Abstract
Water pollution, driven by industrial wastewater, agricultural runoff, and domestic sewage, introduces organic pollutants (e.g., dyes and antibiotics) and heavy metal ions (e.g., Pb2⁺ and Cr(VI)), threatening ecosystems and human health. Although traditional water treatment technologies have now matured, they still have some deficiencies in terms of specific pollutants. Metal–organic frameworks (MOFs), particularly zeolite imidazole frame-67 (ZIF-67)—a cobalt-based MOF with high surface area, tunable pores, and robust chemical stability—show excellent adsorption capacity for pollutants and have emerged as promising candidates for water treatment due to their efficacy in adsorption, catalysis, and photocatalysis. This review examines ZIF-67’s synthesis, functionalization strategies, and applications in removing organic pollutants and heavy metals. It explores its mechanisms, composite designs, and recyclability, while highlighting challenges and future directions for developing efficient, sustainable water treatment technologies.
1. Introduction
Water pollution is one of the major environmental problems facing the world today. Its harm is extensive, persistent, and complex, and has far-reaching impacts on ecosystems, human health, the economy, and social stability [1,2,3]. In recent years, scientific research and monitoring data show that new industrial pollutants, including organic synthetic dyes, daily chemical additives, and artificial drug active ingredients, are constantly detected in the water environment. This kind of emerging organic pollutant generally shows high physicochemical stability characteristics, and there are photolysis and hydrolysis resistance functional groups in its molecular structure, resulting in conventional biodegradation, flocculation precipitation, and other traditional wastewater treatment processes making it difficult to achieve effective degradation. What is more serious is that some pollutants may generate more toxic secondary metabolites in the conventional treatment process, forming the risk of persistent environmental organic pollutants [4,5,6,7]. Since the large-scale application of antibiotics as anti-infective drugs in clinical and animal husbandry fields, human beings have made breakthroughs in controlling the spread of pathogenic bacteria. However, research data show that about 90% of the maternal compounds or metabolites of antibiotics enter the water and soil environment through patient excretion, aquaculture wastewater, and other ways [8]. Water pollution caused by antibiotics has become a global environmental problem. The overuse of antibiotics may not only pose health risks, but may also lead to the development of drug-resistant bacteria, posing a serious threat to ecosystems and public health [9,10,11,12,13]. Common water pollutants include antibiotics, dyes, and heavy metals, etc. According to a number of studies, antibiotics are widely present in all aspects of the urban water cycle, including surface water, groundwater, drinking water, and wastewater. In addition, the concentration of lead detected in domestic wastewater was close to 6 mg/L [14,15,16,17,18]. Such pollutants have persistent residual characteristics in the environment, which can be amplified step by step along the food chain through bioaccumulation effects, causing pathological effects on organisms such as DNA damage and cell dysfunction, and inducing multiple toxic effects such as teratogenesis, mutagenicity, and carcinogenesis. More seriously, high toxicity threshold characteristics enable irreversible long-term harm to aquatic ecosystems and human health, even under low concentration exposure conditions [19,20,21,22]. Effective methods to remove harmful chemicals from water have become a hot topic in the research field and have attracted wide attention. To address the problem of organic pollutants in contaminated water, a variety of treatment technologies have been developed and applied, including but not limited to filtration and coagulation, individual coagulation, ozonation, chemical precipitation, adsorption, reverse osmosis, ion exchange, enzyme-catalyzed repair, and advanced oxidation processes [23,24,25,26,27,28,29,30,31,32]. In addition, converting pollutants into non-toxic substances remains a major challenge. Treating pollutants via adsorption or photocatalysis is not only more economical, but also more energy efficient. From an economic and energy efficiency point of view, the use of photocatalytic processes or adsorption technologies to remove pollutants may be more promising, especially in emerging and developing countries [33,34,35,36,37,38,39]. In order to develop photocatalysts and adsorbents with high contaminant removal capabilities, chemists are actively exploring new materials. With the development of industries such as textiles, papermaking, and clothing, there are a large amount of organic dyes in production wastewater, which leads to environmental pollution. Dye wastewater contains very harmful and carcinogenic compounds, which may pose a serious threat to human health [40]. In addition to antibiotics and organic dyes, heavy metal pollution is also a common type of water resource pollution. Heavy metals are the main pollutants in mining wastewater, such as electroplating, mineral processing, and extraction. They mainly include chromium (Cr), lead (Pb), and mercury (Hg), etc. These heavy metals are not biodegradable, causing cancer or neurological diseases in humans [41]. The main cause of heavy metal pollution is the rapid development of industrialization and urbanization. This leads to the accelerated diffusion of heavy metals in the environment [42].
For the above-mentioned pollutants, common removal methods include physical methods, chemical methods, and biological methods. Chemical methods may cause secondary pollution, and the growth and metabolism conditions of microorganisms in biological methods are strict and have certain limitations. Physical adsorption is simple to operate, has a wide range of applications, and is less likely to cause secondary pollution. With these advantages, physical adsorption is a green and energy-saving method for removing pollutants from water. Common adsorbents include activated carbon, ion exchange resins, and microporous, mesoporous, and metal–organic framework materials. Although the first few types all have certain adsorption effects, the adsorption selectivity of activated carbon for pollutants is relatively poor and its adsorption capacity is limited. Ion exchange resins are prone to being clogged by contaminants, causing secondary pollution. The pore size of microporous materials (such as zeolites) is usually less than 2 nm, which limits their adsorption or catalytic capacity for macromolecules. Moreover, their structure is highly rigid, making it difficult to adjust the pore size and surface properties through chemical modification. Although the pore size of mesoporous materials is relatively large, the control over the ordered fineness ratio and pore size distribution of the structure is not as flexible as that of MOFs. Zeolites and mesoporous materials are mostly brittle crystals or amorphous states, prone to cracking and with low mechanical strength. Metal–organic framework materials have the advantages of an extremely high specific surface area and porosity and good adsorption selectivity and stability, etc. Table 1 presents the adsorption capacities of various commonly used adsorbents.
Among these emerging materials, metal–organic frameworks (MOFs) have attracted considerable attention due to their crystalline porous structure. These structures inherently integrate photocatalytic activity and adsorption functions through precisely designed metal cluster nodes and organic linkers. MOFs are a relatively novel class of porous materials that can be functionalized in a simple and cost-effective manner for use as photocatalysts and adsorbents [43]. MOFs exhibit unique structural designability and outstanding pollutant adsorption performance compared with traditional activated carbon adsorbents. MOFs are formed by the self-assembly of metal nodes and organic ligands to create a highly ordered three-dimensional porous network. Their specific surface area (typically 1000–6000 m2/g) significantly exceeds that of activated carbon (500–1500 m2/g) through the abundant coordination of unsaturated metal sites, functionalized organic groups, and adjustable surface charges on the surface of MOFs. It is endowed with an efficient recognition ability for target pollutants (such as heavy metals, organic dyes, and volatile organic compounds), and its adsorption capacity is generally 1 to 5 times higher than that of activated carbon. Due to its structural properties, it can be functionalized in a simple and cost-effective way, and then used as an efficient photocatalyst and adsorbent in many fields [44,45]. Recently, a subclass of metal–organic frameworks with a zeolite structure has been successfully synthesized and widely used in the field of water purification. Among them, the zeolite imidazolate skeleton (ZIF) has attracted much attention due to its unique properties, such as excellent selective adsorption capacity, efficient electron transfer properties, sensitive detection of contaminants, and potential for biomedical and antimicrobial applications. In addition, ZIF exhibits high energy, a monodisperse microporous structure, and excellent chemical and thermal stability. ZIF consists of transition metal cations (such as Zn and Co) and imidazolate ligands via tetrahedral coordination [46,47,48,49,50,51,52,53,54].
