Abstract
The persistent challenge of achieving cost-effective total phosphorus (TP) removal in wastewater treatment necessitates innovative coagulant development. While polyaluminum chloride (PAC) demonstrates efficacy in eliminating total nitrogen (TN), ammonia nitrogen (NH4+-N), suspended solids (SSs), and pH stabilization, its limitations in attaining economical TP removal remain unresolved. This study introduces a novel FeSO4-Al2(SO4)3 composite coagulant to address PAC’s shortcomings through systematic formulation optimization. Utilizing single-variable experiments and response surface methodology (RSM), we determined the optimal reagent combinations under simulated high-efficiency sedimentation tank conditions. The results revealed that the FeSO4-Al2(SO4)3 composite achieved a TP removal efficiency approximately 40% greater than the PAC at equivalent dosages. A cost–benefit analysis indicated an approximate 50% reduction in the chemical expenditure relative to conventional PAC-based systems. The optimized formulation demonstrated synergistic effects between the Fe2+ and Al3+ ions, enhancing the charge neutralization and sweep flocculation mechanisms. These findings establish FeSO4-Al2(SO4)3 as a technically and economically viable alternative for TP-centric wastewater treatment, with implications for process sustainability. Further investigations should validate the long-term operational stability across diverse water matrices and assess the environmental impacts of residual metal ions.
1. Introduction
Conventional wastewater treatment technologies, including the Conventional Activated Sludge (CAS) [1,2], Anaerobic–Anoxic–Oxic (A2/O) [3], and Membrane Bioreactor (MBR) processes [4,5,6], have been extensively implemented in urban wastewater treatment plants across China. These mature technologies demonstrate robust performances in practical applications, achieving removal efficiencies of 40–60% for chromaticity [7,8], 80–90% for biochemical oxygen demand (BOD5) [9,10,11], and over 90% for chemical oxygen demand (COD) [12,13,14] while simultaneously addressing nitrogen and phosphorus removal [15,16,17,18]. However, the further enhancement of organic matter and nutrient removal efficiencies (e.g., nitrogen and phosphorus) poses significant challenges under the existing operational frameworks. To comply with the increasingly stringent discharge standards for suspended solids (SSs), total nitrogen (TN), ammonia nitrogen (NH3-N), and total phosphorus (TP), wastewater treatment plants (WWTPs) must adopt advanced physicochemical methods for tertiary effluent treatments [19].
Traditional tertiary treatment systems predominantly rely on coagulation–sedimentation–filtration processes to reduce high-molecular-weight organic compounds, turbidity, heavy metals, and chromaticity [20,21,22]. However, the limited sedimentation efficiency of colloidal particles and planktonic microorganisms in coagulation-generated flocs often fails to meet the requirements for advanced wastewater purification. To address this limitation, the integration of high-efficiency sedimentation tanks (HESTs) with polyacrylamide (PAM) [23,24,25], a high-molecular-weight flocculant, has been widely adopted in Chinese WWTPs. This approach enhances floc settleability, thereby improving the COD, SSs, and TP removal rates [25]. HESTs offer distinct advantages, including high treatment efficiency, a compact footprint, cost-effectiveness, and operational resilience to fluctuations in water quality, temperature, pH, and dissolved oxygen levels.
Structurally, HESTs comprise rapid mixing (coagulation), slow mixing (flocculation), reaction (pre-sedimentation), inclined-tube sedimentation, effluent discharge, and sludge return/residual discharge systems. During the operation, coagulants destabilize colloidal particles in the rapid mixing zone, forming microflocs through mechanical agitation. These microflocs aggregate into larger, denser flocs in the slow mixing zone via polymer bridging. The subsequent sedimentation in the inclined-tube modules separates the flocs from the clarified effluent, with the settled sludge partially recycled and the remainder processed for disposal. Coagulants such as aluminum sulfate, ferric chloride, polyaluminum chloride (PAC), polyferric sulfate (PFS), and PAM are critical to the solid–liquid separation in the primary, secondary, and tertiary treatment stages, effectively removing the SSs, TP, pathogens, emulsified oils, and heavy metals.
Among these, PAC is widely favored for its rapid coagulation kinetics, pH adaptability, and operational safety. Its mechanisms include charge neutralization, adsorption–bridging flocculation, and complexation with free hazardous ions [26,27]. However, the PAC efficacy is highly pH-dependent, with optimal performance in neutral to slightly acidic conditions (pH 5–7). Deviations impair hydrolysis, yielding unstable aluminum hydroxide colloids [28,29]. Temperature fluctuations, competing ions (e.g., Ca2+, Mg2+), and organic contaminants (e.g., surfactants, hydrophilic organics) further compromise the PAC efficiency, necessitating pH adjustment, pretreatment, or supplementary flocculants like PAM [28].
Phosphorus, primarily originating from detergents, agricultural runoff, and industrial discharges, exists in wastewater as orthophosphates (H2PO4−/HPO42−), polyphosphates, and organic phosphorus. Orthophosphate dominates in typical municipal wastewater (pH 6–9) and is the primary target for removal [30,31]. While biological phosphorus removal transfers > 90% of the influent phosphorus to sludge, anaerobic digestion releases the soluble phosphorus into return streams, exacerbating the treatment loads [32]. Consequently, chemical precipitation using metal salts (e.g., PAC, PAM) has become indispensable in meeting strict TP standards. However, conventional coagulants exhibit limitations, including high costs, secondary pollution risks, and biological toxicity, underscoring the need for eco-friendly, cost-effective alternatives.
This study investigates the efficacy of novel composite and environmentally friendly phosphorus removal agents in HEST systems under varying operational conditions. The key objectives include the following: (1) optimizing the coagulant formulations to enhance the phosphorus removal efficiency; (2) identifying the critical operational parameters (e.g., mixing intensity, sludge return rate); and (3) developing sustainable strategies to minimize the chemical costs and environmental impacts. By advancing both material innovation and process optimization, this work aims to provide practical solutions for achieving regulatory compliance while improving the economic viability of WWTP operations.
