Fischer–Tropsch Biofuel Production from Supercritical Water Gasification of Lignocellulosic Biomass: Process Modelling and Life-Cycle Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Types
2.2. Process Modelling of FT Biofuel Production from SCWG of Lignocellulosic Biomass
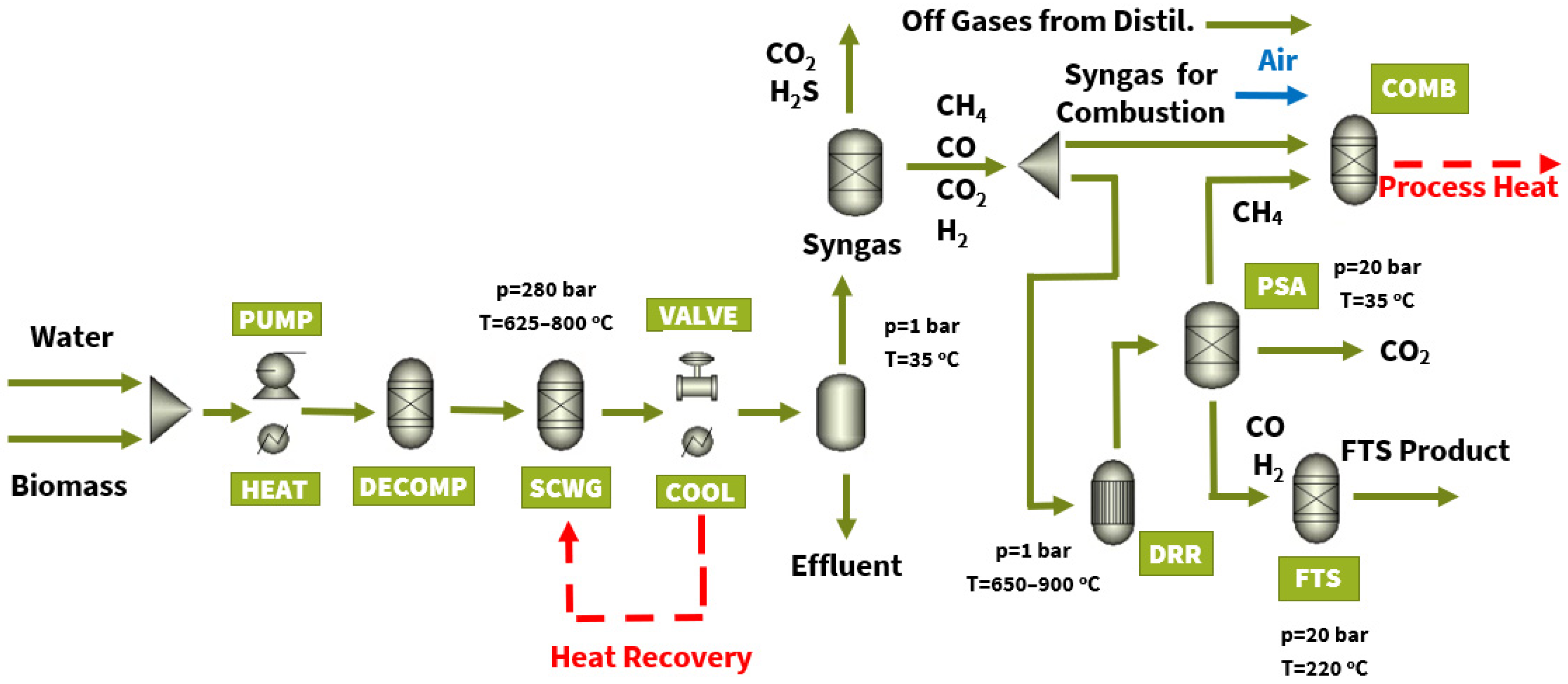
2.2.1. FT Biofuel Production Section (Incl. Supercritical Water Gasification, Dry Reforming and Fischer—Tropsch Synthesis Reactors)
