoremediation. Int. J. Environ. Sci. Technol. 2014, 11, 843–872. [Google Scholar] [CrossRef]
- Masotti, F.; Garavaglia, B.S.; Gottig, N.; Ottado, J. Bioremediation of the Herbicide Glyphosate in Polluted Soils by Plant-Associated Microbes. Curr. Opin. Microbiol. 2023, 73, 102290. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Burguete, Y.; de la Luz Pérez-Rea, M.; Ledesma-García, J.; Campos-Guillén, J.; Ramos-López, M.A.; Guzmán, C.; Rodríguez-Morales, J.A. Global Situation of Bioremediation of Leachate-Contaminated Soils by Treatment with Microorganisms: A Systematic Review. Microorganisms 2023, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Ali, S.S.; El-Sheekh, M. A Comprehensive Review on the Potential of Microbial Enzymes in Multipollutant Bioremediation: Mechanisms, Challenges, and Future Prospects. J. Environ. Manag. 2023, 334, 117532. [Google Scholar] [CrossRef] [PubMed]
- Redfern, L.K.; Gardner, C.M.; Hodzic, E.; Ferguson, P.L.; Hsu-Kim, H.; Gunsch, C.K. A New Framework for Approaching Precision Bioremediation of PAH Contaminated Soils. J. Hazard. Mater. 2019, 378, 120859. [Google Scholar] [CrossRef] [PubMed]
- Rigoletto, M.; Calza, P.; Gaggero, E.; Malandrino, M.; Fabbri, D. Bioremediation Methods for the Recovery of Lead-Contaminated Soils: A Review. Appl. Sci. 2020, 10, 3528. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S. Bioremediation of Hazardous Pollutants from Agricultural Soils: A Sustainable Approach for Waste Management towards Urban Sustainability. Environ. Pollut. 2022, 120031. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent Advances in Glyphosate Biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of Co-Contaminated Soil with Heavy Metals and Pesticides: Influence Factors, Mechanisms and Evaluation Methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Jabbar, N.M.; Alardhi, S.M.; Mohammed, A.K.; Salih, I.K.; Albayati, T.M. Challenges in the Implementation of Bioremediation Processes in Petroleum-Contaminated Soils: A Review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100694. [Google Scholar] [CrossRef]
- Hamoudi-Belarbi, L.; Hamoudi, S.; Belkacemi, K.; Nouri, L.; Bendifallah, L.; Khodja, M. Bioremediation of Polluted Soil Sites with Crude Oil Hydrocarbons Using Carrot Peel Waste. Environments 2018, 5, 124. [Google Scholar] [CrossRef]
- Sagarkar, S.; Nousiainen, A.; Shaligram, S.; Björklöf, K.; Lindström, K.; Jørgensen, K.S.; Kapley, A. Soil Mesocosm Studies on Atrazine Bioremediation. J. Environ. Manag. 2014, 139, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Shah, M.P.; Verma, P. Bioreactors: A Biological and Bioengineering Prodigy. In Biological Treatment of Industrial Wastewater; The Royal Society of Chemistry: London, UK, 2021; pp. 87–104. [Google Scholar]
- Xia, J.; Wang, G.; Lin, J.; Wang, Y.; Chu, J.; Zhuang, Y.; Zhang, S. Advances and Practices of Bioprocess Scale-Up. In Bioreactor Engineering Research and Industrial Applications II; Springer: Berlin/Heidelberg, Germany, 2015; pp. 137–151. [Google Scholar]
- Krishania, M.; Sindhu, R.; Binod, P.; Ahluwalia, V.; Kumar, V.; Sangwan, R.S.; Pandey, A. Design of Bioreactors in Solid-State Fermentation. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 83–96. [Google Scholar]
- Arora, S.; Rani, R.; Ghosh, S. Bioreactors in Solid State Fermentation Technology: Design, Applications and Engineering Aspects. J. Biotechnol. 2018, 269, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, E.; Gálvez, J.; Calleja, C.; Calvo, V.; Cantero, J.; Videla, S.; Medaura, M.C.; DiPaola, M. Extensive Evaluation of Aerated Accumulation Technique for Soil Treatment. In Proceedings of the SPE Latin American and Caribbean Petroleum Engineering Conference, Caracas, Venezuela, 15–18 April 2001; Society of Petroleum Engineers Inc.: Buenos Aires, Argentina, 2001; pp. 1–6. [Google Scholar]
