Smart Food Packaging Films Based on a Poly(lactic acid), Nanomaterials, and a pH Sensitive Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Smart PLA Films
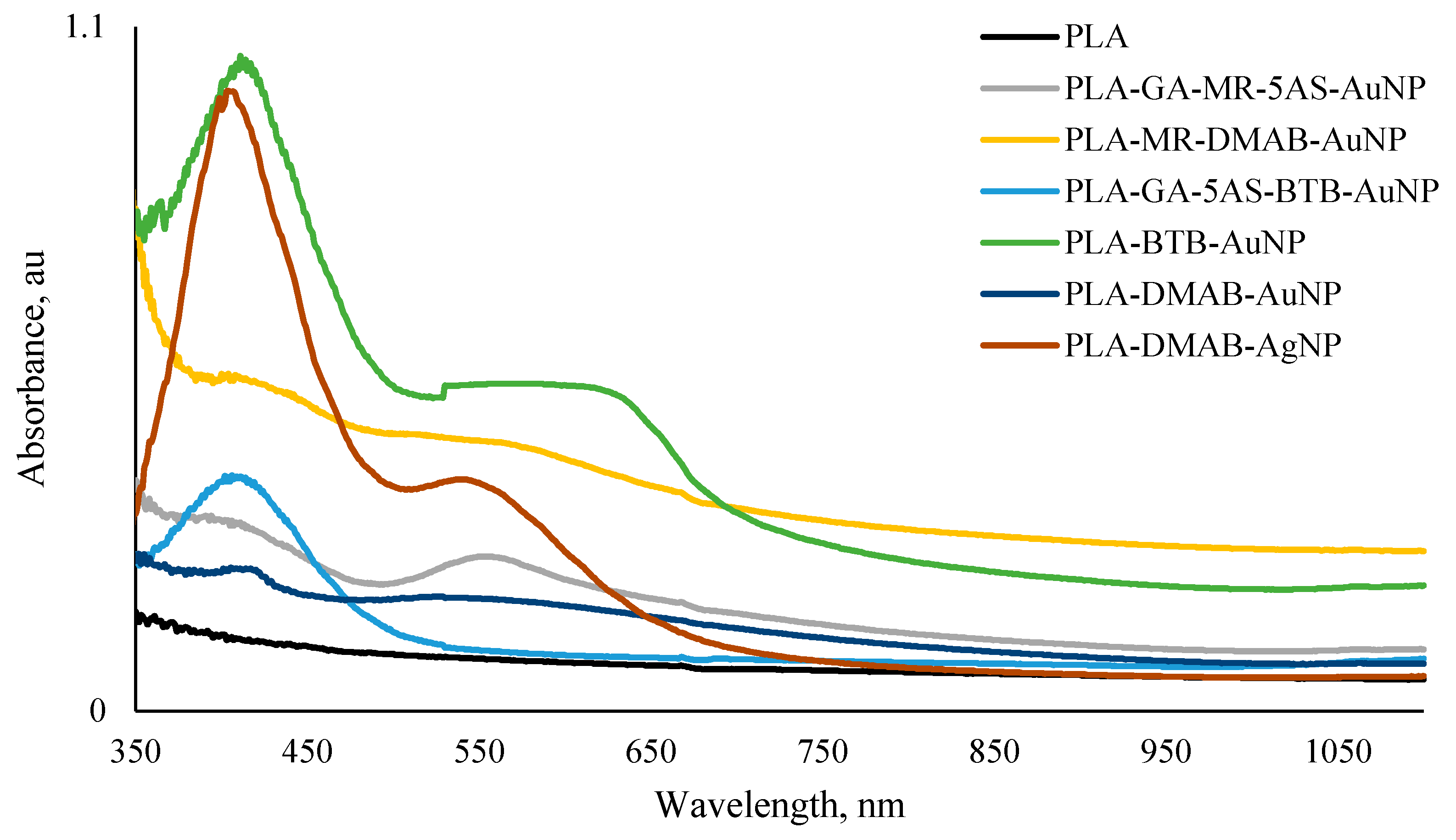
2.3. Optical Characterization
2.4. Solvent Resistance of the PLA Films
2.5. Physical Characterization
2.6. Microbiological Characterization
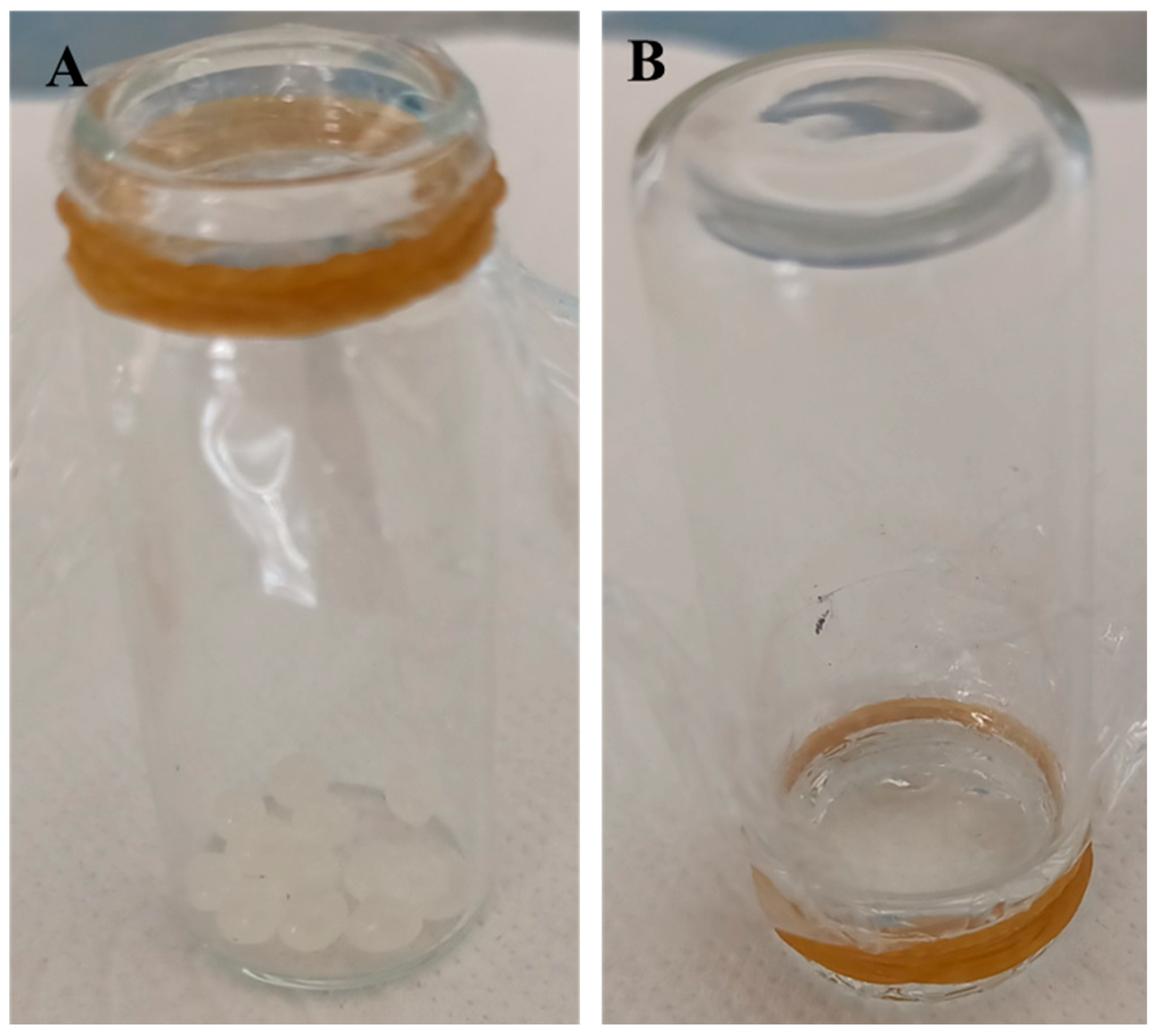
2.7. Real Sample Application
3. Results and Discussions
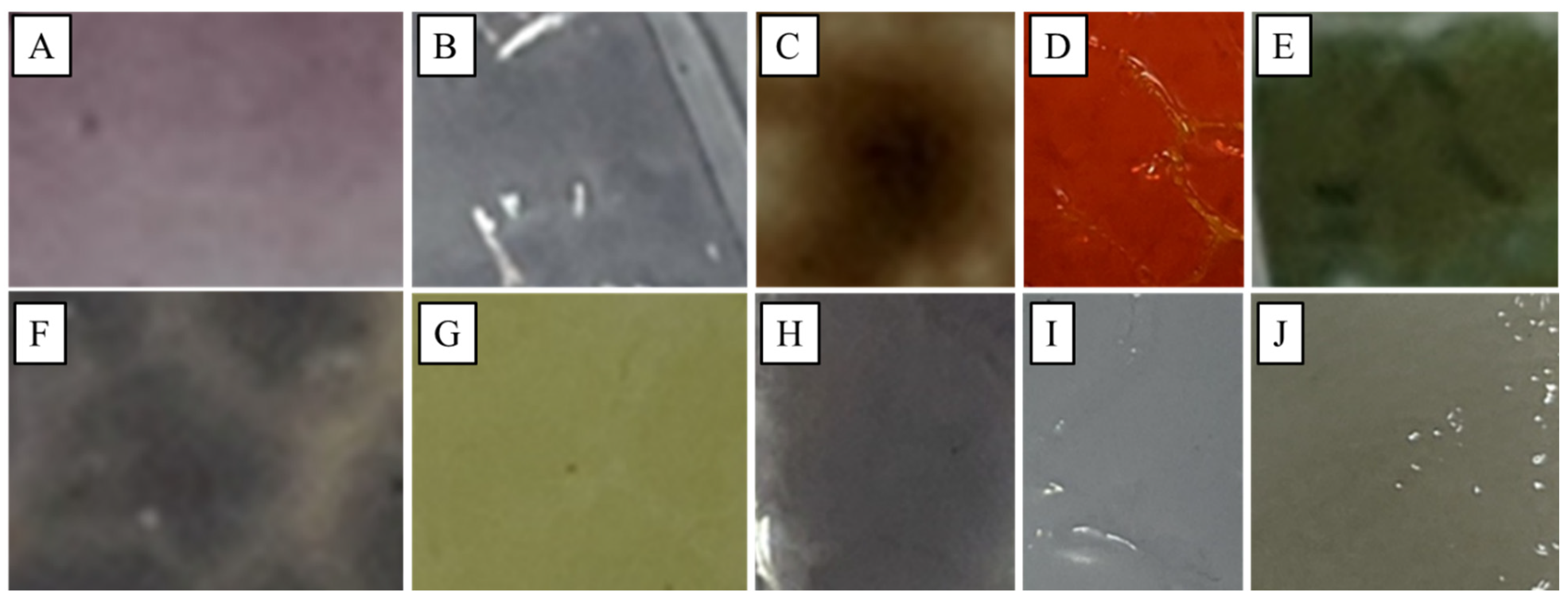
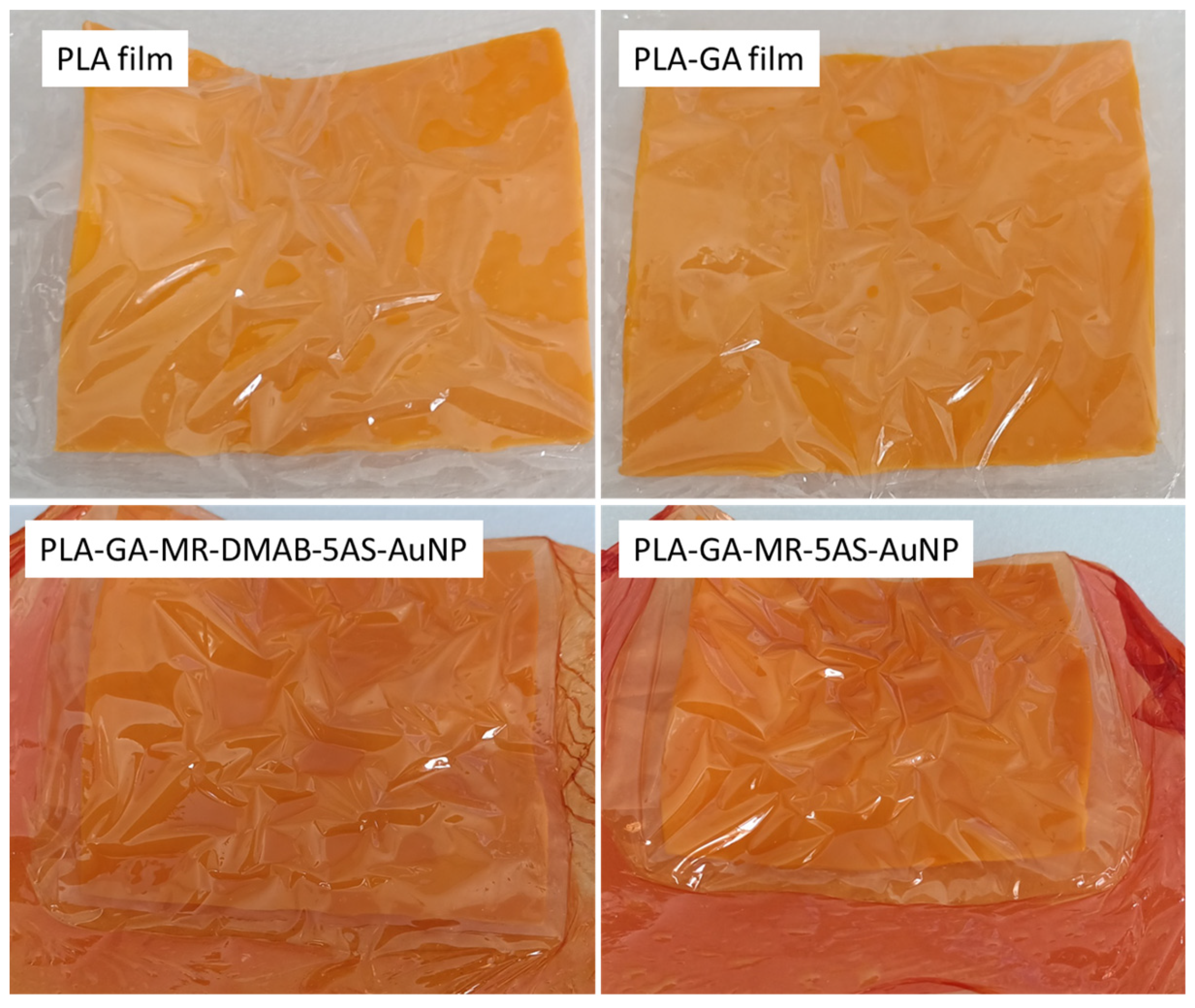
3.1. Smart PLA Film Synthesis
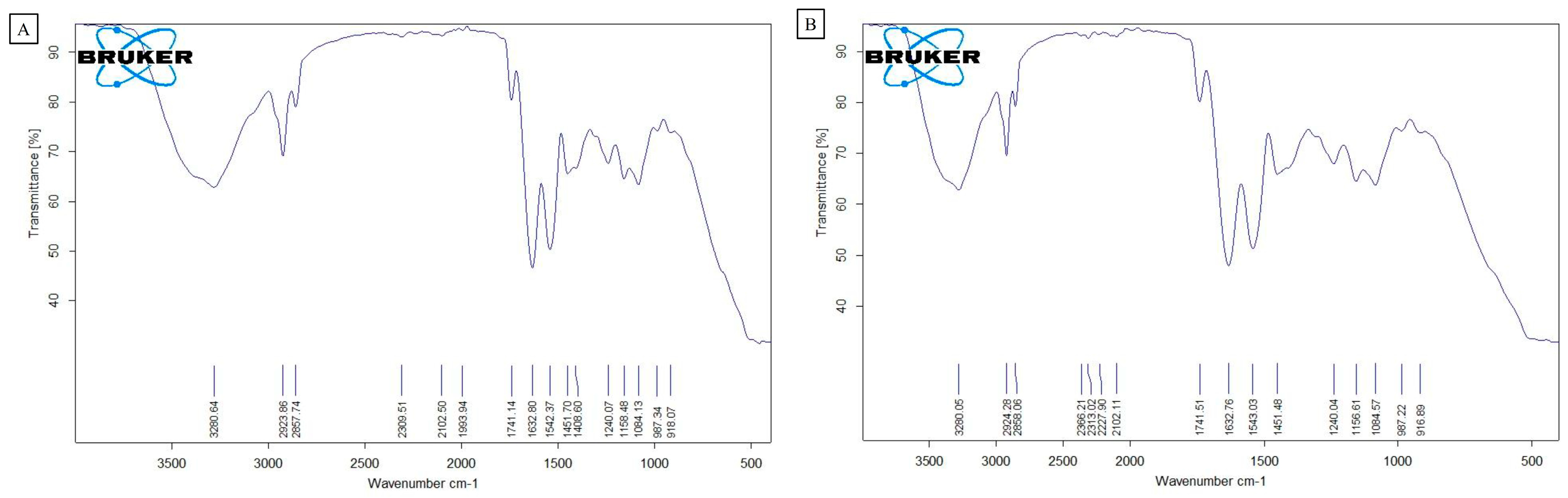
3.2. Physical and Chemical Characterization of the PLA Films
3.2.1. Contact Angle
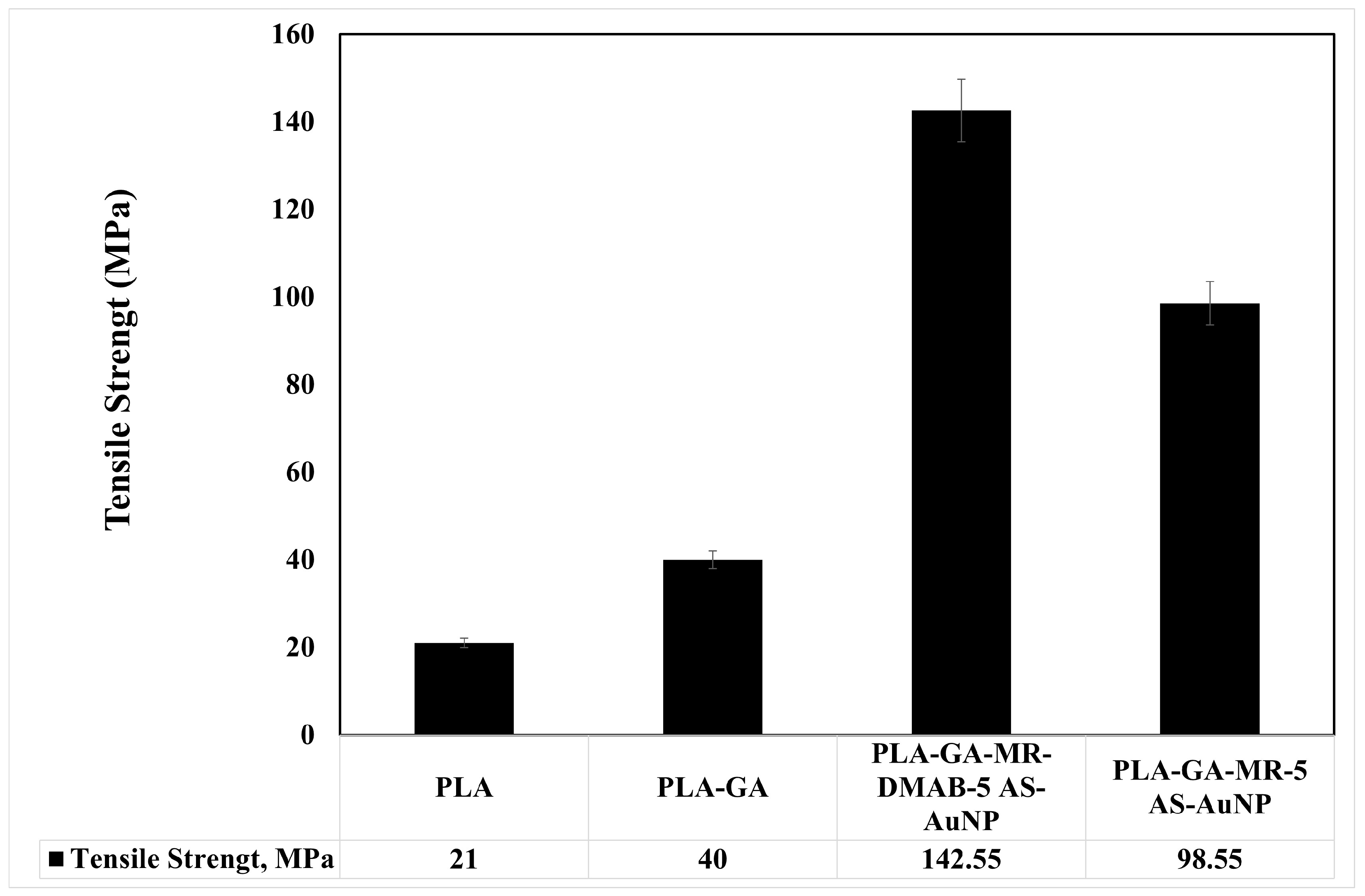
3.2.2. Tensile Strength
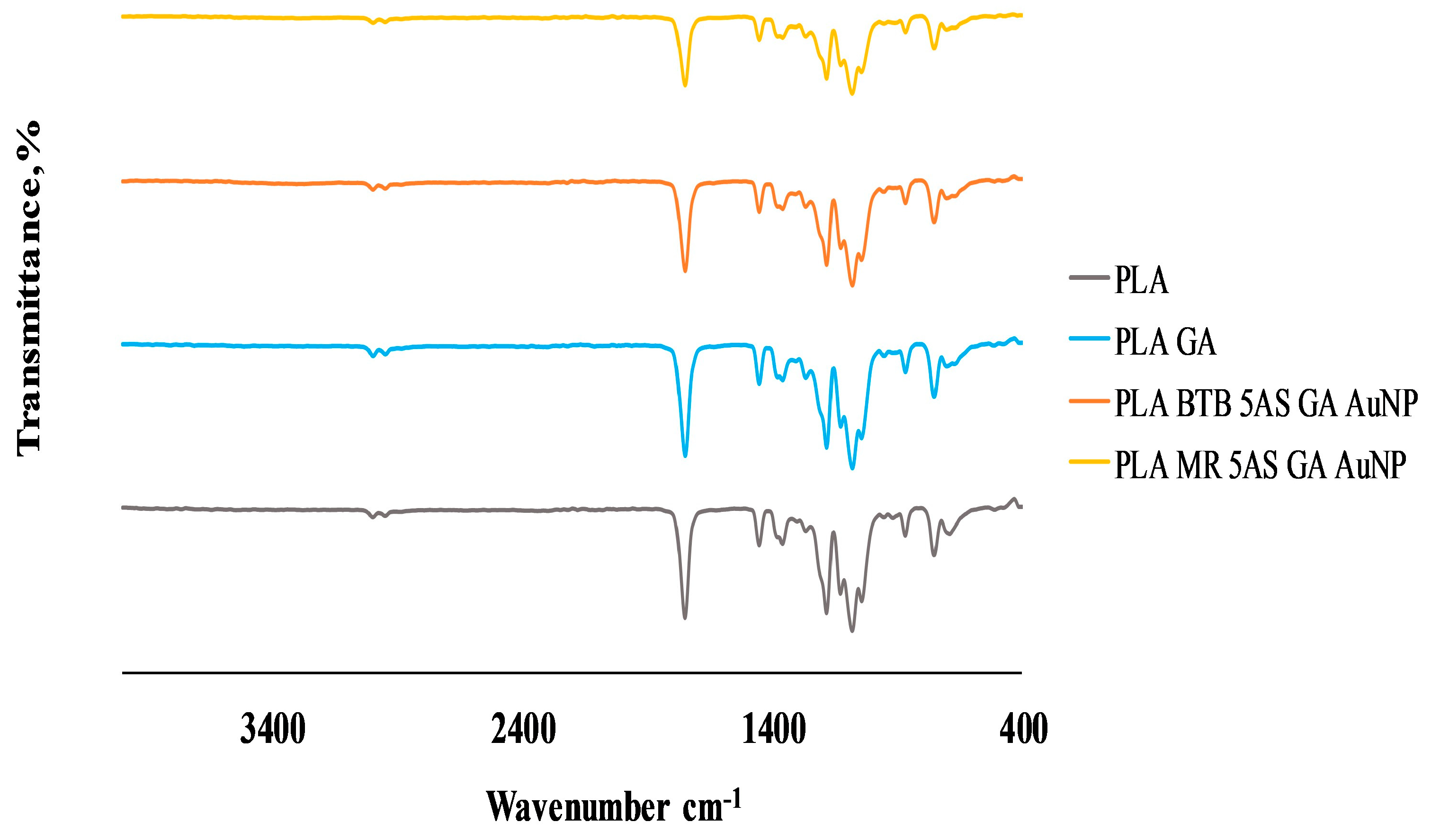
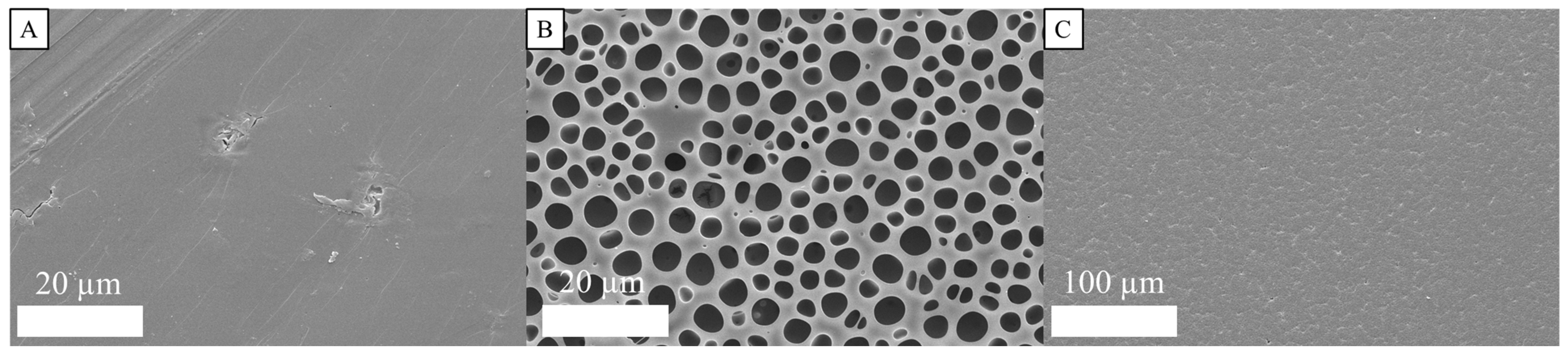
