Abstract
Wound healing remains a critical challenge in healthcare, especially with the increasing prevalence of diabetes and its associated complications, such as diabetic foot ulcers (DFUs). Delayed wound healing in diabetic patients is attributed to several factors, with a pathophysiology that is diverse and multifaceted, including decreased immune responses, microvascular dysfunction, oxidative stress and impaired collagen synthesis. Additionally, the development of an infection at the wound site further complicates and hinders the healing process, especially in DFUs. Epigallocatechin gallate (EGCG), a potent bioactive compound found in green tea, has shown promising effects in modulating various stages of wound healing by its antioxidant, anti-inflammatory and anti-bacterial properties in vitro and in vivo. This review discusses delayed wound healing in diabetic wounds, while highlighting the therapeutic potential of GT/EGCG in different stages of wound healing, including in diabetic wounds both in vitro and in vivo. Novel applications such as GT-/EGCG-loaded wound dressings have demonstrated significant positive impacts on wound healings, including in diabetic wounds, both in in vitro and in vivo studies. Despite its therapeutic potential, the commercial application of green tea in wound care faces significant challenges, including issues of bioavailability, stability, cytotoxicity, production costs, the lack of in-depth and complete in vivo studies and, most importantly, the lack of clinical trials. By consolidating current knowledge and identifying gaps, this review aims to inspire future research and innovations in using green tea for effective wound management and potential use in diabetic patients and DFUs, if the positive results from animal in vivo studies are equally effective in human clinical studies.
1. Introduction
The many benefits of secondary metabolites produced by different plants have been studied extensively. Of these, polyphenols have gained attention in recent years as a possible means of enhancing human health. A review paper by Marino et al. discussed numerous clinical trials highlighting the diverse biological and pharmacological benefits of polyphenolic compounds [1]. As green tea is known to have high phenolic content, in addition to being widely consumed, most studies have been conducted to assess its use as preventive measure in health [2]. Green tea leaves contain a variety of compounds, such as polysaccharides, volatile oils, vitamins, minerals, purines and alkaloids in addition to high polyphenols [3], with catechins being the most notable of all the polyphenols [4]. Catechins form 15–27% of the dry weight of green tea [5,6,7]. The four major catechins are (–)-epigallocatechin gallate (EGCG) (40–69%), followed by (–)-epigallocatechin (EGC) (12–23%), (–)-epicatechin gallate (ECG) (13–21%) and (–)-epicatechin (EC) (5–9%) [8,9,10]. Studies have also identified some green tea varieties with o-methylated forms of EGCG and epigallocatechin-3-o-(4-o-methyl) gallate [11,12,13].
The capabilities of green tea in metabolizing fat and optimizing metabolic activity have made it a popular daily food additive for health purposes [14,15]. Among the bioactive compounds present in green tea, researchers are highly interested in catechins, especially EGCG, which has been found to have effective antioxidant, anti-inflammatory and anti-carcinogenic properties [16,17]. In addition, according to some in vivo diabetic mouse model studies, green tea polyphenols considerably reduced fasting glucose concentrations and levels of glycated hemoglobin (HbA1c), resulting in improved blood glucose levels due to green tea’s ability to imitate anti-diabetic drugs [18,19,20]. Green tea supplementation was also found to significantly reduce both systolic and diastolic blood pressures when compared to the placebo group (p < 0.01) in a clinical trial involving obese and hypertensive people [21]. The study also reported significant (p < 0.01) drops in fasting serum glucose as well as a substantial (p < 0.05) decrease in total and low-density lipoprotein cholesterol [21], supporting its benefit in helping obesity and heart disease. In addition, EGCG, a key tea polyphenol in green tea, has also shown vaso-protective [22], tissue-protective, anti-remodulation [23] and anti-fibrotic properties, promoting wound healing [24]. The wide-range impact of tea polyphenols makes them a promising agent for health and disease prevention, potentially acting as a natural alternative to pharmaceutical medications [25].
This review analyzes the therapeutic benefits of green tea in different stages of wound healing, including in diabetic wounds, focusing on its polyphenols, specifically EGCG in green tea. This review also describes the mechanism of actions of green tea in wound management, through its potential in promoting tissue regeneration, reducing oxidative stress, anti-microbial and anti-inflammatory properties, and its potential use in DFUs. Furthermore, this review discusses some challenges in the development of green tea-based treatments on wound healing and the opportunities for further research on the use of green tea in wound care.
2. Prevalence and Complications of Diabetes and Diabetic Foot Ulcers
Diabetes has become one of the most significant and pressing health challenges worldwide, with its prevalence rising at an alarming rate [26]. The growing prevalence of diabetes reflects not only the increased exposure to risk factors such as obesity and poor nutrition but also underscores the urgent need for better prevention, diagnosis and management strategies to address this global health crisis effectively [27].
The International Diabetes Federation (IDF) in December 2021 revealed that, currently, more than half a billion adults worldwide have diabetes. This is an alarming increase of 16%, representing 74 million, compared with previous predictions made by the IDF in 2019 [28]. The IDF reports that 537 million individuals are living with diabetes, accounting for 10.5% of the global population and costing USD 966 billion in healthcare [28,29]. This healthcare expense is expected to exceed USD 1054 billion by 2045 [28]. It is concerning that the prevalence of diabetes is expected to rise to 11.3% (643 million) by 2030 and 12.2% (783 million) by 2045 (Figure 1) [28,30]. Figure 1 shows the increasing global prevalence of diabetes among adults aged 20–79 years with a forecast through to the year 2045.
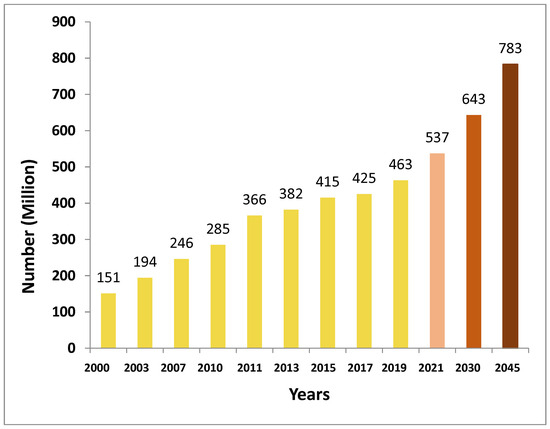
Figure 1.
Incidence of diabetes (millions) of people aged 20 to 79 worldwide with anticipated figures for 2030 and 2045 (data extracted from International Diabetes Federation Diabetes Atlas [28]).
With the increasing incidence of diabetes, there is also a surge in related complications, and diabetic foot ulcers (DFUs) are one of them. The International Working Group on the Diabetic Foot (IWGDF) define DFUs as a collection of symptoms secondary to either current or past diabetes, such as skin blisters, ulcerations, destruction of foot tissues or infection [31,32]. These ulcerations and infections are usually accompanied by neuropathy and/or peripheral artery disease (PAD) [33]. It has been reported that 9.1 to 26.1 million individuals with diabetics worldwide experience DFUs each year [34]. People with diabetes run a 25% risk of developing DFUs, and unfortunately, too many patients are ultimately forced to undergo amputation as a final resort [35]. Despite this, DFUs are preventable even though it has a huge negative impact on patients’ comfort and medical resources. Effective management of DFUs involves adopting a balanced lifestyle as prevention, early diagnosis and receiving high-quality therapy from a multidisciplinary team of professionals [36].
Management of Diabetic Foot Ulcers: Standards, Benefits and Disadvantages
The current gold standard of care for DFUs is a multidisciplinary approach which encompasses treatment of the wound by debridement, wound dressings for infection management and revascularization, along with various methods of offloading to prevent the formation of ulcers [37,38]. The offloading of pressure applied on an ulcer is facilitated by total contact casts (TCCs) or other appropriately designed footwear gear [39]. The benefits of this approach include reduced infection risk, improved wound healing, and lower amputation rates as TCCs distribute pressure points evenly and well, while aiding in better ulcer resolution rates. However, high costs, restricted availability in resource-poor settings and patient non-compliance with offloading devices are the disadvantages of TCCs [40]. Other interventions aside from these are revascularization either through surgical or endovascular techniques. These are performed to reinstate blood supply in ischemic ulcers [38,41]. As it requires surgical intervention, there is a high risk of infection and associated complications. As a result, the recurrence rates are high, and it involves further monitoring and follow-up care; hence, it places long-lasting burdens on health services and patients [42,43]. Debridement is one of the treatments for DFU management that involves the removal of dead tissue or infected tissue and is an important procedure for wound healing and prevention of infection [44]. Newer therapies such as growth factors and stem cell treatments have also been proposed, but more research is needed to discover the best type of growth factors and stem cells for therapy, their safety, the appropriate dose and the most viable administration route [45]. Moreover, there are advanced dressings including hydrogels, foams, films, gauze and alginate dressings for wound healing, while hydrocolloids, hydrogels, foams and films are commonly used for DFUs [46,47]. These dressings help to promote wound healing by enhancing tissue regeneration, infection control and providing optimal moist wound environment [48,49]. However, they also have some disadvantages that might limit their applications. Hydrogels, though possessing good moisture retention that is crucial for wound healing, can cause maceration of the surrounding skin and are not ideal for highly exudative wounds such as infected wounds. While films provide a protective barrier, they are ineffective for wounds with high exudate and might cause damage to skin when removed. Foam dressings, while absorbent, can dry out wounds with little exudate and can be expensive. Gauze, while affordable and adaptable, is frequently unable to maintain an ideal moist environment, increases the risk of infection and can cause damage to tissues during dressing replacements. Alginate dressings are highly absorbent; however, they can dry up low-exudate wounds and need a secondary dressing for effective adhesion [46,47,50]. Therefore, there is no one dressing that is ‘one-size-fits-all’. In addition to that, dressings with active ingredients in them may help with wound healing. Since green tea polyphenols have shown anti-microbial, anti-inflammatory, angiogenetic and antioxidant properties, it is important to explore the potential of green tea for healing wounds, including diabetic wounds.
