Promoting CO2 Methanation Performance over NiO@TiO2 Nanoparticles via Oxygen Vacancies Enriched Fe-Oxide Modifiers Assisted Surface and Interface Engineering
Abstract
1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Structural Characterizations
2.3. Catalytic Activity Measurement
3. Results and Discussion
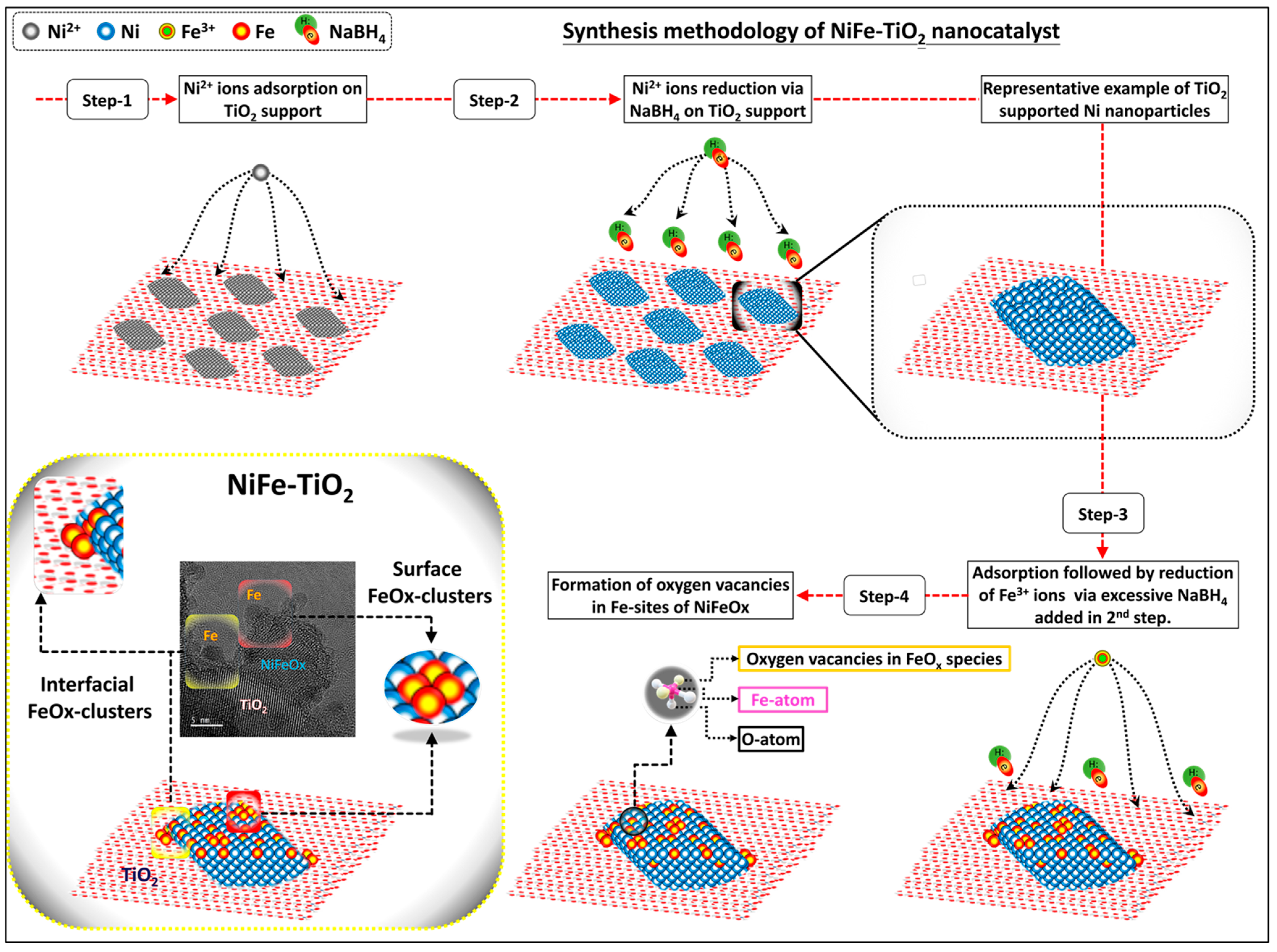
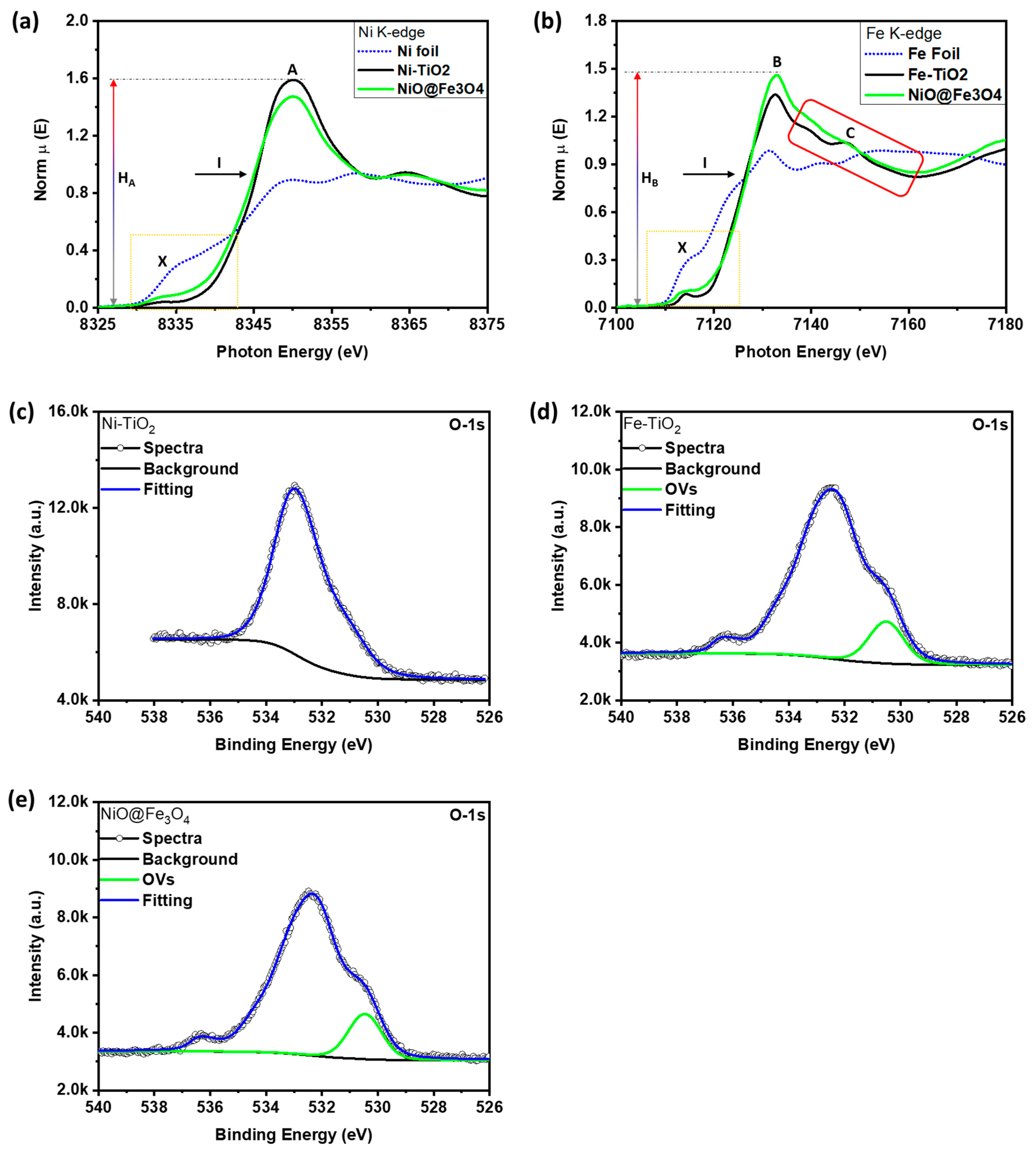
3.1. Structural Characterizations
3.2. Catalytic Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molinet-Chinaglia, C.; Shafiq, S.; Serp, P. Low Temperature Sabatier CO2 Methanation. ChemCatChem 2024, 16, e202401213. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Mohd Ridzuan, N.D.; Shaharun, M.S.; Anawar, M.A.; Ud-Din, I. Ni-Based Catalyst for Carbon Dioxide Methanation: A Review on Performance and Progress. Catalysts 2022, 12, 469. [Google Scholar] [CrossRef]
- Medina, O.E.; Amell, A.A.; López, D.; Santamaría, A. Comprehensive review of nickel-based catalysts advancements for CO2 methanation. Renew. Sustain. Energy Rev. 2025, 207, 114926. [Google Scholar] [CrossRef]
- Lv, C.; Xu, L.; Chen, M.; Cui, Y.; Wen, X.; Li, Y.; Wu, C.-E.; Yang, B.; Miao, Z.; Hu, X.; et al. Recent Progresses in Constructing the Highly Efficient Ni Based Catalysts with Advanced Low-Temperature Activity Toward CO2 Methanation. Front. Chem. 2020, 8, 269. [Google Scholar] [CrossRef]