- Hincapié-Llanos, G.; Ramírez-Carmona, M. Biodegradation of Residual Polyurethane Using Fermentation in Solid State. Fac. De. Cienc. Agropecu. 2009, 7, 99–101. [Google Scholar]
- Nie, Y.; Yang, X.; Yin, M.; Wang, Z.; Wang, Q.; Dong, B.; Zhao, S. Simulated Bio-Slurry Reactor for Bioremediation of Highly Contaminated Soils by 2,4,6-Trinitrotoluene (TNT). Int. Biodeterior. Biodegrad. 2024, 190, 105789. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Tong, Z.; Zhao, R.; Pei, Y.; Chen, Y.; Zhou, P. Influence of Paddle Parameters on Particle Conveying and Mixing in an Organic Fertilizer Continuous Conveying Device. AgriEngineering 2025, 7, 184. [Google Scholar] [CrossRef]
- Pessoa, D.R.; Finkler, A.T.J.; Machado, A.V.L.; Luz, L.F.L.; Mitchell, D.A. Fluid Dynamics Simulation of a Pilot-Scale Solid-State Fermentation Bioreactor. Chem. Eng. Trans. 2016, 49, 49–54. [Google Scholar] [CrossRef]
- E, D.; Wen, Y.; Wu, Y.; Li, J.; Sun, W.; Liu, Y.; Chen, C.; Cui, J.; Qiu, Y. Hydrodynamics of Heterogeneous Particle Swarms in Gas-Liquid-Solid Stirred Tanks with Free Surface Studied by DEM-VOF. Powder Technol. 2025, 462, 121103. [Google Scholar] [CrossRef]
- Füvesi, B.; Goniva, C.; Luding, S.; Magnanimo, V. Mixing Indices in Up-Scaled Simulations. Powder Technol. 2025, 456, 120775. [Google Scholar] [CrossRef]
- Al-Mashhadani, M. Verification of Dead Zones Generated in Bioreactors as a Proactive Stage in Bioreactor Design. J. Ecol. Eng. 2023, 24, 65–74. [Google Scholar] [CrossRef]
- Emmerink, J.; Hadi, A.; Jovanova, J.; Cleven, C.; Schott, D.L. Parametric Analysis of a Double Shaft, Batch-Type Paddle Mixer Using the Discrete Element Method (DEM). Processes 2023, 11, 738. [Google Scholar] [CrossRef]
- Widhate, P.; Zhu, H.; Zeng, Q.; Dong, K. Mixing of Particles in a Rotating Drum with Inclined Axis of Rotation. Processes 2020, 8, 1688. [Google Scholar] [CrossRef]
- Yaraghi, A.; Ebrahimi, M.; Ein-Mozaffari, F.; Lohi, A. Mixing Assessment of Non-Cohesive Particles in a Paddle Mixer through Experiments and Discrete Element Method (DEM). Adv. Powder Technol. 2018, 29, 2693–2706. [Google Scholar] [CrossRef]
- Zheng, C.; Behjani, M.A.; Hu, J.; Wu, C. Discrete Element Modelling of Pharmaceutical Powder Handling Processes. In Simulations in Bulk Solids Handling; Wiley: Hoboken, NJ, USA, 2023; pp. 199–230. [Google Scholar]
- Ajona, M.; Vasanthi, P. Bioremediation of Petroleum Contaminated Soils—A Review. Mater. Today Proc. 2021, 45, 7117–7122. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Kim, J. New Insights into Bioremediation Strategies for Oil-Contaminated Soil in Cold Environments. Int. Biodeterior. Biodegrad. 2019, 142, 58–72. [Google Scholar] [CrossRef]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary Enzyme Based Technologies for Bioremediation: A Review. J. Environ. Manag. 2018, 210, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Fanaei, F.; Moussavi, G.; Shekoohiyan, S. Enhanced Bioremediation of Oil-Contaminated Soil in a Slurry Bioreactor by H2O2-Stimulation of Oil-Degrading/Biosurfactant-Generating Bacteria: Performance Optimization and Bacterial Metagenomics. Biodegradation 2023, 34, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.R.; Delabona, P.d.S. Strategies for Bioremediation of Pesticides: Challenges and Perspectives of the Brazilian Scenario for Global Application—A Review. Environ. Adv. 2022, 8, 100220. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency Biorremediation. Available online: https://clu-in.org/techfocus/default.focus/sec/Bioremediation/cat/Overview/ (accessed on 14 March 2025).
