Biochemical Methane Production Potential of Different Industrial Wastes: The Impact of the Food-to-Microorganism (F/M) Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Analytical Methods
2.2. Biochemical Methane Potential Tests
2.3. Materials
2.4. Calculations
3. Results and Discussion
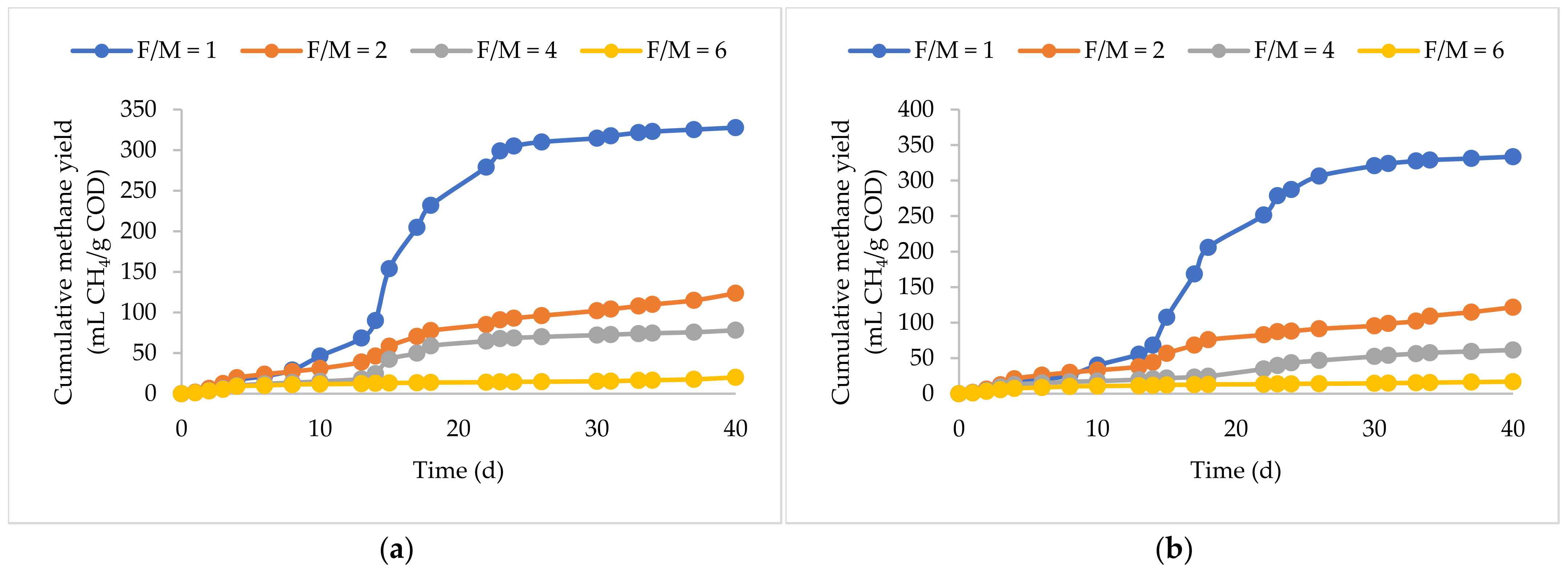
3.1. Methane Yields and Biodegradability
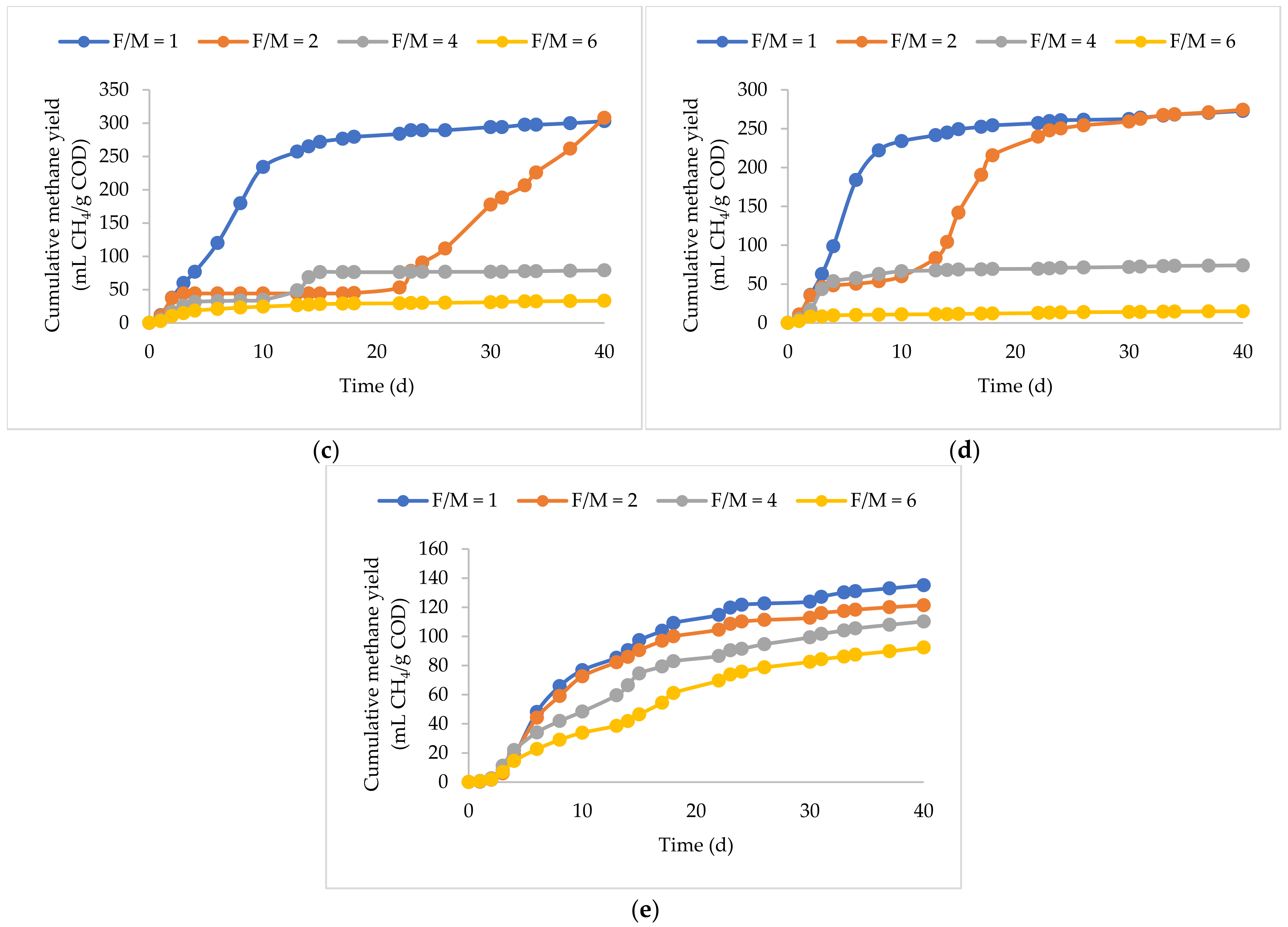
3.2. BP Plus KW
3.3. FOG
3.4. UFMP and PW
3.5. PP
3.6. Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fioreze, M.; Silva, C.M. Anaerobic digestion and pretreatment methods applied to primary and secondary sludge from pulp and paper mills: A review of the development of global research and a case study of a typical brazilian kraft pulp mill. J. Urban Environ. Eng. 2023, 17, 39–50. [Google Scholar]
- Abbasi, T.; Tauseef, S.M.; Abbasi, S.A. Anaerobic digestion for global warming control and energy generation—An overview. Renew. Sustain. Energy Rev. 2012, 16, 3228–3242. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Shekwaga, C.K.O.; Suruagy, M.V.T.; Ross, A.; Valero, M.A.C. Particle size, inoculum-to-substrate ratio and nutrient media effects on biomethane yield from food waste. Renew. Energy 2020, 151, 311–321. [Google Scholar] [CrossRef]
- Xie, A.; Deaver, J.A.; Miller, E.; Popat, S.C. Effect of feed-to-inoculum ratio on anaerobic digestibility of high-fat content animal rendering wastewater. Biochem. Eng. J. 2021, 176, 108215. [Google Scholar] [CrossRef]
- Yulisa, A.; Chairattanawat, C.; Park, S.H.; Jannat, M.A.H.; Hwang, S. Effect of Substrate-to-Inoculum Ratio and Temperatures During the Start-up of Anaerobic Digestion of Fish Waste. Ind. Domest. Waste Manag. 2022, 2, 17–29. [Google Scholar] [CrossRef]
- Kawai, M.; Nagao, N.; Tajima, N.; Niwa, C.; Matsuyama, T.; Toda, T. The effect of the labile organic fraction in food waste and the substrate/inoculum ratio on anaerobic digestion for a reliable methane yield. Bioresour. Technol. 2014, 157, 174–180. [Google Scholar] [CrossRef]
- Veluchamy, C.; Kalamdhad, A.S. Effect of Total Solid Content of Lignocellulose Pulp and Paper Mill Sludge on Methane Production and Modeling. J. Environ. Eng. 2020, 146, 04019121. [Google Scholar] [CrossRef]
