1. Introduction
The use of petroleum-based fuels has significant environmental and climatic consequences, making alternative energy sources increasingly important. One such alternative is synthesis gas, composed primarily of carbon monoxide, hydrogen, and nitrogen, which is expected to contribute significantly to future energy production [
1]. Syngas is a blend of carbon monoxide, carbon dioxide, hydrogen, and trace amounts of methane, derived from sources such as natural gas, coal, oil, biomass, and organic waste [
2]. Due to its potential as a cleaner fuel, investigating syngas flame structures—particularly in relation to pollutant formation—remains a crucial area of research. Studies indicate that hydrogen-rich syngas combustion generates considerably fewer emissions than coal combustion. As a result, syngas technology is being increasingly integrated into energy management systems. Moreover, syngas serves as a feedstock for producing ammonia, methanol, synthetic petroleum, and other fuel sources [
3]. Synthesis gas is used to drive a gas turbine in a moist air cycle. The effects of porous media introduction were examined to predict the thermal behaviour of non-premixed synthesis gas. When compared to a free-space burner, a porous burner produces a shorter flame. As the air vapour drifted more rapidly and the fuel flow was delayed, the maximum temperature was reached closer to the fuel nozzle [
4].
Ref. [
5] examines the non-premixed combustion of CO/H
2 mixtures in a round jet burner. A three-dimensional solution was achieved through direct numerical simulation, incorporating flame-formed manifolds based on detailed chemical kinetics. The findings demonstrated that the H
2/CO ratio in the syngas mixture significantly influences flame characteristics [
6]. Furthermore, experimental studies have analyzed the temperature and composition of unpremixed CO/H
2/N
2 combustion in a round jet burner [
7,
8]. Alavandi and Agrawal [
9] experimentally investigated the lean premixed combustion of CO/H
2/CH
4/air mixture and observed that the CO-H
2 mixture added with CH
4 promoted CO and NOx emissions. Zhang and Yang [
10] used micromixing to study the effect of CO dilution on emissions reduction. In addition to CFD analyses, experimental studies have also been conducted on turbulent unpremixed combustion analyses [
11,
12,
13,
14].
Numerical investigations of syngas combustion in various burner configurations have been conducted to understand the effects of hydrogen content and other gas compositions on flame characteristics and emissions. Studies have shown that increasing the hydrogen concentration in syngas affects the flame structure, decreases radial velocity and strain rate, and significantly reduces NO emissions without substantially impacting temperature [
15,
16]. The addition of diluents like CO
2, H
2O, and N
2 to syngas mixtures can further reduce NO emissions, with CO
2 being the most effective [
16]. Researchers have employed various numerical models, including finite-rate chemistry and assumed-PDF approaches, to simulate turbulent reacting flows and investigate combustion efficiency [
17]. Parameters such as fuel composition, pipe diameter, jet velocity, and co-flow velocity have been analyzed to optimize combustion characteristics and reduce pollutant emissions in non-premixed burners [
18].
Syngas, a mixture of H
2, CO, CO
2, and N
2, is being investigated as a cleaner alternative fuel for internal combustion engines [
19]. The composition of syngas significantly affects its combustion characteristics. Lower H
2/CO ratios lead to a higher flame instability, while CO promotes heat generation with less fluctuation [
20]. Dilution with CO
2 or N
2 reduces the flame propagation velocity, maximum pressure rise, and deflagration index compared to undiluted syngas [
21]. CO
2 dilution results in longer flame lengths and lower reaction rates than N
2 dilution while also increasing CO concentrations in the flame [
22]. The choice of syngas production method, base fuel for reforming, and engine operating conditions are critical factors in determining the advantages of syngas use in internal combustion engines [
19]. These findings contribute to the ongoing research on syngas as a potential environmentally friendly fuel source. In their study, they investigated the flame properties of turbulent premixed jet flames fed with stoichiometric hydrogen/methane mixtures. They examined four conditions with 0, 10, 50, and 80 vol% hydrogen. They found that the addition of hydrogen resulted in a decrease in wrinkling as the flame moved from the thin reaction zone regime to the corrugated flame regime [
23].
