Antimicrobial Effect of Boswellia serrata Resin’s Methanolic Extracts Against Skin Infection Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples Collection and Preparation
2.3. High-Performance Thin-Layer Chromatography
2.3.1. Chemical Profile
2.3.2. Bioautographic Assays
2.4. LC-MS
2.5. Cytotoxicity Assay
2.5.1. Cell Culture
2.5.2. MTT Assay Protocol
3. Results and Discussion
3.1. HPTLC Profiling
3.2. HPTLC Bioassay Profiling
3.3. LC-MS Analysis
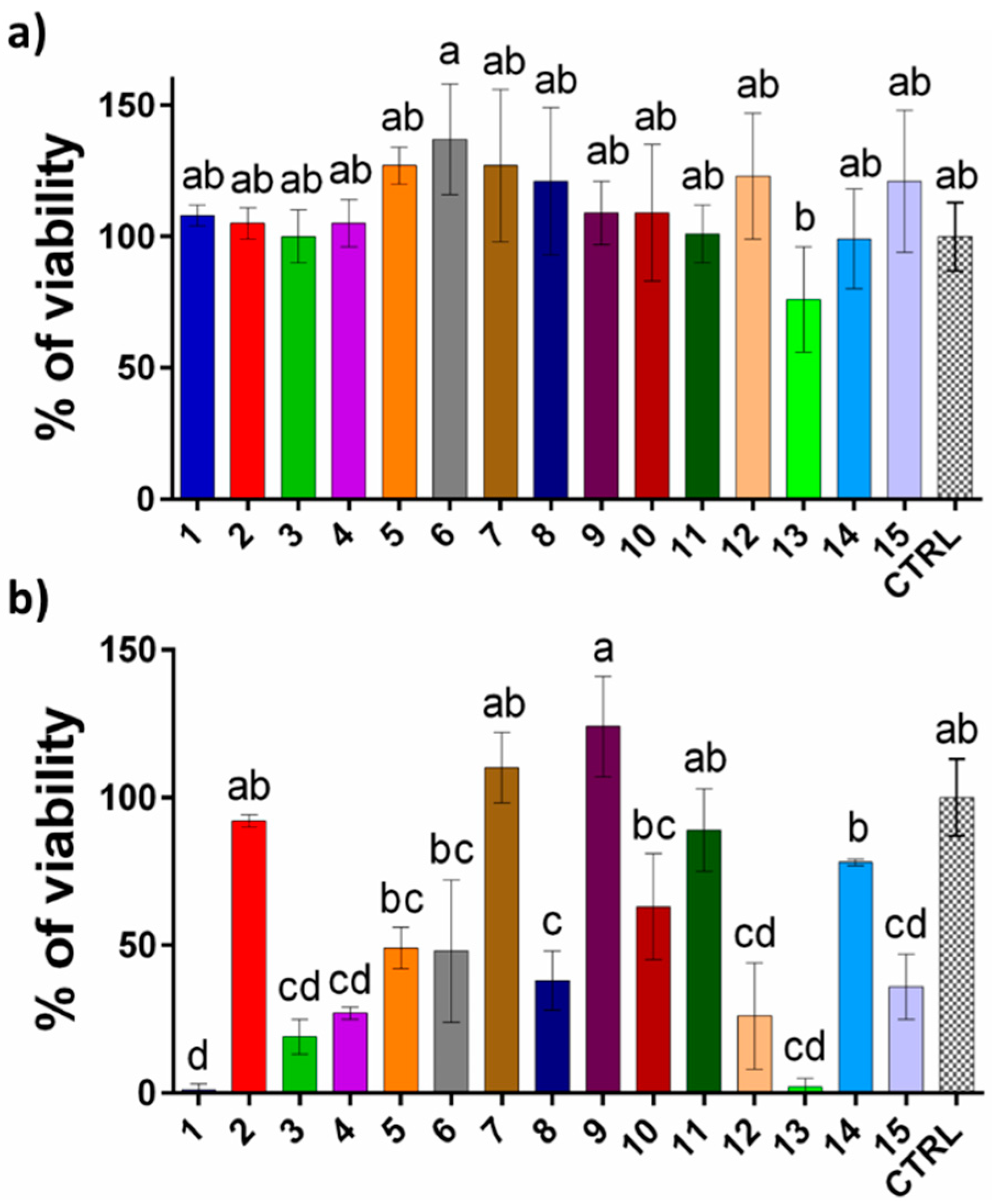
3.4. Cytotoxicity Assessment of Extracts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Warrier, K.C.S.; Muthupandiyan, S. Boswellia serrata Roxb. Ex Coleb.: A Threatened Tree. Int. J. Plant Soil Sci. 2022, 527–534. [Google Scholar] [CrossRef]
- Tironi de Castilho, A.L.T.; de Liori Teixeira, L.d.L.; Costa Lima, V.A.C.; Pesse, V.B.; Rozza, A.L. Chapter 3—Boswellia serrata. In Herbs, Spices and Their Roles in Nutraceuticals and Functional Foods; Amalraj, A., Kuttappan, S., Varma, A.C.K., Matharu, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 31–40. [Google Scholar] [CrossRef]
- Alam, T.; Alam Khan, S.A.; Najam, L. Chemistry, Biological Activities, and Uses of Resin of Boswellia serrata Roxb. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Murthy, H.N., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–43. [Google Scholar] [CrossRef]
- Hafeel, M.; Rizwana, A.A. Medicinal uses of Boswellia serrata Roxb (Kundur) with special reference to its ulcer healing property. J. Pharmacogn. Phytochem. 2021, 10, 34–37. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M. Frankincense (乳香 Rǔ Xiāng; Boswellia Species): From the Selection of Traditional Applications to the Novel Phytotherapy for the Prevention and Treatment of Serious Diseases. J. Tradit. Complement. Med. 2013, 3, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Rahman, K.U.; Padmaja, A.R.; Rahman, S.U. Boswellia serrata Roxb. a Traditional Herb with Versatile Pharma-cological Activity: A Review. Int. J. Pharm. Sci. Res. 2013, 4, 2106–2117. [Google Scholar] [CrossRef]
- Al-Yasiry, A.R.M.; Kiczorowska, B. Frankincense—Therapeutic properties. Postepy Hig. I Med. Doswiadczalnej 2016, 70, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Qurishi, Y.; Hamid, A.; Zargar, M.A.; Singh, S.K.; Saxena, A.K. Potential role of natural molecules in health and disease: Im-portance of boswellic acid. J. Med. Plants Res. 2010, 4, 2778–2785. [Google Scholar]
- Gupta, M.; Verma, S.K.; Singh, S.; Trivedi, L.; Rout, P.K.; Vasudev, P.G.; Luqman, S.; Darokar, M.P.; Bhakuni, R.S.; Misra, L. Anti-proliferative and antibacterial activity of oleo-gum-resin of Boswellia serrata extract and its isolate 3-hydroxy-11-keto-β-boswellic acid. J. Herb. Med. 2022, 32, 100546. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, S.; Kurmi, A.; Luqman, S.; Saikia, D.; Thomas, M.; Rout, P.K. Correlation of boswellic acids with antiproliferative, antioxidant and antimicrobial activities of topographically collected Boswellia serrata oleo-gum-resin. Phytomed. Plus 2022, 2, 100289. [Google Scholar] [CrossRef]
