Co-Producing Xylo-Oligosaccharides, 5-HMF, Furfural, Organic Acids, and Reducing Sugars from Waste Poplar Debris by Clean Hydrothermal Pretreatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Hydrothermal Treatment
2.3. Chemical Composition Analysis
2.4. Enzymatic Hydrolysis
2.5. Accessibility, Hydrophobicity and Surface Lignin Area
2.5.1. Accessibility of Cellulose
2.5.2. Hydrophobicity
2.5.3. Surface Lignin Area
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of PD Between and After Hydrothermal Pretreatment
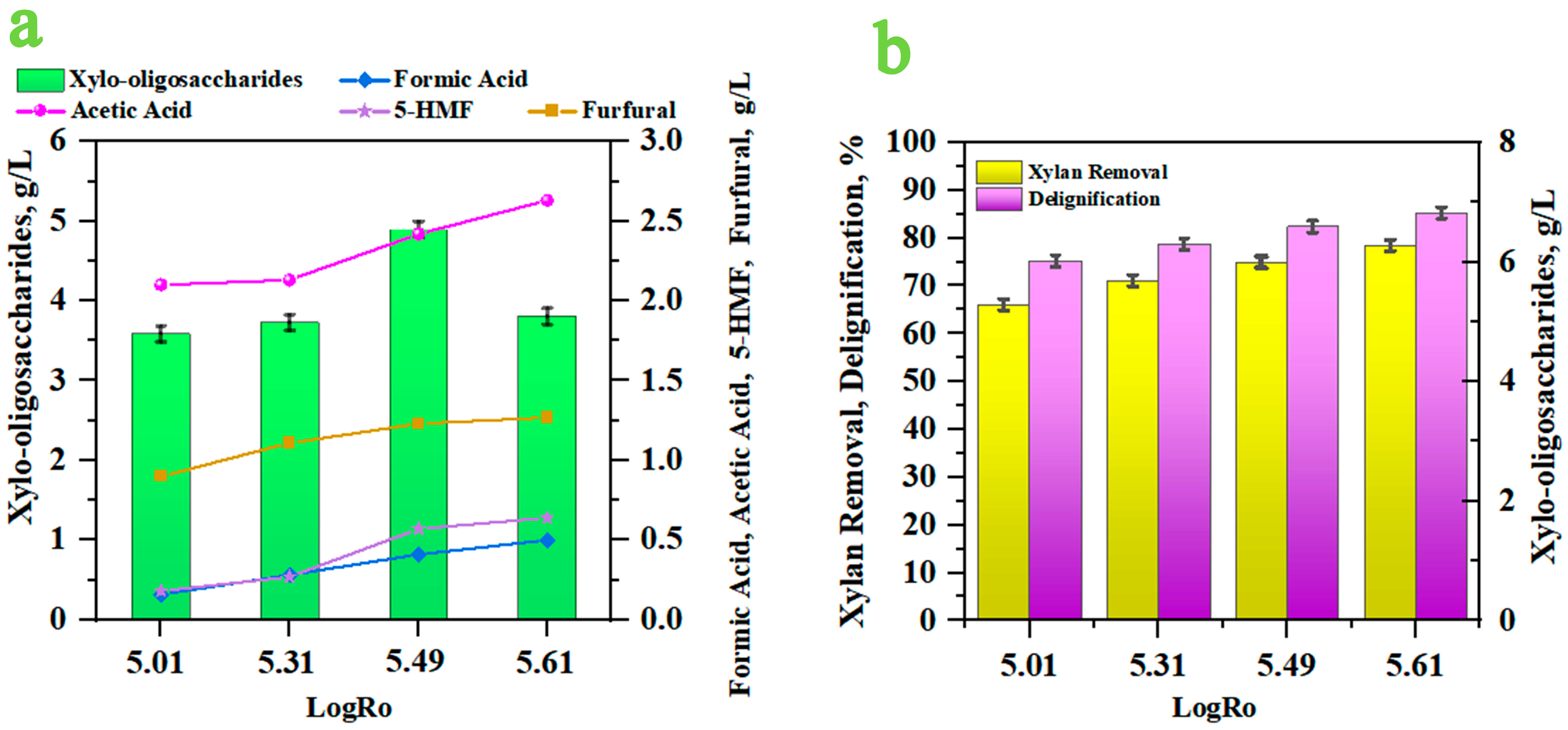
3.2. Chemical Composition of Hydrothermal Treated PD’s Prehydrolysate
3.3. Enzymatic Hydrolysis of Raw and Hydrothermal Treated PD
3.4. Characterization of Raw and Treated PD
3.4.1. Cellulose Accessibility
3.4.2. Surface Properties of Lignin
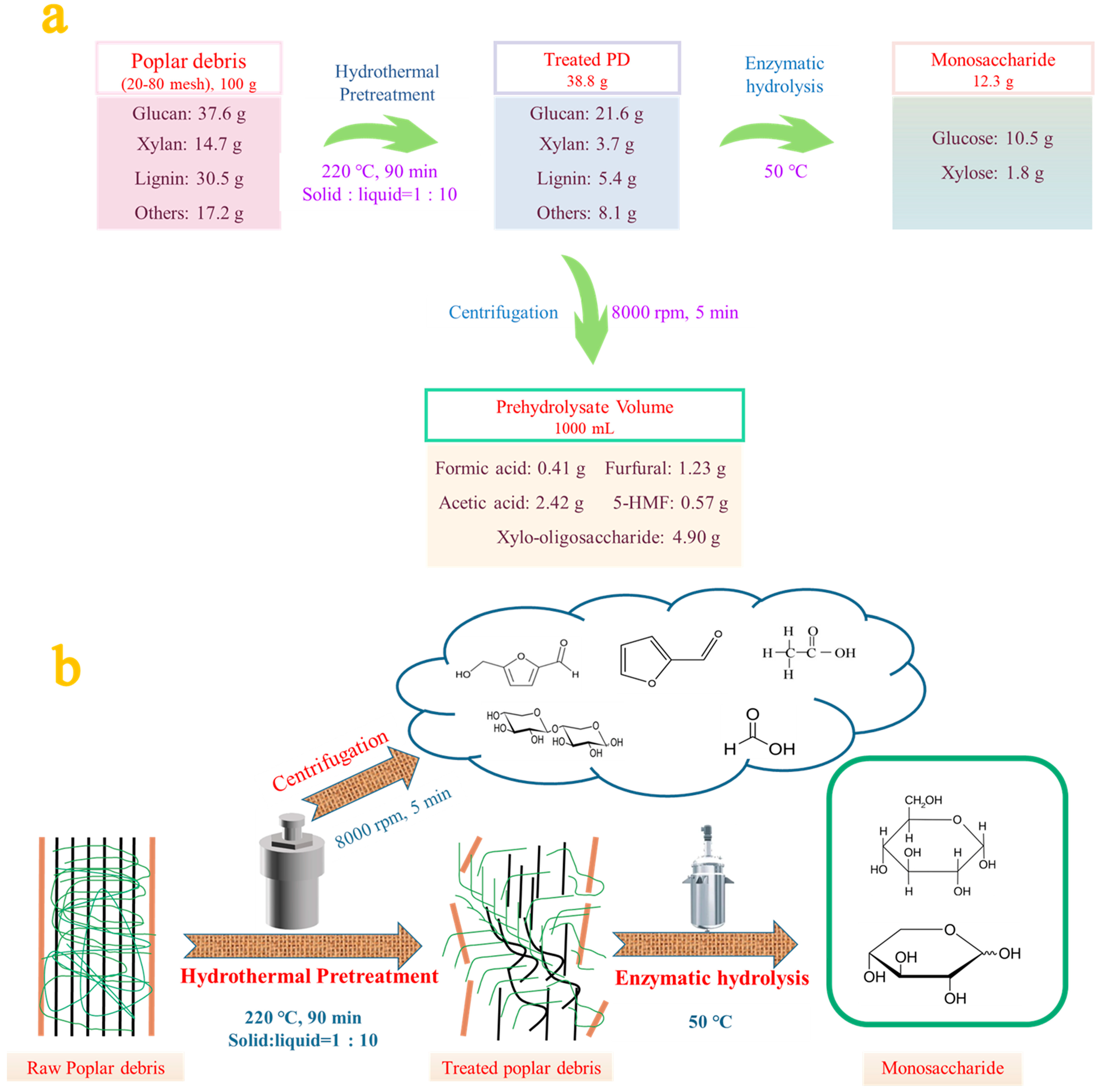
3.5. Mass Balance of Hydrothermal Pretreatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Begum, Y.A.; Kumari, S.; Jain, S.K.; Garg, M.C. A review on waste biomass-to-energy: Integrated thermochemical and biochemical conversion for resource recovery. Environ. Sci. Adv. 2024, 3, 1197–1216. [Google Scholar] [CrossRef]
- Yang, D.; Wen, P.; Ying, W.; He, Y.; Zhang, J. Maximizing carbohydrate recovery and lignin fractionation from bamboo through in-situ synthesis of peracetic acid via mild ethyl acetate-hydrogen peroxide treatment. Sep. Purif. Technol. 2025, 360, 131016. [Google Scholar] [CrossRef]
- Yadav, A.; Sharma, V.; Tsai, M.-L.; Chen, C.-W.; Sun, P.-P.; Nargotra, P.; Wang, J.-X.; Dong, C.-D. Development of lignocellulosic biorefineries for the sustainable production of biofuels: Towards circular bioeconomy. Bioresour. Technol. 2023, 381, 129145. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, Z.; He, Y.-C.; Ma, C. Comprehensive investigation of enhancing enzymatic digestion of maize straw through the synergistic pretreatment of stearyl trimethyl ammonium bromide with ortho-hydroxybenzoic acid. Fuel 2025, 379, 133053. [Google Scholar] [CrossRef]
- Paone, E.; Mauriello, F. Sustainable production of textile fibers, biofuels, and chemicals from poplar wood. Trends Chem. 2023, 5, 1–2. [Google Scholar] [CrossRef]
- Xu, J.; Dai, L.; Gui, Y.; Yuan, L.; Zhang, C.; Lei, Y. Synergistic benefits from a lignin-first biorefinery of poplar via coupling acesul-famate ionic liquid followed by mild alkaline extraction. Bioresour. Technol. 2020, 303, 122888. [Google Scholar] [CrossRef]
- Reyes, L.; Abdelouahed, L.; Mohabeer, C.; Buvat, J.-C.; Taouk, B. Energetic and exergetic study of the pyrolysis of lignocellulosic biomasses, cellulose, hemicellulose and lignin. Energy Convers. Manag. 2021, 244, 114459. [Google Scholar] [CrossRef]