2. Experimental Part
2.1. Reagents and Materials
All of the reagents—including the polyaluminum chloride (PAC, industrial grade), ferrous sulfate (FeSO4·7H2O), and aluminum sulfate (Al2(SO4)3·18H2O)—were procured from Nanchang Industrial and Trade Co., Ltd. (Nanchang, China) through a collaborative wastewater treatment plant (WWTP) to replicate real-world operational conditions. These chemicals were used as received without further purification. Analytical-grade reagents for the phosphorus quantification included the following: sulfuric acid (H2SO4); nitric acid (HNO3); perchloric acid (HClO4); sodium hydroxide (NaOH); sodium peroxodisulfate (Na2S2O8); ascorbic acid (C6H8O6); ammonium molybdate ((NH4)6Mo7O24·4H2O); antimony potassium tartrate (K(SbO)C4H4O6); phosphorus standard solution (KH2PO4, 2.0 μg/mL); and phenolphthalein indicator (C20H14O4).
An MS-M-S4 four-channel magnetic stirrer (IKA, Staufen, Germany) equipped with PTFE-coated stirring bars was employed to simulate the mixing dynamics in WWTPs. All reactions were conducted in 1000 mL borosilicate glass beakers to ensure chemical inertness and thermal stability.
2.2. Wastewater Treatment Procedure
A 1000 mL wastewater sample was collected from the effluent of a high-efficiency sedimentation tank at the Nanchang County Wastewater Treatment Plant (Nanchang, Jiangxi Province, China), which primarily treats domestic wastewater. The sample was stored at 4 °C prior to its analysis and equilibrated to the ambient temperature (25 ± 2 °C) before the experimentation. Polyaluminum chloride (PAC, 30 mg/L) or an equivalent dosage of a composite coagulant was quantitatively dosed into a borosilicate glass beaker. The mixture underwent rapid mixing at 55 rpm for 4 min using a magnetic stirrer to replicate the hydraulic conditions in the rapid mixing zones of sedimentation tanks. Subsequently, polyacrylamide (PAM, 1 mg/L) was introduced as a coagulant aid, followed by slow mixing at 20 rpm for 10 min to simulate flocculation dynamics. After 60 min of quiescent sedimentation, the supernatant was collected for the water quality analysis.
2.3. Analytical Methods
The water quality parameters, including the chemical oxygen demand (COD), total phosphorus (TP), total nitrogen (TN), ammonia nitrogen (NH4+-N), pH, and suspended solids (SSs), were measured according to the standardized methods outlined in China’s Discharge Standard of Pollutants for Municipal Wastewater Treatment Plants (GB 18918-2002). While secondary treatment processes in wastewater treatment plants (WWTPs) effectively remove non-phosphorus contaminants, this study focused exclusively on the phosphorus removal efficiency. The TP concentrations were quantified using a Hach DR3900 spectrophotometer (Hach Company, Loveland, CO, USA). All of the samples were filtered through 0.45 μm membrane filters (Millipore, Burlington, MA, USA) prior to the analysis to eliminate particulate interference.
2.4. Raw Water Characteristics
The raw wastewater exhibited the following properties: COD: 35.66 ± 2.58 mg/L; TP: 0.42 ± 0.01 mg/L; TN: 6.54 ± 0.56 mg/L; NH4+-N: 3.02 ± 0.12 mg/L; pH: 7.12 ± 0.15; SSs: 35 ± 3 mg/L.
3. Results and Discussion
Given that the high-efficiency sedimentation tanks in wastewater treatment plants (WWTPs) are followed by a fine filtration process capable of effectively removing SSs, COD, and other nutrients (e.g., TN, NH4+-N), the primary objective of this study was to evaluate and optimize phosphorus-specific removal agents. Consequently, the experimental analyses were exclusively designed to assess the TP removal efficiency of candidate coagulants, aligning with the critical need for targeted phosphorus control in tertiary wastewater treatment.
3.1. Phosphorus Removal Effectiveness of Single Reagents
The efficacy of various coagulants in TP removal was systematically evaluated under standardized conditions (30 mg/L dosage; mixing speed aligned with conventional wastewater treatment protocols). As delineated in Table 1, polyaluminum chloride (PAC), serving as the control, achieved near-complete TP removal (98% efficiency), reducing the effluent TP concentrations to non-detectable levels. In contrast, the iron-based coagulants exhibited moderate performances: FeSO4 and FeCl3 attained 68% and 87% removal efficiencies, respectively. This discrepancy likely stems from variations in the hydrolysis kinetics and precipitate settling rates, with FeCl3 generating Fe(OH)3 flocs that exhibit superior phosphorus adsorption in acidic environments.

Table 1.
TP removal efficiency by single coagulants.
Aluminum sulfate (Al2(SO4)3) demonstrated comparable efficacy to PAC (99% removal), which is attributable to their analogous hydrolysis pathways yielding highly adsorptive Al(OH)3 precipitates. Polymeric ferric sulfate (PFS) and polyaluminum ferric chloride (PAFC) showed inferior performances (56% and 86% removal, respectively), underscoring the critical role of hydrolyzed metal species in coagulation dynamics.
The operational data from WWTPs reveal a dosage-dependent TP removal relationship: 30 mg/L of PAC typically removes 0.8 mg/L of TP, translating to a removal capacity of 0.02 mg of TP per mg of PAC. For influent TP concentrations averaging 0.42 mg L−1 (typical of municipal WWTPs), theoretical dosage reductions to 15–17 mg/L of PAC could maintain > 95% removal efficiency while lowering chemical costs by 35–48% (Table 2). This aligns with empirical observations at facilities treating low-TP wastewater (0.42 mg/L of initial TP), where the PAC achieved > 95% removal at a 15 mg/L dosage.

Table 2.
TP removal efficiency by reduced single agent.
While PAC and Al2(SO4)3 emerge as optimal choices for high-efficiency TP removal (>98%), iron-based coagulants remain viable for scenarios requiring supplementary treatment or cost-sensitive applications. These findings advocate for a context-specific coagulant selection, prioritizing aluminum-based agents for stringent phosphorus control and iron salts for integrated treatment strategies.