- Kaushik, S.; Roy, A.; Purabiya, P.; Satapathy, T. Anti Bacterial and In Vitro Anti-inflammatory Potential of Boswellia seratta: A Potent Bioactive Compound. Res. J. Pharm. Technol. 2020, 13, 1079. [Google Scholar] [CrossRef]
- Siemoneit, U.; Pergola, C.; Jazzar, B.; Northoff, H.; Skarke, C.; Jauch, J.; Werz, O. On the interference of boswellic acids with 5-lipoxygenase: Mechanistic studies in vitro and pharmacological relevance. Eur. J. Pharmacol. 2009, 606, 246–254. [Google Scholar] [CrossRef]
- Singh, G.B.; Atal, C.K. Pharmacology of an extract of salai guggal ex-Boswellia serrata, a new non-steroidal anti-inflammatory agent. Inflamm. Res. 1986, 18, 407–412. [Google Scholar] [CrossRef]
- Al-Kharousi, Z.S.; Mothershaw, A.S.; Nzeako, B. Antimicrobial Activity of Frankincense (Boswellia sacra) Oil and Smoke against Pathogenic and Airborne Microbes. Foods 2023, 12, 3442. [Google Scholar] [CrossRef]
- Jaroš, P.; Timkina, E.; Michailidu, J.; Maršík, D.; Kulišová, M.; Kolouchová, I.; Demnerová, K. Boswellic Acids as Effective Antibacterial Antibiofilm Agents. Molecules 2022, 27, 3795. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.F.; Ali, F.; Khan, I.A.; Shawl, A.S.; Arora, D.S.; Shah, B.A.; Taneja, S.C. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-β-boswellic acid from Boswellia serrata. BMC Microbiol. 2011, 11, 54. [Google Scholar] [CrossRef]
- Obiștioiu, D.; Hulea, A.; Cocan, I.; Alexa, E.; Negrea, M.; Popescu, I.; Herman, V.; Imbrea, I.M.; Heghedus-Mindru, G.; Suleiman, M.A.; et al. Boswellia Essential Oil: Natural Antioxidant as an Effective Antimicrobial and Anti-Inflammatory Agent. Antioxidants 2023, 12, 1807. [Google Scholar] [CrossRef] [PubMed]
- Hossain, T.J. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Wong, S.; Dolzhenko, A.V.; Gegechkori, V.; Ku, H.; Tucci, J.; Morton, D.W. Evaluation of bioactive compounds from Ficus carica L. leaf extracts via high-performance thin-layer chromatography combined with effect-directed analysis. J. Chromatogr. A 2023, 1706, 464241. [Google Scholar] [CrossRef]
- Choma, I.M.; Jesionek, W. TLC-Direct Bioautography as a High Throughput Method for Detection of Antimicrobials in Plants. Separations 2015, 2, 225–238. [Google Scholar] [CrossRef]
- Ahmad, I.; Husain, F.M.; Maheshwari, M.; Zahin, M. Medicinal Plants and Phytocompounds: A Potential Source of Novel Antibiofilm Agents. In Antibiofilm Agents: From Diagnosis to Treatment and Prevention; Springer: Berlin/Heidelberg, Germany, 2014; pp. 205–232. [Google Scholar] [CrossRef]