At present, in the research literature on ZIF, especially for zeolite imidazole skeleton 67 (ZIF-67), the material has been shown to have significant effects in the treatment of pollutants [55]. As a cobalt-based organic framework material, ZIF-67 shows significant structural advantages and functional characteristics compared with traditional activated carbon adsorbents. Although its preparation cost is higher than that of activated carbon (about 30–50% higher) due to the use of metal precursors and 2-methylimidazole ligands, it demonstrates outstanding performance in treating specific pollutants. Studies have shown that the periodic pore structure (pore diameter ~1.1 nm) of ZIF-67 and its surface coordination active sites can achieve the efficient capture of heavy metal ions (such as Pb2+ and Hg2+) and organic micro-pollutants (such as bisphenol A and tetracycline antibiotics), and its maximum adsorption capacity can reach 2–3 times that of activated carbon. Especially in wastewater systems containing complex matrices, ZIF-67 shows significant selective adsorption advantages (selectivity coefficient > 85%) by means of the pore size sieving effect and metal–ligand synergy, while activated carbon is susceptible to interference from coexisting substances due to its wide pore size distribution (0.5–5 nm) and surface heterogeneity. This performance difference stems from the chemical tuning of ZIF-67. Through ligand functionalization, the specific recognition of target pollutants can be further enhanced, providing a new material strategy for precise environmental remediation. ZIF-67 is a subclass of MOFs, which has the characteristics of MOFs and zeolite imidazole skeleton [56]. It combines the unique advantages of zeolite and metal–organic frameworks through the combination of tetrahedrally coordinated metal cation M (M=Co or Zn) with the organic linker imidazolate, providing innovative opportunities for the development of science and technology. The main characteristics of these materials include the following: ZIF-67 has a large specific surface area, durable porosity, excellent chemical and thermal stability, and an abundance of active sites. By introducing zeolite-like tetrahedral structural units and metal ions or clusters into ZIF-67, materials with well-defined pore structures and tunable chemical functions can be constructed to demonstrate high selectivity and activity for the selective adsorption of antibiotics in wastewater [57].
So far, as many as 150 ZIF materials have been reported, some of which have aroused extensive research attention and practical application interest due to their unique properties. ZIF-67 has attracted attention due to its significant abundance of active sites, mainly due to its high specific surface area (SBET) of over 1700 m2/g, and the presence of microporous structures (pore size 0.34 nm). The microporous structure showed a strong affinity with guest molecules, which effectively promoted the adsorption process of antibiotics. Figure 1 is a schematic diagram of the structure of ZIF-67 [58]. In recent years, the synthesis methods of ZIF-67 and its derivatives have been improved, and the morphology and porosity of ZIF-67 have been accurately regulated. These advances have enabled the researchers to tailor the structural features of ZIF-67 to specific needs. This paper provides a comprehensive review of the properties and recent research progress of ZIF-67-based composites, with a focus on their synthesis methods, adsorption and degradation mechanisms, and application in water pollutant removal. It highlights the unique advantages of ZIF-67 as an adsorbent, summarizes the latest findings in the development and functionalization of ZIF-based materials, and identifies key challenges that remain in the field. Furthermore, this review outlines future research directions, aiming to support the advancement of efficient and sustainable water treatment technologies.
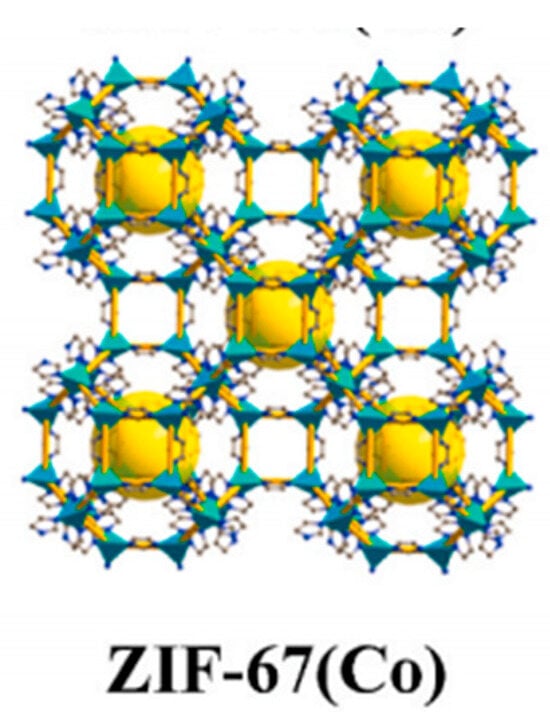
Figure 1.
The structural schematic diagram of ZIF-67 [58].

Table 1.
The adsorption capacity of common adsorbents.
Table 1.
The adsorption capacity of common adsorbents.
| Adsorbent | Pollutant | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|
| Stalk corn activated carbon | Rhodamine B | 5.3 | [59] |
| Rumex abyssinicus activated carbon | Methylene blue | 322 | [60] |
| Wheat straw activated carbon | Pb2+ | 139.44 | [61] |
| Wheat straw activated carbon | Cd2+ | 52.92 | [61] |
| Wheat straw activated carbon | Cu2+ | 31.25 | [61] |
| Powdered activated carbon | Norflkoxacin (NOR) | 124 | [62] |
| Powdered activated carbon | Ciprofloxacin monohydrohloride (CIP) | 127 | [62] |
| Powdered activated carbon | Lomefloxacin hydrochloride (LOM) | 190 | [62] |
| Powdered activated carbon | Sarafloxacin hydrochloride (SAR) | 120 | [62] |
| Powdered activated carbon | Enrofloxacin (ENR) | 93.5 | [62] |
| Powdered activated carbon | Ofloxacin (OPL) | 104 | [62] |
| IGS-300 ion exchange resin | Cr (VI) | 294.11 | [63] |
| Amberlite IRC-748 ion exchange resin | Malachite green | 480.6 | [64] |
| Diaion CR-11 ion exchange resin | Malachite green | 102.1 | [64] |
2. Synthesis Method of ZIF-67
The synthesis route of ZIF-67 is of decisive significance for its large-scale practical application. Over the past decade, several methods have been reported for the synthesis of ZIF-67. The synthesis of ZIF-67 is usually achieved via a reaction between a cobalt precursor and the organic ligand 2-methylimidazole (Hmim). At present, several synthetic strategies have been applied to the preparation of ZIF-67 [65]. Table 2 describes the preparation method of ZIF-67 and its composite materials.

Table 2.
Preparation method of ZIF-67 and its composite materials.
2.1. Solvothermal Method
Solvothermal synthesis is one of the most widely adopted and effective techniques. In this method, cobalt salts and 2-methylimidazole (Hmim) are reacted in a controlled solvent environment under specific temperature and time conditions. This method is known for its simplicity, high efficiency, and ability to produce well-defined ZIF-67 crystals, often even at room temperature. Its scalability and compatibility with diverse application scenarios make it particularly attractive for industrial deployment. For example, Jayashree et al. [66] studied in detail the method of preparing ZIF-67 material with Co(NO3)2·6H2O as a raw material. ZIF-67 material was prepared via a reaction of Co(NO3)2·6H2O with Hmim in a mixed solvent system of dimethylformamide (DMF) and methanol (CH3OH) at 60 degrees Celsius for 4 h. A N2 adsorption–desorption test and scanning electron microscopy (SEM) analysis show that the choice of solvent has a decisive effect on the morphology and structure of the ZIF-67 crystal. The experimental results show that the ZIF-67 synthesized using methanol and DMF as solvents exhibits micropore and fractional pore structure characteristics.
A schematic illustration of the solvothermal synthesis process is shown in Figure 2, where cobalt nitrate and Hmim are dissolved in methanol, followed by heating in an autoclave to facilitate crystal growth.
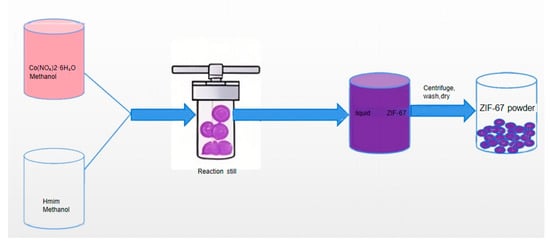
Figure 2.
Schematic diagram of solvothermal synthesis of ZIF-67.
2.2. Surfactant-Assisted Method
By introducing surfactants into the precursor solution, this method not only significantly improves the solubility of the product particles, but also effectively inhibits their agglomeration tendency, thus achieving a better dispersion effect [75]. The type and amount of surfactant have a significant influence on the microstructure and morphological characteristics of the sample [76].