- Mustafa, A.; Faisal, S.; Singh, J.; Rezki, B.; Kumar, K.; Moholkar, V.S.; Kutlu, O.; Aboulmagd, A.; Khamees Thabet, H.; El-Bahy, Z.M.; et al. Converting lignocellulosic biomass into valuable end products for decentralized energy solutions: A comprehensive overview. Sustain. Energy Technol. Assess. 2024, 72, 104065. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Tang, W.; Ma, C.; He, Y.-C. Improved enzymatic saccharification of bulrush via an efficient combination pretreatment. Bioresour. Technol. 2023, 385, 129369. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Qiu, X.; Xu, C. Hydrothermal treatment of lignocellulosic biomass towards low-carbon development: Production of high-value-added bioproducts. EnergyChem 2024, 6, 100133. [Google Scholar] [CrossRef]
- Jomnonkhaow, U.; Imai, T.; Reungsang, A. Microwave-assisted acid and alkali pretreatment of Napier grass for enhanced biohydrogen production and integrated biorefinery potential. Chem. Eng. J. Adv. 2024, 20, 100672. [Google Scholar] [CrossRef]
- Tavanai, T.; Kadivar, M.; Alsharif, M.A. The effect of hydrothermal treatment on the physico-chemical properties of wheat bran and the rheological characteristics of the resulting dough. J. Cereal Sci. 2025, 121, 104098. [Google Scholar] [CrossRef]
- Ge, Q.; Xiao, G.-M.; Wang, L.-Y.; Xu, J.-P.; Hou, C.-L.; Liao, T.-X.; Rao, X.-H.; Mao, J.-W.; Chen, L.-C. Effect of steam explosion pretreatment on the fermentation characteristics of polysaccharides from tea residue. Int. J. Biol. Macromol. 2024, 279, 134920. [Google Scholar] [CrossRef] [PubMed]
- Reis Kemita, L.; França Lopes da Silva, L.; Pratto, B. Optimizing dilute acid pretreatment for enhanced recovery and co-fermentation of hexose and pentose sugars for ethanol and butanol production. Fuel 2024, 372, 132187. [Google Scholar] [CrossRef]
- Sajid, S.; Kudakwashe Zveushe, O.; Resco de Dios, V.; Nabi, F.; Lee, Y.K.; Kaleri, A.R.; Ma, L.; Zhou, L.; Zhang, W.; Dong, F.; et al. Pretreatment of rice straw by newly isolated fungal consortium enhanced lignocellulose degradation and humification during composting. Bioresour. Technol. 2022, 354, 127150. [Google Scholar] [CrossRef]
- Shen, H.; Wang, R.; Bai, J.; Wang, J.; Qi, H.; Luo, A. Utilization of electron beam irradiation pretreatment for the extraction of pectic polysaccharides from Diaphragma juglandis fructus: Structural, physicochemical, and functional properties. Int. J. Biol. Macromol. 2024, 279, 135198. [Google Scholar] [CrossRef]
