Freeze-Dried Liposomes as Carriers of Eugenia pyriformis Cambess Phytoactives for Cosmetic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Pre-Processing of Uvaia Fruits
2.2. Freeze-Drying of Uvaia Pulp
2.3. Extraction of Bioactive Constituents from Freeze-Dried Uvaia Fruit Pulp
2.4. Characterization of Uvaia Pulp Extracts
2.4.1. Total Polyphenol Content
2.4.2. Ascorbic Acid Content
2.4.3. Antioxidant Activity (DPPH Assay)
2.5. Liposome Preparation and Characterization
2.5.1. Liposome Production
2.5.2. Liposome Characterization
2.6. Production and Characterization of Freeze-Dried Liposomes
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Uvaia Pulp Extract
3.2. Liposome Preparation and Characterization
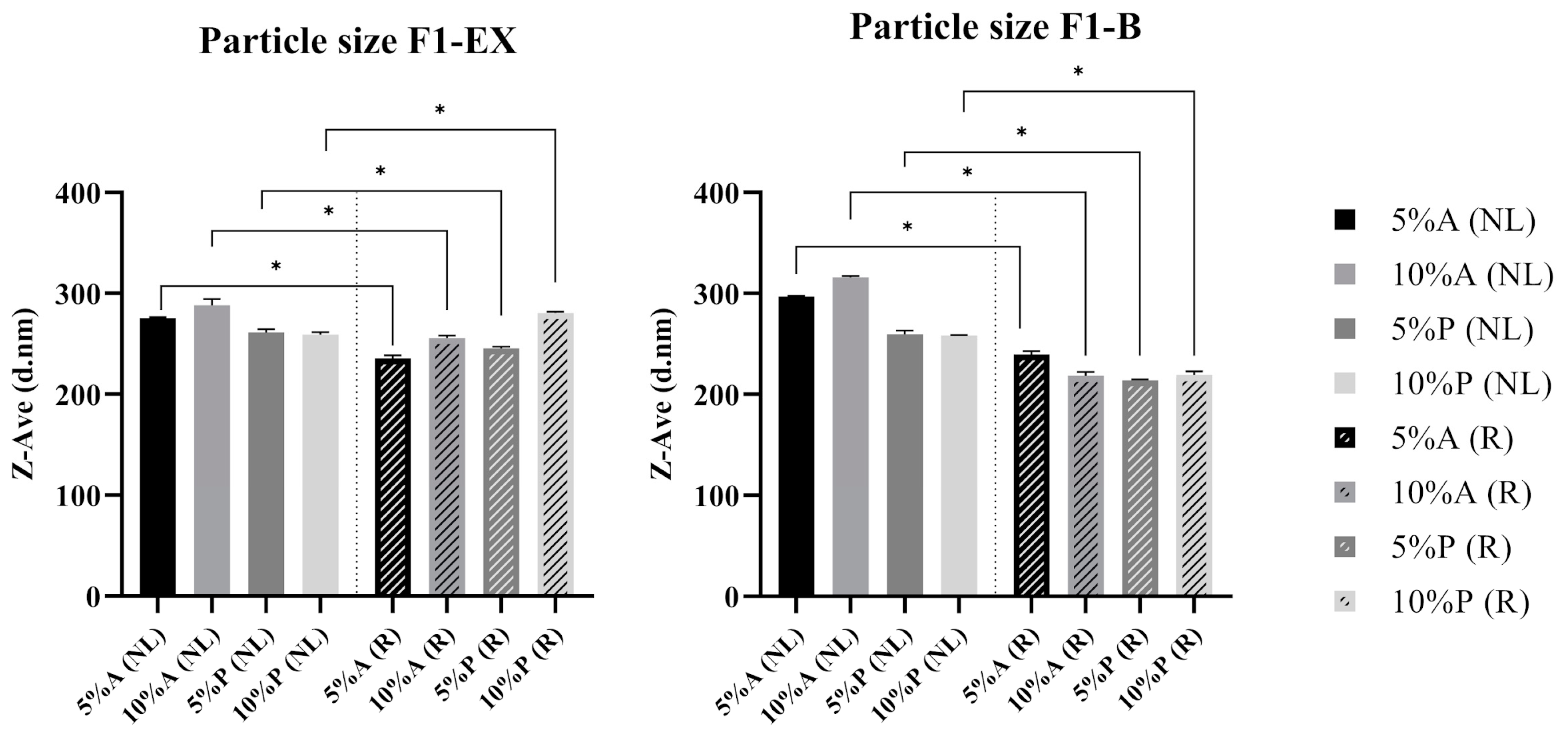
3.3. Impact of Homogenization on the Physicochemical Properties of Liposomes
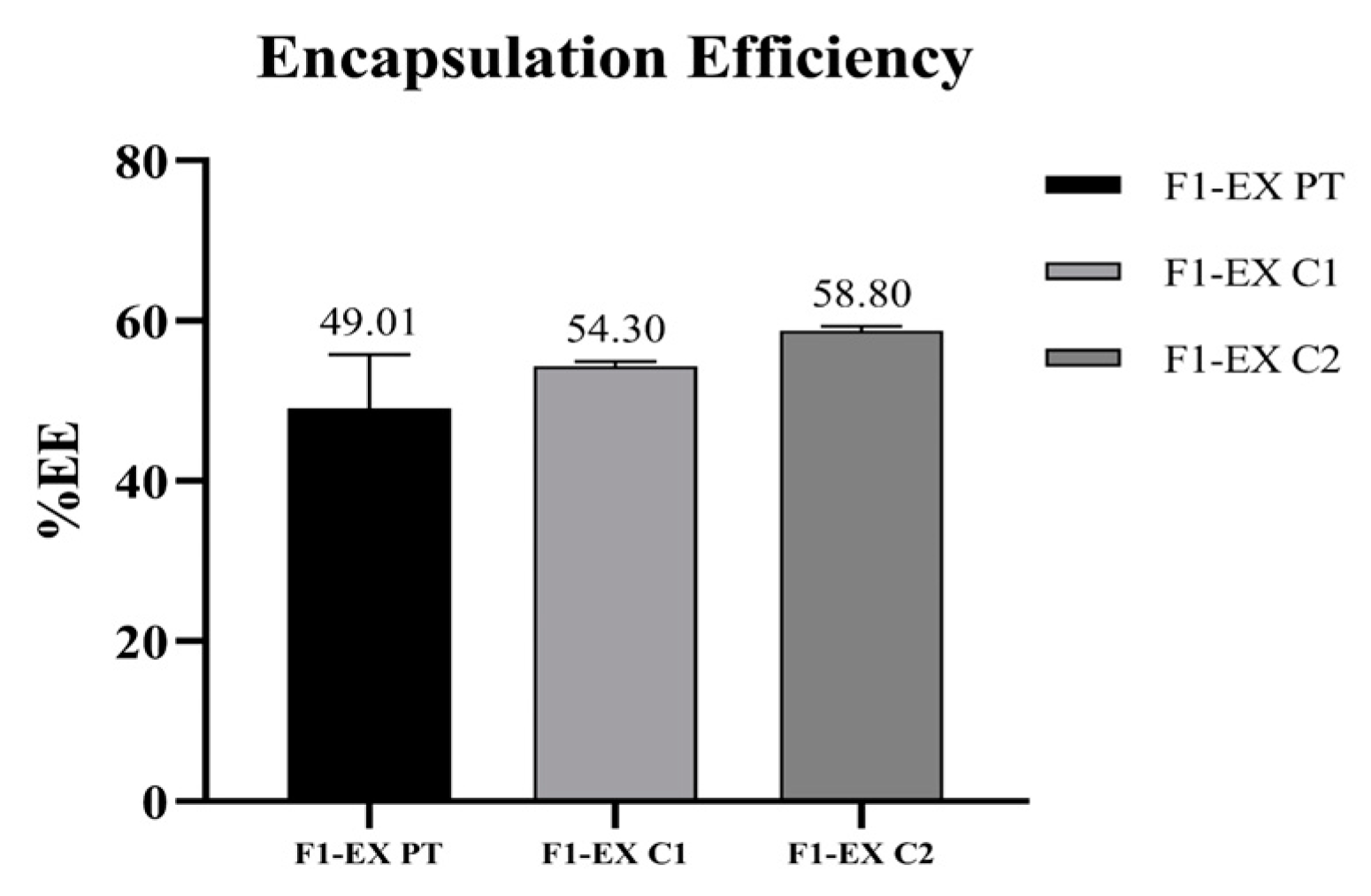
3.4. Production and Characterization of Freeze-Dried Liposomes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, B.D.L.D.A.; Vasconcelos, M.A.D.S.; Batista, K.S.; Batista, F.R.D.C.; Cavalcante, H.C.; Toscano, L.D.L.T.; Aquino, J.D.S. Hepatoprotective, lipid-lowering and antioxidant effects of mangaba powder (Hancornia speciosa) administered to rats fed a high-fat diet. Foods 2024, 13, 3773. [Google Scholar] [CrossRef] [PubMed]
- Guest, P.C. (Ed.) Reviews on Biomarker Studies in Aging and Anti-Aging Research; Springer Nature: Cham, Switzerland, 2019; Volume 1178. [Google Scholar]
- Silva, N.A.D.; Rodrigues, E.; Mercadante, A.Z.; de Rosso, V.V. Phenolic compounds and carotenoids from four fruits native from the Brazilian Atlantic forest. J. Agric. Food Chem. 2014, 62, 5072–5084. [Google Scholar] [CrossRef] [PubMed]