2.2.2. FT Biofuel Upgrade and Refinement Section
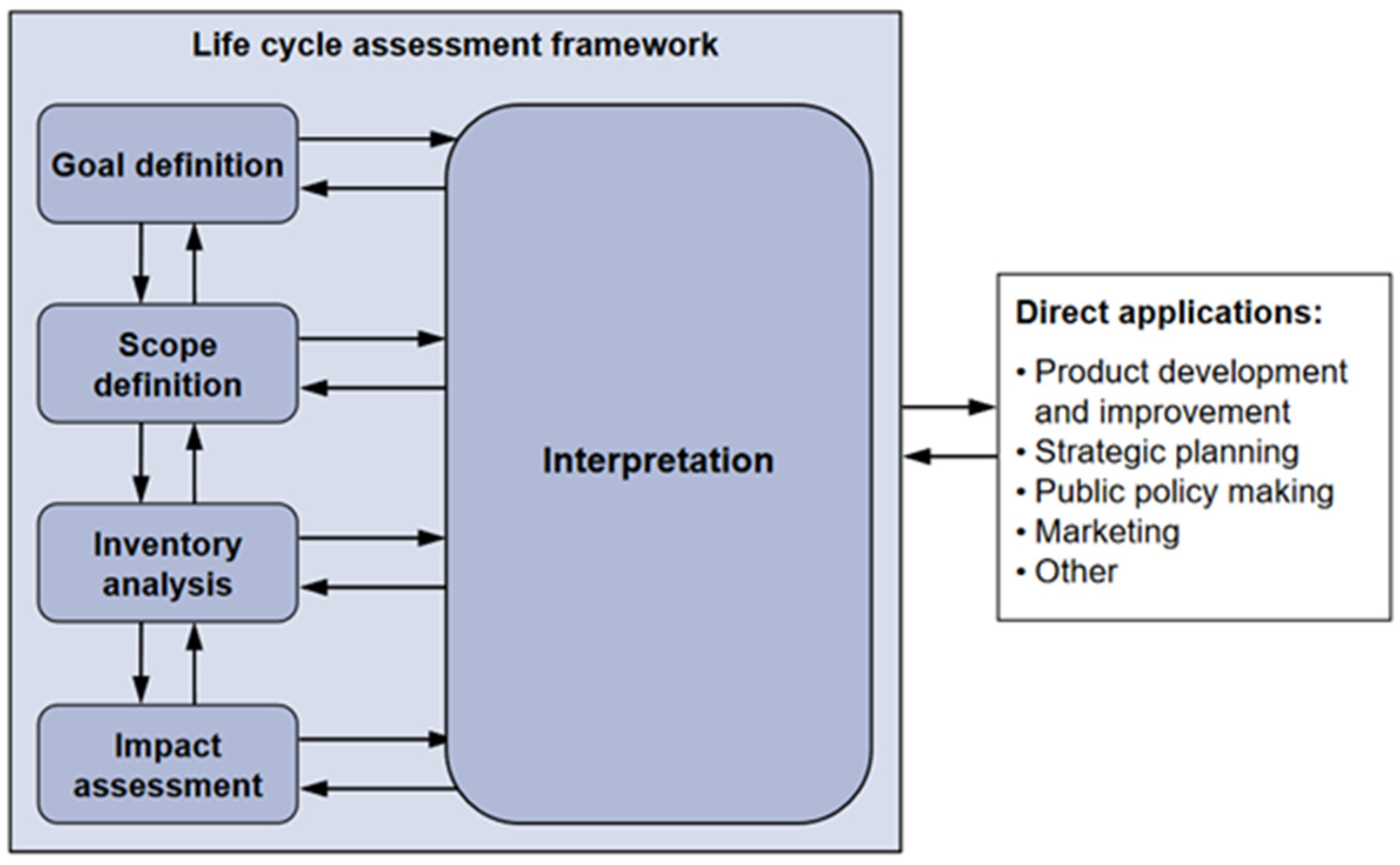
2.3. Life-Cycle Assessment (LCA) Modelling
2.3.1. Goal and Scope Definition
2.3.2. System Description and LCI Modelling
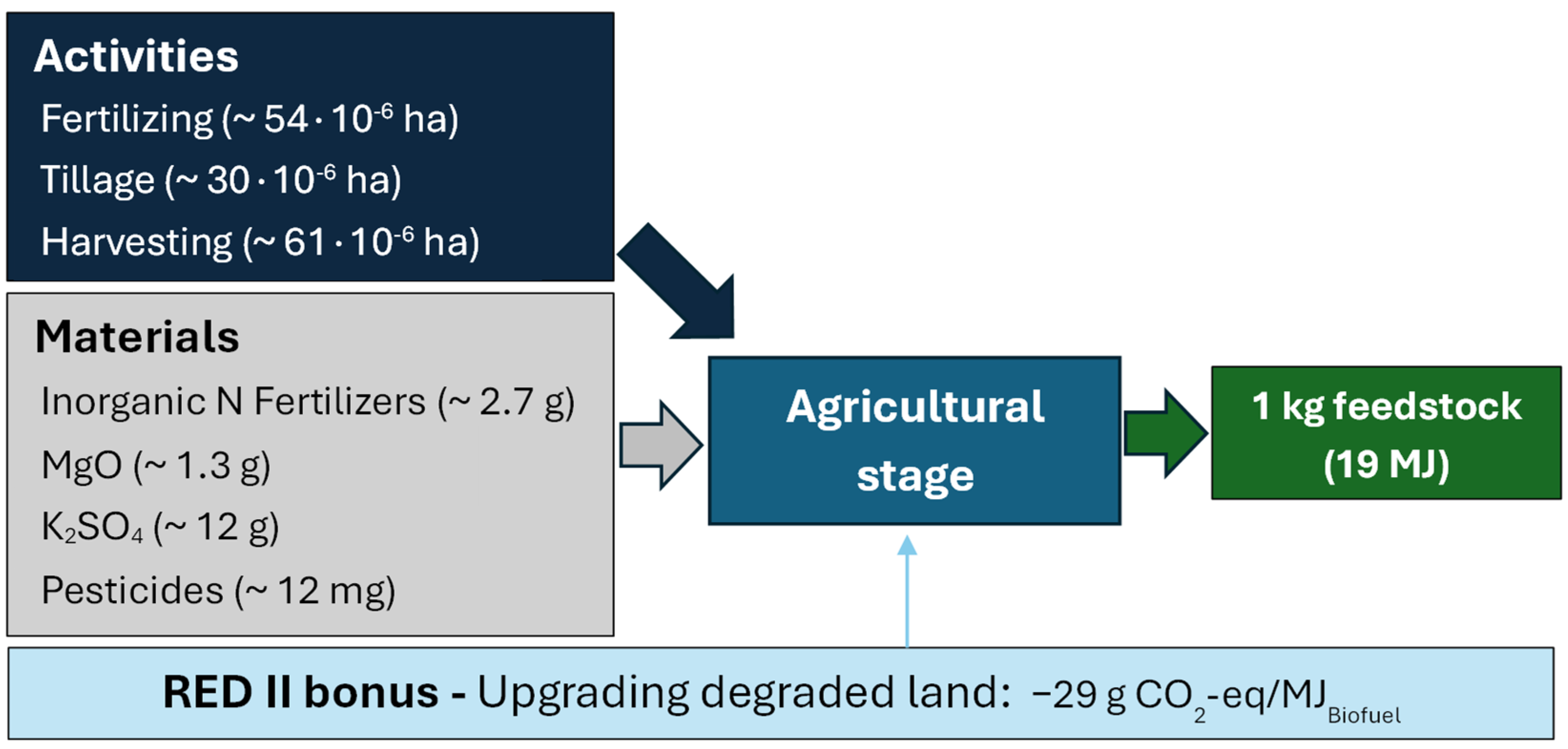
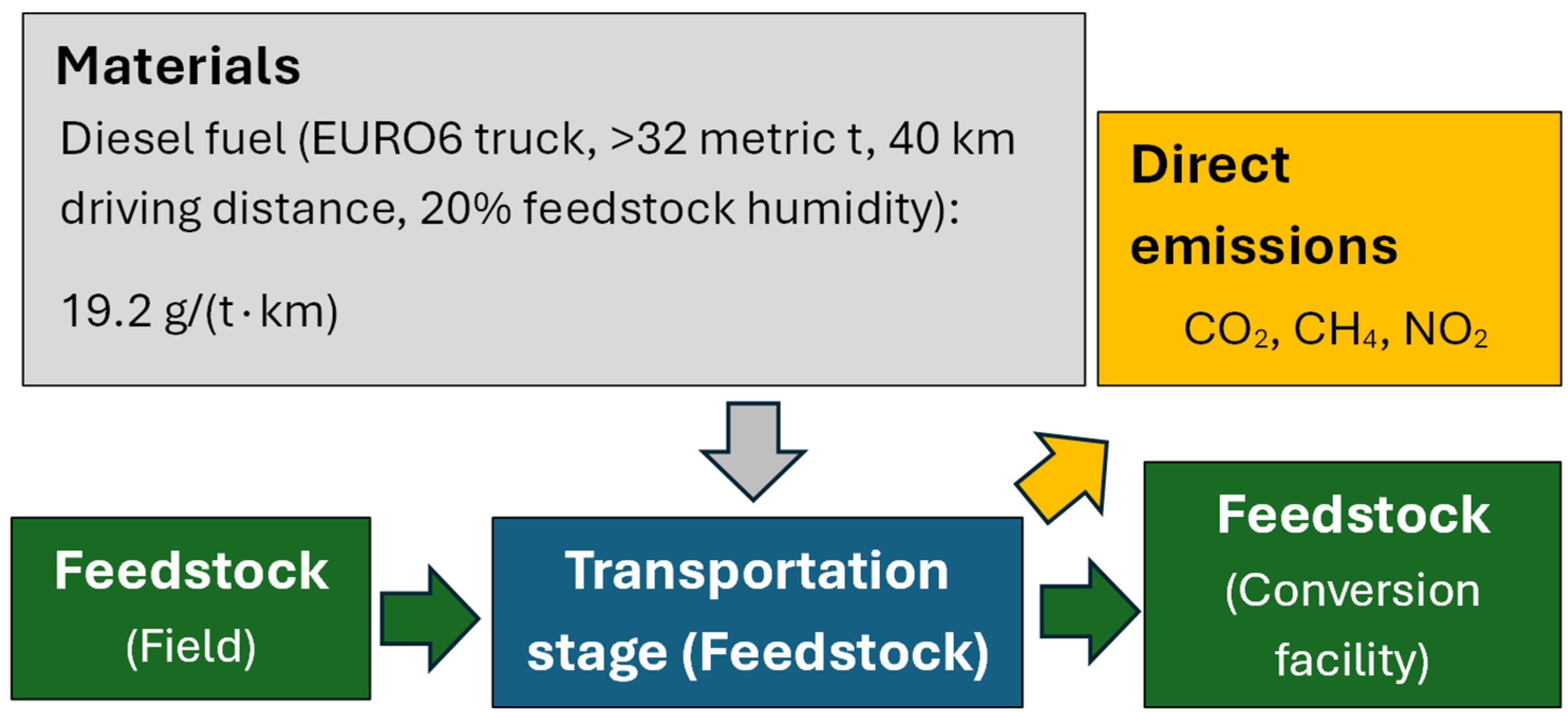
2.3.3. Life-Cycle Inventories
- Agricultural stage (Field-to-feedstock)
- Transportation stage
- Core conversion and upgrading to final fuel.