3.2. Phosphorus Removal Effectiveness of Composite Reagents
The systematic evaluation of the composite coagulants demonstrated superior total phosphorus (TP) removal efficiency compared to single-component reagents (in order to compare with the PAC, the total amount of the composite reagents was 30 mg, 1:1 for each group, such as FeSO4: FeCl3 = 1:1). As summarized in Table 3, the composite formulations incorporating aluminum sulfate (Al2(SO4)3) achieved near-complete TP elimination (100% removal efficiency), significantly outperforming standalone reagents such as PAC (98%), FeCl3 (87%), and FeSO4 (68%). Synergistic interactions between Al2(SO4)3 and iron-based coagulants (e.g., FeSO4, FeCl3) enhanced the floc formation kinetics and phosphate adsorption capacity. For instance, blending the PAC with FeSO4 increased the TP removal from 83% (FeSO4 alone) to 96%, underscoring the critical role of multi-component coordination in coagulation dynamics.

Table 3.
TP removal efficiency by composite reagents (total amount: 30 mg for each group).
Halving the coagulant dosage (30 → 15 mg/L) revealed distinct performance trends (Table 4). While the Al2(SO4)3-based composites retained high TP removal efficiency (e.g., 47.25% for FeSO4-Al2(SO4)3), the PAC-dominated systems exhibited variable efficacy (1–22% removal). Notably, the FeSO4-Al2(SO4)3 composite outperformed the PAC by 12% under reduced dosing conditions, highlighting that the non-linear dose-response relationships were likely influenced by site-specific factors (e.g., influent TP variability, hydraulic retention time).

Table 4.
TP removal efficiency by reduced composite reagents.
Cross-plant validation trials demonstrated the consistent superiority of the FeSO4-Al2(SO4)3 composite across diverse wastewater effluents (TP range: 0.42–1.07 mg/L). This formulation achieved >95% TP removal under varying operational conditions—a performance unreported in prior studies. This study aims to develop an economical and high-efficiency phosphorus removal agent system (single or composite) to replace the traditional PAC formulations. The current literature reveals a notable research gap regarding the systematic application of FeSO4-Al2(SO4)3 composite systems for total phosphorus removal in high-rate sedimentation tanks. A bibliometric analysis of the Web of Science database indicates that recent innovations in phosphorus removal technologies for wastewater treatment plants predominantly focus on novel equipment and advanced processes. In contrast to the current research focus, this investigation emphasizes maintaining the existing wastewater treatment infrastructure and operational processes while developing innovative composite chemical systems to supersede the conventional PAC formulations, thereby achieving enhanced phosphorus removal efficiency. This technical approach not only effectively reduces the retrofitting costs for wastewater treatment facilities but also demonstrates superior compatibility with the existing infrastructure. The mechanisms underlying phosphorus removal by the FeSO4-Al2(SO4)3 composite system in high-rate sedimentation tanks will be systematically investigated in subsequent phases of this research, and a particular emphasis on the mechanistic investigations is warranted to elucidate the synergistic Al3+-Fe2+ interactions, which potentially involve the following: (a) co-precipitation dynamics: the formation of mixed-metal hydroxides (e.g., FeAl(OH)6+) with enhanced phosphate binding; and/or (b) pH stabilization: sulfate counterions buffering the coagulation pH within optimal ranges (6.5–7.5).
3.3. Optimal Conditions for Composite Reagents