- Deza, M.A.; Araujo, M.; Garrido, M.J. Inactivation of Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa and Staphylococcus aureus on stainless steel and glass surfaces by neutral electrolysed water. Lett. Appl. Microbiol. 2005, 40, 341–346. [Google Scholar] [CrossRef]
- Ranjbarnejad, T.; Saidijam, M.; Moradkhani, S.; Najafi, R. Methanolic extract of Boswellia serrata exhibits anti-cancer activities by targeting microsomal prostaglandin E synthase-1 in human colon cancer cells. Prostaglandins Other Lipid Mediat. 2017, 131, 1–8. [Google Scholar] [CrossRef]
- Alharbi, S.A.; Asad, M.; Abdelsalam, K.E.A.; Ibrahim, M.A.; Chandy, S. Beneficial Effect of Methanolic Extract of Frankincense (Boswellia sacra) on Testis Mediated through Suppression of Oxidative Stress and Apoptosis. Molecules 2022, 27, 4699. [Google Scholar] [CrossRef]
- CBS. Analytical Tasks-Efficiently Solved by HPTLC; CAMAG: Pratteln, Switzerland, 2010. [Google Scholar]
- Stojković, D.; Gašić, U.; Uba, A.I.; Zengin, G.; Rajaković, M.; Stevanović, M.; Drakulić, D. Chemical profiling of Anthriscus cerefolium (L.) Hoffm., biological potential of the herbal extract, molecular modeling and KEGG pathway analysis. Fitoterapia 2024, 177, 106115. [Google Scholar] [CrossRef]
- Ivkovic, D.; Cvijetic, I.; Radoicic, A.; Stojkovic-Filipovic, J.; Trifkovic, J.; Krstic Ristivojevic, M.; Ristivojevic, P. NADES-Based Extracts of Selected Medicinal Herbs as Promising Formulations for Cosmetic Usage. Processes 2024, 12, 992. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Wong, S.; Dolzhenko, A.V.; Gegechkori, V.; Morton, D.W. Bioassay-guided detection, identification and assessment of antibacterial and anti-inflammatory compounds from olive tree flower extracts by high-performance thin-layer chromatography linked to spectroscopy. J. Pharm. Biomed. Anal. 2024, 239, 115912. [Google Scholar] [CrossRef]
- Rocamora, C.R.; Ramasamy, K.; Lim, S.M.; Majeed, A.B.A.; Agatonovic-Kustrin, S. HPTLC based approach for bioassay-guided evaluation of antidiabetic and neuroprotective effects of eight essential oils of the Lamiaceae family plants. J. Pharm. Biomed. Anal. 2020, 178, 112909. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Wong, S.; Dolzhenko, A.V.; Gegechkori, V.; Ku, H.; Tan, W.K.; Morton, D.W. Effect directed analysis of bioactive compounds in leaf extracts from two Salvia species by High-performance thin-layer chromatography. J. Pharm. Biomed. Anal. 2023, 227, 115308. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Brüning, G.; Bergmann, J.; Jauch, J. A Thin-layer Chromatography Method for the Identification of Three Different Olibanum Resins (Boswellia serrata, Boswellia papyrifera and Boswellia carterii, respectively, Boswellia sacra). Phytochem. Anal. 2012, 23, 184–189. [Google Scholar] [CrossRef]