3. Results
3.1. Process Modelling
3.1.1. Characterization of Biomass Types
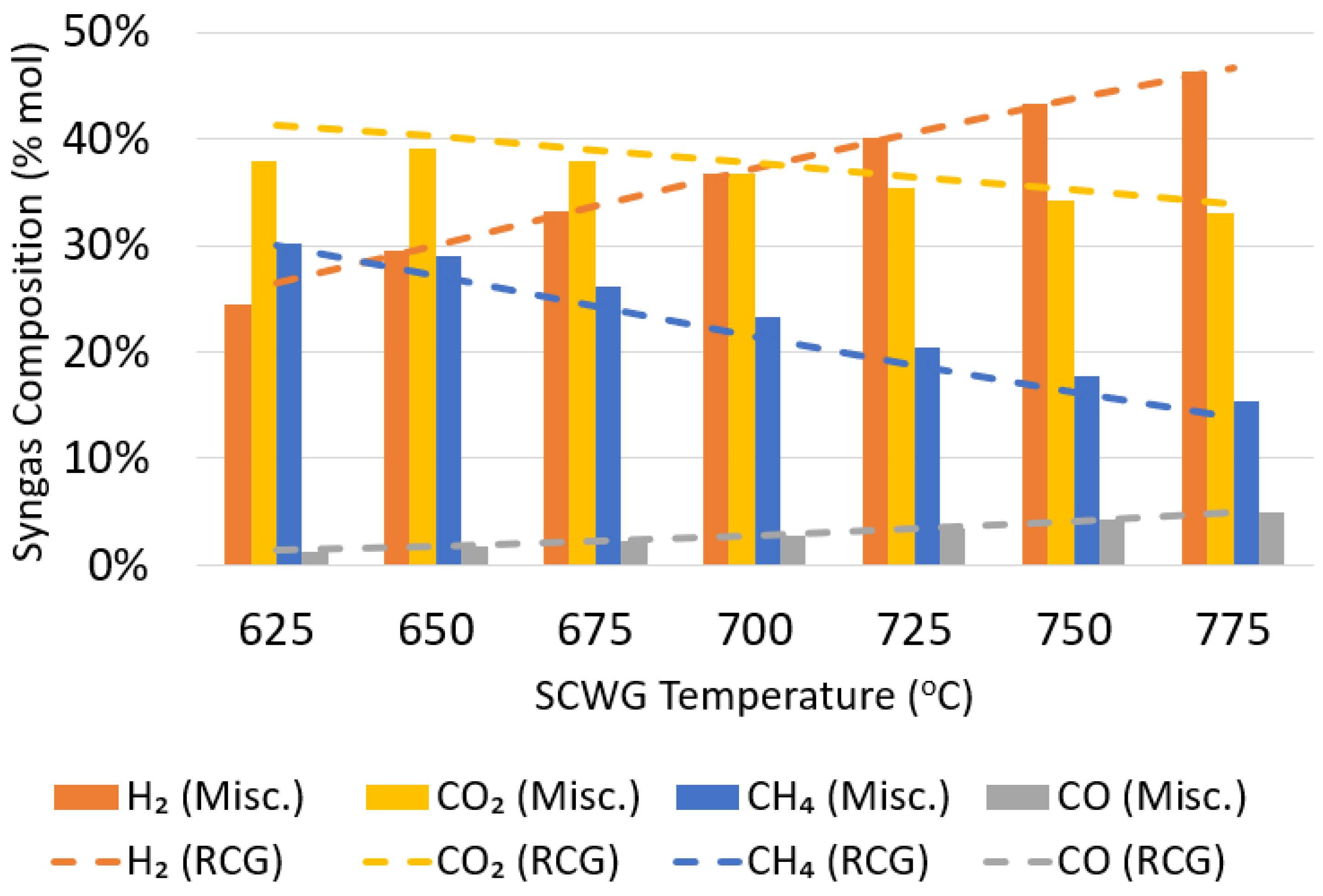
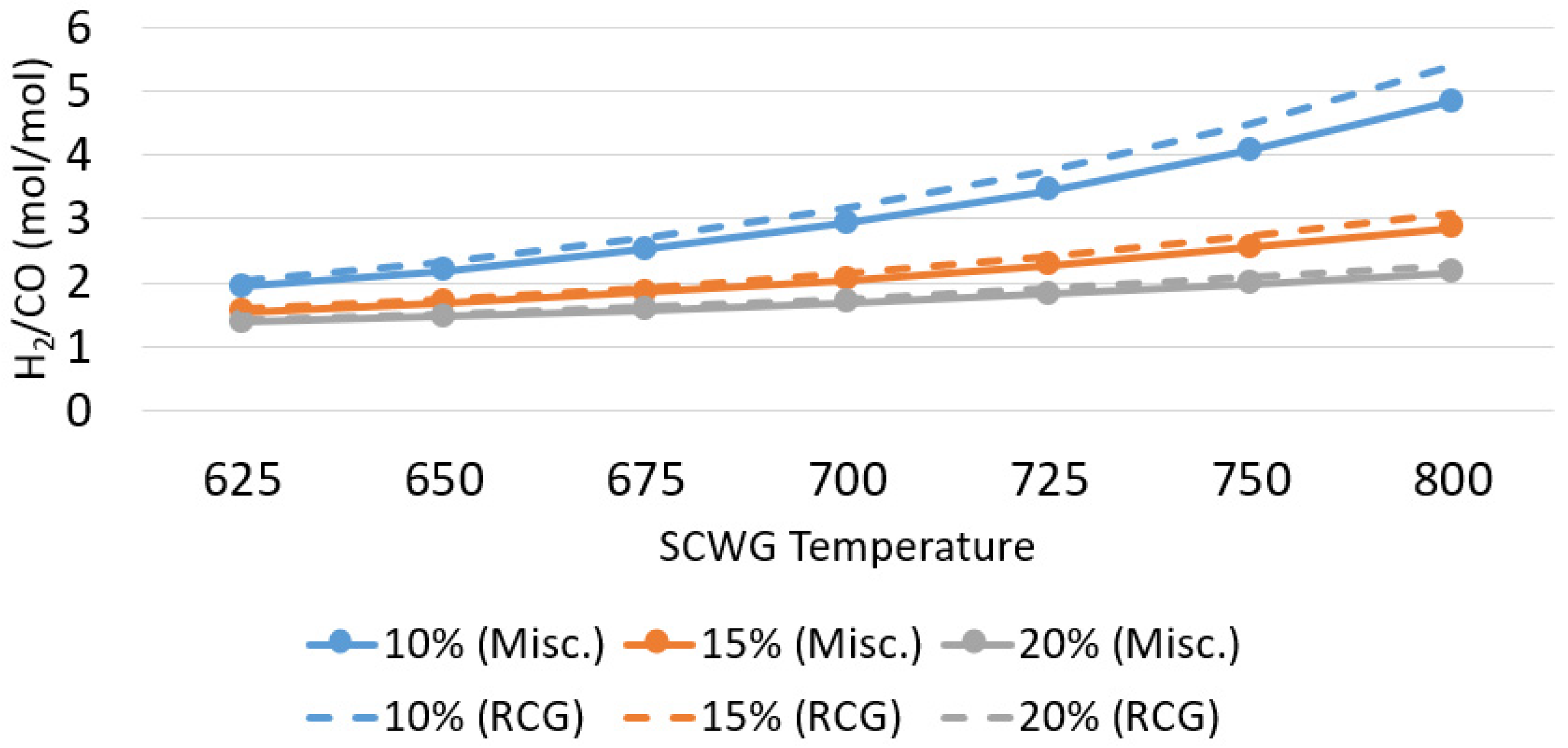
3.1.2. Syngas Composition and Upgrade
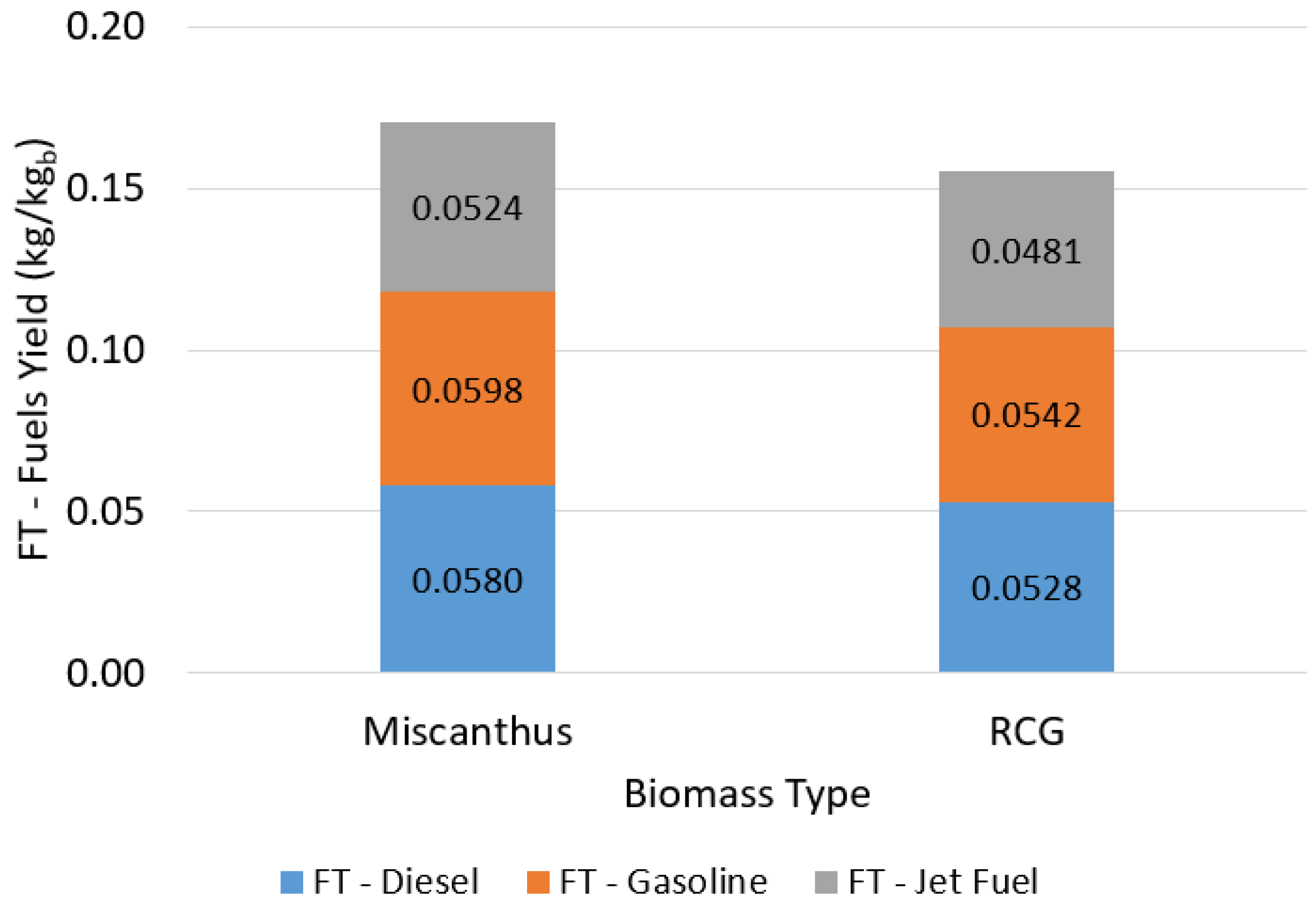
3.1.3. FT Fuel Production: Max-Yield Operating Scenario
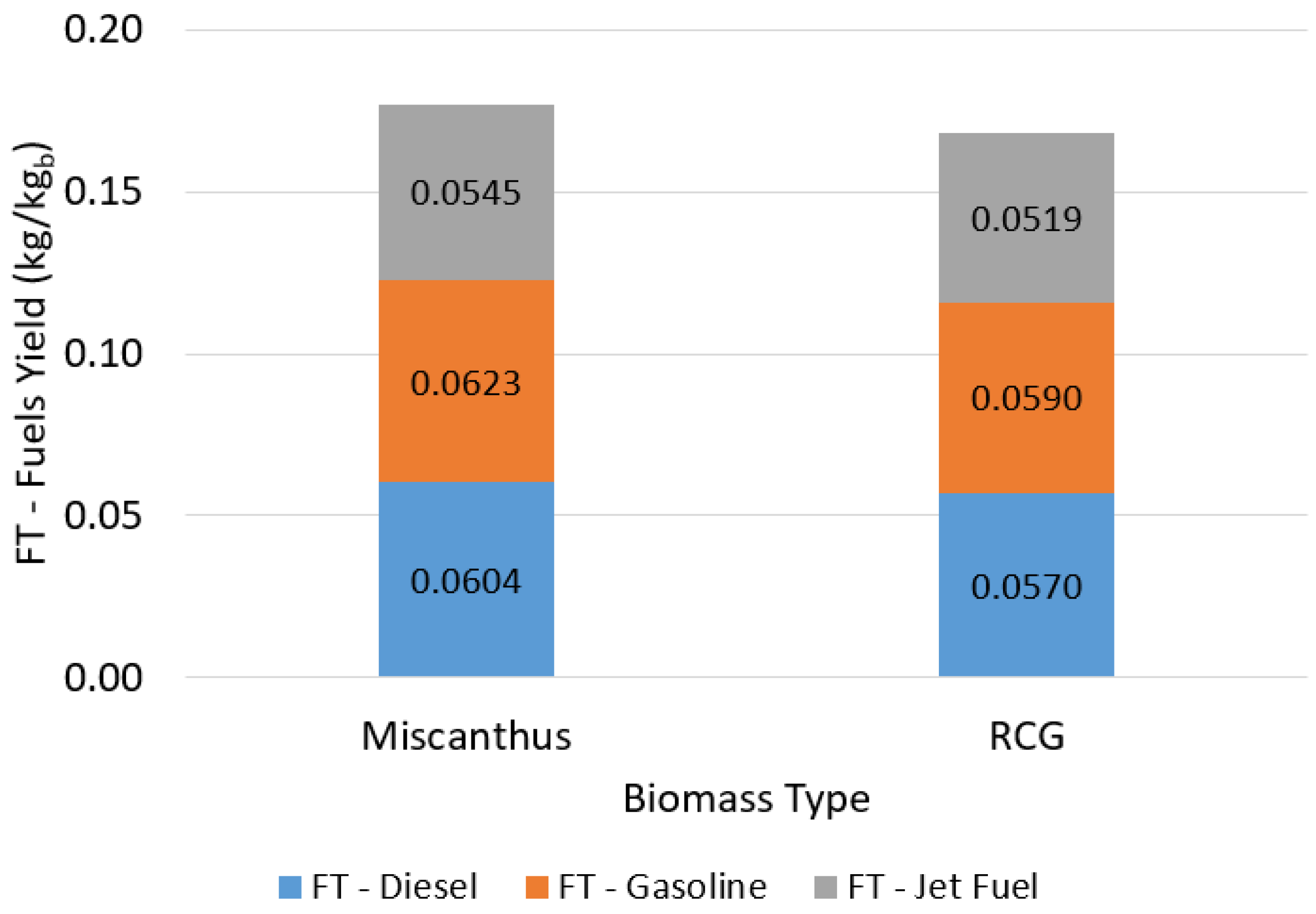
3.1.4. FT Fuel Production: Heat Self-Sufficient Process Operating Scenarios
- HSS-1 (DRR temperature = 900 °C)
- HSS-2 (DRR Temperature = 650 °C)
3.2. Life-Cycle Results
3.2.1. Core Conversion and Upgrading to Final Fuel
3.2.2. Reference Systems
3.2.3. FT Fuel Production
- Max-yield scenario: Maximum yield assumed (Section 3.1.2) and external heat provided by fossil fuel combustion.
- HSS-1 and HSS-2 scenarios: Reflecting the results of Section 3.1.3, where no external heat is required.
- Max-yield operating scenario
- Heat self-sufficient process’s operating scenarios.
4. Discussion
- External heat requirements must be minimal, in order to avoid possible fossil-generated heat inputs. Therefore, it is strongly advised to pursue the integration of the conversion and biofuel-upgrading stages.
- The utilization of waste or renewable heat would greatly lower expected impacts.
- Cultivation energy crops in degraded land can provide the potential of “negative carbon” biofuels, provided that sufficient biomass feedstock yields are achieved.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BTL | Biomass to Liquid | LCI | Life-Cycle Inventory |
| DRR | Dry Reforming Reactor | LHV | Lower Heating Value |
| FTS | Fischer–Tropsch Synthesis | MSW | Municipal Solid Waste |
| GWP | Global Warming Potential | NG | Natural Gas |
| HDR | Hydrocracking Reactor | PSA | Pressure Swing Adsorption |
| HHV | Higher Heating Value | RCG | Reed Canary Grass |
| HSS | Heat Self-Sufficient scenario | RED | Renewable Energy Directive |
| ILCD | International Ref. Life-Cycle Data System | SCWG | Supercritical Water Gasification |
| i-LUC | Indirect Land-Use Change | WGS | Water–Gas Shift |
| LCA | Life-Cycle Assessment |
References
- “The State of European Transport 2024—An Overview of the EU’s Largest Climate Problem”, A Briefing by Transport & Environment 2024. Available online: https://www.transportenvironment.org/uploads/files/TE_SoT_2024_report-1.pdf (accessed on 29 January 2025).