A gradient dosage experiment was conducted to evaluate the TP removal efficiency of the FeSO4-Al2(SO4)3 composite coagulant (1:1 molar ratio) in the influent of the high-efficiency sedimentation tank at the Nanchang County Wastewater Treatment Plant. The total dosages ranged from 5 to 30 mg/L (the total concentration of the composite reagent), with the results summarized in Table 5.

Table 5.
TP removal of different amounts of FeSO4-Al2(SO4)3 composite coagulant.
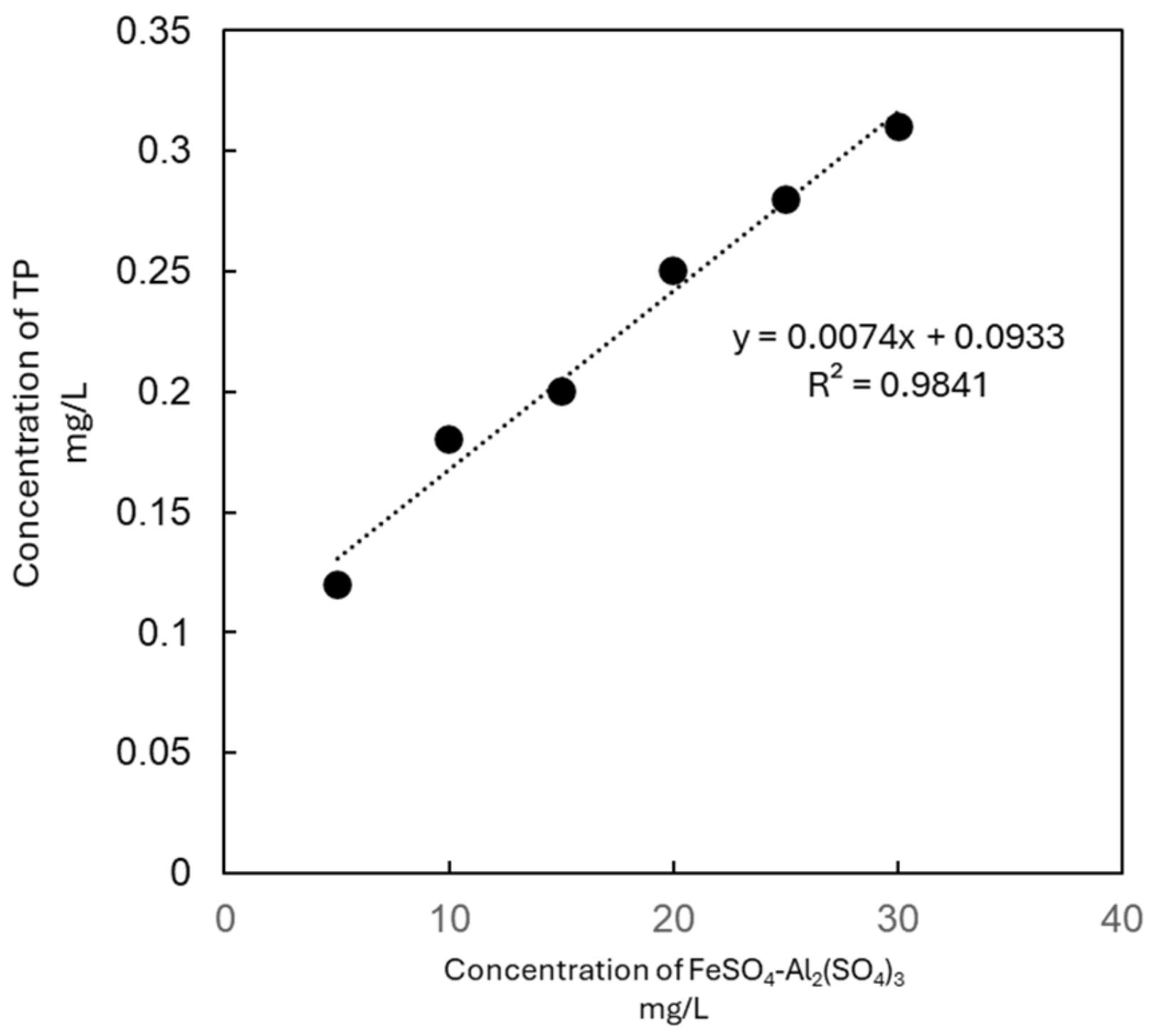
The data revealed a strong linear correlation between the coagulant dosage and the TP removal efficiency (R2 > 0.9, Figure 1). The effluent TP concentrations in all of the experimental groups complied with China’s Class I-A Discharge Standard for Municipal Wastewater Treatment Plants (GB 18918-2002, TP ≤ 0.5 mg/L). Notably, at a dosage of 20 mg/L, the effluent TP concentration (0.15 ± 0.01 mg/L) met the Class III Surface Water Environmental Quality Standard (GB 3838-2002, TP ≤ 0.2 mg/L), demonstrating the composite’s potential for stringent phosphorus control.
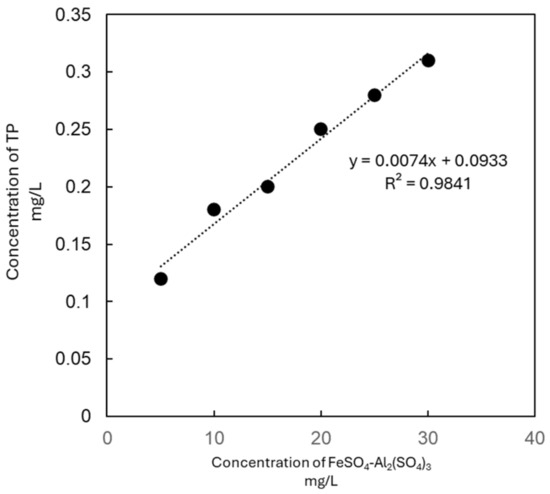
Figure 1.
The correlation between the concentration of FeSO4-Al2(SO4)3 and TP in water.
The gradient experiment was conducted to assess the TP removal efficiency of the FeSO4—Al2(SO4)3 composite reagent (in a 1:1 ratio) in the influent of the high-efficiency sedimentation tank at the Nanchang County sewage treatment plant, with total reagent dosages of 5, 10, 15, 20, 25, and 30 mg/L. The experimental results are shown in Table 5.
An optimization experiment was conducted to evaluate the phosphorus removal efficiency of the FeSO4–Al2(SO4)3 composite reagent at a total dosage of 20 mg/L. Five reagent ratios (1:19, 5:15, 10:10, 15:5, and 19:1) were tested, with a 20 mg/L polyaluminum chloride (PAC) control group. As shown in Table 6, the total phosphorus (TP) removal efficiency exceeded 70% for the ratios between 1:19 and 10:10, significantly outperforming the ratios of 15:5–19:1 (64–62%) and the PAC control group (35%). These results highlight the superior performance of the composite reagent over the conventional PAC formulation, particularly in the lower FeSO4 proportion range. To further refine the reagent ratio, response surface methodology (RSM) was subsequently employed to develop a binomial mathematical model and validate the optimal conditions through experimental verification.

Table 6.
Reagent ratios of FeSO4-Al2(SO4)3 composite reagent.
Building on these findings, a Box–Behnken design (BBD) in the Design Expert 13 software was utilized to establish a response surface model incorporating six independent variables. These variables simulated the operational parameters of the coagulation–flocculation process in the high-efficiency sedimentation basin at the Nanchang County Wastewater Treatment Plant (Jiangxi Province, China) and included the following:
- FeSO4/(FeSO4 + Al2(SO4)3) ratio (0.05–0.5);
- Composite reagent dosage (10–30 mg/L);
- Rapid mixing speed (40–70 rpm) and time (1–7 min);
- Polyacrylamide (PAM) dosage (0.5–1.5 mg/L);
- Slow mixing speed (10–30 rpm) and time (5–15 min).
The central point parameters were based on the plant’s existing coagulation–flocculation process (excluding PAC), with triplicate central point experiments. The total phosphorus (TP) removal efficiency served as the response variable. A total of 59 simulation experiments were conducted (Table 7), followed by validation tests to confirm the optimal conditions for maximizing the TP removal efficiency under the defined operational constraints.

Table 7.
Conditions and results of response surface methodology for high-efficiency sedimentation basin process parameters.