2.3. Microwave/Ultrasonic-Assisted Method
Microwave-assisted synthesis uses the electromagnetic energy of microwave radiation to directly act on polar molecules (such as water and alcohol, etc.) in the reaction system to produce a rapid and uniform heating effect through intermolecular friction and dipole polarization. This energy transfer method can significantly accelerate the nucleation and crystal growth process and shorten the reaction time. The microwave-assisted synthesis method uses high-frequency electromagnetic waves to rapidly and uniformly heat the reaction system, significantly shortening the reaction time (from several hours to several minutes), and reducing side reactions through precise temperature control to obtain ZIF-67 crystals with high crystallinity and uniform morphology. The ultrasonic-assisted synthesis method relies on the local high temperature and high pressure generated by the cavitation effect, accelerates the mixing and nucleation of reactants, and can rapidly synthesize nano-sized ZIF-67 particles at low temperatures and effectively regulate the pore structure and specific surface area. The two methods have the advantages of high efficiency and energy saving, simple operation, and controllable product properties, and overcome the problems of traditional solvothermal methods such as long time, high energy consumption, and particle agglomeration, and provide a new idea for green synthesis and the large-scale preparation of ZIF-67.
2.4. Hydrothermal Process
The hydrothermal method is another effective route for synthesizing ZIF-67 and its composites, offering precise control over the crystal structure and surface characteristics. Khan et al. successfully prepared ZIF-67 and ZIF-67/LaFeO3 nanocomposites using hydrothermal and solvothermal approaches. Their results demonstrated that the hydrothermal method significantly enhanced the specific surface area and porosity of the resulting materials, thereby improving their adsorption and catalytic performance, particularly in the removal of antibiotic contaminants [77]. Similarly, Li et al. reported a simplified hydrothermal synthesis strategy using deionized water as the solvent and various cobalt precursors. This approach promoted the formation of smaller nanocrystals by limiting the excessive coordination between Hmim and metal ions. Remarkably, the method achieved a product yield as high as 94% based on the cobalt content, demonstrating strong potential for scalable production [78].
The size and morphology of the obtained ZIF-67 crystals are affected by many factors, including the choice of solvent, the regulation of the molar ratio of reactants, the difference in cobalt salt type, the setting of synthesis temperature, and the control of aging time. Under different experimental conditions, ZIF-67 crystals can show a variety of structural characteristics such as spherical, granular, or rhombohedral dodecahedrons [79].
3. ZIF-67 Removes Contaminants from Water Bodies
3.1. Adsorption Mechanism
The study of the adsorption mechanism is the key to the analysis of the pollutant removal process. Clarifying the mechanism of action can not only optimize the material design, but also guide the theoretical basis of practical application. In the mechanism exploration, the adsorption kinetics model and isotherm fitting analysis provide quantitative research tools to reveal the action path (such as diffusion control or chemical bonding). At the same time, combined with spectroscopy characterization such as XPS, FT-IR, and SEM/TEM microscopy techniques, the interface behavior of material surface active sites and pollutants can be clarified from the molecular level [80]. Among them, electrostatic interaction is an important role between pollutants (adsorbents) and MOFs (adsorbents) with opposite charges. The net surface charge leads to protonation and deprotonation, which is conducive to the electrostatic interaction between MOFs and pollutants [81]. Furthermore, the interactions between hydrogen atoms in N-H, F-H, and O-H bonds and lone pair electronegative atoms are called hydrogen bonds. Studies have shown that MOFs contain -OH groups and form hydrogen bonds with adsorbents. Acid–base interaction is another mechanism for the adsorption and removal of MOFs [82]. For instance, Hasan et al. [83] used MIL-101 with acidic (-SO3H) and basic (-NH2) groups to remove naproxen and chlorobedic acid from aqueous solutions. The results show that the acid–base interaction is dominant and performs better than bare MIL-101 in acid removal. The interaction between adsorbent and adsorbate includes physical forces (such as van der Waals force and capillary condensation) and chemical bonds (such as coordination bonds and hydrogen bonds). Based on these mechanisms, the adsorption process can be divided into two main types: physical adsorption (mainly weak interaction) and chemical adsorption (involving electron transfer and bond reconstruction) [72,84]. Adsorption can be classified into two categories based on the strength of the action: physical adsorption (weak interactions that are not dominated by chemical bonds) and chemisorption (strong bonds involving the formation of chemical bonds). Physical adsorption is usually achieved via van der Waals forces or intermolecular forces, and adsorbent regeneration can be efficiently accomplished by mild physical means such as solvent displacement, ultrasonic treatment, or calcination. The nature of chemisorption is the formation of chemical bonds (such as covalent bonds and coordination bonds) between adsorbent and adsorbent surface active sites, and its desorption requires the destruction of bond structures, and the difficulty of regeneration is significantly increased [85]. The presence of functional groups on adsorbents and adsorbents was confirmed via FTIR. Figure 3 shows the adsorption behavior of ZIF and antibiotics. These interactions contribute synergistically to the efficient binding of antibiotics onto ZIF frameworks. The figure highlights multiple adsorption pathways, such as size sieving, hydrogen bonding, hydrophobic interactions, and complex formation with metal centers, which together enhance the selectivity and adsorption capacity of ZIF-67.
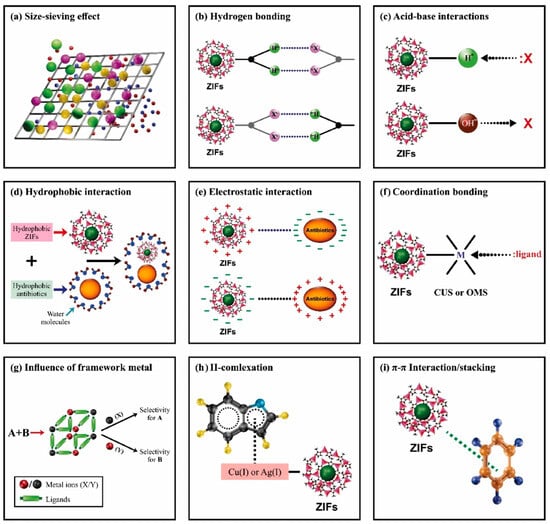
Figure 3.
Schematic representation of adsorption behavior of ZIFs with antibiotics. (a) Size sieving effect, (b) hydrogen bonding, (c) acid–base interaction, (d) hydrophobic interaction, (e) electrostatic interaction, (f) coordination bonding, (g) influence of metal framework, (h) complex formation between adsorbent and adsorbate, and (i) π–π interactions between ZIFs and antibiotics [86].
3.2. Removal of Dyes from Wastewater Using ZIF-67
Synthetic dyes, widely used in the textile, printing, and cosmetic industries, are among the most persistent organic pollutants in wastewater. Their high chemical oxygen demand (COD), strong biochemical oxygen demand (BOD), and resistance to biodegradation make dye effluents extremely challenging to treat. In particular, dyes can alter the optical properties of water, block sunlight penetration, and disrupt aquatic ecosystems by hindering photosynthesis and reducing dissolved oxygen levels. In view of these compound hazards to environmental health, the removal of dye has become an urgent need in the field of environmental engineering to construct an efficient treatment system for this new type of pollutant [87,88]. ZIF-67 has demonstrated excellent performance in removing various organic dyes from aqueous systems, such as methyl orange (MO), malachite green (MG), benzotriazole, phenol, and 1-naphthol. Its high adsorption efficiency is due to its multi-dimensional structural characteristics: its unique surface topological structure provides abundant active sites, its strong coordination capacity of the cobalt metal center enables targeted adsorption, hydrophobic pores promote organic molecule enrichment, the coordination of unsaturated sites enhances binding stability, and it has excellent structural durability itself. Among them, ZIF-67 showed an excellent removal ability of malachite green (MG) and other harmful compounds in water, and successfully realized the adsorption separation of MG. The experimental results showed that the maximum adsorption capacity (qmax) of ZIF-67 for MG was 3226 mg/g at 60 °C. By comparing the fitting results of the Langmuir and Freundlich adsorption isothermal models, it was found that the Langmuir model has a better fitting effect, indicating that the adsorption process conforms to the single-layer adsorption mechanism. The article proposes that the p–p packing interaction between MG and ZIF-67 might be the mechanism for the high adsorption capacity between MG and ZIF-67. Specifically, the maximum adsorption capacity (qmax) of MG on ZIF-67 at 20 °C, 40 °C, and 60 °C is 2500 mg/g, 2941 mg/g, and 3226 mg/g, respectively [89]. Table 3 introduces the performance of the ZIF-67 series in removing various pollutants.

