Abstract
The success of orthopedic implants critically depends on achieving mechanical and biological compatibility with bone tissue. Traditional titanium implants often suffer from high stiffness, which induces stress shielding, a phenomenon that compromises implant integration and accelerates prosthetic loosening. This study introduces an innovative approach to mitigate these limitations by engineering a porous titanium substrate with a controlled microstructure. Utilizing sodium chloride as a spacer holder, an elution and sintering process was applied at 1250 °C under high vacuum conditions to reduce the material’s elastic modulus. By manipulating NaCl volume fractions (20%, 25%, 30%, and 35%), porous titanium samples were created with elastic moduli between 16.37 and 22.56 GPa, closely matching cortical bone properties (4 to 20 GPa). A hydroxyapatite coating applied via plasma thermal spraying further enhanced osseointegration of the material. Comprehensive characterization through X-ray diffraction, scanning electron microscopy, and compression testing validated the material’s structural integrity. In vitro cytotoxicity assessments using osteoblast cells demonstrated exceptional cell viability exceeding 70%, confirming the material’s biocompatibility. These findings represent a significant advancement in biomaterial design, offering a promising strategy for developing next-generation joint prostheses with superior mechanical and biological adaptation to bone tissue.
1. Introduction
Titanium and its alloys have been pivotal materials in aerospace and military industries, prized for their exceptional mechanical strength, low density, and toughness, making them ideal for structural applications [1,2]. The biomedical field has increasingly embraced these materials, particularly in joint prostheses, capitalizing on their outstanding biocompatibility and superior corrosion resistance [3]. Currently, the most commonly used titanium alloys in prostheses include Ti6Al4V, Ti-6Al-7Nb, Ti-15Mo, pure titanium, and nitinol, which exhibit Young’s moduli ranging from approximately 70 to 120 GPa, presenting a significant challenge: the potential for stress shielding [4,5,6].
Bone tissue is a dynamic, living organ with inherent regenerative capacity in response to mechanical stimuli. When an implant material possesses substantially higher stiffness than natural bone, the resulting stress distribution becomes nonhomogeneous. Consequently, this mechanical mismatch can precipitate progressive bone density loss, potentially culminating in prosthetic loosening or even surrounding bone fractures [7,8]. To mitigate the stress shielding phenomenon, researchers have systematically explored several strategies. These approaches include investigating low-density metals, developing specialized alloys [9], and designing innovative composite materials [10] characterized by stiffness more closely aligned with bone tissue. However, these materials frequently compromise mechanical strength, thereby constraining their practical application in demanding joint prosthesis environments.
An alternative methodology for decreasing biomaterial stiffness strategically introducing porosity within the material’s structure composition. It has been demonstrated that controlled porosity can induce significant reductions in material elastic modulus [11,12,13], allowing more appropriate mechanical properties for incorporation into bone tissue. Additive manufacturing (AM), particularly direct metal laser sintering (DMLS), has gained widespread adoption for creating complex, patient-specific geometries [14]. DMLS remains the predominant commercial approach for customized titanium implants due to its unparalleled design freedom. Nevertheless, the modeling and reproduction of small, interconnected pores presents significant challenges, and DMLS processes often suffer from prohibitively long manufacturing times and higher production costs [15,16].
In contrast, the press and sintering (PS) technique combined with the space holder method offers a more cost-effective, scalable alternative, with reduced processing times. While this approach has limitations for patient-specific geometries, it enables precise control over porosity characteristics—including pore size, morphology, and distribution—while maintaining sufficient mechanical strength and effectively mimicking bone structure [17]. PS exhibits certain limitations: restricted geometric complexity that limits direct clinical application for customized implants, susceptibility to pore collapse during compaction, and the requirement for precise sintering cycle control to prevent incomplete densification or contamination from space holder residues. Despite these inherent challenges, PS remains a viable methodology for producing standardized porous structures for fundamental research applications, enabling deeper understanding of material-property relationships that can inform and optimize future implant designs regardless of manufacturing method. The insights gained from PS research provide valuable data on porosity effects that remain applicable to components eventually produced through DMLS or other clinical manufacturing methods.
For enhanced osseointegration, the application of hydroxyapatite (HA), the main mineral component of bone tissue, has been shown to promote osteoconductivity and cellular proliferation, fostering a strong bond between implant and bone [18,19]. Various techniques have been employed to apply hydroxyapatite coatings to metallic substrates. Sputtering enables uniform coating deposition, though hydroxyapatite applications may introduce Ca/P ratio variations potentially compromising biological properties [20]. Physical vapor deposition consists of the condensation of a vapor phase material on a surface, generating dense coatings with strong adhesion but often encounters thickness uniformity challenges [21]. Hydrothermal processes offer homogeneous coating potential for complex geometries, yet struggle with extended reaction times and solvent selection complexities [22]. The sol–gel method presents simplicity and cost-effectiveness but suffers from prolonged experimental durations and elevated raw material expenses [23], while electrochemical deposition provides precise compositional and thickness control of the coating, but frequently requires supplementary surface treatments due to inherent low adhesion limitations [24].
In the present study, the atmospheric plasma spraying (APS) technique was selected for hydroxyapatite deposition. In this process, hydroxyapatite particles are initially heated to a semi-molten state. Subsequently, the particles are accelerated at a high velocity to impact a specific substrate, in this case, porous titanium. Upon surface impact, particles undergo plastic deformation, creating a robust coating layer. This technique is distinguished by its ability to operate at elevated temperatures and projection speeds, which facilitates the deposition of coatings in a relatively simple way. APS distinguishes itself through operational capabilities at elevated temperatures and projection speeds, facilitating relatively straightforward coating deposition. The technique enables precise coating thickness control and generates dense adhesive layers [25]. The resultant hydroxyapatite surface layer actively promotes cellular adhesion and proliferation, significantly improving bone integration and contributing to prosthetic stability within the human physiological environment.
This study aims to develop a porous titanium substrate exhibiting mechanical properties comparable to those of cortical bone, specifically targeting an elastic modulus in the range of 4–20 GPa and a compressive strength of 100–230 MPa, as reported in the literature [26]. Sodium chloride served as a space holder during the elution and sintering processes, systematically optimizing porosity and pore distribution to enhance mechanical compatibility with bone tissue. Furthermore, the material’s bioactivity and osseointegration potential was improved through precise hydroxyapatite coating application via atmospheric plasma spraying. Comprehensive substrate characterization involved microstructural, mechanical, and biological analyses, including in vitro cell viability assessments. The ultimate objective is to develop a biomedical material suitable for joint implants, effectively minimizing the stress-shielding effects while promoting optimal bone integration.