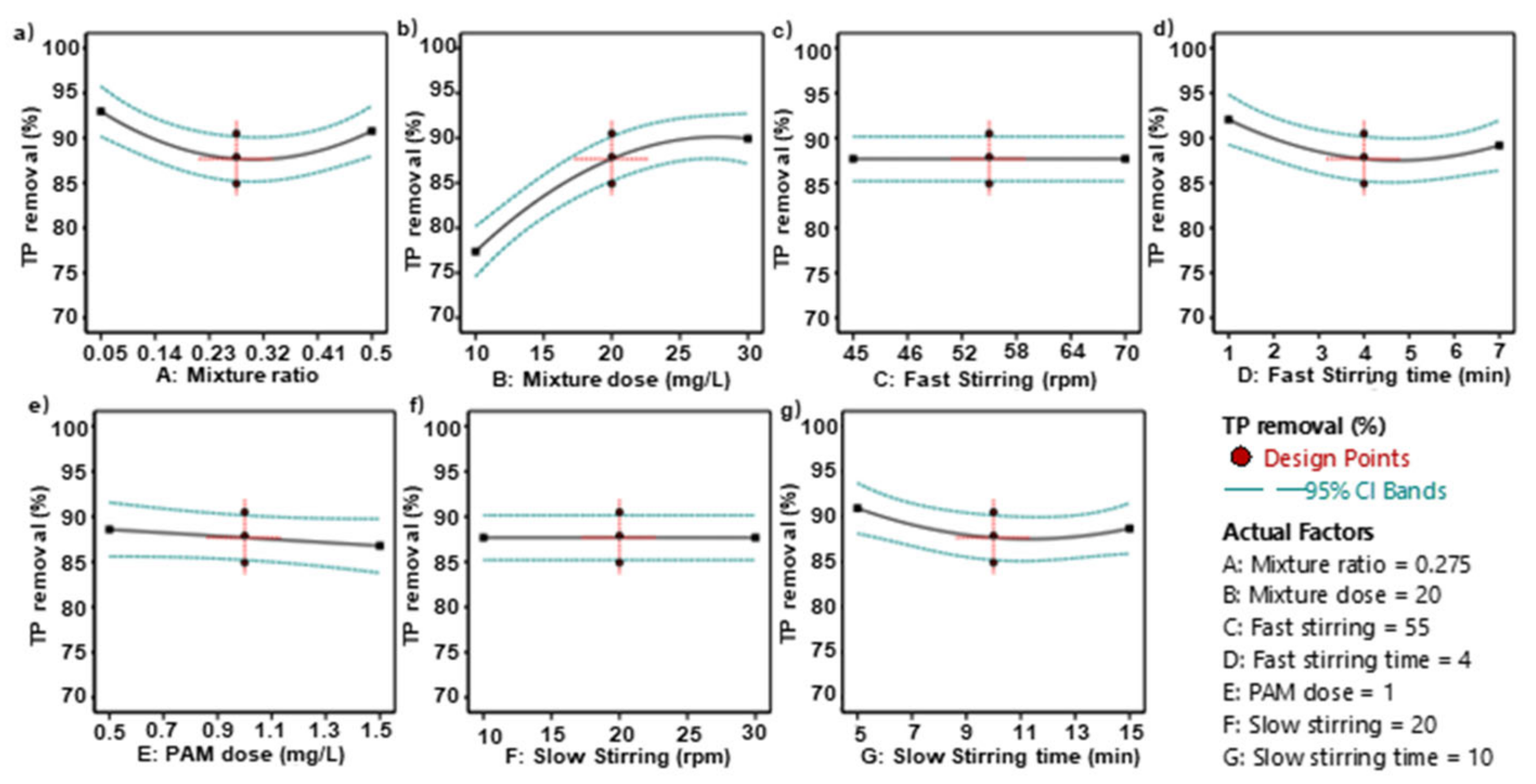
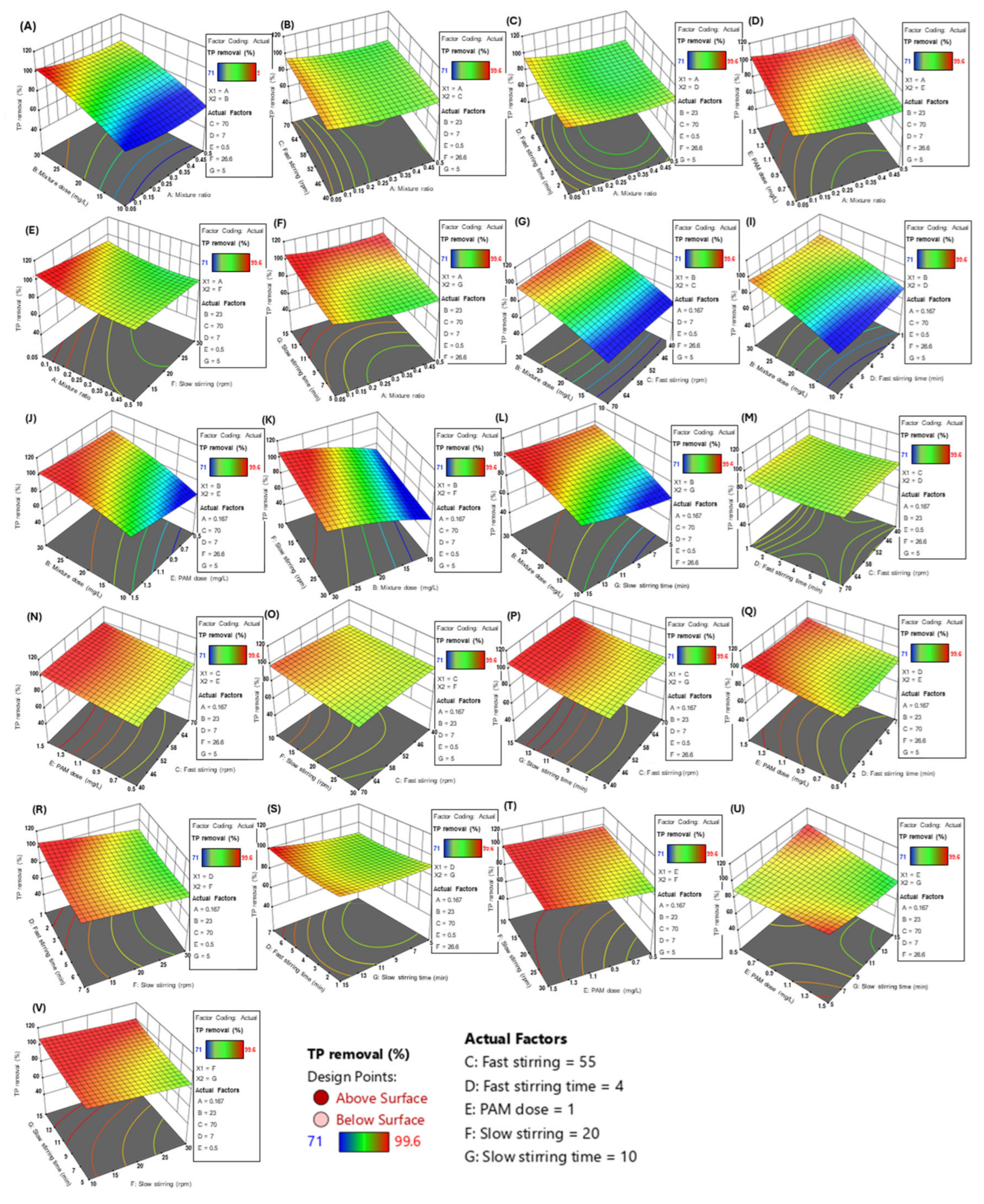
By combining the optimization conditions and experimental results, the software analysis was conducted to determine the impact of each pair of process conditions on the total phosphorus removal efficiency. The results shown in the figures were obtained through comparative experiments and fitting effects.