3. Therapeutic Effects of Green Tea Polyphenols on Different Healing Stages of Wounds, Including Diabetic Wounds
The healing of wounds is a complicated process occurring in an ordered sequence of four overlapping, well-regulated phases: hemostasis, inflammation, proliferation and remodeling [51]. Each of these must occur in the proper sequence and duration for effective wound healing. Any disturbance, deviation or delay in these phases can lead to poor wound healing or wounds becoming chronic [52,53]. Homeostasis is the first phase of wound healing that occurs right after an injury, followed by clotting [54]. The inflammatory phase primarily involves bleeding control. It involves the accumulation of neutrophils, macrophages and monocytes [54]. The inflammatory phase is important in clearing harmful organisms and providing a conducive condition for the following phase of tissue restoration and repair [54,55]. After the inflammation phase, proliferation starts, with an emphasis on repairing the wound site (re-epithelialization), generating granulation tissue and rebuilding the vascular network [55]. Remodeling is the last stage of wound healing, which is described as transitioning from granulation tissue to a scar, and the appearance and strength of the healed tissue are ultimately determined in this phase [56].
In diabetes patients, there is slower blood flow to peripheral regions (peripheral neuropathy), decreased skin cell function and a higher risk of atherosclerosis as a result of hyperglycemic conditions. This often results in reduced wound healing and, in the long term, could impact the morbidity, mortality and quality of life of patients [57,58].
The normal homeostasis phase is generally defined by platelet coagulation at the damage sites, where clotting and growth factors are released, allowing for clot formation and the prevention of more blood loss [59]. Green tea has been shown to have a potential role in homeostasis by constricting tissues, reducing vascularity temporarily and causing blood vessel contractions, thus reducing blood loss. Green tea also stops the further secretion of blood; as a result of albumen coagulation, leading to formation of clot rapidly [60,61,62,63,64]. The hemostatic stage of healing is delayed in DFUs due to vascular dysfunction [60]. Growth factor release and platelet mobilization are lowered as a result of lower blood circulation. This typically causes the fibrin clot to form more slowly [61,62,63]. In diabetic patients, hyperglycemia can also promote disruption of the dissolved clot and may prolong the bleeding time [61]. A study by Sun et al. reported that keratin–catechin nanoparticles (KE-NPs) play a role in hemostasis in vitro and in vivo by quickly activating thrombin and then fibrinogen, which leads to the development of insoluble fibrinogen polymers, hence promoting blood clotting [65]. This could be useful in the management of diabetes wounds including DFU.
In the early stages of the inflammatory phase, invading neutrophils and monocytes release growth factors such as platelet-derived growth factors (PDGFs), transforming growth factor-β (TGF-β) and inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) to clean the wound, while myofibroblasts help the wound to shrink [66,67]. An antagonistic effect of TNF-α on TGF-β has been linked to delayed wound healing, which is characterized by the limited production of granulation tissue and a low capacity for wound contraction. There have been reports of elevated TNF-α levels in diabetic wounds [41] and more recent investigations have found elevated levels of other pro-inflammatory cytokines such as (IL)-1β, IL-6 and IL-8 [67,68,69]. Generally, the inflammatory phase is prolonged in diabetic wounds, which further decreases formation of granulation tissue and reduces the tensile strength of the wound [70]. Such failure to progress properly through the phases of healing can be attributed to vascular compromise, followed by ischemia, as well as other factors such as hypoxia and neuropathy, among others in DFUs [42,57,70]. EGCG has been reported to prevent the movement and attachment of monocytes to the wound site, thus regulating inflammation [71]. EGCG is also well recognized for inhibiting the generation of different pro-inflammatory agents including nitric oxide synthase, peroxynitrite, reactive oxygen/nitrogen species and cyclooxygenase-2, thus promoting wound healing [72,73,74]. Being a strong antioxidant, EGCG is also an excellent scavenger of free radicals. An in vitro study by Klass et al. showed that EGCG regulates the differentiation of dermal fibroblasts by inhibiting the nuclear factor-kβ (NF-kβ) and activator protein 1, proteins responsible for wound contraction in the process of wound healing [75]. Moreover, EGCG, either alone or in combination with other phytonutrients, has also been also found to inhibit the release of pro-inflammatory cytokines such as TNF-α, Interlukin (IL)-1β, IL-8 and IL-6 in skin cells where a positive effect on diabetic wound healing was achieved [54,76,77]. EGCG has also been reported to elevate anti-inflammatory cytokines such as IL-10 [78] that allow for a more structured formation of the extracellular matrix while maintaining biomechanical strength and allow wound repair with appropriate physiologic stability [79,80]. Such properties from EGCG make it a promising candidate for enhancing wound healing during the inflammatory phase with minimized scar formation [75,81] both in normal and diabetic wounds. Figure 2 shows various critical inhibitory, regulatory and stimulatory roles of green tea that promote wound healing and tissue/cell repair [23,82,83,84].
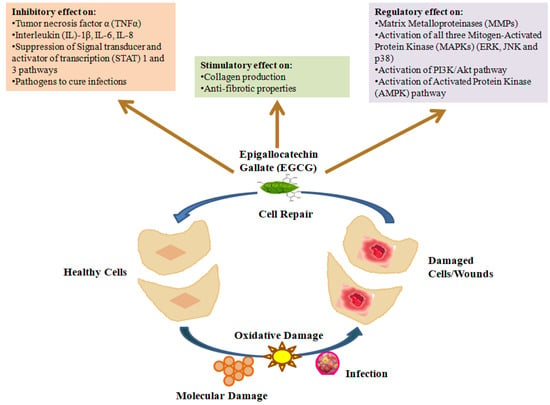
Figure 2.
Schematic presentation of inhibitory, stimulatory and regulatory effects of green tea (adapted from Mokra et al. [23], Chen et al. [82], Viaña-Mendieta et al. [83] and Dunaway et al. [84]).
Re-establishing blood supply is a crucial part of proper wound healing mostly in the proliferation phase [85]. The body concentrates on tissue development during the proliferative phase, which includes the formation of granulation tissue, collagen deposition, epithelialization (skin regeneration) and the development of new blood vessels (angiogenesis) [51]. However, skin cell proliferation and stem and progenitor cell activation are greatly decreased during the proliferative phase within DFUs [86]. Other elements such as protein glycation caused by type 2 diabetes, altered angiogenic functioning and the resulting oxidative stress, all collectively lead to delayed wound healing. Moreover, the differentiation of monocytes towards M2 macrophages is inhibited, and pro-inflammatory effects, i.e., M1 macrophage polarization, is increased in diabetic wounds [86]. EGCG has been demonstrated to augment the activity and expression of transforming growth factor-beta (TGF-β1) [75], which produces multifaceted effects on wound healing, particularly during the proliferation phase, by regulating fibroblast differentiation, proliferation, collagen production, wound contraction, extracellular matrix synthesis and modifying the immunological response [87,88]. In an in vivo study, Hajiaghaalipour et al. reported histological findings of rat models demonstrating that the granulation tissue in the rats treated with tea extract had higher levels of collagen and angiogenesis and reduced inflammation as compared to the positive control rats treated with INTRASITE gel [89]. There was also a broad region of ulceration, likely containing fibrinous exudates, inflammatory cells and a minor degree of vascularization with dermal congestion, and signs of incomplete healing were observed in the control group treated with carboxymethyl cellulose (CMC) in normal saline (2%). In an in vivo study by Shin et al., it was found that hyaluronic acid and EGCG’s synergistic activity significantly aided wound revascularization and epithelialization in diabetic mouse models [90]. In addition to that, green tea polysaccharides were also found to contribute to immunological irradiation protection, anti-blood coagulation, anti-cancer effects and antioxidant actions and inhibit pathogenic bacteria adhesion [91,92,93,94,95]. These showed that green tea has a positive effect on the proliferation stage of wound healing in both normal and diabetic wounds.
The extracellular matrix’s tensile strength is increased during the last remodeling phase to repair the integrity of the skin where the newly developed tissue expands, becomes stronger and reorganizes [96]. However, the delayed healing in diabetes individuals is caused by an imbalance between the buildup of extracellular matrix (ECM) components and their remodeling by matrix metalloproteinase [97]. EGCG is found to decrease the levels of matrix metalloproteinase (MMP) 1 and 2, enzymes which function to regulate extracellular matrix breakdown whose elevated levels are associated with poor wound healing, thus helping in remodeling phase [81]. MMPs are also responsible for increasing the tensile strength of diabetic wounds by collagen remodeling and degradation and secreting a new extracellular matrix (ECM) [98,99]. Tea polyphenols have also been shown to enhance wound morphology and reinstate the type III/I collagen ratio throughout the remodeling phase [56,100]. The role of green tea catechins during different stages of wound healing has been summarized in Table 1.

Table 1.
Role of green tea catechins during different phases of wound healing.
In addition to anti-inflammatory [54,78] and antioxidant properties [106], green tea is also known to have anti-microbial effects [107] and to help in infection control in wounds. Since infected wounds are common in DFUs due to slow healing capacity, therefore, it is important to explore the anti-microbial potential of green tea polyphenols to establish its efficacy for infected wounds such as in DFUs. This holds great potential for wound healing in diabetic patients, possibly in DFUs, and could potentially reduce the associated morbidity and mortality of DFUs.
3.1. Anti-Microbial Properties of Green Tea
Green tea polyphenols have also been found to have anti-bacterial properties [107,108]. Apart from the direct anti-microbial effects of catechins such as destruction to the bacterial cell membrane [109], the inhibition of fatty acid synthesis or the inhibition of enzymatic activity, there are several other effects including inhibitory action on efflux pumps and disruption to bacterial cell functions that could contribute to the general anti-microbial effect in infected individuals [110,111,112,113]. Additionally, studies showed significant in vitro anti-microbial activity against various pathogens, such as Gram-positive and Gram-negative bacteria and fungi, including common multidrug-resistant wound-related pathogens such as Methicillin-resistant Staphylococcus aureus (MRSA), Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Escherichia coli that can delay the wound healing process [107,114,115,116]. However, it is important to discuss the various modes of anti-bacterial mechanism of action of green tea polyphenols as understanding these mechanisms can provide insight into their potential applications in treating bacterial infections and promoting health.
3.1.1. Bacterial Cell Membrane Damage
Macromolecules such as proteins, lipids, carbohydrates and hydrocarbons are strongly attracted to polycyclic structures and phenolic hydroxyl groups, which are abundant in tea [117]. As a result, there is strong affinity between catechins and bacterial cell membranes, resulting in alterations in cell membrane fluidity and instability in bacterial cell structure [118]. Furthermore, it has been reported that the anti-microbial potential of tea polyphenols is also due to the precipitation of bacterial cell membrane proteins caused by their reaction with polyphenols [119,120]. It was also found in other studies that cell membrane fluidity and the perforation of cell membranes were reduced by numerous flavonoids and phenolic acids, ultimately leading to damage to cytoplasmic membranes, making it hard for bacteria to survive [121,122]. Such disruption of the bacterial cell membrane prevents bacteria from adhering to host cells and also from adhering to one another to form biofilms [123], which is significant in bacterial pathogenicity [124,125].