- Haminiuk, C.W.I.; Plata-Oviedo, M.S.V.; de Mattos, G.; Carpes, S.T.; Branco, I.G. Extraction and quantification of phenolic acids and flavonols from Eugenia pyriformis using different solvents. J. Food Sci. Technol. 2014, 51, 2862–2866. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.G.; Sganzerla, W.G.; Jacomino, A.P.; da Silva, E.P.; Xiao, J.; Simal-Gandara, J. Chemical composition, bioactive compounds, and perspectives for the industrial formulation of health products from uvaia (Eugenia pyriformis Cambess–Myrtaceae): A comprehensive review. J. Food Compos. Anal. 2022, 109, 104500. [Google Scholar] [CrossRef]
- Jacomino, A.P.; da Silva, A.P.; de Freitas, T.P.; de Paula Morais, V.S. Uvaia—Eugenia pyriformis Cambess. In Exotic Fruits; Academic Press: London, UK, 2018; pp. 435–438. [Google Scholar]
- Krumreich, F.; D’Avila, R.F.; Freda, S.A.; Chim, J.F.; Chaves, F.C. Análises físico-químicas e estabilidade de compostos bioativos presentes em polpa de uvaia em pó obtidos por métodos de secagem e adição de maltodextrina e goma arábica. Rev. Thema 2016, 13, 4–17. [Google Scholar] [CrossRef]
- Oliveira, E.N.A.; da Costa Santos, D.; de Sousa, F.C.; Martins, J.N.; de Oliveira, S.P.A. Obtenção de ubaia desidratada pelo processo de liofilização. Rev. Bras. De Tecnol. Agroindustrial 2010, 4, 235–242. [Google Scholar] [CrossRef]
- Chong, C.H.; Law, C.L. Drying of Exotic Fruits. In Vegetables and Fruits, 2nd ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 1–42. [Google Scholar]
- Yang, S.; Liu, L.; Han, J.; Tang, Y. Encapsulating plant ingredients for dermocosmetic application: An updated review of delivery systems and characterization techniques. Int. J. Cosmet. Sci. 2020, 42, 16–28. [Google Scholar] [CrossRef]
- Silva, G.S.; Jange, C.G.; Rocha, J.S.; Chaves, M.A.; Pinho, S.C. Characterisation of curcumin-loaded proliposomes produced by coating of micronised sucrose and hydration of phospholipid powders to obtain multilamellar liposomes. Int. J. Food Sci. Technol. 2017, 52, 772–780. [Google Scholar] [CrossRef]