Figure 2a shows the theoretical optimal value of the composite agent ratio when the TP removal efficiency is maximized. Considering the other process conditions, the optimal ratio is between 0.23 and 0.32, with a specific theoretical value of 0.275. Figure 2b shows the optimal dosage of the composite agent, which is theoretically between 20 and 25 mg/L, with the simulated conclusion being 20 mg/L. The process values and theoretical values for the fast mixing speed are both 55 rpm, and the fast mixing time is 4 min (Figure 2c,d). The theoretical optimal dosage for the coagulant PAM (Figure 2e) is 1 mg/L. For the slow speed mixing, the theoretical optimal speed is 20 rpm and the theoretical optimal mixing time is 10 min (Figure 2f,g). The data shown in this series of figures are all the results of single-factor simulations by the software, while the actual wastewater treatment process in sewage treatment plants is influenced by many factors. Therefore, the software optimized and fitted all seven process parameters listed in the table (Table 7), resulting in a 3D surface heat map, as shown in Figure 3. In the figure, blue represents the lowest TP removal rate, and red represents the highest TP removal rate. The AB combination, i.e., considering both the mixing ratio and the dosage of the composite agent, clearly affects the TP removal efficiency. When the dosage is 30 mg/L and the mixing ratio is approximately 0.05, the TP removal rate has a maximum value close to 100%, with the influence range on the TP removal rate being 60~100%. Only the CD combination, i.e., the fast mixing time and speed, has almost no effect on the TP removal rate. In summary, among the 21 total combinations, only the AB combination (composite agent ratio—composite agent dosage), BC combination (composite agent dosage—fast mixing zone speed), BD combination (composite agent dosage—fast mixing time), BE combination (composite agent dosage—coagulant PAM dosage), BF combination (composite agent dosage—slow mixing zone speed), and BG combination (composite agent dosage—slow mixing time) showed significant simultaneous effects on the TP removal rate. This indirectly reflects that the dosage of the composite agent has a significant impact on the TP removal rate.
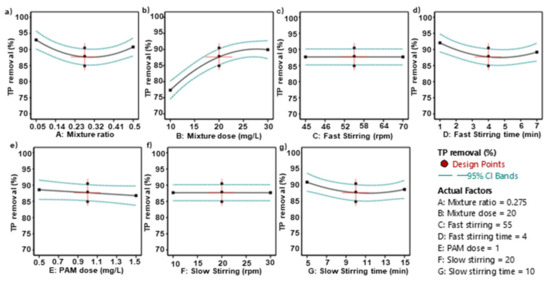
Figure 2.
The effect of a single factor on the TP removal efficiency. (a) relationship between mixture ratio and TP removal; (b) the relationship between mixture dose and TP removal; (c) relationship between fast stirring speed and TP removal; (d) relationship between fast stirring time and TP removal; (e) relationship between PAM dose and TP removal; (f) relationship between slow stirring speed and TP removal and (g) relationship between slow stirring time and TP removal.
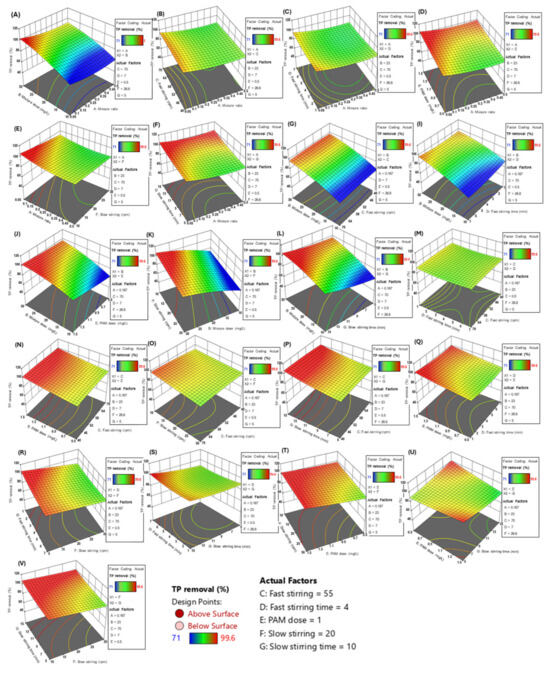
Figure 3.