For both flames, the radial dependence of the conditional means is slightly more pronounced at z/d = 20 than at z/d = 30, while it remains lower at all other measured downstream positions. These findings indicate that assuming radial independence of the conditional means serves as a reasonable approximation for fully coupled simple jet flames, as demonstrated in the present study [
7]. The substantial advancements achieved by artificial intelligence in the domains of supersonic flow and combustion can be categorized into three primary areas. These include intelligent simulation of turbulent combustion, deep learning-based reconstruction of supersonic flow fields, and the smart design of complete flow passages in supersonic engines. In recent years, the turbulent combustion sector has increasingly utilized extensive datasets alongside sophisticated machine learning models, facilitating precise predictions of combustion efficiency and enhancements in the combustion process [
24].
Syngas is a combustible gas mixture produced from solid fuels through gasification technology, which operates at elevated temperatures and pressures. The primary constituents of this flammable gas are hydrogen and carbon monoxide. Syngas is the main gaseous product obtained from thermal conversion processes, predominantly generated through gasification [
25,
26]. It is noteworthy that the pyrolysis process can also yield a gas mixture, commonly known as syngas, alongside tar and char. Demirbas and Arin [
27] are recognized as early contributors to the study of biomass pyrolysis for syngas generation. They found that maintaining high temperatures, modest heating rates, and extended gas residence durations are crucial for pyrolysis to produce the best syngas yields. Kan et al. [
28] examined several factors influencing syngas production, such as biomass type, its inherent characteristics, conversion techniques, and various pretreatment methods, including thermal, physical, biological, and chemical processes. Traditionally, syngas production has been primarily associated with the thermal conversion of fossil fuels like coal, char, natural gas, and petroleum, along with lignocellulosic biomass [
29]. Tangestani et al. [