3.2.3. Surface Characterization
3.2.4. Solvent Resistance
3.2.5. Oil and Water Vapor Transfer Characterization
3.3. Antimicrobial Activity of the PLA Films
3.4. Validation of Smart Packaging Material
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. 2017 Global Initiative on Food Loss and Waste; FAO: Rome, Italy, 2017; p. 6. [Google Scholar]
- Jiang, X.; Valdeperez, D.; Nazarenus, M.; Wang, Z.; Stellacci, F.; Parak, W.J.; Del Pino, P. Future Perspectives towards the Use of Nanomaterials for Smart Food Packaging and Quality Control. Part. Part. Syst. Charact. 2015, 32, 408–416. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Andronescu, E. Smart Food Packaging Designed by Nanotechnological and Drug Delivery Approaches. Coatings 2020, 10, 806. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2019; Moving Forward on Food Loss and Waste Reduction; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; ISBN 978-92-5-131789-1. [Google Scholar]
- Li, T.; Lloyd, K.; Birch, J.; Wu, X.; Mirosa, M.; Liao, X. A Quantitative Survey of Consumer Perceptions of Smart Food Packaging in China. Food Sci. Nutr. 2020, 8, 3977–3988. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, K.A.M.; Kerry, J.P. Consumer Attitudes towards the Application of Smart Packaging Technologies to Cheese Products. Food Packag. Shelf Life 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Vanderroost, M.; Ragaert, P.; Devlieghere, F.; De Meulenaer, B. Intelligent Food Packaging: The next Generation. Trends Food Sci. Technol. 2014, 39, 47–62. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An Overview of the Intelligent Packaging Technologies in the Food Sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Gan, I.; Chow, W.S. Antimicrobial Poly(Lactic Acid)/Cellulose Bionanocomposite for Food Packaging Application: A Review. Food Packag. Shelf Life 2018, 17, 150–161. [Google Scholar] [CrossRef]
- González, A.; Alvarez Igarzabal, C.I. Soy Protein—Poly (Lactic Acid) Bilayer Films as Biodegradable Material for Active Food Packaging. Food Hydrocoll. 2013, 33, 289–296. [Google Scholar] [CrossRef]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-Nanocomposites for Food Packaging Applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Pirsa, S.; Asadi, S. Innovative Smart and Biodegradable Packaging for Margarine Based on a Nano Composite Polylactic Acid/Lycopene Film. Food Addit. Contam. Part A 2021, 38, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Zhang, H. Biodegradable Polylactic Acid Polymer with Nisin for Use in Antimicrobial Food Packaging. J. Food Sci. 2008, 73, M127–M134. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Gonçalves, I.C.; Magalhães, F.D.; Pinto, A.M. Poly(Lactic Acid) Composites Containing Carbon-Based Nanomaterials: A Review. Polymers 2017, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Yazgan, I.; Gümü, A.; Popov, S.; Toprak, M.S. On the Effect of Modified Carbohydrates on the Size and Shape of Gold and Silver Nanostructures. Nanomaterials 2020, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Yazgan, I. Novel Poly (Amic) Acid Membrane Chemistries with Experimentally-Controlled Pore Size, Transport, and Disinfection Properties. Ph.D. Thesis, State University Of Newyork, Binghamton, NY, USA, 2016. [Google Scholar]