- Rudzińska, M.; Grygier, A.; Knight, G.; Kmiecik, D. Liposomes as Carriers of Bioactive Compounds in Human Nutrition. Foods 2024, 13, 1814. [Google Scholar] [CrossRef] [PubMed]
- Bankole, V.O.; Osungunna, M.O.; Souza, C.R.F.; Salvador, S.L.; Oliveira, W.P. Spray-dried proliposomes: An innovative method for encapsulation of Rosmarinus officinalis L. polyphenols. AAPS PharmSciTech 2020, 21, 1–17. [Google Scholar] [CrossRef]
- Hua, Z.Z.; Li, B.G.; Liu, Z.J.; Sun, D.W. Freeze-drying of liposomes with cryoprotectants and its effect on retention rate of encapsulated ftorafur and vitamin A. Dry. Technol. 2003, 21, 1491–1505. [Google Scholar] [CrossRef]
- Kakuda, L.; Maia Campos, P.M.; Oliveira, W.P. Development and Efficacy Evaluation of Innovative Cosmetic Formulations with Caryocar brasiliense Fruit Pulp Oil Encapsulated in Freeze-Dried Liposomes. Pharmaceutics 2024, 16, 595. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.P. (Ed.) Phytotechnology: A Sustainable Platform for the Development of Herbal Products; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Darniadi, S.; Handoko, D.D.; Sunarmani, S.; Widowati, S. Determination of shelf-life using accelerated shelf-life testing (Aslt) method and characterization of the flavour components of freeze-dried durian (Durio zibethinus) products. Food Res. 2021, 5, 98–106. [Google Scholar] [CrossRef]
- Baldim, I.; Souza, C.R.F.; Oliveira, W.P. Encapsulation of Essential Oils in Lipid-Based Nanosystems. In Phytotechnology; CRC Press: Boca Raton, FL, USA, 2021; pp. 197–230. [Google Scholar]
- Zampiér, M.N. Desenvolvimento, Padronização e Avaliação Biológica de Extratos Nebulizados de Dalbergia ecastaphyllum. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2012. [Google Scholar]