30] examined the application of the multilayer perceptron neural network (MLP-NN) for predicting CO conversion in the water–gas shift (WGS) reaction, focusing on various active phase compositions and catalyst supports. Their findings showed that the MLP-NN model effectively estimated CO conversion by incorporating catalyst-specific parameters. Through sensitivity analysis [
25,
26] they identified temperature and H
2 feed concentration as the key factors influencing reaction performance. This work demonstrates the accuracy and reliability of neural network techniques like MLP-NN in modelling CO conversion in WGS reactions. Liu et al. [
31] proposed a smooth gradient approximation neural network for solving nonsmooth nonconvex optimization problems, which are commonly encountered in engineering applications. The method introduces a time-varying control parameter to smooth nonsmooth objective functions and uses a hard comparator function to ensure that the solution stays within nonconvex inequality constraints. The authors prove that any accumulation point of the neural network’s state solution is a stationary point of the nonconvex optimization problem. The proposed neural network outperforms related models by offering simpler algorithm structures and weaker convergence conditions. Simulation results and an optimization application demonstrate the practical effectiveness of the approach.
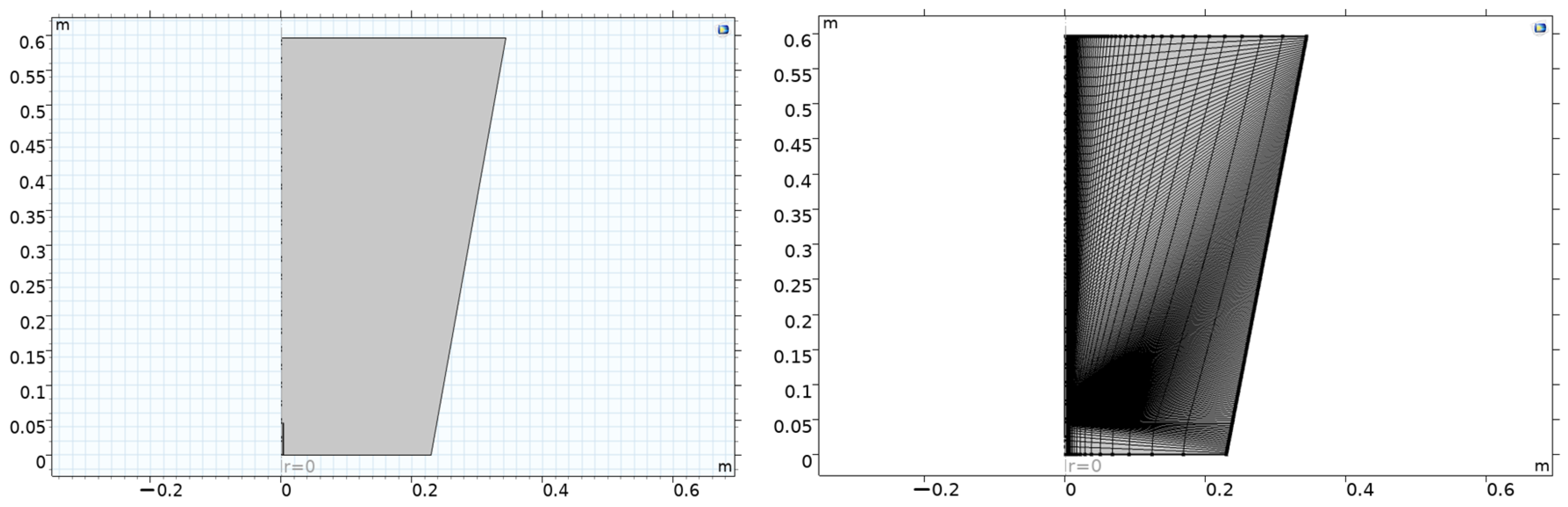
The aim of this study is to compare simultaneously the resulting changes in temperature, velocity, and pressure with the effects of carbon dioxide, water, and oxygen produced after combustion with different proportions of fuel amounts (hydrogen, carbon monoxide, and nitrogen) by volume. The temperature, velocity and pressure changes in the combustion flame obtained for different H2, CO, and N values (30% H2, 30% CO, 40% N2; 30% H2, 40% CO, 30% N2; 50% H2, 20% CO, 30% N2; 50% H2, 30% CO, 20% N2; 70% H2, 10% CO, 20% N2; 70% H2, 20% CO, 10% N2) and the mass change values of CO2, H2O, and O2 were obtained numerically in the Comsol Multiphysics 5.3. programme (COMSOL AB, Stockholm, Sweden, 2017). Additionally, velocity and temperature changes were obtained for z/d = 20 and z/d = 50 values. The internal diameter of the nozzle is represented by d, while z denotes the axial distance measured from the outlet of the nozzle. It was investigated whether there is a radial dependence.
3. Results and Discussion