- Gupta, M.; Rout, P.K.; Misra, L.N.; Gupta, P.; Singh, N.; Darokar, M.P.; Saikia, D.; Singh, S.C.; Bhakuni, R.S. Chemical composition and bioactivity of Boswellia serrata Roxb. essential oil in relation to geographical variation. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2017, 151, 623–629. [Google Scholar] [CrossRef]
- Suhail, M.M.; Wu, W.; Cao, A.; Mondalek, F.G.; Fung, K.-M.; Shih, P.-T.; Fang, Y.-T.; Woolley, C.; Young, G.; Lin, H.-K. Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement. Altern. Med. 2011, 11, 129. [Google Scholar] [CrossRef]
- DeCarlo, A.; Agieb, S.; Johnson, S.; Satyal, P.; Setzer, W.N. Inter-Tree Variation in the Chemical Composition of Boswellia papyrifera Oleo-Gum-Resin. Nat. Prod. Commun. 2022, 17, 1934578X221117411. [Google Scholar] [CrossRef]
- Hussain, H.; Rashan, L.; Hassan, U.; Abbas, M.; Hakkim, F.L.; Green, I.R. Frankincense diterpenes as a bio-source for drug discovery. Expert Opin. Drug Discov. 2022, 17, 513–529. [Google Scholar] [CrossRef]
- Wu, D.C.; Chan, W.W.; Metelitsa, A.I.; Fiorillo, L.; Lin, A.N. Pseudomonas Skin Infection. Am. J. Clin. Dermatol. 2011, 12, 157–169. [Google Scholar] [CrossRef]
- Alkheeqani, B.; Khassaf, A. Detection and Pathogenicity Features of Pseudomonas Aeruginosa in Patients with Skin Infection. Univ. Thi-Qar J. Sci. 2024, 11, 23–29. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef]
- Fady, M.; Irie, Y.; Aljowaie, R.M.; Almutairi, S.M. Effect of β-sitosterol on PEL and PSL of Pseudomonas aeruginosa. J. King Saud Univ. Sci. 2024, 36, 103400. [Google Scholar] [CrossRef]
- Spernovasilis, N.; Psichogiou, M.; Poulakou, G. Skin manifestations of Pseudomonas aeruginosa infections. Curr. Opin. Infect. Dis. 2021, 34, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Pewe, L.L.; Willems, P.; Mayer, R.; Thery, F.; Asselman, C.; Aernout, I.; Verbeke, R.; Eggermont, D.; Van Moortel, L.; et al. Immunopeptidomics Mapping of Listeria monocytogenes T Cell Epitopes in Mice. Mol. Cell. Proteom. 2024, 23, 100829. [Google Scholar] [CrossRef] [PubMed]
- Schlech, W.F., III. Epidemiology and Clinical Manifestations of Listeria monocytogenes Infection. Microbiol. Spectr. 2019, 7, 10-1128. [Google Scholar] [CrossRef]
- Garcia, R.; Alves, E.S.S.; Santos, M.P.; Glória Viégas Aquije, M.F.; Fernandes, A.A.R.; Santos, R.B.D.; Ventura, J.A.; Fernandes, P.M.B. Antimicrobial Activity and Potential use of Monoterpenes as Tropical Fruits Preservatives. Braz. J. Microbiol. 2008, 39, 163–168. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. Essential oils and their major monoterpene alcohols alone and in combination. Molecules 2010, 15, 7825–7839. [Google Scholar] [CrossRef]