- Bhalothia, D.; Li, T.-F.; Beniwal, A.; Bagaria, A.; Chen, T.-Y. Strong Electronic Interaction Between Oxygen Vacancy-Enriched Cobalt-Oxide Support and Nickel-Hydroxide Nanoparticles for Enhanced CO Production. Micro 2025, 5. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.-F. Essential Role of the Support for Nickel-Based CO2 Methanation Catalysts. ACS Catal. 2020, 10, 14581–14591. [Google Scholar] [CrossRef]
- Everett, O.E.; Zonetti, P.C.; Alves, O.C.; de Avillez, R.R.; Appel, L.G. The role of oxygen vacancies in the CO2 methanation employing Ni/ZrO2 doped with Ca. Int. J. Hydrogen Energy 2020, 45, 6352–6359. [Google Scholar] [CrossRef]
- Carrettin, S.; Hao, Y.; Aguilar-Guerrero, V.; Gates, B.C.; Trasobares, S.; Calvino, J.J.; Corma, A. Increasing the Number of Oxygen Vacancies on TiO2 by Doping with Iron Increases the Activity of Supported Gold for CO Oxidation. Chem.—A Eur. J. 2007, 13, 7771–7779. [Google Scholar] [CrossRef]
- Huynh, H.L.; Zhu, J.; Zhang, G.; Shen, Y.; Tucho, W.M.; Ding, Y.; Yu, Z. Promoting effect of Fe on supported Ni catalysts in CO2 methanation by in situ DRIFTS and DFT study. J. Catal. 2020, 392, 266–277. [Google Scholar] [CrossRef]
- Wang, X.; Jin, R.; Yan, W.; Li, H.; Wang, Z.-j. An Al2O3-supported NiFe bimetallic catalyst derived from hydrotalcite precursors for efficient CO2 methanation. Catal. Today 2022, 402, 38–44. [Google Scholar] [CrossRef]
- Messou, D.; Bernardin, V.; Meunier, F.; Ordoño, M.B.; Urakawa, A.; Machado, B.F.; Collière, V.; Philippe, R.; Serp, P.; Le Berre, C. Origin of the synergistic effect between TiO2 crystalline phases in the Ni/TiO2-catalyzed CO2 methanation reaction. J. Catal. 2021, 398, 14–28. [Google Scholar] [CrossRef]