- Raimondo, E.E.; Saez, J.M.; Aparicio, J.D.; Fuentes, M.S.; Benimeli, C.S. Bioremediation of Lindane-Contaminated Soils by Combining of Bioaugmentation and Biostimulation: Effective Scaling-up from Microcosms to Mesocosms. J. Environ. Manag. 2020, 276, 111309. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Ortiz, P.; Tapia-Torres, Y.; Larsen, J.; García-Oliva, F. Glyphosate-Based Herbicides Alter Soil Carbon and Phosphorus Dynamics and Microbial Activity. Appl. Soil. Ecol. 2022, 169, 104256. [Google Scholar] [CrossRef]
- Goma-Tchimbakala, E.J.C.D.; Pietrini, I.; Dal Bello, F.; Goma-Tchimbakala, J.; Lo Russo, S.; Corgnati, S.P. Great Abilities of Shinella Zoogloeoides Strain from a Landfarming Soil for Crude Oil Degradation and a Synergy Model for Alginate-Bead-Entrapped Consortium Efficiency. Microorganisms 2022, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Morillo, E.; Madrid, F.; Lara-Moreno, A.; Villaverde, J. Soil Bioremediation by Cyclodextrins. A Review. Int. J. Pharm. 2020, 591, 119943. [Google Scholar] [CrossRef] [PubMed]
- Poi, G.; Aburto-Medina, A.; Mok, P.C.; Ball, A.S.; Shahsavari, E. Large Scale Bioaugmentation of Soil Contaminated with Petroleum Hydrocarbons Using a Mixed Microbial Consortium. Ecol. Eng. 2017, 102, 64–71. [Google Scholar] [CrossRef]
- Schommer, V.A.; Vanin, A.P.; Nazari, M.T.; Ferrari, V.; Dettmer, A.; Colla, L.M.; Piccin, J.S. Biochar-Immobilized Bacillus Spp. for Heavy Metals Bioremediation: A Review on Immobilization Techniques, Bioremediation Mechanisms and Effects on Soil. Sci. Total Environ. 2023, 881, 163385. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C.; González-López, F.; Cuartas-Uribe, B.; Mendoza-Roca, J.A. Treatment of Water from the Textile Industry Contaminated with Indigo Dye: A Hybrid Approach Combining Bioremediation and Nanofiltration for Sustainable Reuse. Case Stud. Chem. Environ. Eng. 2023, 8, 100498. [Google Scholar] [CrossRef]
- Hassan, A.; Hamid, F.S.; Pariatamby, A.; Suhaimi, N.S.M.; Razali, N.M.b.M.; Ling, K.N.H.; Mohan, P. Bioaugmentation-Assisted Bioremediation and Biodegradation Mechanisms for PCB in Contaminated Environments: A Review on Sustainable Clean-up Technologies. J. Environ. Chem. Eng. 2023, 11, 110055. [Google Scholar] [CrossRef]
- Raffa, C.M.; Chiampo, F. Bioremediation of Agricultural Soils Polluted with Pesticides: A Review. Bioengineering 2021, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Muter, O. Current Trends in Bioaugmentation Tools for Bioremediation: A Critical Review of Advances and Knowledge Gaps. Microorganisms 2023, 11, 710. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Pariatamby, A.; Ossai, I.C.; Ahmed, A.; Muda, M.A.; Wen, T.Z.; Hamid, F.S. Bioaugmentation-Assisted Bioremediation and Kinetics Modelling of Heavy Metal-Polluted Landfill Soil. Int. J. Environ. Sci. Technol. 2022, 19, 6729–6754. [Google Scholar] [CrossRef]
- Hassan, A.; Periathamby, A.; Ahmed, A.; Innocent, O.; Hamid, F.S. Effective Bioremediation of Heavy Metal–Contaminated Landfill Soil through Bioaugmentation Using Consortia of Fungi. J. Soils Sediments 2020, 20, 66–80. [Google Scholar] [CrossRef]