Table 3.
The performance of the ZIF-67 series in removing various pollutants.
3.3. Removal of Antibiotics from Wastewater Using ZIF-67
The widespread use of antibiotics in human medicine and animal husbandry has led to their frequent detection in water environments, raising serious concerns about the development of antibiotic-resistant bacteria and the spread of resistance genes. These pollutants not only threaten aquatic ecosystems, but also compromise public health [96]. ZIF-67 and its functionalized derivatives have demonstrated broad-spectrum adsorption properties in the field of pollution control, and studies have confirmed that ZIF-67 has high efficiency in removing multiple pollutants, including sulfonamides, tetracycline, and other broad-spectrum antibiotics. The application advantages of this material are reflected in two dimensions: from the perspective of the process economy, its adsorption process for antibiotics shows significant cost advantages compared with traditional adsorption technology. From the perspective of material engineering, ZIF-67 can be used as a functional component to construct high-performance composite adsorption materials due to its three-dimensional through pore structure, accurately regulated pore size distribution (0.34–3.4 nm), excellent aqueous chemical stability (stable structure in the range of pH 2–12), and simple preparation process (room temperature synthesis). It can also be used as a template agent to develop new multistage porous materials.
For example, the ZIF-67/V-BiOIO3 heterostructure (ZxVy) system was successfully constructed via in situ solvent thermal synthesis, and its process parameters were controlled to crystallization at 180 °C for 12 h to achieve precise interface coupling. The ZIF-67 component exhibits a high specific surface area of 1339.0 m2/g (BET method), and its functional advantage is reflected in a triple mechanism of action: (1) the porous frame structure specifically captures aromatic pollutants through π-π stacking effect; (2) the exposed Co2+ metal coordination center forms a stable chelate bond with the target; and (3) the three-dimensional pore network provides molecular mass transfer channels. This multiple synergistic mechanism results in the adsorption-catalytic synergistic effect of ZxVy heterojunction in the ciprofloxacin (CIP) enrichment process. According to the experimental data, the compositive optimized Z10V5 samples (ZIF-67 and V-BiOIO3molar ratio 10:5) achieved a CIP removal rate of 98.4%, which was due to the optimization of electron transport paths and the maximization of active site density at the heterogeneous interface [97].
3.4. Removal of Heavy Metal Ions from Wastewater Using ZIF-67
Heavy metals such as lead (Pb), mercury (Hg), cadmium (Cd), chromium (Cr), and arsenic (As) pose serious environmental and health hazards due to their high toxicity, persistence, and bioaccumulative nature. Even at trace concentrations, these metals can cause irreversible damage to ecosystems and human health, necessitating efficient removal strategies [98].
A more advanced design involved the development of a SA-ME@ZIF-67 composite, synthesized via stepwise solvothermal assembly (80 °C, 12h) followed by surface functionalization using 2.5 vol% APTES. This material achieved an exceptionally high adsorption capacity of 634.99 mg/g for Pb2+ at pH 5.0, and retained over 92% efficiency after five regeneration cycles. The enhanced performance of SA-ME@ZIF-67 can be attributed to a synergistic triple mechanism: (1) specific coordination chelation between the open metal site (OMSs) of the ZIF-67 skeleton and Pb2+; (2) grafted amino groups capture heavy metals through ion exchange; and (3) the hierarchical pore structure (mesoporous 2.8 nm/macroporous 50 nm) provides a rapid mass transfer path. The dynamic cycling test showed that after 0.1M of HNO solution was elution and regenerated, the material retained an initial adsorption efficiency of 92.3% in the fifth cycle, and its excellent mechanical stability (BET specific surface area attenuation < 8%) and chemical tolerance (metal dissolution < 0.2 ppm) provided a reliable guarantee for engineering applications [99].
3.5. Research and Analysis of the ZIF-67 Adsorption Isotherm
The adsorption isotherm model is a type of model that describes the interaction mechanism between adsorbents and adsorbents at a constant temperature by utilizing the adsorption performance and dynamic equilibrium of adsorbents and adsorbents. The establishment of this model depends on the physical properties of the adsorbate, adsorbent, adsorbate, and solution. The adsorption isotherm model can provide information on the maximum adsorption capacity, which is of great significance for evaluating the performance of adsorbents and exploring the adsorption mechanism.
For example, in order to understand the effects of different initial concentrations (100–1300 mg/L) of CTC and DOX on adsorption behavior, the Freundlich, Langmuir, Temkin, and Sips models were applied to fit the experimental data. The fitting results are shown in Figure 4a,b. At 293 K, 303 K, and 313 K, the adsorption capacity increases with the rise in temperature, indicating that the increase in temperature is conducive to the reaction and that the reaction is endothermic [100]. The Langmuir model assumes that the surface of the adsorbent is covered by a monolayer of adsorption and that there is no interaction between adsorbent molecules. In contrast, the Freundlich model is more suitable for describing the adsorption process in heterogeneous systems. According to the fitting results, the Sips model describes the adsorption process of CTC and DOX on ZIF-67 more accurately, indicating that the adsorption process of CTC and DOX on ZIF-67 is a complex adsorption process, including both monolayer adsorption at uniform binding sites and adsorption on non-uniform surfaces. The Temkin model has a good fitting effect on the adsorption processes of ZIF-67 with CTC and DOX, indicating that there is chemical adsorption between ZIF-67 and CTC and DOX molecules.
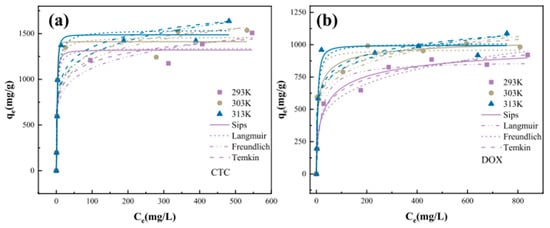
Figure 4.
The data fit the curve to the isotherm model of (a) CTC; (b) DOX [100].
4. Desorption and Reusability
The regenerative capacity and reusability of ZIF-67 (zeolite imidazolate framework-67) are the core elements of its economy and sustainability in water treatment applications. The essence of the regeneration process, which is achieved via heat treatment or chemical elution, is to efficiently remove adsorbed contaminants and restore the active site of the material while maintaining the stability of the skeleton structure. Regeneration via heat treatment, decomposition, or the desorption of contaminants at high temperatures, such as the carbonization of organic dyes or oxidation of heavy metals is carried out with precise temperature control to avoid structural failure due to the collapse of the ZIF-67 skeleton or migration of cobalt ions. In the treatment of water pollutants, ZIF membranes demonstrate unique advantages. Their high porosity and adjustable pore size enable ZIF membranes to effectively retain and remove pollutants in water, such as organic dyes, antibiotics, and heavy metal ions. In addition, ZIF membranes possess chemical stability, thermal stability, and reusability, enabling them to maintain stable performance under harsh water conditions [101,102]. Chemical elution uses acid–base solutions or organic solvents (such as ethanol and hydrochloric acid) to remove contaminants through ion exchange, coordination bond breaking, or dissolution, but needs to balance elution efficiency and material chemical stability to prevent skeleton dissolution or functional group loss. During the regeneration process, the degree of recovery of porosity, specific surface area, and surface chemical properties of ZIF-67 directly determine its reuse performance. Studies have shown that after five cycles, its adsorption capacity for dyes or heavy metals can still maintain the initial value of 80% to 90%, but repeated regeneration may lead to micropore blockage or the passivation of the active site. Mechanical strength and resistance to contamination should be improved via composite modification (e.g., combination with carbon-based materials or surface functionalization). Table 4 summarizes the results of the different sorbents of the ZIF-67 series in terms of desorption and reusability.