2. Materials and Methods
Figure 1 illustrates the comprehensive methodology approach employed to obtain the substrate-coating composite. The process involved a systematic sequence: initial preparation of porous titanium substrate involved utilizing titanium and NaCl powders as primary materials, followed by homogenization, cold pressing to obtain green samples, elution for NaCl removal, and subsequent sintering. The hydroxyapatite coating was then applied via atmospheric plasma spraying, resulting in the final porous titanium substrate with bioactive surface properties.
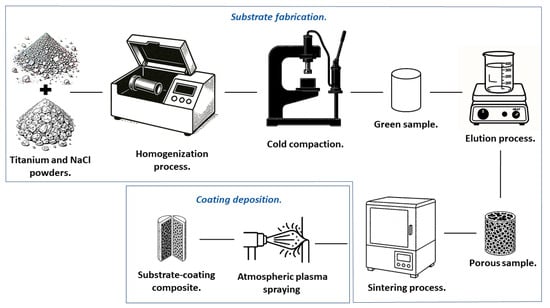
Figure 1.
Schematic of the methodology for obtaining the substrate-coating composite.
2.1. Fabrication and Characterization of Porous Titanium
2.1.1. Raw Materials and Sintering Process
High-purity titanium powder (99.5%, 44 μm particle size) was obtained from Alfa Aesar (Ward Hill, MA, USA). Sodium chloride (NaCl) with 99% purity, sourced from Fermont (Monterrey, NLE, Mexico), was employed as the space-holder material. The NaCl, initially 2.54 cm in size, underwent controlled size reduction to 150 μm through manual crushing in an agate mortar and subsequent sieving for precise particle classification.
The Ti and NaCl powders were combined in carefully controlled volumetric fractions, as detailed in Table 1. Precise proportioning was ensured using a high-precision analytical balance. Homogenization was achieved through high-energy milling (SPEX model 8000M, Metuchen, NJ, USA) conducted for 1 h. Notably, no milling media were introduced during this process to preserve the powders’ morphological integrity while ensuring uniform distribution throughout the mixture.

Table 1.
Composition of the samples.
The homogenized mixtures underwent cold compaction using a 6.5 mm diameter die. Sample consolidation was achieved through uniaxial pressing at 1200 MPa, maintained for 1 min on each side to ensure uniform density distribution throughout the green compact.
To generate controlled porosity within the titanium structure, the compacted specimens underwent an optimized elution process. The specimens were immersed in distilled water maintained at 80 °C under continuous agitation for 2 h, ensuring complete dissolution and removal of the NaCl space-holder material. Afterward, the specimens were thoroughly dried at 100 °C for 12 h to eliminate residual moisture before the sintering stage.
For the sintering process, samples were carefully placed inside quartz ampoules specifically designed to withstand high temperatures under high-vacuum conditions. To ensure an oxygen-free environment critical for maintaining titanium’s mechanical properties, the ampoules were evacuated using a vacuum pump and hermetically sealed by fusion with an oxyacetylene flame. The encapsulated samples underwent sintering process in a Terlab furnace (Guadalajara, Jalisco, Mexico), with controlled heating to 1250 °C for 1 h. A precisely regulated heating rate of 10 °C/min was employed to ensure gradual and uniform thermal distribution, minimizing thermal stress and promoting optimal particle coalescence and densification. These parameters were selected based on preliminary experimental trials conducted by our research group [27].
2.1.2. Characterization of the Microstructure
Phase identification of post-processed materials was conducted using X-ray diffraction (XRD) analysis with an X’Pert3 MRD diffractometer (Panalytical, Almelo, The Netherlands). Cu-Kα monochromatic radiation (λ = 0.1542 nm) was utilized, with diffraction patterns collected across the 2θ range of 20° to 90°. This analysis assessed the efficiency of the elution process by comparing the resulting diffraction patterns with that of pure titanium, verifying the potential presence of NaCl residues.
Comprehensive porosity characterization was performed using scanning electron microscopy (SEM) with a Hitachi microscope (Tokyo, Japan). Quantitative porosity values were determined following the Archimedes method, in accordance with the ASTM B962 standard [28], providing precise volumetric porosity measurements.
2.1.3. Evaluation of Mechanical Properties
Mechanical performance was assessed through compression tests carried out using an Instron Model 3382 Universal Testing Machine (Norwood, MA, USA), following the ASTM E9 standard [29]. The tests were conducted with a 100 kN load cell at a precisely controlled deformation rate of 0.06 mm/min. Cylindrical specimens measuring 6 mm in diameter and 12 mm in height were utilized to ensure standardized testing conditions and reliable comparative analyses.
2.2. Application of the Coating by Atmospheric Plasma Spraying
To improve the osseointegration potential of the porous titanium substrate, a hydroxyapatite (HA) coating was applied via atmospheric plasma spraying (APS).
Prior to coating deposition, substrate surfaces underwent a surface activation treatment via grit blasting with corundum particles approximately 1.3 mm in diameter, creating optimal surface roughness for coating adhesion. The samples were then ultrasonically cleaned in isopropyl alcohol for 30 min followed by thorough drying in an oven at 100 °C for 2 h to remove any residual moisture.
The coating deposition process was conducted using an SG100 plasma thermal projection system (Praxair Surface Technologies, Indianapolis, IN, USA). The precisely controlled deposition parameters are summarized in Table 2.

Table 2.
Atmospheric plasma spraying (APS) parameters.
2.3. Cell Viability
Cell viability, defined as the proportion of metabolically active cells within a population, was evaluated through enzymatic activity assays. A critical parameter in this assessment is cytotoxicity, which refers to the impairment of essential cellular functions by potentially toxic agents that may lead to irreversible cellular damage [30,31].
The biocompatibility assessment employed in vitro testing protocols focused on cytotoxicity and cellular activity metrics. Cytotoxicity analysis was conducted using the MTT assay, which relies on the reduction of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) to formazan, an insoluble blue compound. Reduced MTT serves as a reliable indicator of mitochondrial functionality and overall cell viability [32].