Other than disrupting membrane integrity, treatment with green tea polyphenols resulted in the different expression of 17 genes; 9 genes were upregulated and 8 were downregulated in Escherichia coli [126]. One of the main outcomes of this alteration in gene regulation is the damage to the bacterial cell membrane [126]. Moreover, it has been found that the minimum inhibitory concentration (MIC) of EGCG is 8 to 16 times more (less potent) for Gram-negative bacteria as compared to Gram-positive bacteria [127]. A possible reason behind this could be the protective effect of outer membranes and lipopolysaccharides in Gram-negative bacteria, causing repulsion between the negatively charged catechins with the lipopolysaccharide layer, therefore making it difficult for catechins to show similar anti-bacterial effects shown by Gram-positive bacteria [114].
Other consequences of bacterial membrane damage by green tea polyphenols include the inability of bacteria to secrete toxins [128]. The synthesis of fatty acids is directly connected with the homeostasis of bacterial cell membranes, which when disrupted, leads to cell lysis, causing bacterial cell death [129]. In addition to cell membrane disruption, the inhibition of fatty acid synthesis in bacteria also halts various crucial processes necessary for bacterial survival.
3.1.2. Inhibition of Fatty Acid Synthesis
One notable effect that green tea catechins can have on most bacteria is their capacity to limit bacterial fatty acid production by inhibiting enzymes required in the biosynthetic pathway. Considering that this is a critical pathway for most bacteria, scientists are targeting this mechanism of action in new anti-microbial drug discovery pathways. Fatty acids are essential for the formation of cell membranes, serving as a source of energy and contributing to the synthesis of harmful metabolites produced by bacteria [130,131]. In addition to being an important source of energy, fatty acids in bacteria also act as a building block of phospholipid cellular membranes [125]. It has been found that green tea compounds, especially EGCG, is a mixed-type inhibitor comprising β-ketoacyl-ACP reductase (FabG) and a competitive inhibitor of trans-2-enoyl-ACP reductase (FabI) in bacterial type II synthesis of fatty acids. It has been indicated that EGCG interacts with the ligand-free forms of FabG and FabI, preventing nicotinamide adenine dinucleotide phosphate (NAD(P)H) binding [132]. The galloyl moiety of EGCG is deemed essential for the inhibitory action of FabG and FabI [131,132]. It has been reported that bacterial type II fatty acid synthase is the target of EGCG’s anti-bacterial action, and exposing bacterial cells to EGCG resulted in the inhibition of fatty acid production [132,133]. It has also been speculated that the hypolipidemic and proapoptotic properties of green tea extracts and EGCG come from the suppression of fatty acid production in bacterial cells [94,112,134,135]. Besides that, the suppression of fatty acid production by green tea also inhibits the production of harmful by-products by bacteria that could otherwise compromise the host’s immune system [136].
3.1.3. Inhibition of Several Other Enzyme Activities
Green tea has also been found to affect other essential bacterial enzymes activities, in addition to the enzymes involved in the synthesis of fatty acids. In certain anaerobic oral bacteria such as Prevotella intermedia and Porphyromonas gingivalis, green tea catechins have been found to inhibit the action of enzymes such as protein tyrosine phosphatase (an important regulator of bacterial cell growth, division and metabolism) [137,138] as well as cysteine proteinases (involved in protein processing and catabolic functions) [130,139,140]. Green tea catechins have also demonstrated the ability to inhibit deoxyribonucleic acid (DNA) synthesis by interacting with DNA gyrase enzyme and inhibiting its activity, thus hindering the replication of DNA. Green tea catechins interact with the adenosine triphosphate (ATP) binding site on the gyrase B subunit of bacterial DNA gyrase, leading to bacterial cell death since gyrases are essential bacterial proteins for DNA replication [141]. Moreover, it has also been reported that the activity of bacterial F1-ATPase and ATP production was almost equally inhibited by bioflavonoids, including those from green tea, resulting in the compromised production of energy in bacteria [142]. Another target of green tea catechin is the folate biosynthesis pathway regulated by enzyme dihydrofolate reductase (DHFR). DHFR is known to be a target of inhibitory effects from several sulfa antibiotic drugs, and EGCG has also been found to suppress DHFR activity, leading to the disruption of DNA synthesis, thus disturbing cell growth [25,130,143].
3.1.4. Inhibitory Effects on Efflux Pumps and Other Bacterial Cell Functions
Green tea catechins have also been reported to inhibit the activity of efflux pumps in bacterial cells. Efflux pumps are responsible for maintaining bacteria’s internal environment by the extrusion of various harmful substances including metal ions, metabolites and antibiotics, thus helping bacteria to survive and develop anti-bacterial resistance [144,145]. In addition to allowing bacteria to pump out anti-bacterial compounds, efflux pumps play a role in bacterial stress response, pathogenicity, biofilm formation and host physiology alteration [146]. A study by Sudano-Roccaro et al. reported a decrease in the activity of Tet(K), a tetracycline-resistant efflux pump in Staphylococci when treated with green tea catechins [113]. This type of activity can also take place with other efflux pumps, potentially increasing the potential of some antibiotics to have an anti-bacterial impact. In Gram-negative bacteria with resistance–nodulation–cell division (RND)-type efflux pumps, the potential of green tea catechins to impede the activity of bacterial efflux pumps serves as a major role as an additive anti-bacterial effect for tea catechins and many anti-microbial drugs [130,147]. In addition to this, an increase in antibiotic action and the anti-microbial activity of EGCG has been observed by the inhibition of efflux pumps (MexAB-OprM) in clinical isolates of Pseudomonas aeruginosa [148]. EGCG has also been reported to interfere with the activities of various antibiotic efflux pumps, such as for ciprofloxacin efflux in β-lactamase-producing Klebsiella pneumonia [149] and tetracycline efflux in Staphylococci [113].
Moreover, researchers have also reported the potential of green tea catechins to inhibit several other bacterial functions. One such impact is in inhibiting the formation of Penicillin-Binding Protein 2 (PBP2) in MRSA [150]. As a result of this inhibition, the resistance of MRSA to β-lactam drugs was reversed [150,151]. In a study, Zhao et al. reported that significant levels of MRSA resistance to all beta-lactam antibiotics, including ampicillin, cephalexin, benzylpenicillin, oxacillin and methicillin, were reversible by the addition of 25 µg/mL EGCG with β-lactam antibiotics [152]. Furthermore, in Escherichia coli, the conjugative transfer of the R plasmid was inhibited by green tea catechins [153]. This resulted in the decreased ability of bacteria to share anti-microbial genes, thus reducing bacterial resistance [153].
Based on the above, it is clear that green tea polyphenols have huge effects on the different mechanisms of anti-microbial activity and, in some cases, could work synergistically with current antibiotics against some antibiotic-resistant bacteria. This effect, along with their role in different phases of wound healing, showed that they have great potential to be incorporated as a treatment for wounds, especially for chronic wounds such as DFUs.
4. Unveiling the Potential of Green Tea Dressings for Wound Healing
As mentioned, even though there are numerous benefits and positive effects of green tea catechins in wound healing, their usage for wound healing is somewhat limited owing probably to the various problems associated with the direct delivery of bioactive polyphenols to wounds. Tea polyphenols have limited bioavailability and are unstable, which may compromise their antioxidant properties when exposed to light, pH, oxygen or temperature [154,155]. Polyphenols also have low water solubility, which would impact their bioavailability and absorption, hindering their direct application [156].
Therefore, instead of direct application, incorporating green tea into dressings and other materials may overcome these barriers of direct application. It is equally important to discuss the in vitro and in vivo animal studies and clinical studies conducted so far using green tea or their polyphenols to further evaluate the potential application of green tea in wound dressings. Table 2 shows various studies that have incorporated either green tea/polyphenols into wound dressings, along with their outcomes on wound healing and their pros and cons.

Table 2.
Properties and wound healing potential of green tea polyphenol-based wound dressings.
Since hemostasis is the first phase of wound healing, an in vitro and in vivo rat model study by Sun et al. proposed that cellulose/keratin–catechin composite hydrogel (KEC) can provide rapid blood coagulation and shows strong hemadsorption and adhesion [65]. Another in vivo mouse model study by Kim et al. highlighted the efficacy of EGCG/chitosan hydrogels in tissue regeneration; however, the efficacy of the dressing during the hemostatic and inflammation phase of wound healing is unclear [158]. Although these studies proved good blood clotting potential in vitro and in vivo, there is no information regarding their potential in later stages of wound healing, which is useful since all the four stages of wound healing are crucial for proper healing. In addition, the study by Kim et al. primarily focused on highly exuding wounds, but it did not mention if there is potential use in low exudating/dry wound applications [158].
Another promising study presented gelatin/chitosan/EGCG nanoparticles loaded into a poly (c-glutamic acid)/gelatin hydrogel containing gentamicin and activated carbon fibers [107]. The dressing successfully suppressed the targeted bacterial strains (Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus) in vitro. An in vivo analysis revealed that the hydrogel was easy to remove from the wound, promoted wound tissue regeneration and sped up the healing process. Even though the dressing showed a complete release of EGCG in 12 h, it was also found to cause cytotoxicity within the 12 h it was released. The cytotoxicity issue highlighted by Minnelli et al. [163] and low sustained release (within 12 h) reported by Lin et al. [107] could be solved by maintaining a controlled release of EGCG, whereby only sufficient ECGC is released over a period of time to enhance wound healing without toxicity effects.
Another in vivo study by Kim et al. presented the promising potential of EGCG and poly (lactic-co-glycolic acid) (PLGA) electrospun membranes in promoting wound healing during later stages by accelerating cell infiltration, re-epithelialization and angiogenesis at low concentration (1 wt% EGCG) [104]. The release profile of electrospun membranes showed sustained release for 12 days, which is a promising finding of the study, with potentially low cytotoxic effects, as is highlighted by Lin et al. and Minnelli et al. [107,163]. However, higher concentration of 5 wt% EGCG showed cytotoxicity effects on human dermal fibroblasts (HDFs), supporting that high % EGCG is cytotoxic.