- Wu, M.; Liu, Z.; Gao, H.; Gao, J.; Xu, Y.; Ou, Y. Assessment of Bioremediation Potential of Petroleum-Contaminated Soils from the Shanbei Oilfield of China Revealed by QPCR and High Throughput Sequencing. Chemosphere 2022, 308, 136446. [Google Scholar] [CrossRef] [PubMed]
- Martinez Alvarez, L.; Bolhuis, H.; Mau, G.K.; Kok-Gan, C.; Sing, C.C.; Mac Cormack, W.; Ruberto, L. Identification of Key Bacterial Players during Successful Full-Scale Soil Field Bioremediation in Antarctica. Int. Biodeterior. Biodegrad. 2022, 168, 105354. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Tang, C.; Liu, B.; Zou, H.; Li, W.; Zhang, H. Mixing Performance Analysis and Optimal Design of a Novel Passive Baffle Micromixer. Micromachines 2024, 15, 182. [Google Scholar] [CrossRef] [PubMed]
- Jadidi, B.; Ebrahimi, M.; Ein-Mozaffari, F.; Lohi, A. Effect of the Mixer Design Parameters on the Performance of a Twin Paddle Blender: A DEM Study. Processes 2023, 11, 733. [Google Scholar] [CrossRef]
- Benmoussa, A. Agitation of Viscoplastic Fluid in a Rotating Vessel Using Close Clearance Agitators. Enginerring 2023, 4, 2525–2541. [Google Scholar] [CrossRef]
- Triozzi, M.; Binetti, M.S.; Campanale, C.; Uricchio, V.F.; Massarelli, C. An Integrated Approach to Assess Smart Passive Bioventing as a Sustainable Strategy for the Remediation of a Polluted Site by Persistent Organic Pollutants. Sustainability 2023, 15, 3764. [Google Scholar] [CrossRef]
- Castrejón-Godínez, M.L.; Tovar-Sánchez, E.; Valencia-Cuevas, L.; Rosas-Ramírez, M.E.; Rodríguez, A.; Mussali-Galante, P. Glyphosate Pollution Treatment and Microbial Degradation Alternatives, a Review. Microorganisms 2021, 9, 2322. [Google Scholar] [CrossRef] [PubMed]
- Karimi, H.; Mahdavi, S.; Asgari Lajayer, B.; Moghiseh, E.; Rajput, V.D.; Minkina, T.; Astatkie, T. Insights on the Bioremediation Technologies for Pesticide-Contaminated Soils. Environ. Geochem. Health 2022, 44, 1329–1354. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, J.; Remiszewska-Skwarek, A.; Duda, S.; Łagód, G. Aeration Process in Bioreactors as the Main Energy Consumer in a Wastewater Treatment Plant. Review of Solutions and Methods of Process Optimization. Processes 2019, 7, 311. [Google Scholar] [CrossRef]
- Webb, C. Design Aspects of Solid State Fermentation as Applied to Microbial Bioprocessing. J. Appl. Biotechnol. Bioeng. 2017, 4, 511–532. [Google Scholar] [CrossRef]
- Finkler, A.T.J.; Biz, A.; Pitol, L.O.; Medina, B.S.; Luithardt, H.; Luz, L.F.d.L.; Krieger, N.; Mitchell, D.A. Intermittent Agitation Contributes to Uniformity across the Bed during Pectinase Production by Aspergillus Niger Grown in Solid-State Fermentation in a Pilot-Scale Packed-Bed Bioreactor. Biochem. Eng. J. 2017, 121, 1–12. [Google Scholar] [CrossRef]
- Salari, M.; Rahmanian, V.; Hashemi, S.A.; Chiang, W.-H.; Lai, C.W.; Mousavi, S.M.; Gholami, A. Bioremediation Treatment of Polyaromatic Hydrocarbons for Environmental Sustainability. Water 2022, 14, 3980. [Google Scholar] [CrossRef]
- Hussein, S.H.; Qurbani, K.; Ahmed, S.K.; Tawfeeq, W.; Hassan, M. Bioremediation of Heavy Metals in Contaminated Environments Using Comamonas Species: A Narrative Review. Bioresour. Technol. Rep. 2024, 25, 101711. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Balaganesh, P.; Vasudevan, M.; Natarajan, N.; Chauhan, A.; Arora, J.; Ranjan, A.; Rajput, V.D.; Sushkova, S.; Minkina, T.; et al. Bioremediation of Hydrocarbon Pollutants: Recent Promising Sustainable Approaches, Scope, and Challenges. Sustainability 2023, 15, 5847. [Google Scholar] [CrossRef]