Primary osteoblasts were isolated from Wistar rats using enzymatic digestion with collagenase [33] and used in this study, which was approved by the Research Ethics Committee (approval number CEI-2023-2-148). Cells were cultivated in α-MEM medium supplemented with fetal bovine serum (FBS) and antibiotics (penicillin–streptomycin) under standard culture conditions. For each experimental condition, 5000 cells were precisely seeded per sample, with cell viability evaluated at two exposure times points (24 and 48 h) to assess both immediate and sustained biocompatibility.
Each test was conducted with six replicate samples per composition (Ti80, Ti75, Ti70, and Ti65) to ensure statistical validity. At each evaluation time point, the culture medium was carefully removed, and a solution containing 50 μL MTT and 450 μL α-MEM was added and incubated for 3 h under controlled conditions.
Subsequently, 400 μL dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. Absorbance measurements were taken at the appropriate wavelength using a Benchmark Plus microplate spectrophotometer (Waltham, MA, USA).
The percentage of cell viability was calculated using Equation (1).
Here, and correspond to the absorbance values of the sample and control, respectively.
3. Results and Discussion
3.1. Porous Titanium
Scanning electron microscopy (SEM) analysis provided comprehensive insights into the morphological characteristics and particle distribution of the raw materials used in this study. Figure 2a illustrates the titanium (Ti) powder morphology, revealing notable heterogeneity in particle size distribution. In contrast, Figure 2b presents sodium chloride (NaCl) particles, which demonstrate significant variations in both dimensional and morphological parameters.

Figure 2.
Raw material SEM micrographs: (a) titanium and (b) NaCl powders.
Comparative analysis between the two powder types reveals that NaCl particles substantially exceed titanium particles in size, with a calculated diameter ratio of . This deliberate size disparity serves a dual function; it facilitates enhanced titanium particle coalescence during the sintering process while simultaneously promoting more uniform NaCl distribution throughout the titanium matrix. These characteristics are fundamental for achieving a controlled, reproducible, and interconnected porous structure following the removal of the space-holder material [11].
X-ray diffraction analysis (XRD) revealed distinct crystallographic structures for each material investigated, as depicted in Figure 3. The diffraction pattern of the titanium exhibits sharp, well-defined peaks characteristic of the α-phase of Ti, confirming its hexagonal close-packed (HCP) crystal structure. The intensity and precise positioning of these reflections validate the high crystallinity of the material, a crucial attribute for ensuring mechanical stability and biological suitability in biomedical applications.
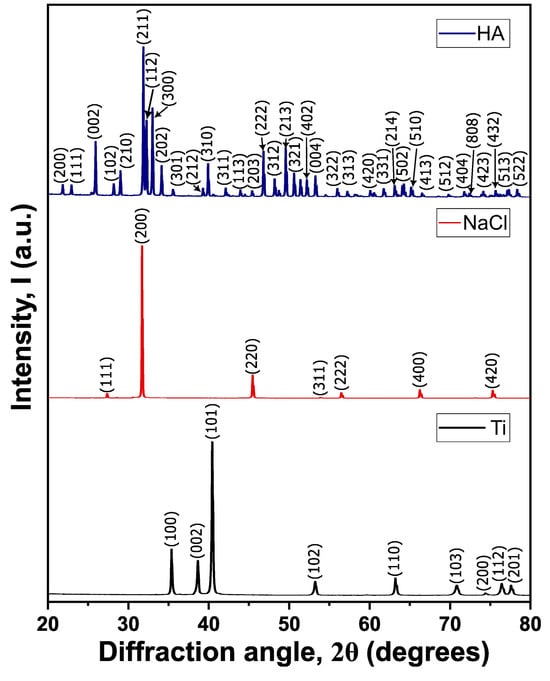
Figure 3.
Diffractograms of the precursor materials: titanium (Ti), NaCl, and hydroxyapatite (HA).
The diffractogram of NaCl presents characteristic peaks corresponding to the cubic crystal system, with prominent reflections associated with the (111), (200), (220), and higher-order planes, confirming its face-centered cubic (FCC) symmetry. This structure is typically associated with high ionic conductivity and structural stability, properties relevant to its function as a space-holder in this application. The diffraction pattern of hydroxyapatite (HA), which exhibits distinctive peaks corresponding to its hexagonal phase. The well-defined reflections confirm its highly ordered crystalline structure, an essential characteristic for its bioactivity and osteoconductive properties in biological environments. The distinct diffraction patterns observed for each material confirm their crystallographic integrity, which is critical for their specific roles in biomedical and structural applications.
Figure 4 presents a comparative analysis of XRD patterns between pure titanium and a porous titanium sample obtained after the NaCl elution process. Both diffractograms exhibit characteristic sharp peaks indexed to the α-phase of titanium, confirming the preservation of the HCP structure. The remarkable similarity in peak positions between pure and porous titanium samples demonstrates that the elution process does not induce significant phase transformations within the titanium lattice, maintaining its intrinsic crystallographic properties.
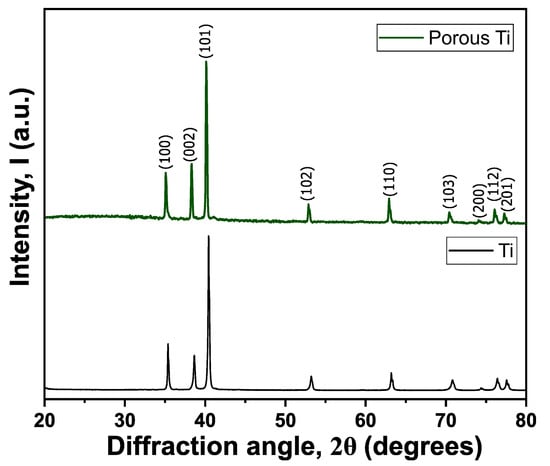
Figure 4.
Diffraction patterns of titanium and porous titanium after the elution process.
The enhanced surface area and modified microstructure of the porous Ti are particularly relevant for biomedical applications, as these characteristics can substantially improve bone integration and mechanical interlocking with bone tissue. In the post-elution titanium sample, only diffraction peaks corresponding to titanium were detected, with a notable absence of NaCl-related peaks. This observation confirms two critical aspects; first, that no chemical reactions occurred between Ti and NaCl during the sintering process, and second, that the elution procedure achieved complete removal of the space-holder material, ensuring effective porosity generation throughout the titanium matrix.