For example, NaCl solutions can also regenerate ZIF materials through electrostatic attraction, which can be used for the electrostatic adsorption of pollutants. Seo et al. used a high concentration of NaCl solution (15 wt%) as an eluent when cleaning used ZIF-67. The results showed that rinsing with NaCl solution could make ZIF material show good and stable regeneration performance in multiple analysis cycles [103]. Nazir et al. compared the reuse performance of ZIF-67 and ZIF-67@LDH. After the adsorption experiment was completed, the adsorbent was washed with ethanol at room temperature and re-applied to the dye adsorption process. The regeneration process was repeated four times. The results showed that the adsorption capacity of ZIF-67 and ZIF-67@LDH decreased slightly after many cycles [72].

Table 4.
Results of desorption and reuse of different sorbents of ZIF-67 series.
Table 4.
Results of desorption and reuse of different sorbents of ZIF-67 series.
| Materials | Pollution | Eluent | Cycle Number | Qmax Before Recycling (mg/g) | Ref. |
|---|---|---|---|---|---|
| ZIF-67 @CoAl-LDH | MO | Ethanol | 4 | 180.5 | [72] |
| Ag@ZIF-67 | MO, MC | Ethanol and HCL | 5 | 994.6, 938.4 | [104] |
| Fabrication of sodium alginate-melamine@ZIF-67 | Pb(II) | HCL | 5 | 634.99 | [99] |
5. Conclusions and Prospect
In recent years, ZIFs have shown great potential in the field of water treatment due to their unique physical and chemical properties, among which cobalt-based ZIF-67 has a high specific surface area (up to 1700 m2/g), adjustable pore structure (pore size 0.34–3.4 nm), and excellent chemical stability (pH 2–12 stable). It has become a research hotspot of the adsorption and catalytic degradation of pollutants. In this paper, the synthesis method, functional modification strategy, and application mechanism of ZIF-67 in removing organic pollutants (such as dyes and antibiotics) and heavy metal ions in water were reviewed, and the regeneration and reusability of ZIF-67 were discussed.
5.1. Greening and Scale of Synthesis Process
Existing synthesis methods mostly rely on organic solvents (such as DMF and methanol), which may cause secondary pollution. The development of aqueous synthesis technologies (such as the strategy of using deionized water as a solvent) and low-energy processes (such as rapid synthesis at room temperature) are key to large-scale applications. In addition, biological templates or waste-derived feedstocks (such as eggshell membrane ESM) need to be explored to reduce material costs and improve sustainability.
5.2. Enhancement of Material Stability
The structural collapse of ZIF-67 at an extreme pH or high temperature restricts its long-term use. In the future, its chemical stability can be improved by surface coating (e.g., silica layer), doping metals (e.g., Zn and Fe), or constructing core–shell structures (e.g., ZIF-8@ZIF-67). At the same time, the development of self-healing ZIFs to repair micro-cracks using dynamic bonds (such as the reversible recombination of coordination bonds) is worth exploring.
5.3. Selective Adsorption in Complex Water Bodies
The coexistence of multiple pollutants (such as antibiotics and heavy metals) in actual wastewater may induce competitive adsorption. The design of ZIF-67 composite materials with hierarchical pores (micromesopia macropores) and multiple functional groups (such as -SH and -NH2) can realize the specific identification of pollutants. For example, the selective adsorption of Pb2+ using amino-functionalized ZIF-67 has been demonstrated and could be further enhanced in the future by incorporating molecular imprinting techniques.
5.4. Optimization and Low Energy Consumption of Regenerative Technology
Current regeneration processes have high energy consumption (such as heat treatment) and secondary pollution (such as pickling waste). The development of photothermal regeneration (using solar energy to drive pollutant desorption) or electrochemical regeneration (using electric fields to regulate the adsorption–desorption balance) can reduce energy consumption and improve environmental friendliness. In addition, studying the transformation paths of contaminants during regeneration, such as the degree of mineralization of antibiotics, is critical to assessing the safety of the technology.
5.5. Environmental and Health Risk Assessment
The potential ecotoxicity of ZIF-67 nanoparticles, such as cobalt ion leaching, needs to be systematically evaluated. In the future, long-term exposure experiments should be carried out to study their migration and transformation in natural water bodies and impact on aquatic organisms, and standardized risk assessment models should be established. At the same time, the development of degradable ZIFs (such as the introduction of ester bonds or pH response groups) to achieve controlled decomposition after use is an important trend in green material design.
Author Contributions
Conceptualization, L.C., S.Z. and K.L.; methodology, W.P., Y.L. and Z.L.; writing—original draft preparation, K.L. and P.L.; writing—review and editing, K.L. and P.L.; visualization, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology development plan project of Jilin Province (20240601029RC).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Pouramini, Z. The VOCs catalytic combustion by perovskite catalysts: A mini-review. Adv. Appl. NanoBio-Technol. 2022, 3, 23–30. [Google Scholar]
- Khan, N.A.; Salma, S.; Tayyaba, N.; Ahmad, S.S.S.; Sufyan, J.M.; Altaf, N.M.; Ejaz, H.; Asma, S.; Shahid, H.; Ashfaq, M. Efficient removal of norfloxacin by MOF@GO composite: Isothermal, kinetic, statistical, and mechanistic study. Toxin Rev. 2021, 40, 915–927. [Google Scholar] [CrossRef]
- Nazir, M.A.; Bashir, M.A.; Najam, T.; Javed, M.S.; Suleman, S.; Hussain, S.; Kumar, O.P.; Shah, S.S.A.; ur Rehman, A. Combining structurally ordered intermetallic nodes: Kinetic and isothermal studies for removal of malachite green and methyl orange with mechanistic aspects. Microchem. J. 2021, 164, 105973. [Google Scholar] [CrossRef]
- Onaizi, S.A. Statistical analyses of the effect of rhamnolipid biosurfactant addition on the enzymatic removal of Bisphenol A from wastewater. Biocatal. Agric. Biotechnol. 2021, 32, 101929. [Google Scholar] [CrossRef]
- Al-Sakkaf, M.K.; Basfer, I.; Iddrisu, M.; Bahadi, S.A.; Nasser, M.S.; Abussaud, B.; Drmosh, Q.A.; Onaizi, S.A. An up-to-date review on the remediation of dyes and phenolic compounds from wastewaters using enzymes immobilized on emerging and nanostructured materials: Promises and challenges. Nanomaterials 2023, 13, 2152. [Google Scholar] [CrossRef]
- Massé, D.I.; Cata Saady, N.M.; Gilbert, Y. Potential of biological processes to eliminate antibiotics in livestock manure: An overview. Animals 2014, 4, 146–163. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Reardon, S. Antibiotic resistance sweeping developing world: Bacteria are increasingly dodging extermination as drug availability outpaces regulation. Nature 2014, 509, 141–143. [Google Scholar] [CrossRef]