- Nieto, P.P.; Hidalgo, D.; Irusta, R.; Kraut, D. Biochemical methane potential (BMP) of agro-food wastes from the Cider Region (Spain). Water Sci. Technol. 2012, 66, 1842–1848. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005; p. 1220. [Google Scholar]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Gallipoli, A.; Braguglia, C.M.; Gianico, A.; Montecchio, D.; Pagliaccia, P. Kitchen waste valorization through a mild-temperature pretreatment to enhance biogas production and fermentability: Kinetics study in mesophilic and thermophilic regimen. J. Environ. Sci. (China) 2020, 89, 167–179. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, J. Influence of feed/inoculum ratios and waste cooking oil content on the mesophilic anaerobic digestion of food waste. Waste Manag. 2018, 73, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Kafle, G.K.; Bhattarai, S.; Kim, S.H.; Chen, L. Effect of feed to microbe ratios on anaerobic digestion of Chinese cabbage waste under mesophilic and thermophilic conditions: Biogas potential and kinetic study. J. Environ. Manag. 2014, 133, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Hamza, R.A.; Elbeshbishy, E. Enhancement of denitrification efficiency using municipal and industrial waste fermentation liquids as external carbon sources. Sci. Total Environ. 2022, 816, 151578. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Bio/Technol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Pereira, M.A.; Sousa, D.Z.; Mota, M.; Alves, M.M. Mineralization of LCFA associated with anaerobic sludge: Kinetics, enhancement of methanogenic activity, and effect of VFA. Biotechnol. Bioeng. 2004, 88, 502–511. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, D.; Yan, J.; Qiao, W.; Wang, W.; Zhu, T. Effects of lipid concentration on anaerobic co-digestion of municipal biomass wastes. Waste Manag. 2014, 34, 1025–1034. [Google Scholar] [CrossRef]
- Palatsi, J.; Laureni, M.; Andrés, M.V.; Flotats, X.; Nielsen, H.B.; Angelidaki, I. Strategies for recovering inhibition caused by long chain fatty acids on anaerobic thermophilic biogas reactors. Bioresour. Technol. 2009, 100, 4588–4596. [Google Scholar] [CrossRef]
- Hwu, C.S.; Donlon, B.; Lettinga, G. Comparative toxicity of long-chain fatty acid to anaerobic sludges from various origins. Water Sci. Technol. 1996, 34, 351–358. [Google Scholar] [CrossRef]