The hydroxyapatite particles were characterized using SEM and energy-dispersive X-ray spectroscopy (EDS) (Figure 5). SEM analysis revealed predominantly spherical particles with an average diameter of 50 μm, while EDS confirmed the presence of essential hydroxyapatite constituent elements, including oxygen, calcium, and phosphorus, in stoichiometric proportions. According to the literature, a particle size between 10 and 100 μm represents the optimal range for achieving strong adhesion in coatings deposited via thermal plasma spraying [34]. This specific particle size range ensures sufficient plastic deformation upon impact, significantly improving adherence to the substrate.
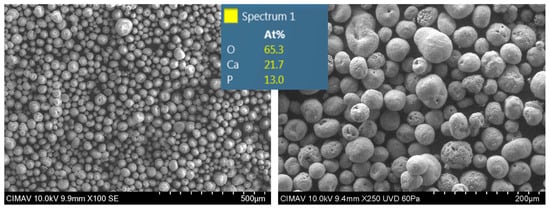
Figure 5.
SEM images of HA particles.
Additionally, the spherical morphology observed facilitates uniform heating during the spraying process, reduces interparticle gaps, and enhances overall coating adhesion [35]. Furthermore, this spherical shape substantially improves powder flowability, ensuring homogeneous dispersion during deposition onto the porous titanium substrate, a critical factor for coating uniformity and performance.
The calcium-to-phosphorus (Ca/P) ratio of the hydroxyapatite was precisely determined using inductively coupled plasma emission spectrometry (ICP), yielding a value of 2.14. This specific ratio indicates optimal bioactivity potential through controlled release of Ca2+ and PO43− ions, which play crucial roles in promoting bone growth and integration. It is noteworthy that reported Ca/P ratios in adult bone tissue range from 0.58 to 2.34, varying with factors such as age, health status, and anatomical location [36], placing our material within the physiologically relevant range.
XRD was employed to evaluate the hydroxyapatite coating (Figure 6), with the aim of identifying any potential phase transformations induced by the high temperatures reached during the atmospheric plasma spraying (APS) process. The analysis revealed the presence of hydroxyapatite (HA), β-tricalcium phosphate (β-TCP), and calcium oxide (CaO), indicating that the thermal conditions during APS promoted partial decomposition of HA. This transformation can be described by the following reaction:
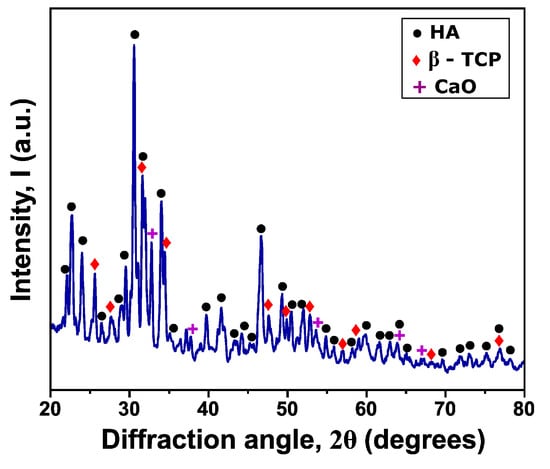
Figure 6.
Diffraction pattern of HA coating.
The formation of β-TCP and CaO is consistent with previous reports and reflects HA thermal instability at temperatures above 800–1000 °C. However, the brief exposure time during the APS process prevents complete HA decomposition, allowing significant retention of the original phase. This coexistence of phases is commonly observed in thermally sprayed calcium phosphate coatings.
From a biological standpoint, both HA and β-TCP are well known for their biocompatibility and bioactivity. HA supports cell attachment and osteoconduction due to its similarity to the mineral component of bone, while β-TCP enhances bone regeneration and exhibits moderate osteoinductive properties. Although CaO is less bioactive, its presence in minor quantities is often reported under high-temperature processing conditions and does not significantly compromise the biological performance of the coating [35,37].
Sample density was determined using the Archimedes method through immersion in distilled water and density calculations based on volumetric displacement. As expected, increasing NaCl content resulted in decreasing density values due to greater porosity (Figure 7). The density of pure titanium (4.5 g/cm3) was significantly reduced across all compositions, even in the Ti80 sample. This density reduction represents a substantial advantage, as it results in a lighter biomaterial while maintaining the desired mechanical properties critical for load-bearing applications.
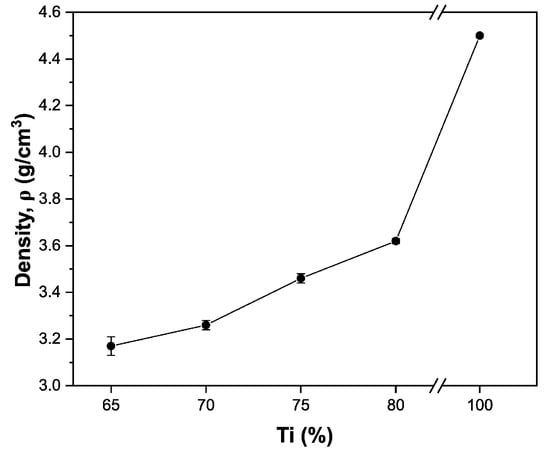
Figure 7.
Sample density with different compositions.
The reduced density is particularly beneficial for prosthetic applications, as it minimizes the overall implant weight without compromising performance, thereby improving patient comfort and reducing stress at the bone–implant interface.
SEM micrographs of sintered titanium samples (Figure 8) reveal a hierarchical porous structure characterized by the presence of macropores (originating from the removal of the NaCl space-holder agent) and micropores (likely resulting from residual interparticle spaces not fully consolidated during sintering). The potential presence of smaller space-holder particles may have contributed to the microporosity, creating a biomimetic structure similar to natural bone.
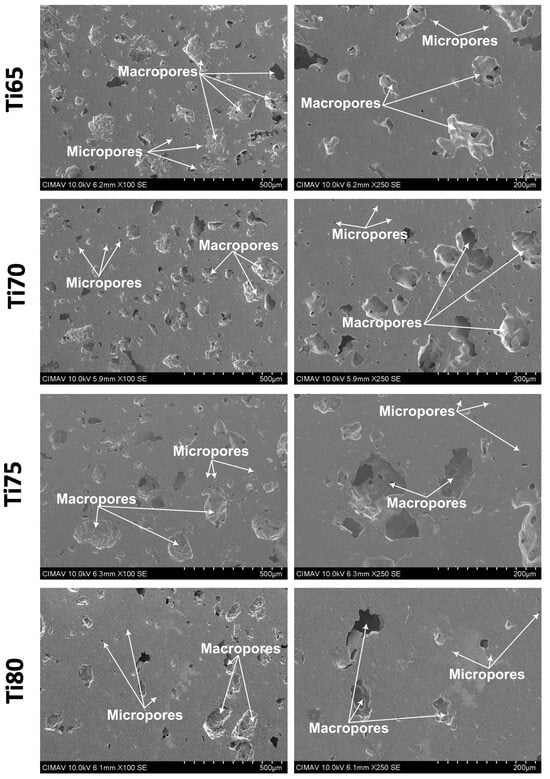
Figure 8.