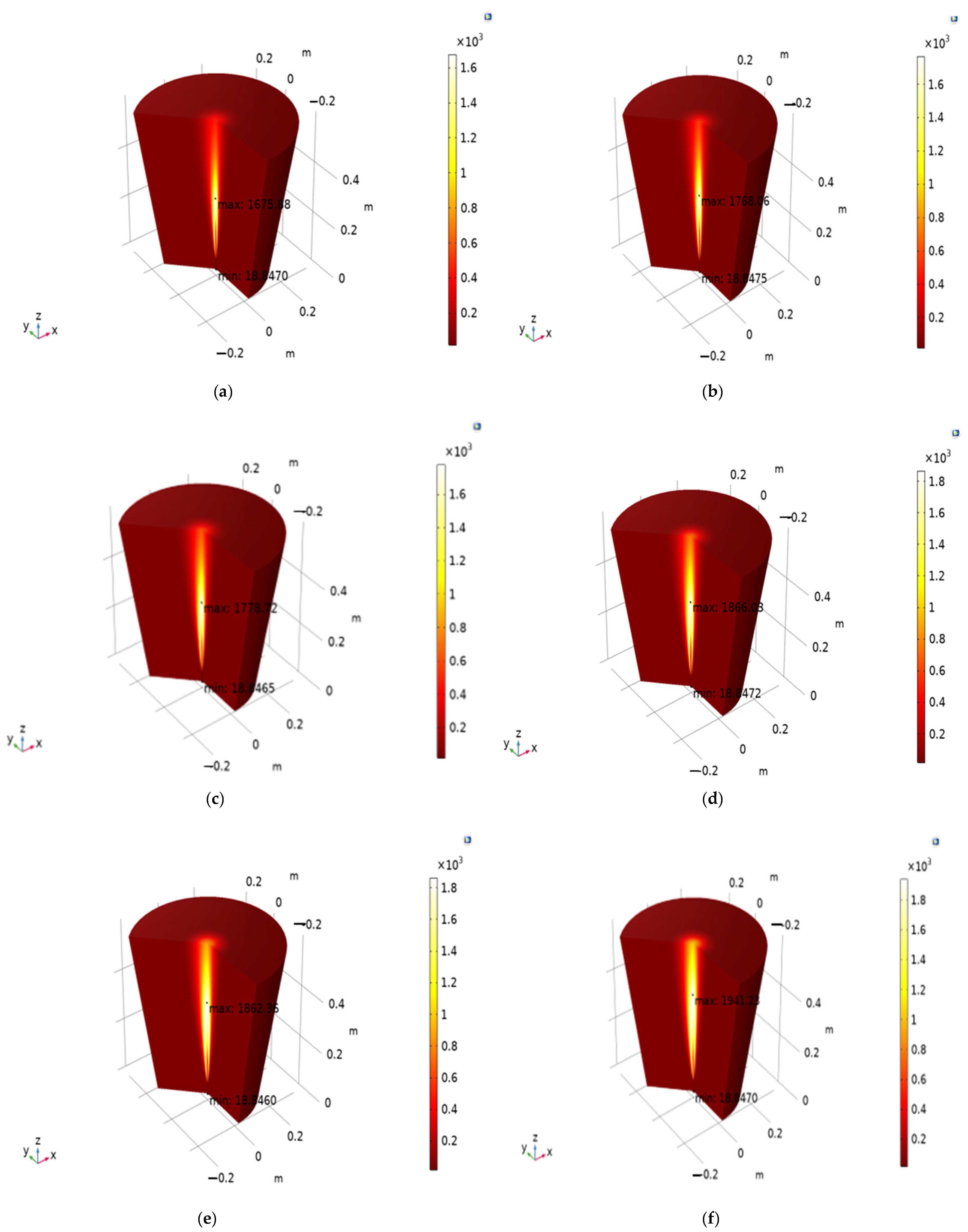
Figure 4 gives simulation results for different gas mixture ratios shown in subpoints a–f. Each subplot represents the temperature distribution of a specific mixture of hydrogen (H
2), carbon monoxide (CO), and nitrogen (N
2). This mixture (30% H
2, 30% CO, 40% N
2) contains equal amounts of hydrogen and carbon monoxide, with nitrogen being the dominant component. The temperature distribution appears relatively even, with a moderate maximum temperature compared to the other mixtures. Here (30% H
2, 40% CO, 30% N
2), the CO concentration is higher than in
Figure 4a, while H
2 and N
2 are equal. The maximum temperature increases compared to
Figure 4a, suggesting that a higher CO content contributes to higher peak temperatures., This mixture (50% H
2, 20% CO, 30% N
2) has a higher hydrogen content and a lower CO content compared to
Figure 4a,b.
The peak temperature increases significantly, indicating that hydrogen has a more substantial impact on raising the temperature. In the mixture of 50% H
2, 30% CO, and 20% N
2, with equal amounts of H
2 and CO and a reduced N
2 content, the temperature distribution shows a high peak temperature. The results suggest that reducing N
2 and increasing H
2 and CO leads to higher temperatures. This mixture (70% H
2, 10% CO, 20% N
2) is dominated by hydrogen, with a very low CO content and moderate N
2. The highest maximum temperature is observed in this scenario, highlighting the significant role of hydrogen in raising the temperature. The mixture of 70% H
2, 20% CO, and 10% N
2 is similar to
Figure 4e but with a higher CO content and lower N
2. The temperature distribution is slightly more uniform, but the peak temperature remains high, confirming the influence of hydrogen.
The increase in hydrogen content leads to a significant rise in the maximum temperature. This is evident from
Figure 4c–f, where mixtures with higher hydrogen percentages show higher peak temperatures. Increasing CO content also contributes to higher temperatures but not as significantly as hydrogen. This is observed by comparing
Figure 4a,b and
Figure 4c,d. Nitrogen appears to act as a diluent, reducing the overall peak temperature. Mixtures with a higher nitrogen content (like in
Figure 4a,b) show lower temperatures compared to those with a reduced nitrogen content (like in
Figure 4e,f).