- Usman Khan, M.; Kiaer Ahring, B. Anaerobic digestion of biorefinery lignin: Effect of different wet explosion pretreatment conditions. Bioresour. Technol. 2020, 298, 122537. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, N.; Wang, S.; Meng, Y.; Sun, Y.; Ye, J.; Li, W.; Xu, S.; Wu, T.; Li, J.; et al. Organic solvent-assisted ethylenediamine pretreatment to improve the high-value utilization efficiency of corn stalk. Chem. Eng. J. 2024, 495, 153341. [Google Scholar] [CrossRef]
- Gonçalves, I.S.; Franco, T.T.; Forte, M.B.S. Cello-oligosaccharides and fermentable sugars production: An integrated deacetylation-delignification process to biomass pretreatment by using a mixture of protic ionic liquids. Food Bioprod. Process. 2024, 148, 95–107. [Google Scholar] [CrossRef]
- Wang, N.; Liu, K.; Hou, Z.; Zhao, Z.; Li, H.; Gao, X. The comparative techno-economic and life cycle assessment for multi-product biorefinery based on microwave and conventional hydrothermal biomass pretreatment. J. Clean. Prod. 2024, 474, 143562. [Google Scholar] [CrossRef]
- Wijaya, Y.P.; Putra, R.D.D.; Widyaya, V.T.; Ha, J.-M.; Suh, D.J.; Kim, C.S. Comparative study on two-step concentrated acid hydrolysis for the extraction of sugars from lignocellulosic biomass. Bioresour. Technol. 2014, 164, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.D.; Mishra, P.K.; Darani, K.K.; Agarwal, A.; Paul, V. Hydrothermal treatment of lignocellulose waste for the production of polyhydroxyalkanoates copolymer with potential application in food packaging. Trends Food Sci. Technol. 2022, 123, 233–250. [Google Scholar] [CrossRef]
- Lee, E.-J.; Shin, Y.-J.; Kim, H.; Lee, J.-W. Sequential pretreatment of lignocellulosic biomass employing hydrothermal treatment and ball milling to improve the efficiency of enzymatic hydrolysis. Ind. Crops Prod. 2024, 222, 120119. [Google Scholar] [CrossRef]
- Oladzad, S.; Fallah, N.; Mahboubi, A.; Afsham, N.; Taherzadeh, M.J.; Toghyani, J. Comparison of acid and hydrothermal pretreatments of date waste for value creation. Sci. Rep. 2024, 14, 18056. [Google Scholar] [CrossRef]
- Wang, S.; Sun, J.; Chen, M.; Hou, X.; Gao, Z. Effect of Fenton pretreatment on enzymatic hydrolysis of poplar. BioResources 2021, 16, 1980–1987. [Google Scholar] [CrossRef]
- Zhang, L.; You, T.; Zhou, T.; Zhang, L.; Xu, F. Synergistic effect of white-rot fungi and alkaline pretreatments for improving enzymatic hydrolysis of poplar wood. Ind. Crops Prod. 2016, 86, 155–162. [Google Scholar] [CrossRef]
- Ziaei-Rad, Z.; Pazouki, M. Cost-effective ionic liquid pretreatment of poplar wood for biofuel production: An optimization study using response surface methodology. Ind. Crops Prod. 2024, 219, 119036. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Lei, F.; Yang, S.; Jiang, J. Coproduction of xylooligosaccharides and fermentable sugars from sugarcane bagasse by seawater hydrothermal pretreatment. Bioresour. Technol. 2020, 309, 123385. [Google Scholar] [CrossRef]