- Meshkini-Far, R.; Dyachenko, A.; Gaidai, S.; Bieda, O.; Filonenko, M.; Ischenko, O. Catalytic Properties of Ni-Fe Systems in the Reaction of CO2 Methanation at Atmospheric Pressure. Acta Phys. Pol. A 2018, 133, 1088–1090. [Google Scholar] [CrossRef]
- Hussain, I.; Tanimu, G.; Ahmed, S.; Aniz, C.U.; Alasiri, H.; Alhooshani, K. A review of the indispensable role of oxygen vacancies for enhanced CO2 methanation activity over CeO2-based catalysts: Uncovering, influencing, and tuning strategies. Int. J. Hydrogen Energy 2023, 48, 24663–24696. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C.-J. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B Environ. 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Kim, M.G.; Kang, J.M.; Lee, J.E.; Kim, K.S.; Kim, K.H.; Cho, M.; Lee, S.G. Effects of Calcination Temperature on the Phase Composition, Photocatalytic Degradation, and Virucidal Activities of TiO2 Nanoparticles. ACS Omega 2021, 6, 10668–10678. [Google Scholar] [CrossRef]
- Taffa, D.H.; Brim, E.; Rücker, K.K.; Hayes, D.; Lorenz, J.; Bisen, O.; Risch, M.; Harms, C.; Richards, R.M.; Wark, M. Influence of Annealing Temperature on the OER Activity of NiO(111) Nanosheets Prepared via Microwave and Solvothermal Synthesis Approaches. ACS Appl. Mater. Interfaces 2024, 16, 62142–62154. [Google Scholar] [CrossRef]
- Zhong, Y.; Yu, L.; Chen, Z.-F.; He, H.; Ye, F.; Cheng, G.; Zhang, Q. Microwave-Assisted Synthesis of Fe3O4 Nanocrystals with Predominantly Exposed Facets and Their Heterogeneous UVA/Fenton Catalytic Activity. ACS Appl. Mater. Interfaces 2017, 9, 29203–29212. [Google Scholar] [CrossRef]