Another in vivo study by Kim et al. incorporated different concentrations of EGCG (10, 100 and 1000 ppm) to a collagen sponge (CS) to examine its effect on diabetic mouse wounds [157]. After 14 days, the mice administered with 10 ppm EGCG-incorporated collagen sponge (E-CS) showed a considerably greater reduction in residual wound size as a result of increased angiogenesis, re-epithelialization, and promoting myofibroblast activity as compared to mice injected with CS alone or those with higher EGCG-CS concentrations of 100 and 1000 ppm. This showed similar effects to those mentioned by Kim et al., [104], Lin et al. [107] and Minnelli et al. [163], whereby higher concentrations of EGCG have toxicity effects and reduce wound healing while lower concentrations exhibit wound healing properties. As there is no release profile made by Kim et al. and the study did not demonstrate any anti-microbial effects of these concentrations, it is hard to conclude the full wound healing capability of this dressing [157].
In a separate study, acrylamide EGCG-complex-based polyacrylamide hydrogel (EACPA hydrogel) was found to have better wound closure in vivo when compared to commercially available Tegaderm™ dressing [159]. The EACPA hydrogel also showed good tissue adhesion, self-healing potential and mechanical features. This study also presented the promising anti-microbial potential of EACPA hydrogel against Escherichia coli and Staphylococcus aureus, which fills the gaps in Kim et al. [104] and Lin et al.’s [107] studies. The study by Zhao et al. also provided a detailed analysis of the mechanism of action of EACPA hydrogel [163]. However, further evaluation in terms of efficacy of wound dressings in infected wounds is required.
Another promising in vivo study by Jia et al. [161] presented some striking findings in terms of the release profile of EGCG as the concentration of ECGC has been a great concern due to their cytotoxicity as highlighted by Kim et al. [104], Lin et al. [107], Kim et al. [157] and Zhao et al. [159]. A multifunctional ROS-scavenging EGCG-loaded acrylamide, meth-acrylamide and borax (EGCG/PHEMAA/PAM) hydrogel exhibited excellent wound healing featuring hemostatic activity, self-healing and tissue adhesive ability. The hydrogel exhibited sustained release and was fully dissolved after four days, reducing the need for painful removal of the dressing, making it a promising solution. However, information on the anti-microbial potential, cytotoxicity and efficacy of dressing during different phases of wound healing are not presented.
Chen et al. developed green tea polyphenol nanospheres loaded into a polyvinyl alcohol (PVA)/alginate hydrogel (TPNH) for better stability and for convenient application [160]. It was found that TPN@H showed promising wound healing in vivo by modulating the PI3K/AKT signaling pathway. However, the preparation of tea polyphenols and their effectiveness as an anti-microbial agent have not been described comprehensively in terms of curbing their usage for diabetic wound applications as previously emphasized by Sun et al. [65], Kim et al. [104], Kim et al. [157] and Zhao et al. [159]. Moreover, the study focused specifically on the PI3K/AKT signaling pathway, which is crucial in the inflammation and proliferations phases of wound healing, but it did not elaborate their potential or lack of potential in other stages of wound healing, which few studies have demonstrated [107,157].
Another review by Xiong et al. [106] has also highlighted the immunomodulatory role of different hydrogel formulations that could be potentially used in the management of DFUs, similarly to this review, where EGCG-based hydrogel has been reported to promote wound healing in in vivo diabetic wounds. The multifunctional properties of EGCG including antioxidative and proangiogenic effects are responsible for the positive effects on various stages of wound healing, including that of diabetic wounds. The positive impacts of green tea, especially EGCG, may help to solve the underlying pathological problems of DFUs by facilitating cellular regeneration, reducing oxidative damage and improving vascularization, hence providing a promising intensive treatment for DFUs [106]. However, even though there have been numerous animal studies conducted to establish these positive effects, no clinical studies have been performed to confirm that these effects are equally effective in humans or in DFUs. This is the biggest gap in the research on commercializing a green tea wound dressing, especially for use in diabetic wounds or DFUs. Though many studies have demonstrated the positive impacts of EGCG in vitro and in vivo in diabetic wound healing, more validation of their release profile, cytotoxicity and anti-microbial properties and in-depth analysis of wound healing mechanisms need to be performed before clinical usage. This could be the reason why there are not many clinical studies conducted using green tea wound dressings. One study by Shahrahmani et al. was performed using green tea ointment with a Vaseline and Eucerin base, which was tested on 33 primiparous women [164]. It was found that green tea with Vaseline and Eucerin effectively reduced episiotomy pain and promoted wound healing compared to the placebo ointment and routine episiotomy care in primiparous women (n = 33 in each group). However, this is the only study using human trials and in a completely different wound, making it hard to draw conclusions on the effectiveness of green tea in diabetic wound healing for human use. More research with a sizable cohort in clinical trials and a comparable approach is required to confirm the effect of using green tea in human/clinical trials, in diabetic wounds and possibly DFUs. However, further investigations on toxicity are needed to determine the treatment’s possible effects as well as the safety and effectiveness of various dosages in order to make progress. Once the toxicity profile of green tea has been well established in animal models, human studies should be carried out due to biological differences, and there might be contrasting findings.
5. Current Challenges with the Commercialization of a Green Tea Wound Dressing
A thorough analysis of the above-mentioned studies has introduced new insights into the potential of using green tea polyphenols as a wound dressing and in diabetic wound healing. However, there are still no green tea wound dressings in the market or any clinical research on green tea wound dressings. There are many challenges in commercializing a green tea wound dressing, especially for diabetic wounds or DFUs.
The main challenge is regulatory approval. In new drug discovery, only a small portion of research goes into clinical trials, with fewer successfully passing the clinical trial stage due to strict regulatory control. In addition to that, extensive cost, planning and organization are involved in running a clinical trial. Even after clinical trials, regulatory bodies approve less than 10% of the medications that go through clinical trials [165]. This is attributed to inadequate drug-like qualities (10–15%), uncontrollable toxicity (30%), lack of clinical efficacy (40–50%), inadequate strategic planning and absence of commercial need (10%) [166,167], which further highlight the need to have in-depth complete in vivo studies ready before clinical trials.
Moreover, the application for regulatory approval is also a long, stressful process. The EU medical device regulation uses a risk-based system to classify medical devices (wound dressings), considering both the equipment’s possible dangers and the human body’s vulnerability. This method classifies devices using a series of criteria that can be integrated in many ways, such as the length of time the object was in contact with the body, the level of invasion in the body, the local versus systemic effect, the possible toxicity, the body part that is impacted by the device’s operation and whether it requires an energy source. The standards can then be used for a wide variety of medical technology and equipment and are outlined in Annex VIII of Regulation (EU) 2017/745 on medical devices (MDR), known as the “classification rules”. They mostly align with the classification guidelines set forth by the guidelines published by the International Medical Device Regulators Forum (IMDRF) in GHTF/SG1/N77:2012 [168]. As dressings used for DFUs are designed to be used in chronic wound management, which entails hazards and necessitates certain functionality, they are usually categorized as Class III devices, which would require the strictest specifications/standards [168]. According to the European Union’s (EU) safety, health and environmental protection standards, products would need to bear the CE mark, which is a regulatory accreditation. Getting the CE certification is essential for medical-grade equipment and anti-microbial wound dressings for DFUs to enter the European Economic Area (EEA) market, which is another limiting factor when seeking to commercialize an anti-microbial wound dressing. In addition to this, even though there are promising in vivo studies on diabetic wounds at different stages of wound healing, it is hard to establish if these dressings would be equally effective in humans, let alone against DFUs, especially as it is hard to mimic DFUs in animal models. This is making it harder to develop a dressing for DFU use unless human clinical trials can be performed using DFU patients, which are currently lacking in research. However, due to the high incidence and cost associated with DFU treatment, there is a need to develop a treatment that could help with wound healings in DFUs along with the prevention of recurrence of DFUs in diabetic patients.
As mentioned before, many in vitro and in vivo studies have shown that green tea dressings are effective in wound healing and in some cases in diabetic wounds. However, it is crucial to understand the complex mechanism and mode of action of green tea in wound healing in order to establish its efficacy when used in humans. Most studies lack in-depth analysis of all parameters that are needed before clinical trials, such as anti-microbial activity, efficacy, release profile, cytotoxicity, mechanism of actions on various wound healing stages, stability and cost analysis. Though the clinical trial by Shahrahmani et al. [164] successfully reported the potential of green tea ointment in wound healing, it was only one study, was conducted on a small sample size and is not applicable on other studies that use different polymer/dressing materials. The types of wounds studied are also different from those in other studies. There are still many questions that need to be answered and researched on before commercializing this product in the market.
Other than that, green tea polyphenols have low bioavailability and stability [154,155,156]; therefore, direct application of the product is restricted. There is the need for a carrier such as one in the form of nanosystems that could protect them from the damage caused by heat, light or pH. However, the biocompatibility and safety of the materials used for preparing the nanosystems should also be taken into consideration to ensure they do not provoke any adverse reaction within the body, but would be beneficial if they also improve wound healing. The addition of polyphenolic chemicals to the phytosomal delivery system has been shown to significantly improve the polyphenol’s properties, resulting in improved bioavailability, absorption and penetration through the biological membranes [169,170]. Therefore, phytosomes could provide a promising platform for introducing green tea polyphenols to wound sites. Additionally, phytosomes could be coupled with another polymer to improve their stability and bioavailability. The choice of polymer with potential wound healing properties would be an added benefit as it will also ultimately contribute to wound healing. In addition, the selection of biocompatible, natural and cost-effective polymers like cellulose, chitosan, collagen and PVA as employed in Kim et al. [157], Sun et al. [65], Kim et al. [158] and Chen et al. [160] is recommended instead of using polymers that are synthetic as these are more likely to cause adverse reactions and are costly.