- Espirito Santo, M.C.; Fockink, D.H.; Pellegrini, V.O.A.; Guimaraes, F.E.G.; deAzevedo, E.R.; Ramos, L.P.; Polikarpov, I. Physical techniques shed light on the differences in sugarcane bagasse structure subjected to steam explosion pretreatments at equivalent combined severity factors. Ind. Crops Prod. 2020, 158, 113003. [Google Scholar] [CrossRef]
- Gao, Q.; Tang, Z.; He, Y.-C. Valorization of wheat straw through enhancement of cellulose accessibility, xylan elimination and lig-nin removal by choline chloride:p-toluenesulfonic acid pretreatment. Int. J. Biol. Macromol. 2025, 301, 140335. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, C.; Yin, J.; Fan, B.; He, Y.-C.; Ma, C. Valorization of rapeseed straw through the enhancement of cellulose accessi-bility, lignin removal and xylan elimination using an n-alkyltrimethylammonium bromide-based deep eutectic solvent. Int. J. Biol. Macromol. 2025, 301, 140151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Tang, W.; Fan, B.; He, Y.-C.; Ma, C. Implementing efficient and sustainable pretreatment of Sorghum stalks for delignifica-tion and xylan separation with a ternary deep eutectic solvent under mild conditions. Int. J. Biol. Macromol. 2025, 303, 140417. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, L.; Liu, C.; Xu, A. Pretreatment of corn straw using the alkaline solution of ionic liquids. Bioresour. Technol. 2018, 260, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Labidi, J.; Gullón, P. Hydrothermal treatments of walnut shells: A potential pretreatment for subsequent product obtaining. Sci. Total Environ. 2021, 764, 142800. [Google Scholar] [CrossRef]
- He, J.; Huang, C.; Lai, C.; Huang, C.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yong, Q. The effect of lignin degradation products on the generation of pseudo-lignin during dilute acid pretreatment. Ind. Crops Prod. 2020, 146, 112205. [Google Scholar] [CrossRef]
- Batista, G.; Souza, R.B.A.; Pratto, B.; dos Santos-Rocha, M.S.R.; Cruz, A.J.G. Effect of severity factor on the hydrothermal pretreatment of sugarcane straw. Bioresour. Technol. 2019, 275, 321–327. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Huang, C.; Ling, Z.; Lai, C.; Yong, Q. Revealing migration discipline of lignin during producing fermentable sugars from wheat straw through autohydrolysis. Ind. Crops Prod. 2021, 171, 113849. [Google Scholar] [CrossRef]