- Dhar, P.; Chan, P.; Cohen, D.T.; Khawam, F.; Gibbons, S.; Snyder-Leiby, T.; Dickstein, E.; Rai, P.K.; Watal, G. Synthesis, Antimicrobial Evaluation, and Structure–Activity Relationship of α-Pinene Derivatives. J. Agric. Food Chem. 2014, 62, 3548–3552. [Google Scholar] [CrossRef]
- Hancock, R.E. The bacterial outer membrane as a drug barrier. Trends Microbiol. 1997, 5, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Vakayil, R.; Murugesan, K.; Gnanasekaran, A.; Radhakrishnan, A.; Ranjith, M.; Bakthavatchalam, P.; Velmurugan, P.; Sivasubramanian, K.; Arumugam, N.; Almansour, A.I.; et al. Determination of phyto-moieties from the traditional therapeutic plant Boswellia serrata Roxb. extract against nosocomial pathogens: In vitro and silico approaches. J. Herb. Med. 2024, 43, 100823. [Google Scholar] [CrossRef]
- Bhutada, S.A.; Farhan, M.M.; Dahikar, S.B. Preliminary phytochemical screening and antibacterial activity of resins of Boswellia serrata Roxb. J. Pharmacogn. Phytochem. 2017, 6, 182–185. [Google Scholar]
- Balgoon, M.J.; Alghamdi, A.M. Biochemical Assessment of Boswellic Acid Enrich-Frankincense Extract and its Antioxidant, Antibacterial, Anticancer and Anti-inflammatory Potential in Ameliorating the GlycerolToxicity in Rats. Pak. Veter. J. 2024, 44, 1023–1032. [Google Scholar] [CrossRef]
- Xavier, S. Study of Antibacterial Activity of Resins of Boswellia serrata Roxb ex Colebr., Commiphora mukul (Hooks ex-Stocks) engl., Gardenia resinifera roth. and Shorea robusta gaertn. Int. J. Pharm. Pharm. Sci. 2016, 8, 29–31. [Google Scholar]
- Alshafei, M.M.; Mabrouk, A.M.; Hanafi, E.M.; Ramadan, M.M.; Korany, R.M.; Kassem, S.S.; Mohammed, D.M. Prophylactic supplementation of microencapsulated Boswellia serrata and probiotic bacteria in metabolic syndrome rats. Food Biosci. 2023, 51, 102325. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Reis, S.F.; Rai, D.K.; Abu-Ghannam, N. Water at room temperature as a solvent for the extraction of apple pomace phenolic compounds. Food Chem. 2012, 135, 1991–1998. [Google Scholar] [CrossRef]
- Sultan, F.I. Phytochemical Analysis and Antibacterial Activities of Frankincense of Boswellia serrata. Plant Arch. 2020, 20, 5219–5226. [Google Scholar]
- Plyuta, V.; Zaitseva, J.; Lobakova, E.; Zagoskina, N.; Kuznetsov, A.; Khmel, I. Effect of plant phenolic compounds on biofilm formation by Pseudomonas aeruginosa. APMIS 2013, 121, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Zamuz, S.; Munekata, P.E.; Dzuvor, C.K.; Zhang, W.; Sant’Ana, A.S.; Lorenzo, J.M. The role of phenolic compounds against Listeria monocytogenes in food. A review. Trends Food Sci. Technol. 2021, 110, 385–392. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [PubMed]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests For In Vitro Cytotoxicity. Tanzania Bureau of Standards: Dar es Salaam, Tanzania, 2009.