- European Union. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources. Off. J. Eur. Union 2018, 1–128. Available online: https://eur-lex.europa.eu/eli/dir/2018/2001/oj/eng (accessed on 29 January 2025).
- European Union. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC 2009. Off. J. Eur. Union 2009, 11, 1–47. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:260:0005:0019:EN:PDF (accessed on 29 January 2025).
- European Union. Directive (EU) 2023/2413 of the European Parliament and of the Council of 18 October 2023 amending Directive (EU) 2018/1999 and Directive 98/70/EC as Regards the promotion of Energy from Renewable Sources, and Repealing Council Directive (EU) 2015/652. Off. J. Eur. Union 2023, 1–77. Available online: https://eur-lex.europa.eu/eli/dir/2023/2413/oj/eng (accessed on 29 January 2025).
- European Union. REPowerEU: A Plan to Rapidly Reduce Dependence on Russian Fossil Fuels and Fast Forward the Green Transition. Press Release 2022. Available online: https://ec.europa.eu/commission/presscorner/api/files/document/print/en/ip_22_3131/IP_22_3131_EN.pdf (accessed on 29 January 2025).
- Boukis, N.; Katharina Stoll, I. Gasification of Biomass in Supercritical Water, Challenges for the Process Design—Lessons Learned from the Operation Experience of the First Dedicated Pilot Plant. Processes 2021, 9, 455. [Google Scholar] [CrossRef]
- Dutzi, J.; Boukis, N.; Sauer, J. Supercritical Water Gasification of Heavy Metal Contaminated Plants with Focus on Separation of Heavy Metal Contaminants. Biomass Bioenergy 2024, 182, 107059. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J. Biofuel Production from Supercritical Water Gasification of Sustainable Biomass. Energy Convers. Manag. X 2022, 14, 100164. [Google Scholar] [CrossRef]
- Louw, J.; Schwarz, C.E.; Knoetze, J.H.; Burger, A.J. Thermodynamic Modelling of Supercritical Water Gasification: Investigating the Effect of Biomass Composition to Aid in the Selection of Appropriate Feedstock Material. Bioresour. Technol. 2014, 174, 11–23. [Google Scholar] [CrossRef]
- Wang, C.; Jin, H.; Peng, P.; Chen, J. Thermodynamics and LCA Analysis of Biomass Supercritical Water Gasification System Using External Recycle of Liquid Residual. Renew Energy 2019, 141, 1117–1126. [Google Scholar] [CrossRef]
- Dutzi, J.; Boukis, N.; Sauer, J. Investigating Salt Precipitation in Continuous Supercritical Water Gasification of Biomass. Processes 2024, 12, 935. [Google Scholar] [CrossRef]
- Wang, S.; Xu, D.; Guo, Y. Supercritical Water Processing Technologies for Environment, Energy and Nanomaterial Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Kladisios, P.; Stegov Sagia, A. A Review on Supercritical Water Gasification of Biomass. Bioenergy Bioresour. 2022, 3, 7–10. [Google Scholar] [CrossRef]
- Gasafi, E.; Reinecke, M.Y.; Kruse, A.; Schebek, L. Economic Analysis of Sewage Sludge Gasification in Supercritical Water for Hydrogen Production. Biomass Bioenergy 2008, 32, 1085–1096. [Google Scholar] [CrossRef]
- Mi, Z.; Wang, S.; Huang, X.; Yang, C.; Zhang, F.; Li, Y. Review of the Supercritical Water Gasification System: Components, Challenges and Sustainability. Energy Convers. Manag. 2025, 323, 119169. [Google Scholar] [CrossRef]
- Antal, M.J.; Allen, S.G.; Schulman, D.; Xu, X.; Divilio, R.J. Biomass Gasification in Supercritical Water. Ind. Eng. Chem. Res. 2000, 39, 4040–4053. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Gorman, D.J.; Tiwari, K.; Rasmussen, E.G.; Kramlich, J.C.; Reinhall, G.; Novosselov, I. V Supercritical Water Gasification: Practical Design Strategies and Operational Challenges for Lab-Scale, Continuous Flow Reactors. Heliyon 2019, 5, 1269. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical Water Gasification of Biomass for Hydrogen Production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Hu, J.; Yu, F.; Lu, Y. Application of Fischer-Tropsch Synthesis in Biomass to Liquid Conversion. Catalysts 2012, 2, 303–326. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Alencar, A.C. Biomass-Derived Syngas Production via Gasification Process and Its Catalytic Conversion into Fuels by Fischer Tropsch Synthesis: A Review. Int. J. Hydrogen Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Swanson, R.M.; Platon, A.; Satrio, J.A.; Brown, R.C. Techno-Economic Analysis of Biomass-to-Liquids Production Based on Gasification. Fuel 2010, 89, S11–S19. [Google Scholar] [CrossRef]
- Bressanin, J.M.; Klein, B.C.; Chagas, M.F.; Watanabe, M.D.B.; de Mesquita Sampaio, I.L.; Bonomi, A.; de Morais, E.R.; Cavalett, O. Techno-Economic and Environmental Assessment of Biomass Gasification and Fischer-Tropsch Synthesis Integrated to Sugarcane Biorefineries. Energies 2020, 13, 4576. [Google Scholar] [CrossRef]
- Snehesh, A.S.; Mukunda, H.S.; Mahapatra, S.; Dasappa, S. Fischer-Tropsch Route for the Conversion of Biomass to Liquid Fuels—Technical and Economic Analysis. Energy 2017, 130, 182–191. [Google Scholar] [CrossRef]
- Ostadi, M.; Rytter, E.; Hillestad, M. Boosting Carbon Efficiency of the Biomass to Liquid Process with Hydrogen from Power: The Effect of H2/CO Ratio to the Fischer-Tropsch Reactors on the Production and Power Consumption. Biomass Bioenergy 2019, 127, 105282. [Google Scholar] [CrossRef]
- Dossow, M.; Dieterich, V.; Hanel, A.; Spliethoff, H.; Fendt, S. Improving Carbon Efficiency for an Advanced Biomass-to-Liquid Process Using Hydrogen and Oxygen from Electrolysis. Renew. Sustain. Energy Rev. 2021, 152, 111670. [Google Scholar] [CrossRef]
- Gruber, H.; Groß, P.; Rauch, R.; Reichhold, A.; Zweiler, R.; Aichernig, C.; Müller, S.; Ataimisch, N.; Hofbauer, H. Fischer-Tropsch Products from Biomass-Derived Syngas and Renewable Hydrogen. Biomass Convers. Biorefinery 2021, 11, 2281–2292. [Google Scholar] [CrossRef]
- Rahbari, A.; Shirazi, A.; Venkataraman, M.B.; Pye, J. A Solar Fuel Plant via Supercritical Water Gasification Integrated with Fischer–Tropsch Synthesis: Steady-State Modelling and Techno-Economic Assessment. Energy Convers Manag. 2019, 184, 636–648. [Google Scholar] [CrossRef]