- Bresolin, J.D.; Hubinger, S.Z. Metodologia para determinação de ácido ascórbico em sucos de citrus utilizando cromatografia líquida de alta eficiência. In Proceedings of the Simpósio Nacional de Instrumentação Agropecuária, São Carlos, Brazil, 18–21 November 2014; pp. 497–500. [Google Scholar]
- Fernandes, M.R.V.; Dias, A.L.T.; Carvalho, R.R.; Souza, C.R.F.; Oliveira, W.P.D. Antioxidant and antimicrobial activities of Psidium guajava L. spray dried extracts. Ind. Crops Prod. 2014, 60, 39–44. [Google Scholar] [CrossRef]
- Silveira, A.C.d.; Kassuia, Y.S.; Domahovski, R.C.; Lazzarotto, M. Método de DPPH adaptado: Uma ferramenta para analisar atividade antioxidante de polpa de frutos da erva-mate de forma rápida e reprodutível. Embrapa Florestas. Comun. Técnico 2018, 421, 11. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/188244/1/CT-421-1642-final.pdf (accessed on 24 February 2025).
- Magalhães, W.L.E.; De Matos, M.; Lourençon, T. Metodologia Científica: Determinação da Capacidade Antioxidante de Lignina Pela Captura do Radical Livre DPPH; Embrapa Florestas: Colombo, Brazil, 2018. [Google Scholar]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]
- Baldim, I.; Tonani, L.; Kress, M.R.v.Z.; Oliveira, W.P. Lippia sidoides essential oil encapsulated in lipid nanosystem as an anti-Candida agent. Ind. Crops Prod. 2019, 127, 73–81. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.F.; Gil, G.A.; Moraes, L.N.; Furtado, F.B.; Kakuda, L.; Grotto, R.M.T.; Oliveira, W.P. Nanostructured lipid carriers loaded with essential oils: A strategy against SARS-CoV-2. J. Microencapsul. 2024, 41, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Farrell, E.; Brousseau, J.L. Guide for DLS sample preparation. Brookhaven Instrum. 2014, 1, 1–3. [Google Scholar]
- Malvern. User Manual: Zetasizer Nano; Malvern: Malvern, UK, 2013. [Google Scholar]
- ISO 14887; Sample Preparation—Dispersing Procedures for Powders in Liquids. International Organization for Standardization: Geneva, Switzerland, 2000.