Furthermore, understanding the cytotoxicity of tea polyphenols is equally important to minimize the risk of further tissue damage. In addition to that, instead of focusing on one key polyphenol (EGCG) as is the case in many studies [65,74,104,107,158,159,161,163], there is need to investigate all green tea polyphenols or green tea extract as a whole [160,162,171,172] since the other bioactive compounds in green tea may also promote wound healing or show synergistic effects that would be beneficial for wound healing. Even though pure EGCG has shown promising wound healing properties, there are studies that have shown its cytotoxicity at high concentrations. Therefore, it would be crucial to understand the complex interactions between different tea polyphenols and their potential cytotoxicity in wound healing. A detailed release profile of the dressing alongside its toxicity is important for establishing this. Understanding the detailed mechanism of action of the major polyphenols within green tea extract is essential because the synergistic effect of all compounds can result in better (or worse) anti-microbial and wound healing potential, and thus the major polyphenols can be a viable and cost-effective natural alternative to the costly EGCG or the high cost of purifying EGCG from crude green tea. However, using crude green tea extract has disadvantages as well. Crude green tea could have inconsistent biological activity due to different concentrations of the active ingredients present in different types of green tea samples, seasonal effects and the use of different extraction techniques. Therefore, a standardized method needs to be developed to ensure that the bioactives extracted from green tea for medical use have a consistent range of activity, such as those used by honey-based or other natural products.
With all the potential hurdles in the way of commercializing green tea-based wound dressings, it is important to understand the significance they hold. The widespread use of antibiotics, which is largely the reason for the emergence of anti-microbial resistance (AMR), is making it harder to treat infections, particularly diabetic foot infections [173]. The fulfilment of the Sustainable Development Goals (SDGs) is threatened by AMR, which is frequently referred to as a silent pandemic due to its substantial effects on the economy and public health worldwide [174]. Green tea-based wound dressings could effectively control AMR, including in wounds and in DFUs, which could lessen the dependency on antibiotics in treating wound infections. This would be in line with SDG 3 (good health and well-being), specifically 3.3, to combat AMR. In addition to that, the development of wound dressings could also address SGD 3.4: non-communicable diseases, of which diabetes is one of them. Poorly managed DFUs can result in serious complications, such as sepsis and amputations, but studies have shown that green tea-based wound dressings promote healing and stop infections, but only in vitro and in vivo. Furthermore, in relation to SDG 9, specifically 9.5, the production of biomaterials or green tea anti-microbial-based biomaterials will encourage sustainability and innovation in medical devices. Moreover, it will promote partnerships for sustainable innovation (SDG 17, target 17.6) through cooperation between industry, academics, governments and healthcare providers. In a nutshell, the incorporation of green tea extract/polyphenols can bring numerous advantages addressing the UN Sustainable Development Goals in addition to establishing an advanced treatment regimen for wound healing, especially in diabetes.
6. Conclusions
Green tea is widely recognized for its health benefits, including its antioxidant, anti-inflammatory and anti-microbial properties, primarily attributed to its high catechin content, particularly EGCG. This review highlighted the positive effects of green tea polyphenols at various stages of skin wound healing, including their potential in diabetic wounds, along with their anti-bacterial properties both in vitro and in vivo. This review also discussed the incorporation of green tea/EGCG into wound dressings for wound treatment where numerous in vivo and in vitro studies demonstrated that GT-/EGCG-based dressings support wound healing through their antioxidant, anti-inflammatory, anti-microbial, angiogenic and anti-fibrotic properties, as well as their modulation of inflammation-related signaling pathways, both in normal and diabetic wounds.
Despite their effectiveness both in vitro and in vivo, there are no GT/EGCG wound dressings in the market currently. This is possibly due to their poor bioavailability, stability, high associated cost and their unknown cytotoxicity, along with the lack of in-depth in vivo studies and lack of DFU animal models that could be translatable to human clinical trials. Therefore, more in-depth studies are needed to check these properties when a green tea wound dressing is developed. In addition to this, there are other challenges, such as the high cost of clinical trials and approval by regulatory bodies, that deter companies from going down this route. Therefore, researchers, medical device companies and governments should work together to conduct extensive research that could lead to clinical trials to prove the efficacy of green tea wound dressings for human use and bring these onto the market.
Funding
This research was funded by the WIT Presidents PhD Scholarship Programme 2022 Department of Science, South East Technological University (SETU) grant (WD_2022_23_WSCH).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Marino, M.; Del Bo’, C.; Martini, D.; Porrini, M.; Riso, P. A review of registered clinical trials on dietary (poly) phenols: Past efforts and possible future directions. Foods 2020, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Sinija, V.R.; Mishra, H.N. Green tea: Health benefits. J. Nutr. Environ. Med. 2008, 17, 232–242. [Google Scholar] [CrossRef]
- Alsabagh, A.M.; Migahed, M.A.; Abdelraouf, M.; Khamis, E.A. Utilization of green tea as environmentally friendly corrosion inhibitor for carbon steel in acidic media. Int. J. Electrochem. Sci. 2015, 10, 1855–1872. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Bhagwat, S.; Haytowitz, D.; Holden, J.; Eldridge, A.L.; Beecher, G.; Aladesanmi, J. Major flavonoids in dry tea. J. Food Compos. Anal. 2005, 18, 487–501. [Google Scholar] [CrossRef]
- Liang, Y.R.; Liu, Z.S.; Xu, Y.R.; Hu, Y.L. A study on chemical composition of two special green teas (Camellia sinensis). J. Sci. Food Agric. 1990, 53, 541–548. [Google Scholar] [CrossRef]
- Lee, L.S.; Kim, S.H.; Kim, Y.B.; Kim, Y.C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Nie, Y.; Gao, Y.; Huang, B.; Ye, J.H.; Lu, J.L.; Liang, Y.R. Screening the cultivar and processing factors based on the flavonoid profiles of dry teas using principal component analysis. J. Food Compos. Anal. 2018, 67, 29–37. [Google Scholar] [CrossRef]
- Amrouche, Z.; Blecker, C.; Laribi-Habchi, H.; Fauconnier, M.L.; El-Hadi, D. Antioxydant activity, oxidative stability properties of Colza oil, comparison of mechanical agitated and ultrasonic extraction on green tea catechins of Camellia sinensis. Alger. J. Env. Sci. Technol. 2021, 7, 2167–2176. [Google Scholar]
- Wang, H.; Provan, G.J.; Helliwell, K. Tea flavonoids: Their functions, utilisation and analysis. Trends Food Sci. 2000, 11, 152–160. [Google Scholar] [CrossRef]
- Nain, C.W.; Mignolet, E.; Herent, M.F.; Quetin-Leclercq, J.; Debier, C.; Page, M.M.; Larondelle, Y. The catechins profile of green tea extracts affects the antioxidant activity and degradation of catechins in DHA-rich oil. Antioxidants 2022, 11, 1844. [Google Scholar] [CrossRef]
- Feng, L.C.; Jen, K.L. HPLC Analysis of Naturally Occurring MethylatedCatechins, 3”-and 4”-Methyl-epigallocatechin Gallate. Various Fresh Tea Leaves and Commercial Teas and Their Potent Inhibitory Effects on Inducible Nitric Oxide Synthase in Macrophages. J. Agric. Food Chem. 2005, 53, 7035–7042. [Google Scholar]
- Sano, M.; Suzuki, M.; Miyase, T.; Yoshino, K.; Maeda-Yamamoto, M. Novel antiallergic catechin derivatives isolated from oolong tea. J. Agric. Food Chem. 1999, 47, 1906–1910. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yoshino, K.; Maeda-Yamamoto, M.; Miyase, T.; Sano, M. Inhibitory effects of tea catechins and O-methylated derivatives of (−)-epigallocatechin-3-O-gallate on mouse type IV allergy. J. Agric. Food Chem. 2000, 48, 5649–5653. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, J. The effects of green tea on obesity and type 2 diabetes. Diabetes Metab. J. 2013, 37, 173–175. [Google Scholar] [CrossRef]
- Baranwal, A.; Aggarwal, P.; Rai, A.; Kumar, N. Pharmacological actions and underlying mechanisms of catechin: A review. Mini-Rev. Med. Chem. 2022, 22, 821–833. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; Arif, M.; Kakar, M.U.; Manzoor, R.; Abd El-Hack, M.E.; Alagawany, M.; Tiwari, R.; Khandia, R.; Munjal, A. Green tea (Camellia sinensis) and l-theanine: Medicinal values and beneficial applications in humans—A comprehensive review. Biomed. Pharmacother. 2017, 95, 1260–1275. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, R.; Wang, B.K.; Shi, L.Y.; Zhu, J.D.; Mi, M.T. Effect of green tea on glucose control and insulin sensitivity: A meta-analysis of 17 randomized controlled trials. Am. J. Clin. Nutr. 2013, 98, 340–348. [Google Scholar] [CrossRef]
- Babu, P.V.A.; Sabitha, K.E.; Shyamaladevi, C.S. Effect of green tea extract on advanced glycation and cross-linking of tail tendon collagen in streptozotocin induced diabetic rats. Food Chem. Toxicol. 2008, 46, 280–285. [Google Scholar] [CrossRef]