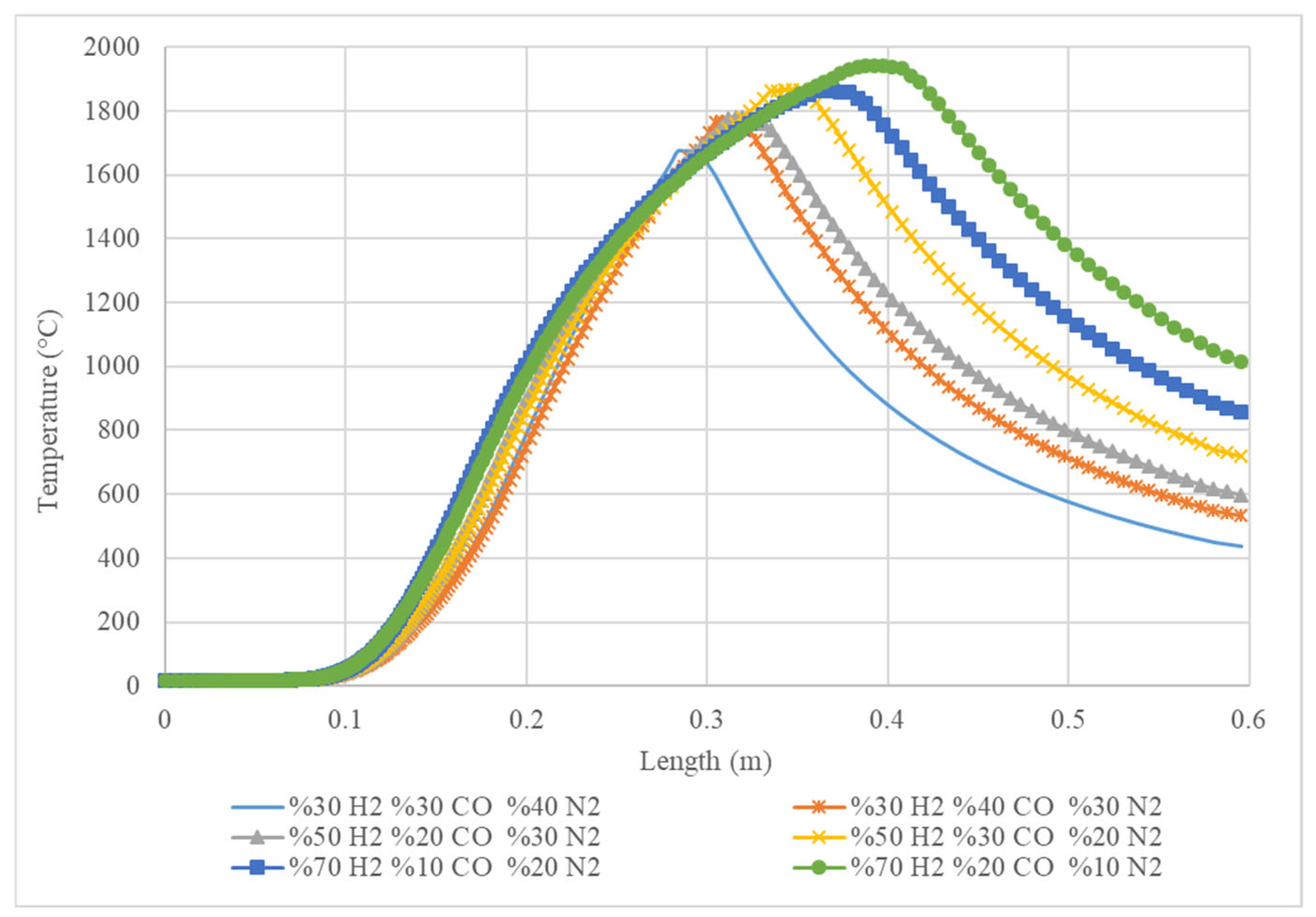
Figure 5 shows the temperature profile along a certain length for different gas compositions. The x-axis represents the length in metres, and the y-axis represents the temperature in degrees Celsius. Each line on the graph represents a different gas mixture, with the percentages of hydrogen (H
2), carbon monoxide (CO), and nitrogen (N
2) given in the legend. The graph shows that the temperature increases along the length, reaching a peak before decreasing again. The peak temperature varies depending on the gas mixture, with the highest temperature achieved by the mixture with 70% H
2, 20% CO, and 10% N
2 (green line). The graph suggests that the gas mixture has a significant impact on the temperature profile. The mixture with the highest percentage of hydrogen and the lowest percentage of nitrogen has the highest peak temperature.