A 3D heatmap of the effect of two factors on the TP removal efficiency. (A) Effects of mixture dose and mixture ratio on TP removal; (B) Effects of fast stirring speed and mixture ratio on TP removal; (C) Effects of fast stirring time and mixture ratio on TP removal; (D) Effects of PAM dosage and mixture ratio on TP removal; (E) Effects of slow stirring speed and mixture ratio on TP removal; (F) Effects of slow stirring time and mixture ratio on TP removal; (G) Effects of mixture dose and fast stirring speed on TP removal; (I) Effects of mixture dose and fast stirring speed on TP removal; (J) Effects of Mixture dose and PAM dosage on TP removal; (K) Effects of Mixture dose and slow stirring speed on TP removal; (L) Effects of mixture dose and slow stirring time on TP removal; (M) Effects of fast stirring time and fast stirring speed on TP removal; (N) Effects of PAM dose time and fast stirring speed on TP removal; (O) Effects of fast stirring speed and slow stirring speed on TP removal; (P) Effects of fast stirring speed and slow stirring time on TP removal; (Q) Effects of fast stirring time and PAM dose on TP removal; (R) Effects of fast stirring time and slow stirring speed on TP removal; (S) Effects of fast stirring time and slow stirring time on TP removal; (T) Effects of PAM dose and slow stirring speed on TP removal; (U) Effects of slow stirring speed and PAM dose on TP removal, and (V) Effects of Effects of slow stirring speed and slow stirring time on TP removal.
Based on the above simulation and actual experimental values (the last column in Table 7), a formula that can quickly calculate the TP removal rate was fitted:
where (A): mixture ratio; (B): mixture dose (mg/L); (C): fast stirring (rpm); (D): fast stirring time (min); (E): PAM dose (mg/L); (F): slow stirring (rpm); and (G): slow stirring time (min).
TP removal = 37.0976 − 50.3848 × A + 3.2258 × B − 4.91305 × D + 41.6917 × E + 2.31742 × G + 0.09125 × BD − 0.7 × BE − 0.065 × BG − 2.95 × EG + 82.8208 × A2 − 0.040322 × B2 + 0.326284 × D2 + 0.0854621 × G2
Accordingly, based on the particular process of a sewage treatment plant, one can quickly determine the change in TP removal efficiency when a particular variable is changed and promptly adjust the process parameters.
4. Conclusions
The principal objective of wastewater treatment facilities lies in achieving the cost-effective purification of municipal sewage to meet mandated discharge standards through optimized operational protocols. In practical operations, the supplementation of coagulants proves critical for enhancing the sedimentation efficiency of biochemical reaction-derived suspended solids and improving the removal efficacy of nutrients such as nitrogen and phosphorus. While polyaluminum chloride (PAC) remains a conventional flocculant choice, its substantial production costs and required dosage levels necessitate the exploration of alternative formulations or composite reagents as strategic approaches for process optimization.
Through the systematic evaluation of individual and composite flocculant configurations, this investigation establishes an optimized FeSO4-Al2(SO4)3 composite formulation. The laboratory analyses, coupled with computational simulations, demonstrate that substituting traditional PAC-polyacrylamide (PAM) combinations with the proposed iron-aluminum sulfate composite (mass ratio 1:1.5, dosage 25 mg/L) enhances the TP removal efficiency by approximately 40% compared to the conventional treatments. The economic assessments reveal that the potential operational cost reductions exceed 50% through this substitution strategy.
Notably, this study represents pioneering research into the application of FeSO4-Al2(SO4)3 composites as high-efficiency sedimentation agents in wastewater treatment contexts, addressing a notable gap in the existing literature. The findings provide valuable technical references for both scientific investigations and engineering applications, particularly regarding sustainable cost-reduction strategies in municipal wastewater management. Future research directions should encompass long-term stability assessments of the composite flocculant and evaluations of its interactions with diverse wastewater matrices.
Author Contributions
Conceptualization, J.T., Y.Z. and Y.L (Yiqiang Luo).; methodology, J.T. and K.W.; software, K.W. and X.M.; validation, J.T., L.C. and Y.L (Yiping Li).; formal analysis, X.C.; investigation, Y.Z.; resources, Q.C.; writing—original draft preparation, Y.L. and J.T.; writing—review and editing, J.T. and Y.L. (Yiqiang Luo); visualization, T.C.; supervision, L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Science and Technology Innovation Program Project of Jiangxi Hongcheng Waterworks Environmental Co., Ltd.”.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Nanchang Industrial and Trade Co., Ltd. and Jun Qian from Nanchang University for their support in this research.
Conflicts of Interest
Authors Jiancheng Tu, Yanping Zhang, Liling Chen, Xin Chen, Yiping Li, Xiaohong Min, Qiu Chen, Tao Chen and Yiqiang Luo was employed by the company Jiangxi Hongcheng Waterworks Environmental Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Jiangxi Hongcheng Waterworks Environmental Co., Ltd. was involved in all stages of the research process, including study design, data collection and analysis, interpretation of results, manuscript writing, and decision to publish the findings.