SEM images showing the porosity obtained using the space holder technique.
It is important to note that any residual NaCl encapsulated within the titanium matrix does not pose a biological risk for two primary reasons. Firstly, the encapsulation prevents significant NaCl release into surrounding tissues; secondly, NaCl is inherently biocompatible and does not cause adverse reactions in the human body [38,39]. The SEM images further confirm effective titanium particle coalescence, indicating that the cold consolidation and sintering processes facilitated robust interparticle bonding, thereby enhancing structural integrity. The degree of pore interconnectivity demonstrates a direct relationship with NaCl content, with higher spacer content leading to increased interconnectivity. These findings provide critical insights into the relationship between processing parameters, resultant porosity, and mechanical properties in porous titanium structures.
As presented in Table 3, porosity values for samples with varying NaCl spacer content demonstrate a direct correlation between NaCl concentration and resultant porosity, with minor variations observed. These fluctuations can be attributed to sintering-induced effects, which promote particle coalescence after initial porous structure formation. During the sintering process, this coalescence mechanism leads to reduced equivalent pore diameters and enhances interparticle necking, resulting in a net decrease in overall porosity. This particle coalescence phenomenon is crucial for the material’s performance, as it significantly enhances structural integrity, ultimately contributing to improved mechanical strength while maintaining the desired porosity level.

Table 3.
Porosity of samples with different compositions.
Several factors may contribute to experimental variability, primarily the distribution and compaction dynamics of NaCl particles, which significantly influence pore morphology, interconnectivity, and spatial arrangement throughout the titanium matrix. Despite these potential sources of variation, the total porosity measurements demonstrated remarkable consistency across all specimens, confirming good reproducibility of bulk porosity characteristics. Through precise calibration of NaCl content as a space-holder material, researchers can strategically engineer porosity parameters and corresponding mechanical properties of the titanium matrix. This controlled customization enables the development of biomaterials with optimized performance profiles specifically tailored for orthopedic implants, dental prostheses, and various other sophisticated engineering applications requiring specific porosity–mechanical property relationships.
Figure 9a presents the stress–strain curves for the different sample compositions under compressive loading. All samples exhibit characteristic initial elastic deformation followed by plastic deformation regions, with elastic modulus values demonstrating clear composition dependence. Notably, compositions Ti65 through Ti80 exhibit elastic modulus values ranging between 15.55 and 22.56 GPa, well within the target range for cortical bone (4–20 GPa), as shown in Figure 9b. This mechanical compatibility is essential for minimizing stress shielding effects at bone–implant interfaces.
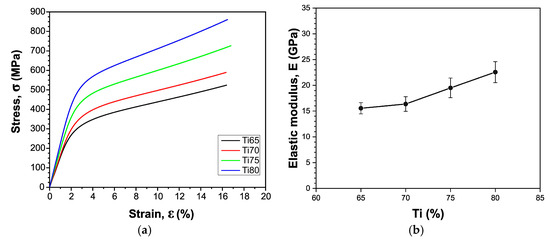
Figure 9.
(a) Stress–strain curves from the compression test and (b) influence of composition on elastic modulus.
Table 4 summarizes the yield and maximum stress values for each titanium composition. In all formulations, the yield stress exceeds the typical range for cortical bone (120–160 MPa), confirming that the material will not undergo plastic deformation under physiological loading conditions. This superior mechanical performance enhances stability and longevity, substantially reducing the likelihood of prosthesis failure and subsequent replacement requirements. Additionally, the mechanical properties effectively mitigate stress shielding effects, which can compromise long-term bone integrity and implant stability.

Table 4.
Yield stress and maximum stress of samples with different compositions.
These results clearly demonstrate the suitability of Ti65 to Ti80 compositions for biomedical applications, as their mechanical performance profile closely aligns with that of natural bone tissue while providing necessary strength reserves for load-bearing applications. To correlate these mechanical properties with porosity, it is noteworthy that the Ti80 sample, with a porosity of 19.55%, exhibited a compressive strength of 860 MPa, whereas the Ti75 sample, which had a slightly higher porosity of 23.11%, demonstrated a lower compressive strength of 726 MPa. These findings clearly show that as porosity decreases, the maximum compressive stress increases, which is consistent with the well-established inverse relationship between porosity and mechanical performance in porous materials.
3.2. Hydroxyapatite Coating
SEM cross-sectional analysis of the coated samples (Figure 10) reveals a uniform hydroxyapatite (HA) layer successfully deposited onto the porous titanium substrate. The distinctive lamellar microstructure observed is characteristic of coatings produced via plasma thermal spraying (APS), resulting from the rapid deposition and solidification kinetics of semi-molten HA particles upon impact with the substrate surface.

Figure 10.
SEM micrographs of the sintered and HA-coated porous titanium samples.
Coating thickness was measured at multiple randomly selected points across each sample to calculate an average thickness per specimen. This analysis showed coating thicknesses ranging from approximately 240 to 391 μm, with variations primarily influenced by the spraying parameters, particularly projection time. While these values correspond to relatively thick coatings, as reported in the literature [18], previous studies have demonstrated that such thicknesses can still achieve favorable performance, provided the processing conditions promote strong adhesion and reduce residual stresses. For instance, coatings up to 310 µm in thickness have exhibited satisfactory mechanical properties under optimized APS conditions [40].
Notably, thicker coatings can offer advantageous surface topographies and enhanced bioactivity, fostering improved interactions at the bone–implant interface. This, in turn, contributes to greater implant stability and in vivo performance. Additionally, the surface roughness produced by the HA deposition process is beneficial for osteoblast adhesion, proliferation, and differentiation—key factors for the long-term clinical success of biomedical implants used in orthopedic and dental applications.
Nevertheless, the APS process is inherently sensitive to variations in operational parameters. Fluctuations in plasma temperature, particle velocity, or feed rate may affect both the uniformity and thickness of the coatings. Although these parameters were carefully monitored and controlled during the experiments, some degree of variability remains inherent to the technique. However, given the controlled conditions employed, the coating performance is expected to remain within acceptable and functional limits.