- Zeng, G.; Chen, M.; Zeng, Z. Risks of neonicotinoid pesticides. Science 2013, 340, 1403. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, X.; Jia, L.; Zhou, T.; Ma, T.; Xu, Z.; Cao, J.; Ge, Z.; Bi, N.; Zhu, T.; et al. A novel visual ratiometric fluorescent sensing platform for highly-sensitive visual detection of tetracyclines by a lanthanide- functionalized palygorskite nanomaterial. J. Hazard. Mater. 2018, 342, 158–165. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W. Water Cleaning Adsorptive Membranes for Efficient Removal of Heavy Metals and Metalloids. Water 2022, 14, 2718. [Google Scholar] [CrossRef]
- Sharma, B.M.; Bečanová, J.; Scheringer, M.; Sharma, A.; Bharat, G.K.; Whitehead, P.G.; Klánová, J.; Nizzetto, L. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India. Sci. Total Environ. 2019, 646, 1459–1467. [Google Scholar] [CrossRef]
- Jurado, A.; Walther, M.; Díaz-Cruz, M.S. Occurrence, fate and environmental risk assessment of the organic microcontaminants included in the Watch Lists set by EU Decisions 2015/495 and 2018/840 in the groundwater of Spain. Sci. Total Environ. 2019, 663, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Vickers, N.J. Animal Communication: When I’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [PubMed]
- TaheriAshtiani, N.; Ayati, B. Using chitosan-based heterogeneous catalyst for degradation of Acid Blue 25 in the effective electro-Fenton process with rotating cathodes. J. Electroanal. Chem. 2022, 905, 115983. [Google Scholar] [CrossRef]
- Yang, Z.-h.; Cao, J.; Chen, Y.-p.; Li, X.; Xiong, W.-p.; Zhou, Y.-y.; Zhou, C.-y.; Xu, R.; Zhang, Y.-r. Mn-doped zirconium metal-organic framework as an effective adsorbent for removal of tetracycline and Cr(VI) from aqueous solution. Microporous Mesoporous Mater. 2019, 277, 277–285. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.; Xu, Z.; Zhang, L.; Cheng, B.; Ho, W. Hierarchical porous Ni/Co-LDH hollow dodecahedron with excellent adsorption property for Congo red and Cr(VI) ions. Appl. Surf. Sci. 2019, 478, 981–990. [Google Scholar] [CrossRef]
- Ismail, U.M.; Onaizi, S.A.; Vohra, M.S. Aqueous Pb (II) removal using ZIF-60: Adsorption studies, response surface methodology and machine learning predictions. Nanomaterials 2023, 13, 1402. [Google Scholar] [CrossRef]
- Bahadi, S.A.; Iddrisu, M.; Al-Sakkaf, M.K.; Elgzoly, M.A.A.; Al-Amrani, W.A.; Ahmed, U.; Zahid, U.; Drmosh, Q.A.; Onaizi, S.A. Chemically versus thermally reduced graphene oxide: Effects of reduction methods and reducing agents on the adsorption of phenolic compounds from wastewater. Emergent Mater. 2024, 7, 533–545. [Google Scholar] [CrossRef]
- Ismail, U.M.; Onaizi, S.A.; Vohra, M.S. Novel MgCuAl-layered triple hydroxide for aqueous selenite and selenate treatment. Emergent Mater. 2024, 7, 521–532. [Google Scholar] [CrossRef]
- Cheng, Y.; He, H.; Yang, C.; Zeng, G.; Li, X.; Chen, H.; Yu, G. Challenges and solutions for biofiltration of hydrophobic volatile organic compounds. Biotechnol. Adv. 2016, 34, 1091–1102. [Google Scholar] [CrossRef]
- Aoudj, S.; Khelifa, A.; Drouiche, N.; Belkada, R.; Miroud, D. Simultaneous removal of chromium(VI) and fluoride by electrocoagulation–electroflotation: Application of a hybrid Fe-Al anode. Chem. Eng. J. 2015, 267, 153–162. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Alshabib, M.; Onaizi, S.A. Enzymatic Remediation of Bisphenol A from Wastewaters: Effects of Biosurfactant, Anionic, Cationic, Nonionic, and Polymeric Additives. Water Air Soil Pollut. 2020, 231, 428. [Google Scholar] [CrossRef]
- Kumar, O.P.; Shahzad, K.; Nazir, M.A.; Farooq, N.; Malik, M.; Ahmad Shah, S.S.; Rehman, A.u. Photo-Fenton activated C3N4x/AgOy@Co1−xBi0.1−yO7 dual s-scheme heterojunction towards degradation of organic pollutants. Opt. Mater. 2022, 126, 112199. [Google Scholar] [CrossRef]
- Hezam, A.; Drmosh, Q.; Ponnamma, D.; Bajiri, M.A.; Qamar, M.; Namratha, K.; Zare, M.; Nayan, M.; Onaizi, S.A.; Byrappa, K. Strategies to enhance ZnO photocatalyst’s performance for water treatment: A comprehensive review. Chem. Rec. 2022, 22, e202100299. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, N.C.; Ifeoma, O.P.; Gaya, U.I.; Nazir, M.A.; Ahmad Shah, S.S. Fabrication of zinc incorporated magnetic iron oxide nanoparticles for photocatalytic degradation of methylene blue. Iran. J. Chem. Chem. Eng. Int. Engl. Ed. 2023, 42, 3590–3600. [Google Scholar]
- Onaizi, S.A.; Alshabib, M. The degradation of bisphenol A by laccase: Effect of biosurfactant addition on the reaction kinetics under various conditions. Sep. Purif. Technol. 2021, 257, 117785. [Google Scholar] [CrossRef]
- Eleryan, A.; Hassaan, M.; Nazir, M.A.; Shah, S.S.A.; Ragab, S.; El Nemr, A. Isothermal and kinetic screening of methyl red and methyl orange dyes adsorption from water by Delonix regia biochar-sulfur oxide (DRB-SO). Sci. Rep. 2024, 14, 13585. [Google Scholar] [CrossRef] [PubMed]
- Aghajani, M.; Torabi, S.A.; Heydari, J. A novel option contract integrated with supplier selection and inventory prepositioning for humanitarian relief supply chains. Socio-Econ. Plan. Sci. 2020, 71, 100780. [Google Scholar] [CrossRef]
- Wang, J.; Dong, S.; Yu, C.; Han, X.; Guo, J.; Sun, J. An efficient MoO3 catalyst for in-practical degradation of dye wastewater under room conditions. Catal. Commun. 2017, 92, 100–104. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Zhang, J.; Si, M.; Jiang, L.; Yuan, X.; Yu, H.; Wu, Z.; Li, Y.; Guo, J. Core-shell Ag@nitrogen-doped carbon quantum dots modified BiVO4 nanosheets with enhanced photocatalytic performance under Vis-NIR light: Synergism of molecular oxygen activation and surface plasmon resonance. Chem. Eng. J. 2021, 410, 128336. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Esmaeili, H.; Amani, A.; Mojoudi, F. Synthesis of Fe3O4 Nanoparticles Modifed by Oak Shell for Treatment of Wastewater Containing Ni(II). Acta Chim. Slov. 2018, 65, 750–756. [Google Scholar] [CrossRef]
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Ramli, A.; Abu Bakar, N.H.H.; Saad, B.; Rozaini, M.N.H.; Isiyaka, H.A.; Jagaba, A.H.; Aldaghri, O. A critical review on metal-organic frameworks and their composites as advanced materials for adsorption and photocatalytic degradation of emerging organic pollutants from wastewater. Polymers 2020, 12, 2648. [Google Scholar] [CrossRef]
- Cao, J.; Sun, S.; Li, X.; Yang, Z.; Xiong, W.; Wu, Y.; Jia, M.; Zhou, Y.; Zhou, C.; Zhang, Y. Efficient charge transfer in aluminum-cobalt layered double hydroxide derived from Co-ZIF for enhanced catalytic degradation of tetracycline through peroxymonosulfate activation. Chem. Eng. J. 2020, 382, 122802. [Google Scholar] [CrossRef]
- Ismail, U.M.; Onaizi, S.A.; Vohra, M.S. Crystal violet removal using ZIF-60: Batch adsorption studies, mechanistic & machine learning modeling. Environ. Technol. Innov. 2024, 33, 103456. [Google Scholar]
- Lanjwani, M.F.; Tuzen, M.; Khuhawar, M.Y.; Saleh, T.A. Trends in photocatalytic degradation of organic dye pollutants using nanoparticles: A review. Inorg. Chem. Commun. 2024, 159, 111613. [Google Scholar] [CrossRef]