- Kisić, I.; Hrenović, J.; Zgorelec, Ž.; Durn, G.; Brkić, V.; Delač, D. Bioremediation of Agriculture Soil Contaminated by Organic Pollutants. Energy 2022, 15, 1561. [Google Scholar] [CrossRef]
- Yaman, C. Performance and Kinetics of Bioaugmentation, Biostimulation, and Natural Attenuation Processes for Bioremediation of Crude Oil-Contaminated Soils. Processes 2020, 8, 883. [Google Scholar] [CrossRef]
- Banat, I.M.; Carboué, Q.; Saucedo-Castañeda, G.; de Jesús Cázares-Marinero, J. Biosurfactants: The Green Generation of Speciality Chemicals and Potential Production Using Solid-State Fermentation (SSF) Technology. Bioresour. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, S.; Ali, M.; Song, X.; Tang, Z.; Zhang, Z.; Zhang, M.; Luo, Y. Thermally Enhanced Bioremediation: A Review of the Fundamentals and Applications in Soil and Groundwater Remediation. J. Hazard. Mater. 2022, 433, 128749. [Google Scholar] [CrossRef] [PubMed]
- Sanscartier, D.; Reimer, K.; Zeeb, B.; Koch, I. The Effect of Temperature and Aeration Rate on Bioremediation of Diesel-Contaminated Soil in Solid-Phase Bench-Scale Bioreactors. Soil. Sediment. Contam. An. Int. J. 2011, 20, 353–369. [Google Scholar] [CrossRef]
- El Salamony, D.H.; Salah Eldin Hassouna, M.; Zaghloul, T.I.; Moustafa Abdallah, H. Valorization of Chicken Feather Waste Using Recombinant Bacillus Subtilis Cells by Solid-State Fermentation for Soluble Proteins and Serine Alkaline Protease Production. Bioresour. Technol. 2024, 393, 130110. [Google Scholar] [CrossRef] [PubMed]
- Amândio, M.S.T.; Rocha, J.M.S.; Xavier, A.M.R.B. Fed-Batch SSF with Pre-Saccharification as a Strategy to Reduce Enzyme Dosage in Cellulosic Ethanol Production. Fuel 2024, 357, 129842. [Google Scholar] [CrossRef]
- Afedzi, A.E.K.; Parakulsuksatid, P. Recent Advances in Process Modifications of Simultaneous Saccharification and Fermentation (SSF) of Lignocellulosic Biomass for Bioethanol Production. Biocatal. Agric. Biotechnol. 2023, 54, 102961. [Google Scholar] [CrossRef]
- Caro, I.; Marzo-Gago, C.; Díaz, A.B.; Blandino, A. Modelling and Optimization of Simultaneous Saccharification and Fermentation of Agro-Food Residues. J. Environ. Chem. Eng. 2024, 12, 111862. [Google Scholar] [CrossRef]
- Ramírez, M.E.; Vélez, Y.H.; Rendón, L.; Alzate, E. Brazilian Journal of Biology Potential of Microalgae in the Bioremediation of Water with Chloride Content. Braz. J. Biol. 2018, 78, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C.; González-López, F.; Cuartas-Uribe, B.; Mendoza-Roca, J.A. Efficient Bioremediation of Indigo-Dye Contaminated Textile Wastewater Using Native Microorganisms and Combined Bioaugmentation-Biostimulation Techniques. Chemosphere 2024, 353, 141538. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, K.; Smith, E.; Palanisami, T.; Mathieson, G.; Srivastava, P.; Megharaj, M.; Naidu, R. Evaluation of Constraints in Bioremediation of Weathered Hydrocarbon-Contaminated Arid Soils through Microcosm Biopile Study. Int. J. Environ. Sci. Technol. 2015, 12, 3597–3612. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).