3.3. Cell Viability and Biocompatibility Assessment
As shown in Figure 11, cell viability demonstrates a significant correlation with substrate porosity and surface characteristics. Spectrophotometric analysis confirmed that all samples maintained viability values exceeding 70% after 24 and 48 h of exposure, fully satisfying the non-cytotoxicity requirements outlined in ISO 10993-5 [41]. These findings align with previous studies evaluating HA coatings applied through different deposition techniques [42,43].
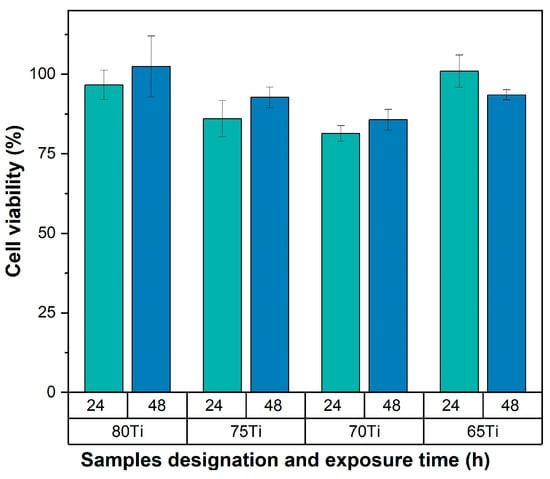
Figure 11.
Graph showing the percentage of cell viability at 24 and 48 h.
A notable progressive increase in cell viability was observed over time, particularly in Ti80, Ti75, and Ti70 samples. This enhanced cellular response can be attributed to the controlled partial degradation of HA coating, which facilitates the regulated release of Ca2+ and PO43− ions into the surrounding microenvironment. However, the evolution and kinetics are controlled by cellular mechanisms [44].
The bioavailable presence of these ions significantly enhances osteogenic cell adhesion, proliferation, and metabolic activity, effectively stimulating biological responses that accelerate the bone regeneration process. This integrated biomaterial approach combining optimized titanium porosity with bioactive hydroxyapatite coating demonstrates considerable potential for enhancing osseointegration in orthopedic implant applications.
4. Conclusions
This comprehensive investigation successfully developed and characterized porous titanium substrates by strategically incorporating sodium chloride as a space holder. This approach enabled the formation of an interconnected porous architecture ideally suited for biomedical applications. The experimental findings conclusively demonstrate that increasing the concentration of sodium chloride directly correlates with higher porosity levels, which in turn predictably reduces material density and modulates mechanical properties.
Notably, despite the systematic decrease in density, all titanium compositions exhibited yield strength and Young’s modulus values that fall within the biomechanical range of cortical bone. This alignment underscores the suitability of these materials for orthopedic implants intended to withstand physiological loading conditions.
The hydroxyapatite (HA) coating, applied via atmospheric plasma spraying (APS), formed a homogeneous lamellar structure with an average thickness between 240 and 391 μm. This microstructural configuration enhanced osseointegration by establishing a bioactive surface conducive to cell adhesion and bone tissue integration. Moreover, the controlled release of calcium (Ca2+) and phosphate (PO43−) ions from the HA coating significantly enhanced cell proliferation and viability, with values consistently exceeding 70% at both 24 and 48 h of exposure. These outcomes meet the stringent biocompatibility criteria established in ISO 10993-5:2009, confirming the material’s safety for biological applications.
XRD analysis revealed partial thermal decomposition of HA phase during the APS process, resulting in the formation of β-tricalcium phosphate (β-TCP) and minor amounts of calcium oxide (CaO). However, the short exposure time preserved a significant proportion of the HA phase. The coexistence of HA and β-TCP contributes synergistically to the coating’s bioactivity, further reinforcing its biomedical potential.
The integration of optimized titanium porosity with bioactive hydroxyapatite coating presents a synergistic approach to addressing the dual challenges of stress shielding and inadequate osseointegration that have historically limited titanium implant performance. The porosity-induced reduction in elastic modulus effectively minimizes the mechanical mismatch between implant and bone tissue, while the hydroxyapatite coating provides a bioactive surface that actively promotes cellular adhesion and osteogenic differentiation.
Collectively, these findings establish that titanium compositions ranging from Ti65 to Ti80 present an optimal balance of porosity, mechanical performance, and bioactivity, positioning them as promising candidates for next-generation biomedical implants. The material design strategy demonstrated in this work offers a viable approach to developing orthopedic implants with superior biomechanical compatibility and enhanced osseointegration potential.
Future research directions should evaluate coating adhesion strength under physiological conditions, investigate mechanical behavior under cyclic loading that mimics in vivo patterns, and analyze long-term degradation characteristics in physiological environments. Additionally, in vivo studies examining bone integration in animal models would provide valuable translational insights. Such investigations will be instrumental in further validating the clinical potential of these biomaterials, ultimately contributing to enhanced patient outcomes in bone repair, reconstruction, and joint replacement procedures.