- Samavati, Z.; Samavati, A.; Goh, P.S.; Ismail, A.F.; Abdullah, M.S. A comprehensive review of recent advances in nanofiltration membranes for heavy metal removal from wastewater. Chem. Eng. Res. Des. 2023, 189, 530–571. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.M.Z.; Hossain, M.M. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Alshabib, M.; Onaizi, S.A. A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes: Current status and potential challenges. Sep. Purif. Technol. 2019, 219, 186–207. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal–organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- Maurin, G.; Serre, C.; Cooper, A.; Férey, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.; Han, Y.; Liao, Z.; Lu, P.; Nezamzadeh-Ejhieh, A.; Liu, J.; Peng, Y. Recent advances in Al (iii)/In (iii)-based MOFs for the detection of pollutants. New J. Chem. 2022, 46, 19577–19592. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, F.; Luo, D.; Huang, J.; Ouyang, J.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Peng, Y. Recent advances in Ti-based MOFs in biomedical applications. Dalton Trans. 2022, 51, 14817–14832. [Google Scholar] [CrossRef]
- Pouramini, Z.; Mousavi, S.M.; Babapoor, A.; Hashemi, S.A.; Lai, C.W.; Mazaheri, Y.; Chiang, W.-H. Effect of Metal Atom in Zeolitic Imidazolate Frameworks (ZIF-8 & 67) for Removal of Dyes and Antibiotics from Wastewater: A Review. Catalysts 2023, 13, 155. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Bahrani, S.; Mosleh, S.; Chiang, W.-H.; Yousefi, K.; Ramakrishna, S.; Wei, L.C.; Omidifar, N. Hybrid of sodium polytungstate polyoxometalate supported by the green substrate for photocatalytic degradation of auramine-O dye. Environ. Sci. Pollut. Res. 2022, 29, 56055–56067. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D.; Zhang, J. The application of ZIF-67 and its derivatives: Adsorption, separation, electrochemistry and catalysts. J. Mater. Chem. A 2018, 6, 1887–1899. [Google Scholar] [CrossRef]
- Cheng, N.; Ren, L.; Xu, X.; Du, Y.; Dou, S.X. Recent development of zeolitic imidazolate frameworks (ZIFs) derived porous carbon based materials as electrocatalysts. Adv. Energy Mater. 2018, 8, 1801257. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, G.; Liao, D.; Chen, X.; Lu, C.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Pan, Y.; Dai, Z. Recent Advances of Silver-Based Coordination Polymers on Antibacterial Applications. Molecules 2022, 27, 7166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, H.; Cheng, M.; Liu, Z.; Huang, D.; Zhang, G.; Shao, B.; Liang, Q.; Luo, S.; Wu, T.; et al. The application of Zeolitic imidazolate frameworks (ZIFs) and their derivatives based materials for photocatalytic hydrogen evolution and pollutants treatment. Chem. Eng. J. 2021, 417, 127914. [Google Scholar] [CrossRef]
- Tan, Y.-X.; Wang, F.; Zhang, J. Design and synthesis of multifunctional metal–organic zeolites. Chem. Soc. Rev. 2018, 47, 2130–2144. [Google Scholar] [CrossRef]
- Duan, C.; Yu, Y.; Hu, H. Recent progress on synthesis of ZIF-67-based materials and their application to heterogeneous catalysis. Green Energy Environ. 2022, 7, 3–15. [Google Scholar] [CrossRef]
- Fard, N.E.; Ali, N.S.; Saady, N.M.C.; Albayati, T.M.; Salih, I.K.; Zendehboudi, S.; Harharah, H.N.; Harharah, R.H. A review on development and modification strategies of MOFs Z-scheme heterojunction for photocatalytic wastewater treatment, water splitting, and DFT calculations. Heliyon 2024, 10, e32861. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Kamarehie, B.; Almasi, A.; Darvishmotevalli, M.; Salari, M.; Moradnia, M.; Azimi, F.; Ghaderpoori, M.; Neyazi, Z.; Karami, M.A. Removal of Rhodamine B from aqueous solution by stalk corn activated carbon: Adsorption and kinetic study. Biomass Convers. Biorefinery 2023, 13, 7927–7936. [Google Scholar] [CrossRef]
- Fito, J.; Abewaa, M.; Mengistu, A.; Angassa, K.; Ambaye, A.D.; Moyo, W.; Nkambule, T. Adsorption of methylene blue from textile industrial wastewater using activated carbon developed from Rumex abyssinicus plant. Sci. Rep. 2023, 13, 5427. [Google Scholar] [CrossRef]
- Qi, G.; Pan, Z.; Zhang, X.; Chang, S.; Wang, H.; Wang, M.; Xiang, W.; Gao, B. Microwave biochar produced with activated carbon catalyst: Characterization and adsorption of heavy metals. Environ. Res. 2023, 216, 114732. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, X.; Wang, J.; Lin, P.; Chen, C.; Zhang, X.; Suffet, I.M. Activated carbon adsorption of quinolone antibiotics in water: Performance, mechanism, and modeling. J. Environ. Sci. 2017, 56, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.; Sivalingam, S.; Srinadh, R.V.; Mishra, S. Efficient removal of hexavalent chromium ions from simulated wastewater by functionalized anion exchange resin: Process optimization, isotherm and kinetic studies. Environ. Chem. Ecotoxicol. 2023, 5, 98–107. [Google Scholar] [CrossRef]
- Yanardağ, D.; Edebali, S. Adsorptive removal of malachite green dye from aqueous solution by ion exchange resins. Biomass Convers. Biorefinery 2024, 14, 5699–5710. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Bandal, H.A.; Ramakrishna, S.; Kim, H. Critical review, recent updates on zeolitic imidazolate framework-67 (ZIF-67) and its derivatives for electrochemical water splitting. Adv. Mater. 2022, 34, 2107072. [Google Scholar] [CrossRef]
- Ethiraj, J.; Palla, S.; Reinsch, H. Insights into high pressure gas adsorption properties of ZIF-67: Experimental and theoretical studies. Microporous Mesoporous materials 2020, 294, 109867. [Google Scholar] [CrossRef]
- Nazir, M.A.; Bashir, M.S.; Jamshaid, M.; Anum, A.; Najam, T.; Shahzad, K.; Imran, M.; Shah, S.S.A.; ur Rehman, A. Synthesis of porous secondary metal-doped MOFs for removal of Rhodamine B from water: Role of secondary metal on efficiency and kinetics. Surf. Interfaces 2021, 25, 101261. [Google Scholar] [CrossRef]
- Lin, D.; Duan, P.; Yang, W.; Liu, Y.; Pan, Q. Facile controlled synthesis of core–shell/yolk–shell/hollow ZIF-67@ Co-LDH/SiO2 via a self-template method. Inorg. Chem. Front. 2020, 7, 1643–1650. [Google Scholar] [CrossRef]
- Saghir, S.; Fu, E.; Xiao, Z. Synthesis of CoCu-LDH nanosheets derived from zeolitic imidazole framework-67 (ZIF-67) as an efficient adsorbent for azo dye from waste water. Microporous Mesoporous Mater. 2020, 297, 110010. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Yang, X.; Zhan, F.; Wang, Y.; Liu, M.; Qiu, X.; Li, J.; Zhan, J.; Li, Q.; et al. Surfactant-assisted controlled synthesis of a metal-organic framework on Fe2O3 nanorod for boosted photoelectrochemical water oxidation. Chem. Eng. J. 2020, 379, 122256. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Zarin, K.; Shahzad, K.; Javed, M.S.; Jamshaid, M.; Bashir, M.A.; Shah, S.S.A.; Rehman, A.U. Enhanced adsorption removal of methyl orange from water by porous bimetallic Ni/Co MOF composite: A systematic study of adsorption kinetics. Int. J. Environ. Anal. Chem. 2023, 103, 4841–4856. [Google Scholar] [CrossRef]
- Nazir, M.A.; Khan, N.A.; Cheng, C.; Shah, S.S.A.; Najam, T.; Arshad, M.; Sharif, A.; Akhtar, S.; ur Rehman, A. Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Appl. Clay Sci. 2020, 190, 105564. [Google Scholar] [CrossRef]