- Campanario, F.J.; Gutiérrez Ortiz, F.J. Fischer-Tropsch Biofuels Production from Syngas Obtained by Supercritical Water Reforming of the Bio-Oil Aqueous Phase. Energy Convers Manag. 2017, 150, 599–613. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J. Techno-Economic Assessment of Supercritical Processes for Biofuel Production. J. Supercrit. Fluids 2020, 160, 104788. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Kruse, A.; Ramos, F.; Ollero, P. Integral Energy Valorization of Municipal Solid Waste Reject Fraction to Biofuels. Energy Convers Manag. 2019, 180, 1167–1184. [Google Scholar] [CrossRef]
- Voultsos, I.; Katsourinis, D.; Giannopoulos, D.; Founti, M. Integrating LCA with Process Modeling for the Energetic and Environmental Assessment of a CHP Biomass Gasification Plant: A Case Study in Thessaly, Greece. Eng.—Adv. Eng. 2020, 1, 2–30. [Google Scholar] [CrossRef]
- Alifieris, O.; Katsourinis, D.; Giannopoulos, D.; Founti, M. Process Simulation and Life Cycle Assessment of Ceramic Pigment Production: A Case Study of Green Cr2o3. Processes 2021, 9, 1731. [Google Scholar] [CrossRef]
- Tasiopoulou, T.; Katsourinis, D.; Giannopoulos, D.; Founti, M. Production-Process Simulation and Life-Cycle Assessment of Metakaolin as Supplementary Cementitious Material. Eng 2023, 4, 761–779. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Pourrut, B.; Mench, M.; Douay, F. Suitability of Miscanthus Species for Managing Inorganic and Organic Contaminated Land and Restoring Ecosystem Services. A Review. J. Environ. Manag. 2014, 143, 123–134. [Google Scholar] [CrossRef]
- Lord, R.A. Reed Canarygrass (Phalaris Arundinacea) Outperforms Miscanthus or Willow on Marginal Soils, Brownfield and Non-Agricultural Sites for Local, Sustainable Energy Crop Production. Biomass Bioenergy 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Roni, M.S.; Cafferty, K.G.; Hess, J.R.; Jacobson, J.J.; Kenney, K.L.; Searcy, E.; Tumuluru, J.S. Biomass Supply Chains for Bioenergy & Biorefining. In Chapter 11: Miscanthus, Switchgrass, Reed Canary Grass, Rye, Giant Reed Etc.; Holm-Nielsen, J.B., Ehimen, E.A., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 271–291. [Google Scholar] [CrossRef]
- Elsayed, S. Gasification of Algal Biomass in Supercritical Water with the Potential of Energy and Nutrients Recovery. Ph.D. Thesis, Karlsruhe Institute of Technology (KIT), Karlsruhe, Germany, 2019. [Google Scholar]
- Suárez París, R.; L’Abbate, M.E.; Liotta, L.F.; Montes, V.; Barrientos, J.; Regali, F.; Aho, A.; Boutonnet, M.; Järås, S. Hydroconversion of Paraffinic Wax over Platinum and Palladium Catalysts Supported on Silica–Alumina. Catal Today 2016, 275, 141–148. [Google Scholar] [CrossRef]
- ISO 14044:2006+A1+A2:2020; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. CEN—European Committee for Standardization: Brussels, Belgium, 2020.
- ISO 14040:2006+A1:2020; Environmental Management—Life Cycle Assessment—Principles and Framework. CEN—European Committee for Standardization: Brussels, Belgium, 2020.
- EC-JRC. International Reference Life Cycle Data System (ILCD) Handbook—General Guide for Life Cycle Assessment—Detailed Guidance, 1st ed.; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar] [CrossRef]
- Muench, S.; Guenther, E. A Systematic Review of Bioenergy Life Cycle Assessments. Appl. Energy 2013, 112, 257–273. [Google Scholar] [CrossRef]
- Zuwała, J. Life Cycle Approach for Energy and Environmental Analysis of Biomass and Coal Co-Firing in CHP Plant with Backpressure Turbine. J Clean Prod. 2012, 35, 164–175. [Google Scholar] [CrossRef]
- Peters, J.F.; Iribarren, D.; Dufour, J. Simulation and Life Cycle Assessment of Biofuel Production via Fast Pyrolysis and Hydroupgrading. Fuel 2015, 139, 441–456. [Google Scholar] [CrossRef]
- Brander, M.; Tipper, R.; Hutchison, C.; Davis, G. Consequential and Attributional Approaches to LCA: A Guide to Policy Makers with Specific Reference to Greenhouse Gas LCA of Biofuels; Technical Paper TP-090403-A; Ecometrica Press: Edinburgh, UK, 2009. [Google Scholar]
- Hölscher, T.; Deinmling, S.; Freiermuth Knuchel, R.; Gaillard, G.; Kägi, T.; Müller-Sämann, K. Energieoptionen Aus Landwirtschaftlicher Biomasse Am Oberrhein—Auswirkungen Für Landwirtschaft Und Umwelt—Endbericht Zum Projekt; Agentur Für Nachhaltige Nutzung von Agrarlandschaften (ANNA): Müllheim, Germany, 2007. [Google Scholar]
- KTBL Energiepflanzen. Daten Für Die Planung Des Energiepflanzenanbaus; Kuratorium Für Technik Und Bauwesen in Der Landwirtschaft: Darmstadt, Germany, 2006; p. 372. [Google Scholar]
- Keller, M.; Wuthrich, P.; Notter, B. Handbook of Emission Factors for Road Transport v3.1; HBEFA, INFRAS: Berne, Switzerland, 2017. [Google Scholar]
- Phyllis2—Database for the Physico-Chemical Composition of (Treated) Lignocellulosic Biomass, Micro- and Macroalgae, Various Feedstocks for Biogas Production and Biochar. Available online: https://phyllis.nl/ (accessed on 29 January 2025).
- Gutiérrez Ortiz, F.J.; Alonso-Fariñas, B.; Campanario, F.J.; Kruse, A. Life Cycle Assessment of the Fischer-Tropsch Biofuels Production by Supercritical Water Reforming of the Bio-Oil Aqueous Phase. Energy 2020, 210, 118648. [Google Scholar] [CrossRef]
- Allam, R.; Martin, S.; Forrest, B.; Fetvedt, J.; Lu, X.; Freed, D.; Brown, G.W.; Sasaki, T.; Itoh, M.; Manning, J. Demonstration of the Allam Cycle: An Update on the Development Status of a High Efficiency Supercritical Carbon Dioxide Power Process Employing Full Carbon Capture. In Proceedings of the Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 114, pp. 5948–5966. [Google Scholar]
- Rogalev, N.; Rogalev, A.; Kindra, V.; Zlyvko, O.; Bryzgunov, P. Review of Closed SCO2 and Semi-Closed Oxy–Fuel Combustion Power Cycles for Multi-Scale Power Generation in Terms of Energy, Ecology and Economic Efficiency. Energies 2022, 15, 9226. [Google Scholar] [CrossRef]
- Kaminski, K.; Znaczko, P.; Kardas-Cinal, E.; Chamier-Gliszczynski, N.; Koscielny, K.; Cur, K. Comparison of the Heat Transfer Efficiency of Selected Counterflow Air-to-Air Heat Exchangers Under Unbalanced Flow Conditions. Energies 2025, 18, 117. [Google Scholar] [CrossRef]
- Campanario, F.J.; Gutiérrez Ortiz, F.J. Techno-Economic Assessment of Bio-Oil Aqueous Phase-to-Liquids via Fischer-Tropsch Synthesis and Based on Supercritical Water Reforming. Energy Convers. Manag. 2017, 154, 591–602. [Google Scholar] [CrossRef]
- IRENA Report. Renewable Energy Prospects for the European Union. ISBN 978-92-9260-007-5. 2018. Available online: https://www.irena.org/publications/2018/Feb/Renewable-energy-prospects-for-the-EU (accessed on 30 January 2025).