References
- Latif, E.F. Applying novel methods in conventional activated sludge plants to treat low-strength wastewater. Environ. Monit. Assess. 2024, 196, 652, Erratum in 2022, 194, 323. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.-K. A comparative analysis of two different wastewater treatment processes in actual wastewater treatment plants. Quant. Bio-Sci. 2018, 37, 19–26. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, G.; Tian, H. Current state of sewage treatment in China. Water Res. 2014, 66, 85–98. [Google Scholar] [CrossRef]
- Jing, H.E.; Xinnan, W.A.N. Application and development of membrane bioreactor in wastewater treatment. Guangdong Weiliang Yuansu Kexue 2006, 13, 16–22. [Google Scholar]
- Rahman, T.U.; Roy, H.; Islam, M.R.; Tahmid, M.; Fariha, A.; Mazumder, A.; Tasnim, N.; Pervez, M.N.; Cai, Y.; Naddeo, V.; et al. The advancement in membrane bioreactor (mbr) technology toward sustainable industrial wastewater management. Membranes 2023, 13, 181. [Google Scholar] [CrossRef]
- Xiaohua, Z.; Cuiyan, F.U.; Jing, L.I.; Hongchao, G.A.O.; Guanghui, Z. Influence factors of membrane bioreactors in wasterwater treatment. Technol. Water Treat. 2008, 34, 7–13. [Google Scholar]
- Han, C.; Cao, M.; Zhang, B. Progress of flocculation applied to dyeing wastewater treatment. Ind. Water Treat. 2006, 26, 5–9. [Google Scholar]
- Manzoor, K.; Batool, M.; Naz, F.; Nazar, M.F.; Hameed, B.H.; Zafar, M.N. A comprehensive review on application of plant-based bioadsorbents for congo red removal. Biomass Convers. Biorefinery 2024, 14, 4511–4537. [Google Scholar] [CrossRef]
- Amor, C.; Marchao, L.; Lucas, M.S.; Peres, J.A. Application of advanced oxidation processes for the treatment of recalcitrant agro-industrial wastewater: A review. Water 2019, 11, 205. [Google Scholar] [CrossRef]
- Derco, J.; Guasova, P.; Legan, M.; Zakhar, R.; Gotvajn, A.Z. Sustainability strategies in municipal wastewater treatment. Sustainability 2024, 16, 9038. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S. Experimental study on advanced treatment of urban wastewater by using combined velocity-changeable bio-filter. China Water Wastewater 2004, 20, 13–16. [Google Scholar]
- Emparan, Q.; Harun, R.; Danquah, M.K. Role of phycoremediation for nutrient removal from wastewaters: A review. Appl. Ecol. Environ. Res. 2019, 17, 889–915. [Google Scholar] [CrossRef]
- Katare, A.K.; Tabassum, A.; Sharma, A.K.; Sharma, S. Treatment of pharmaceutical wastewater through activated sludge process—A critical review. Environ. Monit. Assess. 2023, 195, 1466. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Yang, S.; Li, Y.-Y.; Wen, W.; Wang, X.C.; Chen, R. Application of anaerobic membrane bioreactors to municipal wastewater treatment at ambient temperature: A review of achievements, challenges, and perspectives. Bioresour. Technol. 2018, 267, 756–768. [Google Scholar] [CrossRef]
- Akpor, O.B.; Muchie, M. Bioremediation of polluted wastewater influent: Phosphorus and nitrogen removal. Sci. Res. Essays 2010, 5, 3222–3230. [Google Scholar]
- Khan, S.; Thaher, M.; Abdulquadir, M.; Faisal, M.; Mehariya, S.; Al-Najjar, M.A.A.; Al-Jabri, H.; Das, P. Utilization of microalgae for urban wastewater treatment and valorization of treated wastewater and biomass for biofertilizer applications. Sustainability 2023, 15, 16019. [Google Scholar] [CrossRef]
- Moretti, C.J.; Das, D.; Kistner, B.T.; Gullicks, H.; Hung, Y.-T. Activated sludge and other aerobic suspended culture processes. Water 2011, 3, 806–818. [Google Scholar] [CrossRef]
- Ye, L.; Li, D.; Zhang, J.; Zhang, J.; Zeng, H. Advance of research on the technology of nitrite-denitrifying phosphorus removal. J. Beijing Univ. Technol. 2016, 42, 585–593. [Google Scholar]
- Qiu, Z.; Zhou, Z.; Hu, D. Advances on reject water treatment for wastewater treatment plants. Water Wastewater Eng. 2018, 44, 127–131. [Google Scholar]
- Bai, C.; Wan, C.; Huang, W.; Wei, X.; Yang, C.; Zhai, W. Application of msbr+high efficiency sedimentation tank process in wastewater treatment plant with stringent discharge standard. Technol. Water Treat. 2024, 50, 147–151+156. [Google Scholar]
- Chen, Y.; Zhang, F.; Gan, F. Application optimization of high efficiency settling tank in wastewater advanced treatment project. Water Wastewater Eng. 2022, 48, 46–50+56. [Google Scholar]
- Wang, H.; Yu, X.; Li, Y.; Cui, Y.; Zhang, K. Effect of sludge return ratio on the treatment characteristics of high-efficiency sedimentation tank. Desalination Water Treat. 2014, 52, 5118–5125. [Google Scholar] [CrossRef]
- Xiao, D.; Nan, J.; Zhang, X.; He, W.; Fan, Y.; Lin, X. Pilot-scale study of turbid particle evolutional/removal characteristics during coagulation-sedimentation-filtration (csf): Effects of coagulant dosage and secondary dosing after breakage. J. Water Process Eng. 2024, 68, 106325. [Google Scholar] [CrossRef]
- Rao, M. Experimental Study on Treatment of Low Turbidity and Micropolluted Water by Combined Application of High-Density Sedimentation Tank and Powder Active Carbon. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2009. [Google Scholar]
- Sherman, J.J.; Van Horn, H.H.; Nordstedt, R.A. Use of flocculants in dairy wastewaters to remove phosphorus. Appl. Eng. Agric. 2000, 16, 445–452. [Google Scholar] [CrossRef]
- Liu, T.; Yang, S.; Wei, X.; Guo, S. Application of high-efficiency sedimentation tank and hydro-clear shallow media rapid filter for advanced treatment in a wastewater treatment plant. China Water Wastewater 2021, 37, 82–86. [Google Scholar]
- Lin, M.; Zhao, Z.; Cui, F.; Niu, C.; Wang, Y. Powdered activated carbon adsorption for water works to cope with the sudden pollution of ethylbenzene in raw water. J. Harbin Inst. Technol. 2012, 44, 48–51. [Google Scholar]
- Cao, X.; Liu, Y.; Fu, X.; Yang, J.; Wang, W. The effect of pam and pac compound conditioning on sludge rheology and dewatering. Appl. Chem. Ind. 2023, 52, 2571–2575+2579. [Google Scholar]
- Tang, W.; Zhao, X. Study on the treatment of boiler raw water in winter with flocculants combination. Ind. Water Treat. 2015, 35, 110–112. [Google Scholar]
- Penn, C.; Chagas, I.; Klimeski, A.; Lyngsie, G. A review of phosphorus removal structures: How to assess and compare their performance. Water 2017, 9, 583. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Li, J. Phosphorus pollution and its prevention and control measures. Environ. Prot. Chem. Ind. 2002, 22, 68–70. [Google Scholar]
- Zhang, Z.; Xu, Z.; Yang, L. Removal effect of nitrogen and phosphorus on micro-polluted raw water treatment processess. Technol. Water Treat. 2009, 35, 11–14+38. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).