- Rockenbach, I.I.; Silva, G.L.D.; Rodrigues, E.; Kuskoski, E.M.; Fett, R. Influência do solvente no conteúdo total de polifenóis, antocianinas e atividade antioxidante de extratos de bagaço de uva (Vitis vinifera) variedades Tannat e Ancelota. Food Sci. Technol. 2008, 28, 238–244. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Romanini, E.B.; Silva, E.; Pilau, E.J.; Da Costa, S.C.; Madrona, G.S. Uvaia (Eugenia pyriformis Cambess) residue as a source of antioxidants: An approach to ecofriendly extraction. LWT 2021, 138, 110785. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Farias, P.D.; de Araujo, F.F.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; Catharino, R.R.; Pastore, G.M. Distribution of nutrients and functional potential in fractions of Eugenia pyriformis: An underutilized native Brazilian fruit. Food Res. Int. 2020, 137, 109522. [Google Scholar] [CrossRef] [PubMed]
- Zlatić, N.; Jakovljević, D.; Stanković, M. Temporal, plant part, and interpopulation variability of secondary metabolites and antioxidant activity of Inula helenium L. Plants 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Carrubba, A.; Lazzara, S.; Giovino, A.; Ruberto, G.; Napoli, E. Content variability of bioactive secondary metabolites in Hypericum perforatum L. Phytochem. Lett. 2021, 46, 71–78. [Google Scholar] [CrossRef]
- Abrafrutas. Quais São as Frutas Mais Produzidas No Brasil? Available online: https://abrafrutas.org/2023/04/quais-sao-as-frutas-mais-produzidas-no-brasil/ (accessed on 15 January 2025).
- NEPA—UNICAMP. Tabela Brasileira de Composição de Alimentos; NEPA—Unicamp: Campinas, Brazil, 2011. [Google Scholar]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef] [PubMed]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-aging and brightening effects of a topical treatment containing vitamin C, vitamin E, and raspberry leaf cell culture extract: A split-face, randomized controlled trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- Jagdeo, J.; Kurtti, A.; Hernandez, S.; Akers, N.; Peterson, S. Novel Vitamin C and E and Green Tea Polyphenols Combination Serum Improves Photoaged Facial Skin. J. Drugs Dermatol. JDD 2021, 20, 996–1003. [Google Scholar] [CrossRef]
- Cassol, G.S. Estudo da Aplicação do Ultrassom e de um Agitador de alta Velocidade no Desenvolvimento de Protetores Solares. Bachelor’s Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2016. [Google Scholar]
- Hielscher, T. Ultrasonic Production of Nano-Size Dispersions and Emulsions. In Proceedings of the ENS 2005 Conference, Paris, France, 14–16 December 2005; pp. 138–143. [Google Scholar]
- Chirayil, C.J.; Joy, J.; Mathew, L. Instrumental techniques for the characterization of nanocarriers. In Thermal and Rheological Measurement Techniques for Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–36. [Google Scholar]
- Anton, N.; Vandamme, T.F.; Ubrich, N. Nano-emulsions and nanocapsules by the PIT method: An investigation on the role of the temperature cycling on the emulsion phase inversion. Int. J. Pharm. 2007, 344, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.; de Freitas, L.A.P.; Maia Campos, P.M.B.G. Topical formulation containing beeswax-based nanocarriers improved in vivo skin barrier function. AAPS PharmSciTech 2017, 18, 2505–2516. [Google Scholar] [CrossRef] [PubMed]