- Campos, R.D.; de Oliveira, A.L.M.; Rostas, A.M.; Kuncser, A.C.; Negrila, C.C.; Galca, A.-C.; Félix, C.; Castellano, L.; da Silva, F.F.; dos Santos, I.M.G. TiO2/ZIF-67 nanocomposites synthesized by the microwave-assisted solvothermal method: A correlation between the synthesis conditions and antimicrobial properties. New J. Chem. 2023, 47, 2177–2188. [Google Scholar] [CrossRef]
- Alamgholiloo, H.; Hashemzadeh, B.; Pesyan, N.N.; Sheikhmohammadi, A.; Asgari, E.; Yeganeh, J.; Hashemzadeh, H. A facile strategy for designing core-shell nanocomposite of ZIF-67/Fe3O4: A novel insight into ciprofloxacin removal from wastewater. Process Saf. Environ. Prot. 2021, 147, 392–404. [Google Scholar] [CrossRef]
- Bradshaw, D.; El-Hankari, S.; Lupica-Spagnolo, L. Supramolecular templating of hierarchically porous metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5431–5443. [Google Scholar] [CrossRef]
- Sarawade, P.; Tan, H.; Polshettiwar, V. Shape-and morphology-controlled Sustainable synthesis of Cu, Co, and in metal organic frameworks with high CO2 capture capacity. ACS Sustain. Chem. Eng. 2013, 1, 66–74. [Google Scholar] [CrossRef]
- Zhong, M.; Kong, L.; Zhao, K.; Zhang, Y.H.; Li, N.; Bu, X.H. Recent progress of nanoscale metal-organic frameworks in synthesis and battery applications. Adv. Sci. 2021, 8, 2001980. [Google Scholar] [CrossRef]
- Qian, J.; Sun, F.; Qin, L. Hydrothermal synthesis of zeolitic imidazolate framework-67 (ZIF-67) nanocrystals. Mater. Lett. 2012, 82, 220–223. [Google Scholar] [CrossRef]
- Guo, X.; Xing, T.; Lou, Y.; Chen, J. Controlling ZIF-67 crystals formation through various cobalt sources in aqueous solution. J. Solid State Chem. 2016, 235, 107–112. [Google Scholar] [CrossRef]
- Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive removal of diclofenac sodium from water with Zr-based metal–organic frameworks. Chem. Eng. J. 2016, 284, 1406–1413. [Google Scholar] [CrossRef]
- Tchinsa, A.; Hossain, M.F.; Wang, T.; Zhou, Y. Removal of organic pollutants from aqueous solution using metal organic frameworks (MOFs)-based adsorbents: A review. Chemosphere 2021, 284, 131393. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Devi, N.; Siwal, S.S.; Alsanie, W.F.; Thakur, M.K.; Thakur, V.K. Metal–organic framework-based materials for wastewater treatment: Superior adsorbent materials for the removal of hazardous pollutants. ACS Omega 2023, 8, 9004–9030. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Choi, E.-J.; Jhung, S.H. Adsorption of naproxen and clofibric acid over a metal–organic framework MIL-101 functionalized with acidic and basic groups. Chem. Eng. J. 2013, 219, 537–544. [Google Scholar] [CrossRef]
- Ibrahim, A.; Vohra, M.S.; Bahadi, S.A.; Onaizi, S.A.; Essa, M.H.; Mohammed, T. Heavy metals adsorption onto graphene oxide: Effect of mixed systems and response surface methodology modeling. Desalination Water Treat. 2022, 266, 78–90. [Google Scholar] [CrossRef]
- Ismail, U.M.; Vohra, M.S.; Onaizi, S.A. Adsorptive removal of heavy metals from aqueous solutions: Progress of adsorbents development and their effectiveness. Environ. Res. 2024, 251, 118562. [Google Scholar] [CrossRef]
- Saghir, S.; Zhang, S.; Wang, Y.; Fu, E.; Xiao, Z.; Zahid, A.H.; Pu, C. Review, recent advancements in zeolitic imidazole frameworks-67 (ZIF-67) and its derivatives for the adsorption of antibiotics. J. Environ. Chem. Eng. 2024, 12, 113166. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chang, H.-A. Ultra-high adsorption capacity of zeolitic imidazole framework-67 (ZIF-67) for removal of malachite green from water. Chemosphere 2015, 139, 624–631. [Google Scholar] [CrossRef]
- Kola, D.Y.; Edebali, S. Facile synthesis of zeolitic-imidazole framework-67 (ZIF-67) for the adsorption of indigo carmine dye. Can. J. Chem. Eng. 2024, 103, 2373–2385. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, M.; Li, S.; Zhou, Y.; Zeng, J.; Huang, M.; Sun, Q.; Le, T. Development of Recoverable Magnetic Bimetallic ZIF-67 (Co/Cu) Adsorbent and Its Enhanced Selective Adsorption of Organic Dyes in Wastewater. Molecules 2024, 29, 4860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Hou, B.; Yang, X.; Ren, J.; Shao, C. Efficient removal of oxytetracycline hydrochloride by ZIF-67 coated hollow spherical CuS (CuS-PVP@ ZIF-67) under the synergistic effect of adsorption and PMS activation. Appl. Surf. Sci. 2024, 643, 158663. [Google Scholar] [CrossRef]
- Yang, H.; Wang, S.; Liu, Y.; Hu, Y.; Shen, W. ZIF-67 grows in chitosan-rGO hydrogel beads for efficient adsorption of tetracycline and norfloxacin. Sep. Purif. Technol. 2024, 330, 125208. [Google Scholar] [CrossRef]
- Ghafil, A.J.; Mazloom, G.; Abdi, J.; Tamtaji, M.; Banisharif, F. Ti3C2Tx/ZIF-67 hybrid nanocomposite as a highly effective adsorbent for Pb (II) removal from water: Synthesis and DFT calculations. Appl. Surf. Sci. 2024, 643, 158642. [Google Scholar] [CrossRef]
- Vo, T.K.; Phuong, N.H.Y.; Nguyen, V.C.; Quang, D.T. ZIF-67 grafted-boehmite-PVA composite membranes with enhanced removal efficiency towards Cr (VI) from aqueous solutions. Chemosphere 2023, 341, 139996. [Google Scholar] [CrossRef]
- Mangla, D.; Annu; Sharma, A.; Ikram, S. Critical review on adsorptive removal of antibiotics: Present situation, challenges and future perspective. J. Hazard. Mater. 2022, 425, 127946. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, C.; Wang, T.; Chu, H.; Wang, C.-C. Efficient ciprofloxacin removal over Z-scheme ZIF-67/V-BiOIO3 heterojunctions: Insight into synergistic effect between adsorption and photocatalysis. Sep. Purif. Technol. 2023, 313, 123511. [Google Scholar] [CrossRef]
- Davies, K.M.; Mercer, J.F.; Chen, N.; Double, K.L. Copper dyshomoeostasis in Parkinson’s disease: Implications for pathogenesis and indications for novel therapeutics. Clin. Sci. 2016, 130, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Zhang, T.; Chen, J.; Ji, W.; Wei, Y. Fabrication of sodium alginate-melamine@ ZIF-67 composite hydrogel and its adsorption application for Pb (II) in wastewater. Environ. Sci. Pollut. Res. 2023, 30, 18364–18379. [Google Scholar] [CrossRef]
- Li, K.; Chen, M.; Chen, L.; Zhao, S.; Pan, W.; Li, P. Efficient removal of chlortetracycline hydrochloride and doxycycline hydrochloride from aqueous solution by ZIF-67. Heliyon 2024, 10, e36848. [Google Scholar] [CrossRef]
- Zhang, J.; Han, J.; Chen, X.; Xu, D.; Wen, X.; Zhao, Y.; Huang, Y.; Ding, X.; Chen, G.; Xu, D. Recent Advances in ZIF Membrane: Fabrication, Separation Ability and Its Application. Nanomaterials 2025, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shan, Y.; Zhang, S.; Kong, Q.; Pang, H. Application of metal organic framework in wastewater treatment. Green Energy Environ. 2023, 8, 698–721. [Google Scholar] [CrossRef]
- Seo, P.W.; Song, J.Y.; Jhung, S.H. Adsorptive removal of hazardous organics from water with metal-organic frameworks. Appl. Chem. Eng. 2016, 27, 358–365. [Google Scholar] [CrossRef][Green Version]
- Saghir, S.; Xiao, Z. Synthesis of novel Ag@ ZIF-67 rhombic dodecahedron for enhanced adsorptive removal of antibiotic and organic dye. J. Mol. Liq. 2021, 328, 115323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).