- Vrijburg, W.L.; Moioli, E.; Chen, W.; Zhang, M.; Terlingen, B.J.P.; Zijlstra, B.; Filot, I.A.W.; Züttel, A.; Pidko, E.A.; Hensen, E.J.M. Efficient Base-Metal NiMn/TiO2 Catalyst for CO2 Methanation. ACS Catal. 2019, 9, 7823–7839. [Google Scholar] [CrossRef]
- Bhalothia, D.; Chen, P.-C.; Yan, C.; Wang, K.-W.; Chen, T.-Y. Heterogeneous NiO2-to-Pd Epitaxial Structure Performs Outstanding Oxygen Reduction Reaction Activity. J. Phys. Chem. C 2020, 124, 2295–2306. [Google Scholar] [CrossRef]
- Beniwal, A.; Bhalothia, D.; Chen, Y.-R.; Kao, J.-C.; Yan, C.; Hiraoka, N.; Ishii, H.; Cheng, M.; Lo, Y.-C.; Tu, X.; et al. Incorporation of atomic Fe-oxide triggers a quantum leap in the CO2 methanation performance of Ni-hydroxide. Chem. Eng. J. 2024, 493, 152834. [Google Scholar] [CrossRef]
- Yang, T.; Bhalothia, D.; Chang, H.-W.; Yan, C.; Beniwal, A.; Chang, Y.-X.; Wu, S.-C.; Chen, P.-C.; Wang, K.-W.; Dai, S.; et al. Oxygen vacancies endow atomic cobalt-palladium oxide clusters with outstanding oxygen reduction reaction activity. Chem. Eng. J. 2023, 454, 140289. [Google Scholar] [CrossRef]
- Bhalothia, D.; Hsiung, W.-H.; Yang, S.-S.; Yan, C.; Chen, P.-C.; Lin, T.-H.; Wu, S.-C.; Chen, P.-C.; Wang, K.-W.; Lin, M.-W.; et al. Submillisecond Laser Annealing Induced Surface and Subsurface Restructuring of Cu–Ni–Pd Trimetallic Nanocatalyst Promotes Thermal CO2 Reduction. ACS Appl. Energy Mater. 2021, 4, 14043–14058. [Google Scholar] [CrossRef]