Author Contributions
Conceptualization, K.R.-V. and C.C.-G.; methodology, J.E.L.-S., R.C.-B. and I.O.-A.; formal analysis, A.T.-O. and R.C.-B.; investigation, J.M.H.-R. and K.R.-V.; resources, J.E.L.-S. and V.M.O.-C.; writing—original draft preparation, K.R.-V. and A.T.-O.; writing—review, investigation and editing, J.M.H.-R. and C.C.-G.; supervision, V.M.O.-C. and C.C.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Primary osteoblasts were isolated by I.O.-A. from Wistar rats using enzymatic digestion with collagenase, which was approved by the Research Ethics Committee from Universidad Autónoma de Ciudad Juárez (approval number CEI-2023-2-148).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
K.R.-V. thanks the Secretaria de Ciencia, Humanidades, Tecnologia e Innovacion for the support of her doctoral fellowship (grant SECIHTI 965121). Special thanks are due to K. Campos Venegas and A.I. Gonzalez Jacquez for sharing their expertise and technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AM | additive manufacturing |
| APS | atmospheric plasma spraying |
| DMLS | direct metal laser sintering |
| DMSO | dimethyl sulfoxide |
| EDS | energy-dispersive X-ray spectroscopy |
| FBS | fetal bovine serum |
| FCC | face-centered cubic |
| HA | hydroxyapatite |
| HCP | hexagonal close-packed |
| ICP | inductively coupled plasma emission spectrometry |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide |
| PS | press and sintering |
| SEM | scanning electron microscopy |
| TCP | tricalcium phosphate |
| XRD | X-ray diffraction |
References
- Zhao, Q.; Sun, Q.; Xin, S.; Chen, Y.; Wu, C.; Wang, H.; Xu, J.; Wan, M.; Zeng, W.; Zhao, Y. High-strength titanium alloys for aerospace engineering applications: A review on melting-forging process. Mater. Sci. Eng. A 2022, 845, 143260. [Google Scholar] [CrossRef]
- Amigo, V.; Romero, F.; Salvador, M.; Busquets, D. Matrix-reinforcement reactivity in P/M titanium matrix composites. Rev. De Metal. 2007, 43, 434–447. [Google Scholar]
- Abd-Elaziem, W.; Darwish, M.A.; Hamada, A.; Daoush, W.M. Titanium-Based alloys and composites for orthopedic implants Applications: A comprehensive review. Mater. Des. 2024, 241, 112850. [Google Scholar] [CrossRef]
- Campanelli, L.C. A review on the recent advances concerning the fatigue performance of titanium alloys for orthopedic applications. J. Mater. Res. 2021, 36, 151–165. [Google Scholar] [CrossRef]
- Fellah, M.; Hezil, N.; Leila, D.; Samad, M.A.; Djellabi, R.; Kosman, S.; Montagne, A.; Iost, A.; Obrosov, A.; Weiss, S. Effect of sintering temperature on structure and tribological properties of nanostructured Ti–15Mo alloy for biomedical applications. Trans. Nonferrous Met. Soc. China 2019, 29, 2310–2320. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A. Biomedical applications of titanium alloys: A comprehensive review. Materials 2023, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Yokoya, S.; Harada, Y.; Sumimoto, Y.; Kikugawa, K.; Natsu, K.; Nakamura, Y.; Nagata, Y.; Negi, H.; Watanabe, C.; Adachi, N. Factors affecting stress shielding and osteolysis after reverse shoulder arthroplasty: A multicenter study in a Japanese population. J. Orthop. Sci. 2024, 29, 521–528. [Google Scholar] [CrossRef]
- Shahzamanian, M.; Banerjee, R.; Dahotre, N.B.; Srinivasa, A.R.; Reddy, J. Analysis of stress shielding reduction in bone fracture fixation implant using functionally graded materials. Compos. Struct. 2023, 321, 117262. [Google Scholar] [CrossRef]
- Yamako, G.; Chosa, E.; Totoribe, K.; Hanada, S.; Masahashi, N.; Yamada, N.; Itoi, E. In-vitro biomechanical evaluation of stress shielding and initial stability of a low-modulus hip stem made of β type Ti-33.6 Nb-4Sn alloy. Med. Eng. Phys. 2014, 36, 1665–1671. [Google Scholar] [CrossRef]
- Wei, P.; Fang, J.; Fang, L.; Wang, K.; Lu, X.; Ren, F. Novel niobium and silver toughened hydroxyapatite nanocomposites with enhanced mechanical and biological properties for load-bearing bone implants. Appl. Mater. Today 2019, 15, 531–542. [Google Scholar] [CrossRef]
- Fujii, T.; Murakami, R.; Kobayashi, N.; Tohgo, K.; Shimamura, Y. Uniform porous and functionally graded porous titanium fabricated via space holder technique with spark plasma sintering for biomedical applications. Adv. Powder Technol. 2022, 33, 103598. [Google Scholar] [CrossRef]
- Takata, N.; Uematsu, K.; Kobashi, M. Compressive properties of porous Ti–Al alloys fabricated by reaction synthesis using a space holder powder. Mater. Sci. Eng. A 2017, 697, 66–70. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, J.; Meng, Z.; He, Z.; Zhang, Y.; Jiang, Y.; Zhou, R. Low elastic modulus Ti-Ag/Ti radial gradient porous composite with high strength and large plasticity prepared by spark plasma sintering. Mater. Sci. Eng. A 2017, 688, 330–337. [Google Scholar] [CrossRef]
- Bari, K.; Arjunan, A. Extra low interstitial titanium based fully porous morphological bone scaffolds manufactured using selective laser melting. J. Mech. Behav. Biomed. Mater. 2019, 95, 1–12. [Google Scholar] [CrossRef]
- Zettel, D.; Breitkopf, P.; Cauvin, L.; Nicolay, P.; Willmann, R. Multi-scale mechanical properties of Al–Mg–Zr–Sc alloys fabricated by direct metal laser sintering: Towards single-material composites. J. Mater. Res. Technol. 2025, 35, 2887–2913. [Google Scholar] [CrossRef]
- Ishfaq, K.; Abdullah, M.; Mahmood, M.A. A state-of-the-art direct metal laser sintering of Ti6Al4V and AlSi10Mg alloys: Surface roughness, tensile strength, fatigue strength and microstructure. Opt. Laser Technol. 2021, 143, 107366. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Punset, M.; Calero, J.A.; Gil, F.J.; Ruperez, E.; Manero, J.M. Powder metallurgy with space holder for porous titanium implants: A review. J. Mater. Sci. Technol. 2021, 76, 129–149. [Google Scholar] [CrossRef]
- Nuswantoro, N.F.; Manjas, M.; Suharti, N.; Juliadmi, D.; Kasuma, N.; Yusuf, Y.; Sari, Y.W.; Niinomi, M.; Akahori, T. Effect of hydroxyapatite coating thickness on inflammation and osseointegration of Ti–29Nb–13Ta-4.6 Zr (TNTZ) implants. J. Mater. Res. Technol. 2024, 30, 6210–6217. [Google Scholar] [CrossRef]