- Quine, S.D.; Raghu, P.S. Effects of (-)-epicatechin, a flavonoid on lipid peroxidation and antioxidants in streptozotocin-induced diabetic liver, kidney and heart. Pharmacol. Rep. 2005, 57, 610–615. [Google Scholar]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Protective effect of epigallocatechin gallate on endothelial disorders in atherosclerosis. J. Cardiovasc. Pharmacol. 2020, 75, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic effects of green tea polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) in relation to molecular pathways controlling inflammation, oxidative stress, and apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef]
- Tsai, C.F.; Hsu, Y.W.; Ting, H.C.; Huang, C.F.; Yen, C.C. The in vivo antioxidant and antifibrotic properties of green tea (Camellia sinensis, Theaceae). Food Chem. 2013, 136, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Tanwar, J.; Hameed, S.; Fatima, Z.; Manesar, G. Antimicrobial potential of epigallocatechin-3-gallate (EGCG): A green tea polyphenol. J. Biochem. Pharmacol. Res. 2014, 2, 167–174. [Google Scholar]
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 2 October 2024).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Bus, S.A.; Lavery, L.A.; Monteiro-Soares, M.; Rasmussen, A.; Raspovic, A.; Sacco, I.C.; van Netten, J.J. International Working Group on the Diabetic Foot, Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36, e3269. [Google Scholar] [CrossRef]
- Van Netten, J.J.; Bus, S.A.; Apelqvist, J.; Lipsky, B.A.; Hinchliffe, R.J.; Game, F.; Rayman, G.; Lazzarini, P.A.; Forsythe, R.O.; Peters, E.J.; et al. Definitions and criteria for diabetic foot disease. Diabetes Metab. Res. Rev. 2020, 36, e3268. [Google Scholar] [CrossRef] [PubMed]
- Subrata, S.A.; Phuphaibul, R. Diabetic foot ulcer care: A concept analysis of the term integrated into nursing practice. Scand. J. Caring Sci. 2019, 33, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M.; Manu, C.; Vas, P. The current burden of diabetic foot disease. J. Clin. Orthos. Trauma 2021, 17, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, E.; Adeleye, O.; Gezawa, I.; Okpe, I.; Enamino, M.; Ezeani, I. Predictors of lower extremity amputation in patients with diabetic foot ulcer: Findings from MEDFUN, a multi-center observational study. J. Foot. Ankle. Res. 2019, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hurlow, J.J.; Humphreys, G.J.; Bowling, F.L.; McBain, A.J. Diabetic foot infection: A critical complication’. Int. Wound J. 2018, 15, 814–882. [Google Scholar] [CrossRef]
- Alexiadou, K.; Doupis, J. Management of diabetic foot ulcers. Diabetes Ther. 2012, 3, 1–15. [Google Scholar] [CrossRef]
- Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef]
- Wu, S.C.; Jensen, J.L.; Weber, A.K.; Robinson, D.E.; Armstrong, D.G. Use of pressure offloading devices in diabetic foot ulcers: Do we practice what we preach? Diabetes Care 2008, 31, 2118–2119. [Google Scholar] [CrossRef]
- Kr, A.S. Negative Pressure Wound Therapy Versus Conventional Dressing in the Management of Diabetic Foot Ulcers–A Comparative Study. Master’s Thesis, Rajiv Gandhi University of Health Sciences (India), Bengaluru, India, 2018. [Google Scholar]
- Wang, X.; Yuan, C.X.; Xu, B.; Yu, Z. Diabetic foot ulcers: Classification, risk factors and management. World. J. Diabetes 2022, 13, 1049. [Google Scholar] [CrossRef]
- Alavi, A.; Sibbald, R.G.; Mayer, D.; Goodman, L.; Botros, M.; Armstrong, D.G.; Woo, K.; Boeni, T.; Ayello, E.A.; Kirsner, R.S. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J. Am. Acad. Dermatol. 2014, 70, 1-e1. [Google Scholar]
- Doğruel, H.; Aydemir, M.; Balci, M.K. Management of diabetic foot ulcers and the challenging points: An endocrine view. World. J. Diabetes 2022, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, E.; Tomic-Canic, M.; Kirsner, R.S. The role of surgical debridement in healing of diabetic foot ulcers. Wound Repair Regen. 2010, 18, 433–438. [Google Scholar] [CrossRef] [PubMed]
- El Hage, R.; Knippschild, U.; Arnold, T.; Hinterseher, I. Stem cell-based therapy: A promising treatment for diabetic foot ulcer. Biomedicines 2022, 10, 1507. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.I.; Dias, A.M.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of appropriate wound dressing for various wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef]
- Weller, C.; Team, V. Interactive dressings and their role in moist wound management. In Advanced Textiles for Wound Care; Woodhead Publishing: Cambridge, UK, 2019; pp. 105–134. [Google Scholar]
- Britto, E.J.; Nezwek, T.A.; Popowicz, P.; Robins, M. Wound Dressings; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound dressings: A comprehensive review. Curr. Dermatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of wound healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Fawzy, H.M.; El Shawarby, A.; Kalleny, N.K.; Shaker, S.M. Comparative Study on the Effect of Silver Nanoparticles versus Silver Sulfadiazine in Diabetic Wound Healing in Albino Rat: A Histological Study. Egypt. J. Hosp. Med. 2018, 73, 6042–6051. [Google Scholar] [CrossRef]
- Guo, S.A.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Xu, F.W.; Lv, Y.L.; Zhong, Y.F.; Xue, Y.N.; Wang, Y.; Zhang, L.Y.; Hu, X.; Tan, W.Q. Beneficial Effects of Green Tea EGCG on Skin Wound Healing: A Comprehensive Review. Molecules 2021, 26, 6123. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Mantle, D.; Gok, M.A.; Lennard, T.W. Adverse and beneficial effects of plant extracts on skin and skin disorders. Advers. Drug React. Toxicol. Rev. 2001, 20, 89–103. [Google Scholar]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic wound-healing science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Sorg, C.G. Skin wound healing: Of players, patterns, and processes. Eur. Surg. Res. 2023, 64, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Bardill, J.R.; Laughter, M.R.; Stager, M.; Liechty, K.W.; Krebs, M.D.; Zgheib, C. Topical gel-based biomaterials for the treatment of diabetic foot ulcers. Acta Biomater. 2022, 138, 73–91. [Google Scholar] [CrossRef]
- Li, X.; Weber, N.C.; Cohn, D.M.; Hollmann, M.W.; DeVries, J.H.; Hermanides, J.; Preckel, B. Effects of hyperglycemia and diabetes mellitus on coagulation and hemostasis. J. Clin. Med. 2021, 10, 2419. [Google Scholar] [CrossRef]
- Cheng-lie, Y. Effects of Dan Wei Powder tea bag on aggregation of blood platelet in vivo and in vitro. J. Chin. Med. 2011, 2011, 78–79. [Google Scholar]
- Monaghan, M.G.; Borah, R.; Thomsen, C.; Browne, S. Thou shall not heal: Overcoming the non-healing behaviour of diabetic foot ulcers by engineering the inflammatory microenvironment. Adv. Drug Deliv. Rev. 2023, 203, 115120. [Google Scholar] [CrossRef]
- Al-Safaar, M.A.; Al-Din, A.A.M.; Alwan, Z.H. The Effect of Green Tea (Camilla sinensis L.) on Blood Clotting after Tooth Extractions. Int. J. Sci. Technol. 2013, 143, 1–12. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, X.; Ma, X.; Cui, X.; Yi, Z.; Li, X. Cellulose/keratin–catechin nanocomposite hydrogel for wound hemostasis. J. Mater. Chem. B 2018, 6, 6133–6141. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.T.; Han, Y.P.; Yan, C.; Shaw, M.C.; Garner, W.L. TNF-α suppresses α-smooth muscle actin expression in human dermal fibroblasts: An implication for abnormal wound healing. J. Investig. Dermatol. 2007, 127, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Portou, M.J.; Baker, D.; Abraham, D.; Tsui, J. The innate immune system, toll-like receptors and dermal wound healing: A review. Vasc. Pharmacol. 2015, 71, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef]
- Dasari, N.; Jiang, A.; Skochdopole, A.; Chung, J.; Reece, E.M.; Vorstenbosch, J.; Winocour, S. Updates in diabetic wound healing, inflammation, and scarring. Semin. Plast. Surg. 2021, 35, 153–158. [Google Scholar] [CrossRef]
- Melgarejo, E.; Medina, M.Á.; Sánchez-Jiménez, F.; Urdiales, J.L. Epigallocatechin gallate reduces human monocyte mobility and adhesion in vitro. Br. J. Pharmacol. 2009, 158, 1705–1712. [Google Scholar] [CrossRef]
- Hagiu, A.; Attin, T.; Schmidlin, P.R.; Ramenzoni, L.L. Dose-dependent green tea effect on decrease of inflammation in human oral gingival epithelial keratinocytes: In vitro study. Clin. Oral. Investig. 2020, 24, 2375–2383. [Google Scholar] [CrossRef]
- Fujimura, Y.; Sumida, M.; Sugihara, K.; Tsukamoto, S.; Yamada, K.; Tachibana, H. Green tea polyphenol EGCG sensing motif on the 67-kDa laminin receptor. PLoS ONE 2012, 7, e37942. [Google Scholar] [CrossRef]
- Lee, G.; Ko, Y.G.; Bae, K.H.; Kurisawa, M.; Kwon, O.K.; Kwon, O.H. Green tea catechin-grafted silk fibroin hydrogels with reactive oxygen species scavenging activity for wound healing applications. Biomater. Res. 2022, 26, 1–16. [Google Scholar] [CrossRef]
- Klass, B.R.; Branford, O.A.; Grobbelaar, A.O.; Rolfe, K.J. The effect of epigallocatechin-3-gallate, a constituent of green tea, on transforming growth factor-β1–stimulated wound contraction. Wound Repair Regen. 2010, 18, 80–88. [Google Scholar] [CrossRef]
- Huang, Y.W.; Zhu, Q.Q.; Yang, X.Y.; Xu, H.H.; Sun, B.; Wang, X.J.; Sheng, J. Wound healing can be improved by (-)-epigallocatechin gallate through targeting Notch in streptozotocin-induced diabetic mice. FASEB J. 2019, 33, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, L.; Wang, X.; Liu, J.; Wang, Y.; Zhou, Y.; Ho, W. (-)-Epigallocatechin gallate inhibits endotoxin-induced expression of inflammatory cytokines in human cerebral microvascular endothelial cells. J. Neuroinflamm. 2012, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dong, M.; Bo, L.; Li, C.; Liu, Q.; Li, Y.; Ma, L.; Xie, Y.; Fu, E.; Mu, D.; et al. Epigallocatechin-3-gallate ameliorates seawater aspiration-induced acute lung injury via regulating inflammatory cytokines and inhibiting JAK/STAT1 pathway in rats. Mediators Inflamm. 2014, 2014, 612593. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Balaji, S.; Le, L.D.; Crombleholme, T.M.; Keswani, S.G. Regenerative wound healing: The role of interleukin-10. Adv. Wound Care 2014, 3, 315–323. [Google Scholar] [CrossRef]