- Zoppi, G.; Pipitone, G.; Gruber, H.; Weber, G.; Reichhold, A.; Pirone, R.; Bensaid, S. Aqueous Phase Reforming of Pilot-Scale Fischer-Tropsch Water Effluent for Sustainable Hydrogen Production. Catal. Today 2021, 367, 239–247. [Google Scholar] [CrossRef]









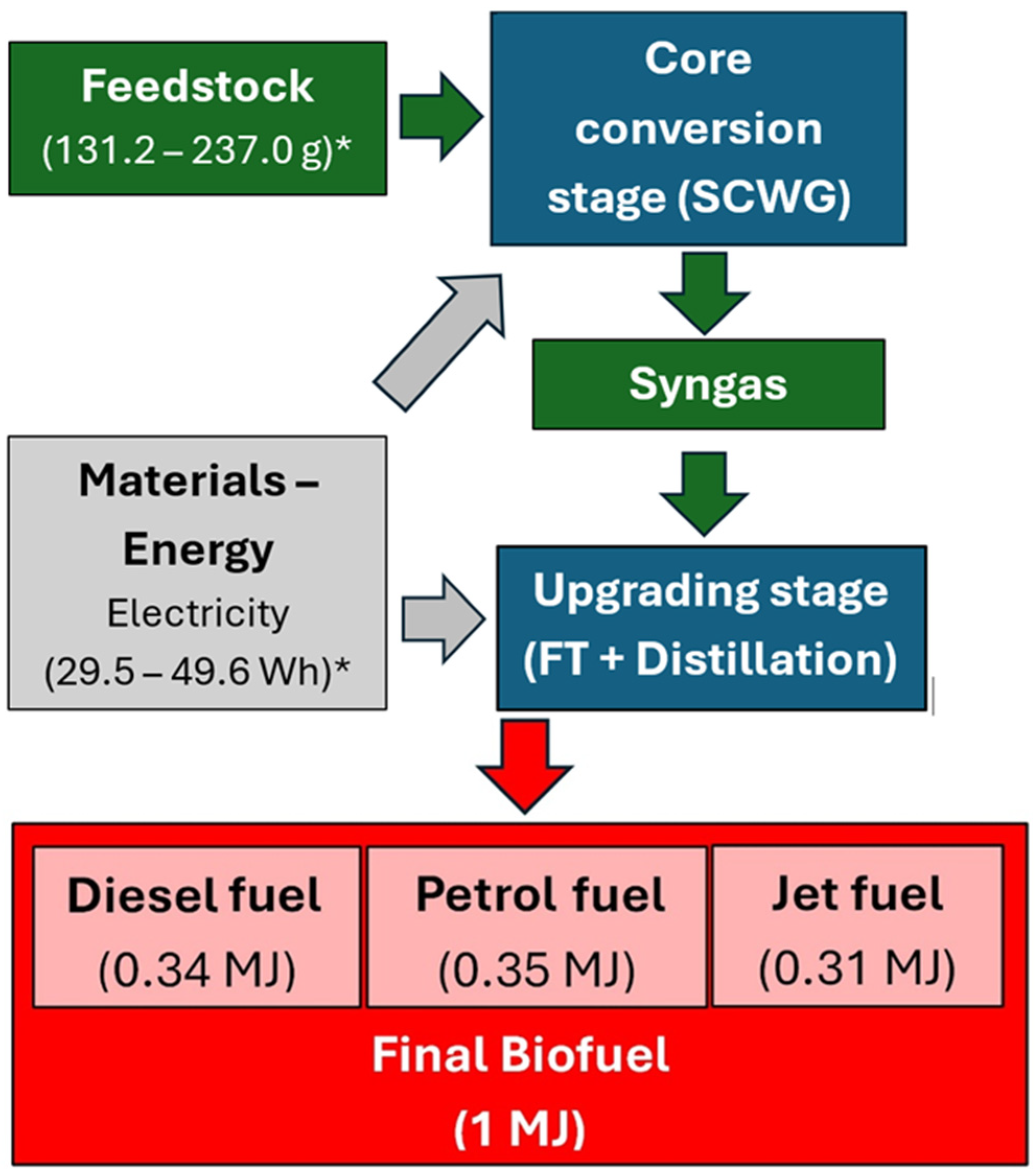
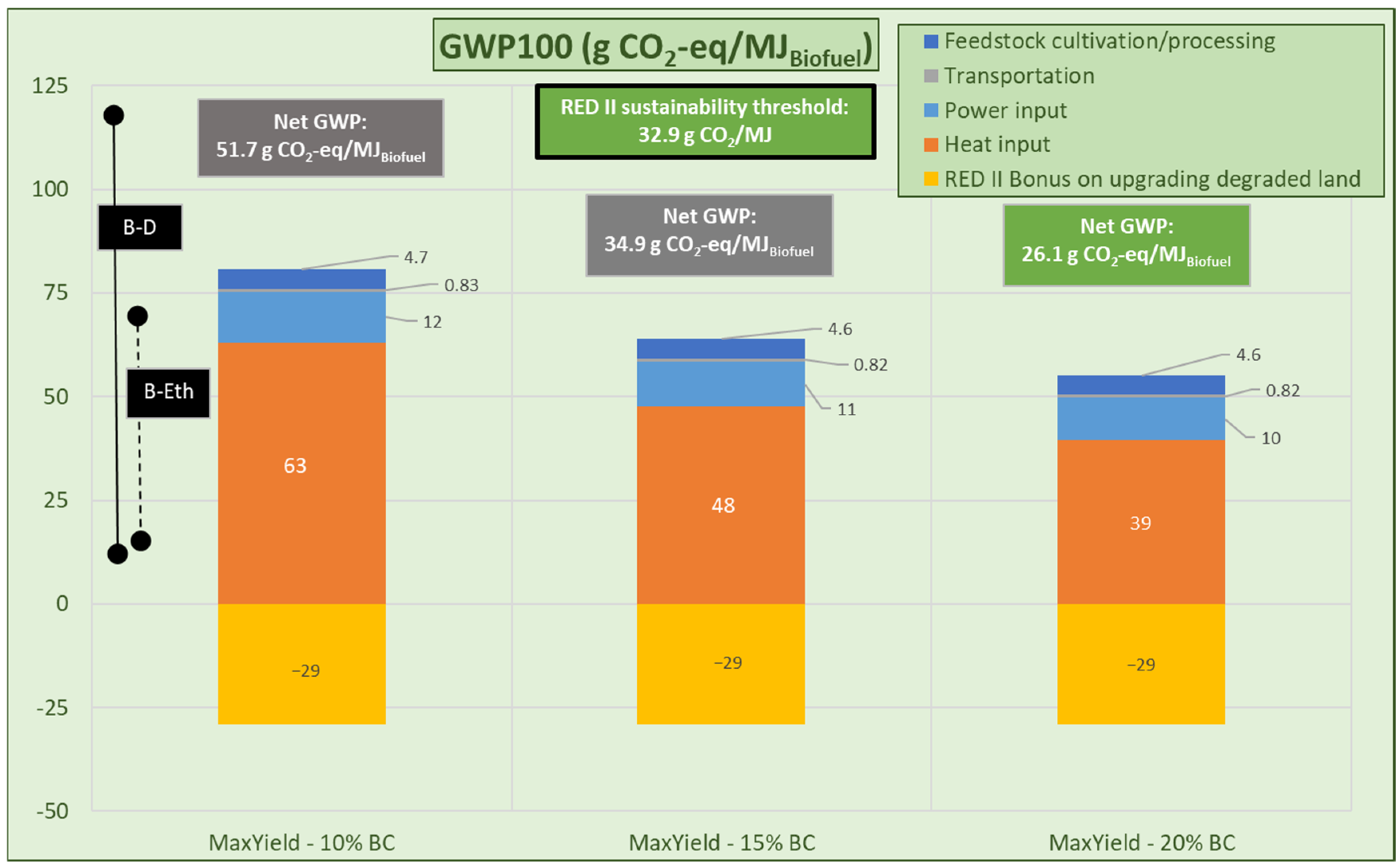

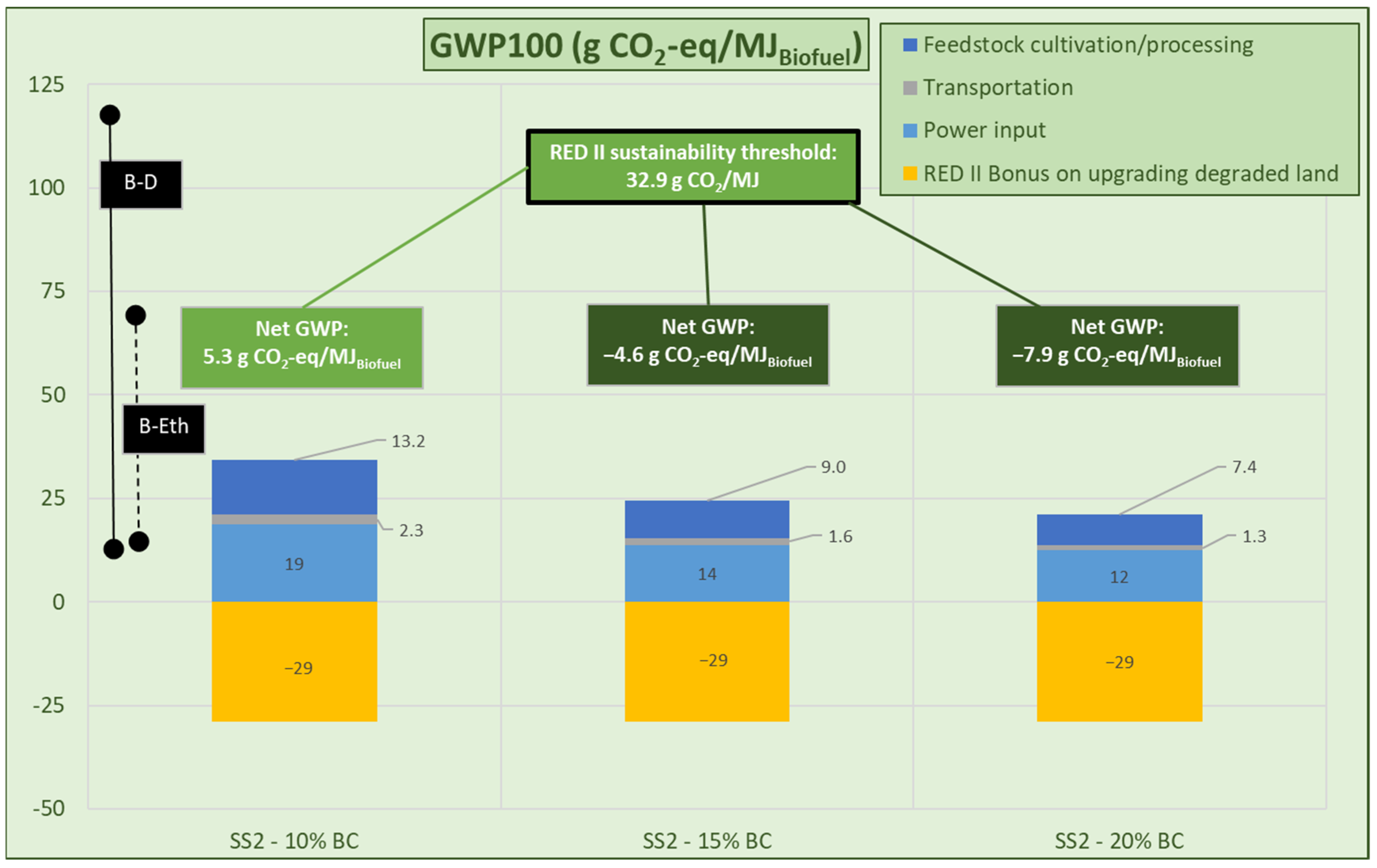
| Analysis | Miscanthus | RCG |
|---|---|---|
| Volatile Matter | 86.50 | 74 |
| Fixed Carbon | 10.09 | 20.4 |
| Ash | 3.41 | 5.6 |
| C | 48.60 | 46.00 |
| H | 6.00 | 5.50 |
| O | 41.07 | 41.84 |
| N | 0.52 | 0.88 |
| S | 0.20 | 0.09 |
| HHV [MJ/kg] (exp) | 19.12 | 18.80 |
| HHV [MJ/kg] (Milne) | 19.48 | 17.76 |
| Phyllis2 ID | 1743 | 2124 |
| Biomass Type | Biomass Concentration (% wt.) | SCWG Temperature (°C) | H2/CO (mol/mol) | Carbon Efficiency (%) |
|---|---|---|---|---|
| Miscanthus | 10 | 640 | 2.08 | 48.7 |
| 15 | 700 | 2.04 | 49.4 | |
| 20 | 760 | 2.05 | 49.3 | |
| RCG | 10 | 630 | 2.08 | 47.4 |
| 15 | 690 | 2.06 | 47.8 | |
| 20 | 750 | 2.08 | 47.4 |
| SCWG + DRR Heat Recovery (%) | Heat Demand (kWh/kgb) |
|---|---|
| 65 | 2.28 |
| 75 | 1.78 |
| 85 | 1.28 |
| Biomass Type | Biomass Concentration (% wt.) | Syngas Combustion (%) | Carbon Efficiency (%) |
|---|---|---|---|
| Miscanthus | 10 | 65 | 17.0 |
| 15 | 50 | 24.7 | |
| 20 | 40 | 29.6 | |
| RCG | 10 | 65 | 16.6 |
| 15 | 50 | 23.9 | |
| 20 | 40 | 28.5 |
| SCWG + DRR Heat Recovery (%) | Syngas Combustion (%) | FT Diesel (kg/kgb) | FT Gasoline (kg/kgb) | FT Jet Fuel (kg/kgb) |
|---|---|---|---|---|
| 65 | 50 | 0.0484 | 0.0499 | 0.0437 |
| 75 | 40 | 0.0580 | 0.0598 | 0.0524 |
| 85 | 30 | 0.0677 | 0.0698 | 0.0611 |
| Biomass Type | Biomass Concentration (% wt.) | SCWG Temperature (°C) | H2/CO (mol/mol) | Syngas Combustion (%) | Carbon Efficiency (%) |
|---|---|---|---|---|---|
| Miscanthus | 10 | 595 | 2.06 | 50 | 18.1 |
| 15 | 650 | 2.06 | 30 | 25.4 | |
| 20 | 700 | 2.06 | 15 | 30.8 | |
| RCG | 10 | 590 | 2.07 | 50 | 18.0 |