- Li, Z.; Liu, T.; Fan, K.; Geng, L.; Wang, P.; Ren, F.; Luo, J. Preparation of PH-Responsive Chitosan Microspheres Containing Aminopeptidase and Their Application in Accelerating Cheese Ripening. J. Dairy Sci. 2024, 107, 3502–3514. [Google Scholar] [CrossRef] [PubMed]
- ASTM E96-1995; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: Conshohocken, PA, USA, 1995.
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Rocculi, P.; Rosa, M.D. Poly(Lactic Acid)-Modified Films for Food Packaging Application: Physical, Mechanical, and Barrier Behavior. J. Appl. Polym. Sci. 2012, 125, E390–E401. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, B.; Zhao, J.; Chen, H. Development and Characterization of Food Packaging Film Fromcellulose Sulfate. Food Hydrocoll. 2014, 35, 476–483. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green Synthesized Silver Nanoparticles: Antibacterial and Anticancer Activities, Biocompatibility, and Analyses of Surface-Attached Proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef] [PubMed]


| PLA Film | Average Contact Angle (°) (n = 3) |
|---|---|
| PLA | 75.89 ± 0.67 |
| PLA-GA (0.5 mg/mL) | 81.10 ± 0.88 |
| PLA-GA (1 mg/mL) | 62.30 ± 0.89 |
| PLA-GA (1 mg/mL)-MR-5AS-AuNP | 84.10 ± 0.78 |
| PLA-GA (1 mg/mL)-5AS-DMAB-AuNP | 83.10 ± 0.76 |
| PLA-GA (1 mg/mL)-BTB-5AS-AuNP | 85.20 ± 0.18 |
| PLA-GA (1 mg/mL)-MR-5AS-AgNP | 83.70 ± 0.77 |
| PLA-GA (1 mg/mL)-MR-DMAB-AgNP | 88.70 ± 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yetgin, S.; Ağırsaygın, M.; Yazgan, İ. Smart Food Packaging Films Based on a Poly(lactic acid), Nanomaterials, and a pH Sensitive Dye. Processes 2025, 13, 1105. https://doi.org/10.3390/pr13041105
Yetgin S, Ağırsaygın M, Yazgan İ. Smart Food Packaging Films Based on a Poly(lactic acid), Nanomaterials, and a pH Sensitive Dye. Processes. 2025; 13(4):1105. https://doi.org/10.3390/pr13041105
Chicago/Turabian StyleYetgin, Senem, Melike Ağırsaygın, and İdris Yazgan. 2025. "Smart Food Packaging Films Based on a Poly(lactic acid), Nanomaterials, and a pH Sensitive Dye" Processes 13, no. 4: 1105. https://doi.org/10.3390/pr13041105
APA StyleYetgin, S., Ağırsaygın, M., & Yazgan, İ. (2025). Smart Food Packaging Films Based on a Poly(lactic acid), Nanomaterials, and a pH Sensitive Dye. Processes, 13(4), 1105. https://doi.org/10.3390/pr13041105