- Gordon, A.; Kozin, E.D.; Keswani, S.G.; Vaikunth, S.S.; Katz, A.B.; Zoltick, P.W.; Favata, M.; Radu, A.P.; Soslowsky, L.J.; Herlyn, M.; et al. Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen. 2008, 16, 70–79. [Google Scholar] [CrossRef]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1β-induced expression of matrix metalloproteinase-1 and-13 in human chondrocytes. J. Pharmacol. Exp. Ther. 2004, 308, 767–773. [Google Scholar] [CrossRef]
- Chen, C.; Yu, R.; Owuor, E.D.; Tony Kong, A.N. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch. Pharmacal Res. 2000, 23, 605–612. [Google Scholar] [CrossRef]
- Viaña-Mendieta, P.; Sánchez, M.L.; Benavides, J. Rational selection of bioactive principles for wound healing applications: Growth factors and antioxidants. Int. Wound J. 2022, 19, 100–113. [Google Scholar] [CrossRef]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural antioxidants: Multiple mechanisms to protect skin from solar radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Durham, J.T.; Herman, I.M. Wound healing angiogenesis: Innovations and challenges in acute and chronic wound healing. Adv. Wound Care 2012, 1, 17–22. [Google Scholar] [CrossRef]
- Fernández-Guarino, M.; Hernández-Bule, M.L.; Bacci, S. Cellular and molecular processes in wound healing. Biomedicines 2023, 11, 2526. [Google Scholar] [CrossRef] [PubMed]
- Kiritsi, D.; Nyström, A. The role of TGFβ in wound healing pathologies. Mech. Ageing Dev. 2018, 172, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Dodsworth, J.; Boxtel, E.V.; Tarnuzzer, R.W.; Horan, M.A.; Schultz, G.S.; Ferguson, M.W. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-β1 levels. Nat. Med. 1997, 3, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghaalipour, F.; Kanthimathi, M.S.; Abdulla, M.A.; Sanusi, J. The effect of Camellia sinensis on wound healing potential in an animal model. Evid.-Based Complement. Altern. Med. 2013, 2013, 386734. [Google Scholar] [CrossRef]
- Shin, Y.C.; Shin, D.M.; Lee, E.J.; Lee, J.H.; Kim, J.E.; Song, S.H.; Hwang, D.Y.; Lee, J.J.; Kim, B.; Lim, D.; et al. Hyaluronic acid/PLGA core/shell fiber matrices loaded with EGCG beneficial to diabetic wound healing. Adv. Healthc. Mater. 2016, 5, 3035–3045. [Google Scholar] [CrossRef]
- Nie, S.P.; Xie, M.Y. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocoll. 2011, 25, 144–149. [Google Scholar] [CrossRef]
- Du, L.L.; Fu, Q.Y.; Xiang, L.P.; Zheng, X.Q.; Lu, J.L.; Ye, J.H.; Li, Q.S.; Polito, C.A.; Liang, Y.R. Tea polysaccharides and their bioactivities. Molecules 2016, 21, 1449. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Q.; Du, X. Effects of molecular characteristics of tea polysaccharide in green tea on glass transitions of potato amylose, amylopectin and their mixtures. Food Hydrocoll. 2011, 25, 486–494. [Google Scholar] [CrossRef]
- Wang, X.; Tian, W. Green tea epigallocatechin gallate: A natural inhibitor of fatty-acid synthase. Biochem. Biophys. Res. Commun. 2001, 288, 1200–1206. [Google Scholar] [CrossRef]
- Yang, G.; Liang, X.; Hu, J.; Li, C.; Hu, W.; Li, K.; Chang, X.; Zhang, Y.; Zhang, X.; Shen, Y.; et al. Feeding tea polysaccharides affects lipid metabolism, antioxidant capacity and immunity of common carp (Cyprinus carpio L.). Front. Immunol. 2022, 13, 1074198. [Google Scholar] [CrossRef]
- Singh, M.R.; Saraf, S.; Vyas, A.; Jain, V.; Singh, D. Innovative approaches in wound healing: Trajectory and advances. Artif. Cells Nanomed. Biotechnol. 2013, 41, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Gooyit, M.; Peng, Z.; Wolter, W.R.; Pi, H.; Ding, D.; Hesek, D.; Lee, M.; Boggess, B.; Champion, M.M.; Suckow, M.A.; et al. A chemical biological strategy to facilitate diabetic wound healing. ACS Chem. Biol. 2014, 9, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: New insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef] [PubMed]
- Edoamodu, C.E.; Nwodo, U.U. Recent advances and future prospects in topical creams from medicinal plants to expedite wound healing: A review. Biotechnol. Biotechnol. Equip. 2022, 36, 798–801. [Google Scholar]
- Zhao, H.; Lou, Z.; Chen, Y.; Cheng, J.; Wu, Y.; Li, B.; He, P.; Tu, Y.; Liu, J. Tea polyphenols (TPP) as a promising wound healing agent: TPP exerts multiple and distinct mechanisms at different phases of wound healing in a mouse model. Biomed. Pharmacother. 2023, 166, 115437. [Google Scholar] [CrossRef]
- Dona, M.; Dell’Aica, I.; Calabrese, F.; Benelli, R.; Morini, M.; Albini, A.; Garbisa, S. Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J. Immun. 2003, 170, 4335–4341. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, H.W.; Thirupathi, K.; Wook, K. Chitosan green tea polyphenol complex as a released control compound for wound healing. Chin. J. Traumatol. 2010, 13, 91–95. [Google Scholar]
- Qin, Y.; Guo, X.W.; Li, L.; Wang, H.W.; Kim, W. The antioxidant property of chitosan green tea polyphenols complex induces transglutaminase activation in wound healing. J. Med. Food. 2013, 16, 487–498. [Google Scholar] [CrossRef]
- Kim, H.L.; Lee, J.H.; Kwon, B.J.; Lee, M.H.; Han, D.W.; Hyon, S.H.; Park, J.C. Promotion of Full-Thickness Wound Healing Using Epigallocatechin-3-O-Gallate/Poly (Lactic-C o-G lycolic Acid) Membrane as Temporary Wound Dressing. Artif. Organs 2014, 38, 411–417. [Google Scholar] [CrossRef]
- Syed, F.; Bagabir, R.A.; Paus, R.; Bayat, A. Ex vivo evaluation of antifibrotic compounds in skin scarring: EGCG and silencing of PAI-1 independently inhibit growth and induce keloid shrinkage. Lab. Invest. 2013, 93, 946–960. [Google Scholar] [CrossRef]
- Xiong, Y.; Feng, Q.; Lu, L.; Zha, K.; Yu, T.; Lin, Z.; Hu, Y.; Panayi, A.C.; Nosrati-Ziahmagi, V.; Chu, X.; et al. Immunomodulatory hydrogels: Advanced regenerative tools for diabetic foot ulcer. Adv. Funct. Mater. 2023, 33, 2213066. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, J.H.; Li, T.S.; Wang, S.H.; Yao, C.H.; Chung, W.Y.; Ko, T.H. Dressing with epigallocatechin gallate nanoparticles for wound regeneration. Wound Repair Regen. 2016, 24, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.A.K.; Saha, K.; Rahman, J.; Munmun, R.A.; Rahman, M.A.; Dey, S.K.; Rahman, M.S.; Islam, S.; Shariare, M.H. Antibacterial activities of green tea crude extracts and synergistic effects of epigallocatechingallate (EGCG) with gentamicin against MDR pathogens. Heliyon 2019, 5, e02126. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.W.; Hamilton-Miller, J.M.T.; Stapleton, P.D. Antimicrobial properties of green tea catechins. Food. Sci. Technol. Bull. Funct. Foods 2005, 2, 71–81. [Google Scholar] [CrossRef]
- Tian, W.X. Inhibition of fatty acid synthase by polyphenols. Curr. Med. Chem. 2006, 13, 967–977. [Google Scholar] [CrossRef]
- Ikeda, I.; Hamamoto, R.; Uzu, K.; Imaizumi, K.; Nagao, K.; Yanagita, T.; Suzuki, Y.; Kobayashi, M.; Kakuda, T. Dietary gallate esters of tea catechins reduce deposition of visceral fat, hepatic triacylglycerol, and activities of hepatic enzymes related to fatty acid synthesis in rats. Biosci. Biotechnol. Biochem. 2005, 69, 1049–1053. [Google Scholar] [CrossRef]
- Wang, X.; Song, K.S.; Guo, Q.X.; Tian, W.X. The galloyl moiety of green tea catechins is the critical structural feature to inhibit fatty-acid synthase. Biochem. Pharmacol. 2003, 66, 2039–2047. [Google Scholar] [CrossRef]
- SudanoRoccaro, A.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob. Agents. Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J.H.; Lee, C.K.; Oh, C.H.; Song, H.J. The antimicrobial activity of (-)-epigallocatehin-3-gallate and green tea extracts against Pseudomonas aeruginosa and Escherichia coli isolated from skin wounds. Ann. Dermatol. 2014, 26, 564–569. [Google Scholar] [CrossRef]
- Ananthi, J.; Sagaya Giri, R. Antibacteria potential of green tea (Camellia sinensis) against urinary tract infections. World J. Pharm. Med. Res. 2018, 4, 181–185. [Google Scholar]
- Osterburg, A.; Gardner, J.; Hyon, S.H.; Neely, A.; Babcock, G. Highly antibiotic-resistant Acinetobacter baumannii clinical isolates are killed by the green tea polyphenol (–)-epigallocatechin-3-gallate (EGCG). Clin. Microbiol. Infect. 2009, 15, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Ignasimuthu, K.; Prakash, R.; Murthy, P.S.; Subban, N. Enhanced bioaccessibility of green tea polyphenols and lipophilic activity of EGCG octaacetate on gram-negative bacteria. LWT 2019, 105, 103–109. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punicagranatum) flour obtained from co-product of juice extraction. Food Control 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, S.; Sarethy, I.P.; Dang, S.; Gabrani, R. Green tea extract: Possible mechanism and antibacterial activity on skin pathogens. Food Chem. 2012, 135, 672–675. [Google Scholar] [CrossRef]
- Blanco, A.R.; Sudano-Roccaro, A.; Spoto, G.C.; Nostro, A.; Rusciano, D. Epigallocatechin gallate inhibits biofilm formation by ocular staphylococcal isolates. Antimicrob. Agents Chemother. 2005, 49, 4339–4343. [Google Scholar] [CrossRef]
- Hara-Kudo, Y.; Yamasaki, A.; Sasaki, M.; Okubo, T.; Minai, Y.; Haga, M.; Kondo, K.; Sugita-Konishi, Y. Antibacterial action on pathogenic bacterial spore by green tea catechins. J. Sci. Food Agric. 2005, 85, 2354–2361. [Google Scholar] [CrossRef]