| Extract Number | Geographical Region of Monk’s Frankincense | Name of the Church/Monastery |
|---|---|---|
| 1 | Subotica, Serbia | St. George’s church |
| 2 | Golubac, Serbia | Monastery Tumane |
| 3 | Kuršumlija, Serbia | St. Nichola’s church |
| 4 | Novi Sad, Serbia | St. George’s church |
| 5 | Valjevo, Serbia | Church of the Holy Mother of God |
| 6 | Banja Luka, Bosnia and Herzegovina | St. Peter and Paul’s church |
| 7 | Bethlehem, Palestine | Church of the Nativity |
| 8 | Mionica, Serbia | St. Nichola’s church |
| 9 | Koceljeva, Serbia | St. George’s church |
| 11 | Kotor, Montenegro | St. Luka’s church |
| 12 | Kruševac, Serbia | Church of the Holy Mother of God |
| 13 | Pljevlje, Montenegro | Church of St. James |
| 14 | Mount Atos, Greece | Monastery Hilandar |
| 15 | Vranje, Serbia | St. George’s church |
| 16 | Standard mixture |
| µg/g | GA | PCA | Caffeic Acid | CGA | SACl | p-CA | FA | SA | K-3-O-G | LT | QCT | NAR | KO | KBA (mg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13.13 ± 0.21 | 28.80 ± 0.55 | 1.53 ± 0.04 | 3.36 ± 0.22 | 3.74 ± 0.10 | 5.50 ± 0.13 | 3.81 ± 0.10 | 5.27 ± 0.45 | NF | NF | NF | 12.45 ± 0.17 | NF | 2.76 ± 0.14 |
| 2 | NF | 280.53 ± 5.33 | NF | NF | 5.37 ± 0.15 | 43.46 ± 0.99 | 9.97 ± 0.26 | 6.46 ± 0.55 | NF | NF | NF | 5.77 ± 0.08 | NF | 3.73 ± 0.19 |
| 3 | 6.03 ± 0.10 | 8.84 ± 0.17 | NF | NF | 3.76 ± 0.10 | 14.82 ± 0.34 | 3.85 ± 0.10 | 4.59 ± 0.39 | NF | NF | NF | 12.96 ± 0.17 | NF | 2.1 ± 0.1 |
| 4 | 10.63 ± 0.17 | 15.77 ± 0.30 | 1.62 ± 0.05 | 3.54 ± 0.23 | 3.72 ± 0.10 | 5.74 ± 0.13 | 8.32 ± 0.22 | 5.12 ± 0.44 | NF | NF | NF | 12.44 ± 0.17 | NF | 2.86 ± 0.14 |
| 5 | 13.13 ± 0.21 | NF | 1.72 ± 0.05 | 3.72 ± 0.24 | 3.75 ± 0.10 | 16.15 ± 0.37 | 10.54 ± 0.28 | NF | 5.94 ± 0.07 | 7.32 ± 0.24 | NF | 22.00 ± 0.30 | 13.00 ± 0.44 | 1.84 ± 0.09 |
| 6 | 110.93 ± 1.76 | NF | 1.68 ± 0.05 | 19.05 ± 1.23 | 3.70 ± 0.10 | 9.29 ± 0.21 | 10.61 ± 0.28 | 4.51 ± 0.38 | 8.35 ± 0.09 | 8.09 ± 0.27 | NF | 36.86 ± 0.50 | 14.84 ± 0.50 | 2.07 ± 0.10 |
| 7 | 56.27 ± 0.89 | 9.35 ± 0.18 | 1.64 ± 0.05 | 10.39 ± 0.67 | 3.76 ± 0.10 | 17.10 ± 0.39 | 4.64 ± 0.12 | 4.64 ± 0.39 | 6.41 ± 0.07 | 7.32 ± 0.24 | NF | 20.35 ± 0.27 | 12.65 ± 0.43 | 2.18 ± 0.11 |
| 8 | 91.34 ± 1.45 | 9.61 ± 0.18 | 1.59 ± 0.04 | 8.79 ± 0.57 | 3.74 ± 0.10 | 10.05 ± 0.23 | 6.61 ± 0.18 | 4.52 ± 0.38 | 5.38 ± 0.06 | NF | NF | 53.13 ± 0.72 | 15.28 ± 0.52 | 3.27 ± 0.16 |
| 9 | 6.26 ± 0.10 | NF | 1.53 ± 0.04 | NF | NF | 8.04 ± 0.18 | 3.85 ± 0.10 | NF | NF | NF | NF | 11.20 ± 0.15 | 10.69 ± 0.36 | 2.50 ± 0.12 |