- Yan, C.; Wang, C.-H.; Lin, M.; Bhalothia, D.; Yang, S.-S.; Fan, G.-J.; Wang, J.-L.; Chan, T.-S.; Wang, Y.-l.; Tu, X.; et al. Local synergetic collaboration between Pd and local tetrahedral symmetric Ni oxide enables ultra-high-performance CO2 thermal methanation. J. Mater. Chem. A 2020, 8, 12744–12756. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.R.; AlMomani, F.; Kumar, A.; Banu, A.; Ashok, A.; Rashid, S.; Khraisheh, M.; Shakoor, A.; al Ashraf, A. Thermochemical splitting of CO2 using solution combustion synthesized LaMO3 (where, M = Co, Fe, Mn, Ni, Al, Cr, Sr). Appl. Surf. Sci. 2020, 509, 144908. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.R.; AlMomani, F. Sol-gel synthesized NixFe3−xO4 for thermochemical conversion of CO2. Appl. Surf. Sci. 2019, 489, 693–700. [Google Scholar] [CrossRef]
- Khamhangdatepon, T.; Tongnan, V.; Hartley, M.; Sornchamni, T.; Siri-Nguan, N.; Laosiripojana, N.; Li, K.; Hartley, U.W. Mechanisms of synthesis gas production via thermochemical cycles over La0.3Sr0.7Co0.7Fe0.3O3. Int. J. Hydrogen Energy 2021, 46, 24666–24675. [Google Scholar] [CrossRef]
- Burra, K.G.; Gupta, A.K.; Kerdsuwan, S. Isothermal Splitting of CO2 to CO Using Cobalt-Ferrite Redox Looping. J. Energy Resour. Technol. 2020, 143, 032303. [Google Scholar] [CrossRef]


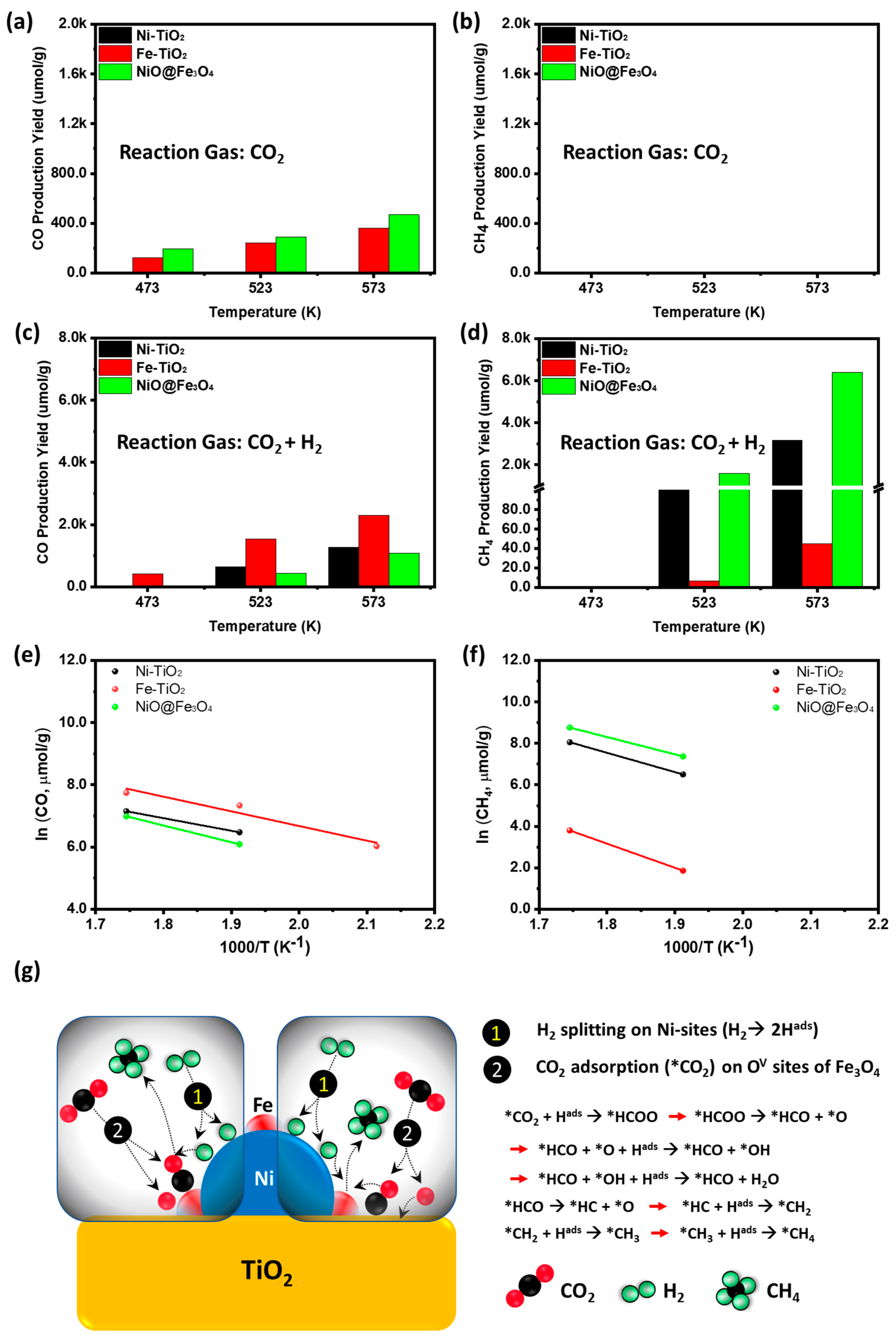
| Sample | Temperature (K) | YieldCH4 (μmol/g) | References |
|---|---|---|---|
| NiO@Fe3O4 | 573 | 6400.50 | This work |