- Cho, Y.S.; Schiller, N.L.; Kahng, H.Y.; Oh, K.H. Cellular responses and proteomic analysis of Escherichia coli exposed to green tea polyphenols. Curr. Microbiol. 2007, 55, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Yoda, Y.; Hu, Z.Q.; Shimamura, T.; Zhao, W.H. Different susceptibilities of Staphylococcus and Gram-negative rods to epigallocatechin gallate. J. Infect. Chemother. 2004, 10, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Stapleton, P.D.; Taylor, P.W. The polyphenol (−)-epicatechin gallate disrupts the secretion of virulence-related proteins by Staphylococcus aureus. Lett. Appl. Microbiol. 2008, 46, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Giordano, N.P.; Cian, M.B.; Dalebroux, Z.D. Outer membrane lipid secretion and the innate immune response to gram-negative bacteria. Infect. Immun. 2020, 88, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S. Recent advances in inhibitors of bacterial fatty acid synthesis type II (FASII) system enzymes as potential antibacterial agents. ChemMedChem 2013, 8, 1589–1608. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Rock, C.O. Evaluation of epigallocatechin gallate and related plant polyphenols as inhibitors of the FabG and FabI reductases of bacterial type II fatty-acid synthase. J. Biol. Chem. 2004, 279, 30994–31001. [Google Scholar] [CrossRef]
- Heath, R.J.; Yu, Y.T.; Shapiro, M.A.; Olson, E.; Rock, C.O. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J. Biol. Chem. 1998, 273, 30316–30320. [Google Scholar] [CrossRef]
- Li, B.H.; Tian, W.X. Inhibitory effects of flavonoids on animal fatty acid synthase. J. Biochem. 2004, 135, 85–91. [Google Scholar] [CrossRef]
- Tian, W.X.; Li, L.C.; Wu, X.D.; Chen, C.C. Weight reduction by Chinese medicinal herbs may be related to inhibition of fatty acid synthase. Life Sci. 2004, 74, 2389–2399. [Google Scholar] [CrossRef]
- Sakanaka, S.; Okada, Y. Inhibitory effects of green tea polyphenols on the production of a virulence factor of the periodontal-disease-causing anaerobic bacterium Porphyromonasgingivalis. J. Agric. Food Chem. 2004, 52, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Menegatti, A.C.O. Targeting protein tyrosine phosphatases for the development of antivirulence agents: Yersinia spp. and Mycobacterium tuberculosis as prototypes. Biochim. Biophys. Acta Proteins Proteom. 2022, 1870, 140782. [Google Scholar] [CrossRef] [PubMed]
- Standish, A.J.; Morona, R. The role of bacterial protein tyrosine phosphatases in the regulation of the biosynthesis of secreted polysaccharides. Antioxid. Redox. Signal. 2014, 20, 2274–2289. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Matthew, M.A.; Yao, C. Roles of cysteine proteases in biology and pathogenesis of parasites. Microorganisms 2023, 11, 1397. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Dixit, R.; Pandey, K.C. Cysteine proteases: Modes of activation and future prospects as pharmacological targets. Front. Pharmacol. 2016, 7, 107. [Google Scholar] [CrossRef]
- Gradišar, H.; Pristovšek, P.; Plaper, A.; Jerala, R. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J. Med. Chem. 2007, 50, 264–271. [Google Scholar] [CrossRef]
- Chinnam, N.; Dadi, P.K.; Sabri, S.A.; Ahmad, M.; Kabir, M.A.; Ahmad, Z. Dietary bioflavonoids inhibit Escherichia coli ATP synthase in a differential manner. Int. J. Biol. Macromol. 2010, 46, 478–486. [Google Scholar] [CrossRef]
- Spina, M.; Cuccioloni, M.; Mozzicafreddo, M.; Montecchia, F.; Pucciarelli, S.; Eleuteri, A.M.; Fioretti, E.; Angeletti, M. Mechanism of inhibition of wt-dihydrofolate reductase from E. coli by tea epigallocatechin-gallate. Proteins Struct., Funct., Bioinf. 2008, 72, 240–251. [Google Scholar] [CrossRef]
- Webber, M.A.; Piddock, L.J. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 2003, 51, 9–11. [Google Scholar] [CrossRef]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Al Razqan, G.S.; Kwon, D.H. Antibacterial activity of epigallocatechin-3-gallate (EGCG) and its synergism with β-lactam antibiotics sensitizing carbapenem-associated multidrug resistant clinical isolates of Acinetobacter baumannii. Phytomedicine 2017, 24, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kanagaratnam, R.; Sheikh, R.; Alharbi, F.; Kwon, D.H. An efflux pump (MexAB-OprM) of Pseudomonas aeruginosa is associated with antibacterial activity of Epigallocatechin-3-gallate (EGCG). Phytomedicine 2017, 36, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Ghosh, S.; Ray, R.; Hazra, B. Polyphenolic secondary metabolites synergize the activity of commercial antibiotics against clinical isolates of β-Lactamase-producing Klebsiella pneumoniae. Phyto. Res. 2016, 30, 272–282. [Google Scholar] [CrossRef]
- Paulin, S. Impact of Epicatechin Gallate on the Structural Integrity of the PBP2/PBP2a Complex in Methicillin Resistant Staphylococcus Aureus; University of London, University College London: London, UK, 2014. [Google Scholar]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q.; Shimamura, T.; Hara, Y. Inhibition by epigallocatechin gallate (EGCg) of conjugative R plasmid transfer in Escherichia coli. J. Infect. Chemother. 2001, 7, 195–197. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Dai, J.; Sameen, D.E.; Zeng, Y.; Li, S.; Qin, W.; Liu, Y. An overview of tea polyphenols as bioactive agents for food packaging applications. LWT 2022, 167, 113845. [Google Scholar] [CrossRef]
- Annunziata, G.; Jiménez-García, M.; Capó, X.; Moranta, D.; Arnone, A.; Tenore, G.C.; Sureda, A.; Tejada, S. Microencapsulation as a tool to counteract the typical low bioavailability of polyphenols in the management of diabetes. Food Chem. Toxicol. 2020, 139, 111248. [Google Scholar] [CrossRef]
- Kim, H.; Kawazoe, T.; Han, D.W.; Matsumara, K.; Suzuki, S.; Tsutsumi, S.; Hyon, S.H. Enhanced wound healing by an epigallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Repair Regen. 2008, 16, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, S.H.; Kim, K.; An, Y.H.; So, K.H.; Kim, B.G.; Hwang, N.S. Enzyme-mediated one-pot synthesis of hydrogel with the polyphenol cross-linker for skin regeneration. Mater.Today. Bio. 2020, 8, 100079. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pei, D.; Yang, Y.; Xu, K.; Yu, J.; Zhang, Y.; Zhang, Q.; He, G.; Zhang, Y.; Li, A.; et al. Green tea derivative driven smart hydrogels with desired functions for chronic diabetic wound treatment. Adv. Funct. Mater. 2021, 31, 2009442. [Google Scholar] [CrossRef]
- Chen, G.; He, L.; Zhang, P.; Zhang, J.; Mei, X.; Wang, D.; Zhang, Y.; Ren, X.; Chen, Z. Encapsulation of green tea polyphenol nanospheres in PVA/alginate hydrogel for promoting wound healing of diabetic rats by regulating PI3K/AKT pathway. Mater. Sci. Eng. C 2020, 110, 110686. [Google Scholar] [CrossRef]
- Jia, G.; Li, Z.; Le, H.; Jiang, Z.; Sun, Y.; Liu, H.; Chang, F. Green tea derivative-based hydrogel with ROS-scavenging property for accelerating diabetic wound healing. Mater. Des. 2023, 225, 111452. [Google Scholar] [CrossRef]
- Sadri, M.; Arab-Sorkhi, S.; Vatani, H.; Bagheri-Pebdeni, A. New wound dressing polymeric nanofiber containing green tea extract prepared by electrospinning method. Fibers Polym. 2015, 16, 1742–1750. [Google Scholar] [CrossRef]
- Minnelli, C.; Stipa, P.; Sabbatini, S.; Mengucci, P.; Mobbili, G.; Galeazzi, R.; Armeni, T.; Romaldi, B.; Celli, A.; Laudadio, E. Insights into PLGA-encapsulated epigallocatechin 3-gallate nanoparticles as a new potential biomedical system: A computational and experimental approach. Eur. Polym. J. 2023, 182, 111723. [Google Scholar] [CrossRef]
- Shahrahmani, H.; Kariman, N.; Jannesari, S.; Rafieian-Kopaei, M.; Mirzaei, M.; Ghalandari, S.; Shahrahmani, N.; Mardani, G. The effect of green tea ointment on episiotomy pain and wound healing in primiparous women: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2018, 32, 522–530. [Google Scholar] [CrossRef]
- Akhondzadeh, S. The importance of clinical trials in drug development. Avicenna J. Med. Biotechnol. 2016, 8, 151. [Google Scholar]
- Harrison, R.K. Phase II and phase III failures: 2013–2015. Nat. Rev. Drug Discov. 2016, 15, 817–818. [Google Scholar] [CrossRef]
- Dowden, H.; Munro, J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 2019, 18, 495–496. [Google Scholar] [CrossRef] [PubMed]
- MDCG 2021-24: Guidance on Classification of Medical Devices. Available online: https://health.ec.europa.eu/system/files/2021-10/mdcg_2021-24_en_0.pdf (accessed on 5 October 2024).
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to enhance the bioavailability and physiological functions of polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Kalita, B. Design and evaluation of phyto-phospholipid complexes (phytosomes) of rutin for transdermal application. J. Appl. Pharm. Sci. 2014, 4, 51–57. [Google Scholar] [CrossRef]
- Hu, K.; Huang, Z.; Tang, Q.; Chen, D.; Chen, L.; Chen, L.; Jiang, G.; Huang, Q.; Chai, L.; Chen, H.; et al. Green Tea Carbon Dots-Based Electrically Active Hydrogel Dressing for Promoting Wound Healing. Adv. Therap. 2024, 7, 2400016. [Google Scholar] [CrossRef]
- Li, H.; Shen, S.; Yu, K.; Wang, H.; Fu, J. Construction of porous structure-based carboxymethyl chitosan/sodium alginate/tea polyphenols for wound dressing. Int. J. Biol. Macromol. 2023, 233, 123404. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Virulence Mech. Bact. Pathog. 2016, 4, 481–511. [Google Scholar]
- Aslam, B.; Asghar, R.; Muzammil, S.; Shafique, M.; Siddique, A.B.; Khurshid, M.; Ijaz, M.; Rasool, M.H.; Chaudhry, T.H.; Aamir, A.; et al. AMR and Sustainable Development Goals: At a crossroads. Glob. Health 2024, 20, 73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).