- Nabarlatz, D.; Ebringerová, A.; Montané, D. Autohydrolysis of agricultural by-products for the production of xylo-oligosaccharides. Carbohydr. Polym. 2007, 69, 20–28. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Xu, H.; Liu, H.; He, Y.-C. Improving saccharification efficiency of corn stover through ferric chloride-deep eutectic solvent pretreatment. Bioresour. Technol. 2024, 399, 130579. [Google Scholar] [CrossRef]
- de Carvalho Silvello, M.A.; Martínez, J.; Goldbeck, R. Increase of reducing sugars release by enzymatic hydrolysis of sugarcane bagasse intensified by ultrasonic treatment. Biomass Bioenergy 2019, 122, 481–489. [Google Scholar] [CrossRef]
- Guo, H.; Chang, Y.; Lee, D.-J. Enzymatic saccharification of lignocellulosic biorefinery: Research focuses. Bioresour. Technol. 2018, 252, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Tang, W.; Ma, C.; He, Y.-C. Efficient co-production of xylooligosaccharides, furfural and reducing sugars from yellow bamboo via the pretreatment with biochar-based catalyst. Bioresour. Technol. 2023, 387, 129637. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, C.; Tang, W.; Ma, C.; He, Y.-C. A novel cetyltrimethylammonium bromide-based deep eutectic solvent pretreatment of rice husk to efficiently enhance its enzymatic hydrolysis. Bioresour. Technol. 2023, 376, 128806. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fang, G.; Yu, L.; Zhou, Y.; Meng, X.; Deng, Y.; Shen, K.; Ragauskas, A.J. Maximizing enzymatic hydrolysis efficiency of bamboo with a mild ethanol-assistant alkaline peroxide pretreatment. Bioresour. Technol. 2020, 299, 122568. [Google Scholar] [CrossRef]
- Jagusiak, A.; Chłopaś, K.; Zemanek, G.; Kościk, I.; Roterman, I. Interaction of Supramolecular Congo Red and Congo Red-Doxorubicin Complexes with Proteins for Drug Carrier Design. Pharmaceutics 2021, 13, 2027. [Google Scholar] [CrossRef]
- Uma Maheswari, R.; Mavukkandy, M.O.; Adhikari, U.; Naddeo, V.; Sikder, J.; Arafat, H.A. Synergistic effect of humic acid on alkali pretreatment of sugarcane bagasse for the recovery of lignin with phenomenal properties. Biomass Bioenergy 2020, 134, 105486. [Google Scholar] [CrossRef]
- Hassan, M.G.; Wassel, M.A.; Gomaa, H.A.; Elfeky, A.S. Adsorption of Rose Bengal dye from waste water onto modified biomass. Sci. Rep. 2023, 13, 14776. [Google Scholar] [CrossRef]
- Hina, Q.; Afshan, K.; Roheena, A.; Mehwish, I.; Daniel, C.H. Overview of Lignocellulolytic Enzyme Systems with Special Reference to Valorization of Lignocellulosic Biomass. Protein Pept. Lett. 2021, 28, 1349–1364. [Google Scholar] [CrossRef]
- Wang, T.; Ai, Y.; Peng, L.; Zhang, R.; Lu, Q.; Dong, C. Pyrolysis characteristics of poplar sawdust by pretreatment of anaerobic fermentation. Ind. Crops Prod. 2018, 125, 596–601. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Karimi, K.; Mirmohamadsadeghi, S. Hydrothermal pretreatment of safflower straw to enhance biogas production. Energy 2019, 172, 545–554. [Google Scholar] [CrossRef]