| 15 | 640 | 2.03 | 25 | 27.3 | |
| 20 | 690 | 2.04 | 15 | 30.8 |
| SCWG + DRR Heat Recovery (%) | Syngas Combustion (%) | FT Diesel (kg/kgb) | FT Gasoline (kg/kgb) | FT Jet Fuel (kg/kgb) |
|---|---|---|---|---|
| 65 | 30 | 0.0497 | 0.0513 | 0.0449 |
| 75 | 15 | 0.0604 | 0.0623 | 0.0545 |
| 85 | 0 | 0.0710 | 0.0733 | 0.0641 |
| Description | Cultivation, Transportation, and Biofuel Production (g CO2-eq/MJbiofuel) | Direct and Indirect Land-Use Change Impact (g CO2-eq/MJbiofuel) | Total (Range) (g CO2-eq/MJbiofuel) | |
|---|---|---|---|---|
| Reference biofuels | Biodiesel (B-D) | Lowest: 11.2 (Waste cooking oil–animal fat) Highest: 63.5 (Palm oil) | Lowest: 0 (Waste cooking oil–animal fat) Highest: 55 (Oil crops) | 11.2–118.5 |
| Bioethanol (B-Eth) | Lowest: 13.5 (Wheat straw) Highest: 56.3 (Corn) | Lowest: 0 Highest: 12 (Cereals and starch-rich crops) | 13.5–68.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsourinis, D.; Giannopoulos, D.; Founti, M. Fischer–Tropsch Biofuel Production from Supercritical Water Gasification of Lignocellulosic Biomass: Process Modelling and Life-Cycle Assessment. Processes 2025, 13, 895. https://doi.org/10.3390/pr13030895
Katsourinis D, Giannopoulos D, Founti M. Fischer–Tropsch Biofuel Production from Supercritical Water Gasification of Lignocellulosic Biomass: Process Modelling and Life-Cycle Assessment. Processes. 2025; 13(3):895. https://doi.org/10.3390/pr13030895
Chicago/Turabian StyleKatsourinis, Dimitrios, Dimitrios Giannopoulos, and Maria Founti. 2025. "Fischer–Tropsch Biofuel Production from Supercritical Water Gasification of Lignocellulosic Biomass: Process Modelling and Life-Cycle Assessment" Processes 13, no. 3: 895. https://doi.org/10.3390/pr13030895
APA StyleKatsourinis, D., Giannopoulos, D., & Founti, M. (2025). Fischer–Tropsch Biofuel Production from Supercritical Water Gasification of Lignocellulosic Biomass: Process Modelling and Life-Cycle Assessment. Processes, 13(3), 895. https://doi.org/10.3390/pr13030895