- Muñoz-Sanchez, E.; Arrieta-Gonzalez, C.; Quinto-Hernandez, A.; Garcia-Hernandez, E.; Porcayo-Calderon, J. Synthesis of hydroxyapatite from eggshell and its electrochemical characterization as a coating on titanium. Int. J. Electrochem. Sci. 2023, 18, 100204. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A Review on Ionic Substitutions in Hydroxyapatite Thin Films: Towards Complete Biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Rau, J.V.; Cacciotti, I.; Laureti, S.; Fosca, M.; Varvaro, G.; Latini, A. Bioactive, nanostructured Si-substituted hydroxyapatite coatings on titanium prepared by pulsed laser deposition. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Nevarez-Rascón, A.; Hurtado-Macías, A.; Esparza-Ponce, H.E.; Nevarez-Rascón, M.M.; González-Hernández, J.; Yacamán, M.J. Nano-structured hydroxyapatite and titanium dioxide enriching PENTA /UDMA adhesive as aesthetic coating for tooth enamel. Dent. Mater. 2021, 37, e290–e299. [Google Scholar] [CrossRef]
- Mohammad, N.F.; Ahmad, R.N.; Rosli, N.L.M.; Manan, M.S.A.; Marzuki, M.; Wahi, A. Sol gel deposited hydroxyapatite-based coating technique on porous titanium niobium for biomedical applications: A mini review. Mater. Today Proc. 2021, 41, 127–135. [Google Scholar] [CrossRef]
- Lu, M.; Chen, H.; Yuan, B.; Zhou, Y.; Min, L.; Xiao, Z.; Zhu, X.; Tu, C.; Zhang, X. Electrochemical Deposition of Nanostructured Hydroxyapatite Coating on Titanium with Enhanced Early Stage Osteogenic Activity and Osseointegration. Int. J. Nanomed. 2020, 15, 6605–6618. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, T.; Liao, Z.; Wei, Y.; Hang, R.; Huang, D. Engineered functional doped hydroxyapatite coating on titanium implants for osseointegration. J. Mater. Res. Technol. 2023, 27, 122–152. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef]
- Tejeda-Ochoa, A.; Rivera-Vicuña, K.G.; Ledezma-Sillas, J.E.; Herrera-Ramírez, J.M.; Carreño-Gallardo, C. The Effect of Space Holder Size on the Mechanical Properties of Porous Titanium. Microsc. Microanal. 2023, 29, 1459–1460. [Google Scholar] [CrossRef]
- ASTM B962; Standard Test Methods for Density of Compacted or Sintered Powder Metallurgy (PM) Products Using Archimedes’ Principle. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM E9; Standard Test Methods of Compression Testing of Metallic Materials at Room Temperature. ASTM International: West Conshohocken, PA, USA, 2025.
- Dias Corpa Tardelli, J.; Lima da Costa Valente, M.; Theodoro de Oliveira, T.; Cândido dos Reis, A. Influence of chemical composition on cell viability on titanium surfaces: A systematic review. J. Prosthet. Dent. 2020, 125, 421–425. [Google Scholar] [CrossRef]
- Arencibia, D.F.; Fernández, L.A.R.; Sánchez, D.L.C. Principal assays that to determine the citotoxicity of a substance, some considerations and their utility. Rev. De Toxicol. En Línea 2009, 42–52. [Google Scholar]
- Escobar M., L.; Rivera, A.; Aristizábal, F.A. Estudio comparativo de los métodos de resazurina y MTT en estudios de citotoxicidad en líneas celulares tumorales humanas. Vitae 2010, 17, 67–74. [Google Scholar] [CrossRef]
- Martel-Estrada, S.A.; Rodríguez-Espinoza, B.; Santos-Rodríguez, E.; Jiménez-Vega, F.; García-Casillas, P.E.; Martínez-Pérez, C.A.; Armendáriz, I.O. Biocompatibility of chitosan/Mimosa tenuiflora scaffolds for tissue engineering. J. Alloys Compd. 2015, 643, S119–S123. [Google Scholar] [CrossRef]
- Hudomalj, U.; Sichani, E.F.; Weiss, L.; Nabavi, M.; Wegener, K. Effect of particle size distribution width on repeatability of coating characteristics in atmospheric plasma spraying. Procedia CIRP 2022, 113, 530–535. [Google Scholar] [CrossRef]
- Lin, W.H.; Tsao, S.Y.; Cheng, T.C.; Shieh, J.; Yang, J.Y. Effect of Morphologies of Hydroxyapatite Powders on Thermal Sprayed Hydroxyapatite Coatings. Surf. Interfaces 2025, 60, 105948. [Google Scholar] [CrossRef]
- Sotiropoulou, P.; Fountos, G.; Martini, N.; Koukou, V.; Michail, C.; Kandarakis, I.; Nikiforidis, G. Bone calcium/phosphorus ratio determination using dual energy X-ray method. Phys. Med. 2015, 31, 307–313. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, S.C.; Freitas, R.B.; Sotiles, A.R.; Schemczssen-Graeff, Z.; De Almeida Miranda, I.M.; Biscaia, S.M.P.; Wypych, F.; da Silva Trindade, E.; Leão, M.P.; Zielak, J.C.; et al. 3D scaffold of hydroxyapatite/β tricalcium phosphate from mussel shells: Synthesis, characterization and cytotoxicity. Heliyon 2025, 11, e41585. [Google Scholar] [CrossRef]
- Jha, N.; Mondal, D.; Majumdar, J.D.; Badkul, A.; Jha, A.; Khare, A. Highly porous open cell Ti-foam using NaCl as temporary space holder through powder metallurgy route. Mater. Des. 2013, 47, 810–819. [Google Scholar] [CrossRef]
- Torres, Y.; Pavón, J.; Rodríguez, J. Processing and characterization of porous titanium for implants by using NaCl as space holder. J. Mater. Process. Technol. 2012, 212, 1061–1069. [Google Scholar] [CrossRef]
- Vahabzadeh, S.; Roy, M.; Bandyopadhyay, A.; Bose, S. Phase stability and biological property evaluation of plasma sprayed hydroxyapatite coatings for orthopedic and dental applications. Acta Biomater. 2015, 17, 47–55. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Foroutan, R.; Mohammadzadeh, A.; Javanbakht, S.; Mohammadi, R.; Ghorbani, M. Alginate/magnetic hydroxyapatite bio-nanocomposite hydrogel bead as a pH-responsive oral drug carrier for potential colon cancer therapy. Results Chem. 2025, 15, 102177. [Google Scholar] [CrossRef]
- Fatimah, I.; Hidayat, H.; Citradewi, P.W.; Tamyiz, M.; Doong, R.-A.; Sagadevan, S. Hydrothermally synthesized titanium/hydroxyapatite as photoactive and antibacterial biomaterial. Heliyon 2023, 9, e14434. [Google Scholar] [CrossRef]
- Busuioc, C.; Voicu, G.; Jinga, S.-I.; Mitran, V.; Cimpean, A. The influence of barium titanate on the biological properties of collagen-hydroxiapatite composite scaffolds. Mater. Lett. 2019, 253, 317–322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).