- Wang, R.; Wang, K.; Zhou, M.; Xu, J.; Jiang, J. Efficient fractionation of moso bamboo by synergistic hydrothermal-deep eutectic solvents pretreatment. Bioresour. Technol. 2021, 328, 124873. [Google Scholar] [CrossRef] [PubMed]
- Özbay, N.; Yaman, E.; Yargıç, A.Ş.; Şahin, R.Z.Y. Hydrothermal vs. dilute acid pre-treatments: Comparison of the biomass properties, distribution of pyrolysis products, and bio-oil characteristics. Biomass Convers. Biorefinery 2023, 13, 739–753. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, Y.; Liu, T.; Sun, S.; Ren, J.; Wu, A.; Li, H. A novel wet-mechanochemical pretreatment for the efficient enzymatic saccharification of lignocelluloses: Small dosage dilute alkali assisted ball milling. Energy Convers. Manag. 2019, 194, 46–54. [Google Scholar] [CrossRef]




| No. | Temperature, °C | Time, min | Glucan, % DM | Xylan, % DM | Lignin, % DM | Recovery, % | Removal, % | ||
|---|---|---|---|---|---|---|---|---|---|
| Solid | Glucan | Xylan | Lignin | ||||||
| Raw | / | / | 37.6 ± 0.2 | 14.7 ± 0.3 | 30.5 ± 0.1 | / | / | / | / |
| 1 | 160 | 30 | 39.4 ± 0.1 | 13.4 ± 0.2 | 20.5 ± 0.1 | 92.7 ± 0.1 | 97.2 ± 0.3 | 15.5 ± 0.1 | 37.7 ± 0.1 |
| 2 | 160 | 60 | 40.2 ± 0.1 | 12.5 ± 0.1 | 20.3 ± 0.1 | 90.6 ± 0.2 | 96.8 ± 0.1 | 22.9 ± 0.1 | 39.8 ± 0.1 |
| 3 | 160 | 90 | 41.9 ± 0.2 | 11.7 ± 0.2 | 17.5 ± 0.1 | 83.7 ± 0.1 | 93.2 ± 0.3 | 33.4 ± 0.2 | 52.1 ± 0.3 |
| 4 | 160 | 120 | 46.5 ± 0.1 | 10.2 ± 0.1 | 15.5 ± 0.1 | 74.2 ± 0.3 | 91.8 ± 0.2 | 48.4 ± 0.1 | 62.2 ± 0.2 |
| 5 | 180 | 30 | 44.8 ± 0.1 | 10.9 ± 0.1 | 17.0 ± 0.2 | 80.9 ± 0.1 | 96.4 ± 0.1 | 39.9 ± 0.1 | 54.9 ± 0.1 |
| 6 | 180 | 60 | 50.5 ± 0.2 | 11.3 ± 0.1 | 16.6 ± 0.1 | 69.6 ± 0.3 | 93.4 ± 0.1 | 46.4 ± 0.12 | 62.1 ± 0.3 |
| 7 | 180 | 90 | 51.8 ± 0.1 | 10.8 ± 0.2 | 15.1 ± 0.1 | 63.0 ± 0.1 | 86.8 ± 0.2 | 53.8 ± 0.1 | 68.9 ± 0.2 |
| 8 | 180 | 120 | 52.4 ± 0.1 | 10.1 ± 0.1 | 14.5 ± 0.1 | 60.5 ± 0.2 | 84.3 ± 0.3 | 58.5 ± 0.2 | 71.2 ± 0.1 |
| 9 | 200 | 30 | 52.1 ± 0.2 | 10.6 ± 0.3 | 14.8 ± 0.1 | 61.8 ± 0.2 | 85.6 ± 0.1 | 55.3 ± 0.1 | 70.1 ± 0.2 |
| 10 | 200 | 60 | 52.7 ± 0.1 | 10.5 ± 0.1 | 14.7 ± 0.1 | 57.6 ± 0.3 | 80.9 ± 0.2 | 58.8 ± 0.2 | 72.2 ± 0.3 |
| 11 | 200 | 90 | 53.6 ± 0.3 | 10.2 ± 0.1 | 15.6 ± 0.2 | 51.4 ± 0.1 | 73.3 ± 0.1 | 64.2 ± 0.1 | 73.8 ± 0.1 |
| 12 | 200 | 120 | 54.1 ± 0.2 | 10.0 ± 0.3 | 14.3 ± 0.1 | 47.3 ± 0.1 | 68.0 ± 0.2 | 67.8 ± 0.1 | 77.8 ± 0.1 |
| 13 | 220 | 30 | 53.9 ± 0.1 | 10.0 ± 0.1 | 15.2 ± 0.1 | 49.7 ± 0.2 | 71.2 ± 0.3 | 66.1 ± 0.2 | 75.3 ± 0.2 |