- Carminati, P.; Gusmini, D.; Pizzera, A.; Catenacci, A.; Parati, K.; Ficara, E. Biogas from mono- and co-digestion of microalgal biomass grown on piggery wastewater. Water Sci. Technol. 2018, 78, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Mainardis, M.; Flaibani, S.; Trigatti, M.; Goi, D. Techno-economic feasibility of anaerobic digestion of cheese whey in small Italian dairies and effect of ultrasound pre-treatment on methane yield. J. Environ. Manag. 2019, 246, 557–563. [Google Scholar] [CrossRef]
- Bella, K.; Rao, P.V. Anaerobic co-digestion of cheese whey and septage: Effect of substrate and inoculum on biogas production. J. Environ. Manag. 2022, 308, 114581. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Okopi, S.I.; Jiang, Y.; Chen, Z.; Meng, L.; Li, Y.; Sun, W.; Li, C. Multi-criteria assessment of food waste and waste paper anaerobic co-digestion: Effects of inoculation ratio, total solids content, and feedstock composition. Renew. Energy 2022, 194, 40–50. [Google Scholar] [CrossRef]
- Rodriguez, C.; Alaswad, A.; El-Hassan, Z.; Olabi, A.G. Mechanical pretreatment of waste paper for biogas production. Waste Manag. 2017, 68, 157–164. [Google Scholar] [CrossRef]
- Haroun, B.M.; Nakhla, G.; Hafez, H.; Nasr, F.A. Impact of furfural on biohydrogen production from glucose and xylose in continuous-flow systems. Renew. Energy 2016, 93, 302–311. [Google Scholar] [CrossRef]
- Veluchamy, C.; Kalamdhad, A.S. Biochemical methane potential test for pulp and paper mill sludge with different food/microorganisms ratios and its kinetics. Int. Biodeterior. Biodegrad. 2017, 117, 197–204. [Google Scholar] [CrossRef]

| Parameters | BP Plus KW | FOG | UFMP | PW | PP Sludge | Inoculum |
|---|---|---|---|---|---|---|
| COD (g/L) | 195 ± 14 | 283 ± 20 | 70 ± 5 | 252 ± 18 | 38 ± 3 | 18 ± 0.5 |
| sCOD (g/L) | 45 ± 3 | 48 ± 3 | 68 ± 5 | 224 ± 16 | 19 ± 1 | 0.8 ± 0.02 |
| VFA (g/L) | 5.6 ± 0.4 | 7 ± 0.5 | 5.3 ± 0.4 | 5.4 ± 0.4 | 0.9 ± 0.1 | – |
| TS (g/L) | 164 ± 11 | 211± 15 | 67 ± 5 | 209 ± 15 | 46 ± 3 | 17 ± 0.5 |
| VS (g/L) | 154 ± 11 | 206 ± 14 | 61± 4 | 191± 13 | 27± 2 | 14 ± 0.4 |
| TSS (g/L) | 148 ± 10 | 192 ±13 | 25 ± 2 | 49 ± 3 | 18 ± 1 | 16 ± 0.5 |