- Filon, F.L.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanocarriers skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Baldim, I. Desenvolvimento Tecnológico e Secagem de Sistemas Lipídicos Incorporando óleo Essencial de Lippia Sidoides. Ph.D. Thesis, Universidade de São Paulo, Ribeirão Preto, Brazil, 2017. [Google Scholar]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, characterization and applications of liposomes: State of the art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, A.; Zhou, S.; Ruan, J.; Qian, C.; Wu, C.; Ye, L. Preparation of VC nanoliposomes by high pressure homogenization: Process optimization and evaluation of efficacy, transdermal absorption, and stability. Heliyon 2024, 10, e29516. [Google Scholar] [CrossRef]
- Oliveira, W.P.; Freitas, L.A.P.; Freire, J.T. Drying of pharmaceutical products. In Transport Phenomena in Particulate Systems; Freire, J.T., Silveira, A.M., Ferreira, M.C., Eds.; Bentham Science: Sharjah, United Arab Emirates, 2012; pp. 148–171. [Google Scholar]
- Labuza, T.P.; Altunakar, B. Water activity prediction and moisture sorption isotherms. In Water Activity in Foods: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2020; pp. 161–205. [Google Scholar]
- Chaves, M.A.; Pinho, S.C. Unpurified soybean lecithins impact on the chemistry of proliposomes and liposome dispersions encapsulating vitamin D3. Food Biosci. 2020, 37, 100700. [Google Scholar] [CrossRef]






| Components | Concentration (%) | |
|---|---|---|
| F1-B | F1-EX | |
| Phospholipon® 90H | 5 | 5 |
| Cholesterol | 1 | 1 |
| Absolute ethanol | 28 | 28 |
| Uvaia pulp extract | - | 1 |
| Milli-Q® water (q.s.p.) | 100 | 100 |
| Sample | Bioactive Concentration | |||||||
|---|---|---|---|---|---|---|---|---|
| Total Polyphenols | Vitamin C (AA) | Antioxidant Activity | ||||||
| GAEeb (µg/mL) | GAEdb (mg/g) | GAEfb (mg/g) | AAeb (µg/mL) | AAdb (mg/g) | AAfb (mg/g) | IC50 (µg/mL) | EC50 (g Extract/100 g DPPH) | |
| EX.AQ | 255.67 ± 5.60 * | 7.67 ± 0.17 | 0.85 ± 0.02 | 139.01 ± 1.59 | 4.17 ± 0.05 | 0.46 ± 0.01 | 219.87 ± 6.00 * | 3.69 |
| EX.ET | 548.09 ± 31.70 * | 16.44 ± 0.95 | 1.83 ± 0.11 | 127.46 ± 7.50 | 3.82 ± 0.23 | 0.43 ± 0.03 | 122.09 ± 5.28 * | 2.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.A.; Kakuda, L.; Oliveira, W.P. Freeze-Dried Liposomes as Carriers of Eugenia pyriformis Cambess Phytoactives for Cosmetic Applications. Processes 2025, 13, 693. https://doi.org/10.3390/pr13030693
Silva GA, Kakuda L, Oliveira WP. Freeze-Dried Liposomes as Carriers of Eugenia pyriformis Cambess Phytoactives for Cosmetic Applications. Processes. 2025; 13(3):693. https://doi.org/10.3390/pr13030693
Chicago/Turabian StyleSilva, Gabriela Alves, Letícia Kakuda, and Wanderley Pereira Oliveira. 2025. "Freeze-Dried Liposomes as Carriers of Eugenia pyriformis Cambess Phytoactives for Cosmetic Applications" Processes 13, no. 3: 693. https://doi.org/10.3390/pr13030693
APA StyleSilva, G. A., Kakuda, L., & Oliveira, W. P. (2025). Freeze-Dried Liposomes as Carriers of Eugenia pyriformis Cambess Phytoactives for Cosmetic Applications. Processes, 13(3), 693. https://doi.org/10.3390/pr13030693