| 14 | 220 | 60 | 54.2 ± 0.1 | 9.8 ± 0.2 | 14.9 ± 0.1 | 43.4 ± 0.1 | 62.5 ± 0.1 | 71.1 ± 0.1 | 78.8 ± 0.3 |
| 15 | 220 | 90 | 55.8 ± 0.2 | 9.5 ± 0.1 | 13.8 ± 0.1 | 38.8 ± 0.2 | 57.6 ± 0.1 | 74.9 ± 0.1 | 82.4 ± 0.1 |
| 16 | 220 | 120 | 57.2 ± 0.3 | 9.1 ± 0.1 | 12.9 ± 0.1 | 34.7 ± 0.1 | 52.7 ± 0.2 | 78.5 ± 0.1 | 85.3 ± 0.2 |
| Test of Intersubjective Effects | ||||||
|---|---|---|---|---|---|---|
| Variance Source | Dependent Variable | Class III Sum of Squares | Degree of Freedom | Mean Square | F | p |
| Modified model | Xylo-oligosaccharide | 86.605a | 16 | 5.413 | 6.22113 × 1032 | <0.001 |
| Relative saccharification | 816.331a | 16 | 51.021 | 1.8325 × 1032 | <0.001 | |
| Intercept | Xylo-oligosaccharide | 15.8 | 1 | 15.8 | 1.81592 × 1033 | <0.001 |
| Relative saccharification | 814.454 | 1 | 814.454 | 2.92525 × 1033 | <0.001 | |
| Temperature | Xylo-oligosaccharide | 40.17 | 3 | 13.39 | 1.53896 × 1033 | <0.001 |
| Relative saccharification | 496.993 | 3 | 165.664 | 5.95013 × 1032 | <0.001 | |
| Time | Xylo-oligosaccharide | 15.873 | 3 | 5.291 | 6.08097 × 1032 | <0.001 |
| Relative saccharification | 146.321 | 3 | 48.774 | 1.7518 × 1032 | <0.001 | |
| Temperature × Time | Xylo-oligosaccharide | 28.263 | 9 | 3.14 | 3.60926 × 1032 | <0.001 |
| Relative saccharification | 54.466 | 9 | 6.052 | 2.17359 × 1031 | <0.001 | |
| Errors | Xylo-oligosaccharide | 2.96 × 10−31 | 34 | 8.70 × 10−33 | ||
| Relative saccharification | 9.47 × 10−30 | 34 | 2.78 × 10−31 | |||
| Total | Xylo-oligosaccharide | 123.401 | 51 | |||
| Relative saccharification | 2713.138 | 51 | ||||
| Corrected Total | Xylo-oligosaccharide | 86.605 | 50 | |||
| Relative saccharification | 816.331 | 50 | ||||
| No. | Temperature, °C | Time, min | Constituents, g/L | |||
|---|---|---|---|---|---|---|
| Glucose | Xylose | Xylo-Oligosaccharides | ||||
| 1 | 160 | 30 | 0.04 ± 0.01 | 0.03 ± 0.01 | 1.00 ± 0.03 | 5.35 |
| 2 | 160 | 60 | 0.05 ± 0.01 | 0.04 ± 0.01 | 1.02 ± 0.05 | 4.37 |
| 3 | 160 | 90 | 0.10 ± 0.01 | 0.15 ± 0.02 | 1.03 ± 0.05 | 3.99 |
| 4 | 160 | 120 | 0.14 ± 0.02 | 0.37 ± 0.01 | 1.34 ± 0.06 | 3.69 |
| 5 | 180 | 30 | 0.06 ± 0.01 | 0.06 ± 0.01 | 1.51 ± 0.05 | 4.20 |
| 6 | 180 | 60 | 0.14 ± 0.02 | 0.09 ± 0.02 | 1.64 ± 0.08 | 3.38 |
| 7 | 180 | 90 | 0.16 ± 0.02 | 0.18 ± 0.01 | 1.76 ± 0.06 | 3.32 |
| 8 | 180 | 120 | 0.20 ± 0.01 | 1.24 ± 0.06 | 1.85 ± 0.08 | 3.39 |
| 9 | 200 | 30 | 0.19 ± 0.02 | 1.84 ± 0.07 | 2.13 ± 0.10 | 3.20 |
| 10 | 200 | 60 | 0.48 ± 0.02 | 2.05 ± 0.09 | 2.43 ± 0.12 | 3.03 |
| 11 | 200 | 90 | 0.73 ± 0.04 | 3.14 ± 0.10 | 2.49 ± 0.11 | 2.98 |
| 12 | 200 | 120 | 1.14 ± 0.05 | 3.29 ± 0.12 | 2.81 ± 0.12 | 2.94 |
| 13 | 220 | 30 | 0.73 ± 0.03 | 2.81 ± 0.11 | 3.59 ± 0.18 | 3.22 |