| CNP-10 | 1719.87 | [23] | |
| CNP-1 | 1651.91 | ||
| CNP- | 1353.68 | ||
| NiPd-TMOS (NiOTPd-T) | 1905.1 | [24] | |
| NiOT-T | 1083.2 | ||
| Pd-T | 92.2 | ||
| LaSrO3 | 1273 | 124.1 | [25] |
| NiFe2O4 | 1273 | 125.9 | [26] |
| La0.3Sr0.7Co0.7Fe0.3O3 (LSCF) | 973 | 705 | [27] |
| Co1.3Fe1.7O4 | 1673 | 750 | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhalothia, D.; Beniwal, A.; Bagaria, A.; Chen, T.-Y. Promoting CO2 Methanation Performance over NiO@TiO2 Nanoparticles via Oxygen Vacancies Enriched Fe-Oxide Modifiers Assisted Surface and Interface Engineering. Processes 2025, 13, 834. https://doi.org/10.3390/pr13030834
Bhalothia D, Beniwal A, Bagaria A, Chen T-Y. Promoting CO2 Methanation Performance over NiO@TiO2 Nanoparticles via Oxygen Vacancies Enriched Fe-Oxide Modifiers Assisted Surface and Interface Engineering. Processes. 2025; 13(3):834. https://doi.org/10.3390/pr13030834
Chicago/Turabian StyleBhalothia, Dinesh, Amisha Beniwal, Ashima Bagaria, and Tsan-Yao Chen. 2025. "Promoting CO2 Methanation Performance over NiO@TiO2 Nanoparticles via Oxygen Vacancies Enriched Fe-Oxide Modifiers Assisted Surface and Interface Engineering" Processes 13, no. 3: 834. https://doi.org/10.3390/pr13030834
APA StyleBhalothia, D., Beniwal, A., Bagaria, A., & Chen, T.-Y. (2025). Promoting CO2 Methanation Performance over NiO@TiO2 Nanoparticles via Oxygen Vacancies Enriched Fe-Oxide Modifiers Assisted Surface and Interface Engineering. Processes, 13(3), 834. https://doi.org/10.3390/pr13030834