| 10 | 26.50 ± 0.42 | 12.17 ± 0.23 | 1.54 ± 0.04 | 5.89 ± 0.38 | 3.70 ± 0.10 | 10.06 ± 0.23 | 5.31 ± 0.14 | 4.53 ± 0.39 | 5.24 ± 0.06 | 7.65 ± 0.26 | NF | 19.39 ± 0.26 | 12.02 ± 0.41 | 2.43 ± 0.12 |
| 11 | NF | 19.48 ± 0.37 | 26.18 ± 0.74 | NF | 3.69 ± 0.10 | NF | 11.06 ± 0.29 | 4.43 ± 0.38 | 4.35 ± 0.05 | 7.34 ± 0.25 | 24.89 ± 0.75 | 3.27 ± 0.04 | NF | 1.84 ± 0.09 |
| 12 | NF | 27.44 ± 0.52 | 1.64 ± 0.05 | NF | 3.75 ± 0.10 | 11.13 ± 0.25 | 6.56 ± 0.17 | 4.91 ± 0.42 | NF | NF | NF | 11.80 ± 0.16 | NF | 3.35 ± 0.17 |
| 13 | 11.15 ± 0.18 | 9.43 ± 0.18 | 1.54 ± 0.04 | 3.15 ± 0.20 | 3.72 ± 0.10 | 10.17 ± 0.23 | 6.06 ± 0.16 | 4.45 ± 0.38 | NF | 7.38 ± 0.25 | NF | 11.25 ± 0.15 | 11.56 ± 0.39 | 2.30 ± 0.12 |
| 14 | NF | 14.43 ± 0.27 | 1.52 ± 0.04 | NF | NF | 115.54 ± 2.63 | 9.13 ± 0.24 | 4.59 ± 0.39 | NF | NF | NF | 2.44 ± 0.03 | NF | 1.66 ± 0.08 |
| 15 | 75.08 ± 1.19 | 18.90 ± 0.36 | NF | NF | 3.78 ± 0.10 | 14.44 ± 0.33 | 8.91 ± 0.24 | 5.07 ± 0.43 | NF | NF | NF | 13.97 ± 0.19 | 11.27 ± 0.38 | 2.82 ± 0.14 |
| 16 | 93.05 ± 1.48 | 16.65 ± 0.32 | 1.57 ± 0.04 | 10.34 ± 0.67 | 3.75 ± 0.10 | 7.88 ± 0.18 | 19.40 ± 0.51 | 5.00 ± 0.43 | 9.43 ± 0.10 | 8.38 ± 0.28 | 39.12 ± 1.19 | 4.37 ± 0.06 | 10.84 ± 0.37 | 0.59 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorović, P.; Krstić Ristivojević, M.; Jović, M.; Ivković, Đ.; Nestorović Živković, J.; Gašić, U.; Dimkić, I.; Stojiljković, I.; Ristivojević, P. Antimicrobial Effect of Boswellia serrata Resin’s Methanolic Extracts Against Skin Infection Pathogens. Processes 2025, 13, 850. https://doi.org/10.3390/pr13030850
Todorović P, Krstić Ristivojević M, Jović M, Ivković Đ, Nestorović Živković J, Gašić U, Dimkić I, Stojiljković I, Ristivojević P. Antimicrobial Effect of Boswellia serrata Resin’s Methanolic Extracts Against Skin Infection Pathogens. Processes. 2025; 13(3):850. https://doi.org/10.3390/pr13030850
Chicago/Turabian StyleTodorović, Petar, Maja Krstić Ristivojević, Marko Jović, Đurđa Ivković, Jasmina Nestorović Živković, Uroš Gašić, Ivica Dimkić, Ivana Stojiljković, and Petar Ristivojević. 2025. "Antimicrobial Effect of Boswellia serrata Resin’s Methanolic Extracts Against Skin Infection Pathogens" Processes 13, no. 3: 850. https://doi.org/10.3390/pr13030850
APA StyleTodorović, P., Krstić Ristivojević, M., Jović, M., Ivković, Đ., Nestorović Živković, J., Gašić, U., Dimkić, I., Stojiljković, I., & Ristivojević, P. (2025). Antimicrobial Effect of Boswellia serrata Resin’s Methanolic Extracts Against Skin Infection Pathogens. Processes, 13(3), 850. https://doi.org/10.3390/pr13030850