| 14 | 220 | 60 | 1.01 ± 0.05 | 3.01 ± 0.15 | 3.73 ± 0.19 | 3.11 |
| 15 | 220 | 90 | 1.67 ± 0.08 | 3.98 ± 0.19 | 4.90 ± 0.24 | 2.99 |
| 16 | 220 | 120 | 2.52 ± 0.12 | 4.43 ± 0.22 | 3.81 ± 0.19 | 2.93 |
| No. | Temperature, °C | Time, min | Formic Acid, g/L | Acetic Acid, g/L | 5-HMF, g/L | Furfural, g/L | |
|---|---|---|---|---|---|---|---|
| 1 | 160 | 30 | 3.24 | N.D. | N.D. | N.D. | N.D. |
| 2 | 160 | 60 | 3.54 | 0.03 ± 0.01 | 0.04 ± 0.01 | N.D. | N.D. |
| 3 | 160 | 90 | 3.72 | 0.06 ± 0.01 | 0.09 ± 0.01 | N.D. | N.D. |
| 4 | 160 | 120 | 3.85 | 0.08 ± 0.01 | 0.22 ± 0.01 | N.D. | N.D. |
| 5 | 180 | 30 | 3.83 | 0.01 ± 0.01 | 0.05 ± 0.01 | N.D. | N.D. |
| 6 | 180 | 60 | 4.13 | 0.06 ± 0.01 | 0.38 ± 0.02 | 0.12 ± 0.01 | N.D. |
| 7 | 180 | 90 | 4.31 | 0.15 ± 0.01 | 0.85 ± 0.01 | 0.22 ± 0.03 | 0.04 ± 0.01 |
| 8 | 180 | 120 | 4.43 | 0.18 ± 0.02 | 1.21 ± 0.05 | 0.32 ± 0.01 | 0.06 ± 0.01 |
| 9 | 200 | 30 | 4.42 | 0.15 ± 0.01 | 0.53 ± 0.02 | 0.03 ± 0.01 | 0.36 ± 0.01 |
| 10 | 200 | 60 | 4.72 | 0.19 ± 0.01 | 1.18 ± 0.05 | 0.19 ± 0.02 | 0.51 ± 0.02 |
| 11 | 200 | 90 | 4.90 | 0.27 ± 0.01 | 2.10 ± 0.11 | 0.42 ± 0.10 | 0.71 ± 0.03 |
| 12 | 200 | 120 | 5.02 | 0.34 ± 0.02 | 2.29 ± 0.10 | 0.60 ± 0.02 | 0.82 ± 0.03 |
| 13 | 220 | 30 | 5.01 | 0.16 ± 0.02 | 2.10 ± 0.09 | 0.18 ± 0.03 | 0.90 ± 0.03 |
| 14 | 220 | 60 | 5.31 | 0.28 ± 0.03 | 2.13 ± 0.13 | 0.27 ± 0.01 | 1.11 ± 0.01 |
| 15 | 220 | 90 | 5.49 | 0.41 ± 0.01 | 2.42 ± 0.12 | 0.57 ± 0.02 | 1.23 ± 0.05 |
| 16 | 220 | 120 | 5.61 | 0.50 ± 0.02 | 2.63 ± 0.13 | 0.64 ± 0.01 | 1.27 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Cui, R.; Tang, W.; Fan, B.; He, Y. Co-Producing Xylo-Oligosaccharides, 5-HMF, Furfural, Organic Acids, and Reducing Sugars from Waste Poplar Debris by Clean Hydrothermal Pretreatment. Processes 2025, 13, 665. https://doi.org/10.3390/pr13030665
Yang Y, Cui R, Tang W, Fan B, He Y. Co-Producing Xylo-Oligosaccharides, 5-HMF, Furfural, Organic Acids, and Reducing Sugars from Waste Poplar Debris by Clean Hydrothermal Pretreatment. Processes. 2025; 13(3):665. https://doi.org/10.3390/pr13030665
Chicago/Turabian StyleYang, Yuheng, Ruibing Cui, Wei Tang, Bo Fan, and Yucai He. 2025. "Co-Producing Xylo-Oligosaccharides, 5-HMF, Furfural, Organic Acids, and Reducing Sugars from Waste Poplar Debris by Clean Hydrothermal Pretreatment" Processes 13, no. 3: 665. https://doi.org/10.3390/pr13030665
APA StyleYang, Y., Cui, R., Tang, W., Fan, B., & He, Y. (2025). Co-Producing Xylo-Oligosaccharides, 5-HMF, Furfural, Organic Acids, and Reducing Sugars from Waste Poplar Debris by Clean Hydrothermal Pretreatment. Processes, 13(3), 665. https://doi.org/10.3390/pr13030665