| VSS (g/L) | 146 ±10 | 188 ±13 | 24 ± 2 | 48 ± 3 | 14 ± 1 | 13 ± 0.4 |
| Ammonia (mg/L) | 80 ± 6 | 30 ± 2 | 3 ± 0.2 | 40 ± 3 | 20 ± 1 | 782 ± 24 |
| pH | 3.68 | 3.78 | 4.32 | 5.42 | 6.61 | 7.2 |
| Alkalinity (g/L as CaCO3) | – | – | – | 5.3 ± 0.4 | 0.5 ± 0 | 4.2 ± 0.1 |
| Substrate | F/M | First-Order Rate (k) (d−1) | Modified-Gompertz Kinetics | ||
|---|---|---|---|---|---|
| P (mL CH4/g COD) | Rm (mL CH4/g COD∙d) | λ (d) | |||
| BP plus KW | 1 | 0.14 | 326 | 31 | 10.32 |
| 2 | 0.13 | 128 | 4 | 2.15 | |
| 4 | 0.12 | 79 | 5 | 6.36 | |
| 6 | 0.12 | 15 | 2 | 0 | |
| FOG | 1 | 0.18 | 326 | 31 | 10.32 |
| 2 | 0.23 | 128 | 4 | 2.15 | |
| 4 | 0.21 | 79 | 5 | 6.36 | |
| 6 | 0.17 | 15 | 2 | 0 | |
| UFMP | 1 | 0.14 | 295 | 27 | 1.16 |
| 2 | 0.13 | 308 | 14 | 17 | |
| 4 | 0.13 | 80 | 5 | 0.00 | |
| 6 | 0.11 | 30 | 4 | 0.00 | |
| PW | 1 | 0.12 | 260 | 39 | 1.32 |
| 2 | 0.13 | 293 | 13 | 3.69 | |
| 4 | 0.11 | 70 | 18 | 0.87 | |
| 6 | 0.09 | 13 | 3 | 0 | |
| PP | 1 | 0.16 | 129 | 8 | 1.53 |
| 2 | 0.14 | 116 | 8 | 1.68 | |
| 4 | 0.13 | 107 | 5 | 0.80 | |
| 6 | 0.13 | 96 | 4 | 1.58 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Sayed, A.; Ismail, A.; Rabii, A.; Hamze, A.; Hamza, R.A.; Elbeshbishy, E. Biochemical Methane Production Potential of Different Industrial Wastes: The Impact of the Food-to-Microorganism (F/M) Ratio. Processes 2025, 13, 802. https://doi.org/10.3390/pr13030802
El Sayed A, Ismail A, Rabii A, Hamze A, Hamza RA, Elbeshbishy E. Biochemical Methane Production Potential of Different Industrial Wastes: The Impact of the Food-to-Microorganism (F/M) Ratio. Processes. 2025; 13(3):802. https://doi.org/10.3390/pr13030802
Chicago/Turabian StyleEl Sayed, Ahmed, Amr Ismail, Anahita Rabii, Abir Hamze, Rania Ahmed Hamza, and Elsayed Elbeshbishy. 2025. "Biochemical Methane Production Potential of Different Industrial Wastes: The Impact of the Food-to-Microorganism (F/M) Ratio" Processes 13, no. 3: 802. https://doi.org/10.3390/pr13030802
APA StyleEl Sayed, A., Ismail, A., Rabii, A., Hamze, A., Hamza, R. A., & Elbeshbishy, E. (2025). Biochemical Methane Production Potential of Different Industrial Wastes: The Impact of the Food-to-Microorganism (F/M) Ratio. Processes, 13(3), 802. https://doi.org/10.3390/pr13030802