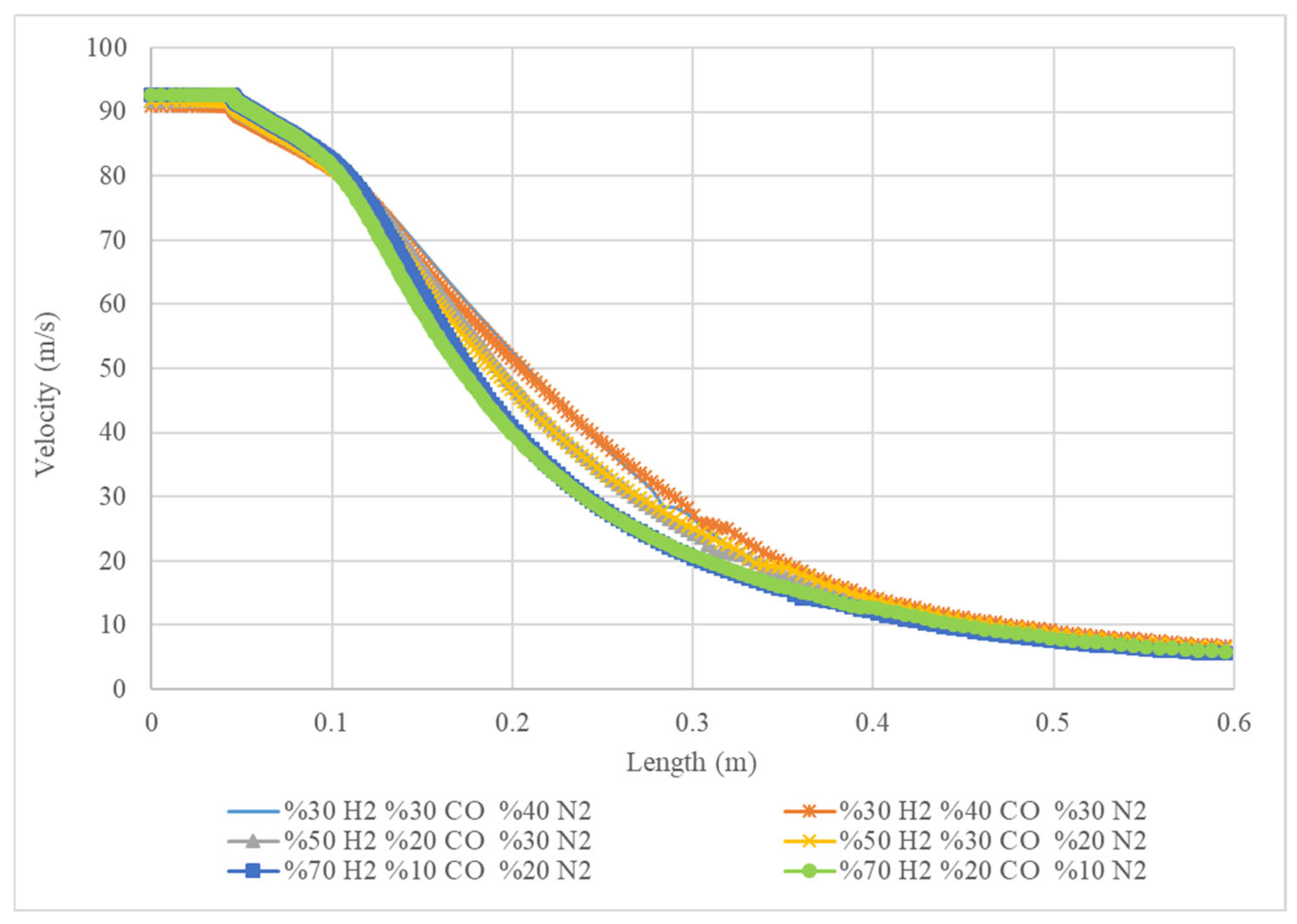
Figure 6 shows the velocity of a gas mixture as it travels along a certain length. The x-axis represents the length in metres, and the y-axis represents the velocity in metres per second (m/s). Each line on the graph represents a different gas mixture, with the percentages of hydrogen (H
2), carbon monoxide (CO), and nitrogen (N
2) given in the legend. The graph shows that the velocity decreases as the gas travels along the length, with the rate of decrease varying depending on the gas composition. The mixture with the highest initial velocity is the one with 70% H
2, 10% CO, and 20% N
2 (blue line), but it also decreases the fastest. The mixture with the lowest initial velocity is the one with 30% H
2, 30% CO, and 40% N
2 (light blue line), but it decreases at a slower rate. An increase in the hydrogen concentration leads to a higher combustion rate and an elevated maximum temperature. The concentration of hydrogen in syngas plays a crucial role in influencing the combustion rate. The mechanism of the chemical reaction that takes place during the simulation of an elevated jet flame, which is a result of hydrogen combustion, and leads to an increase in temperature.
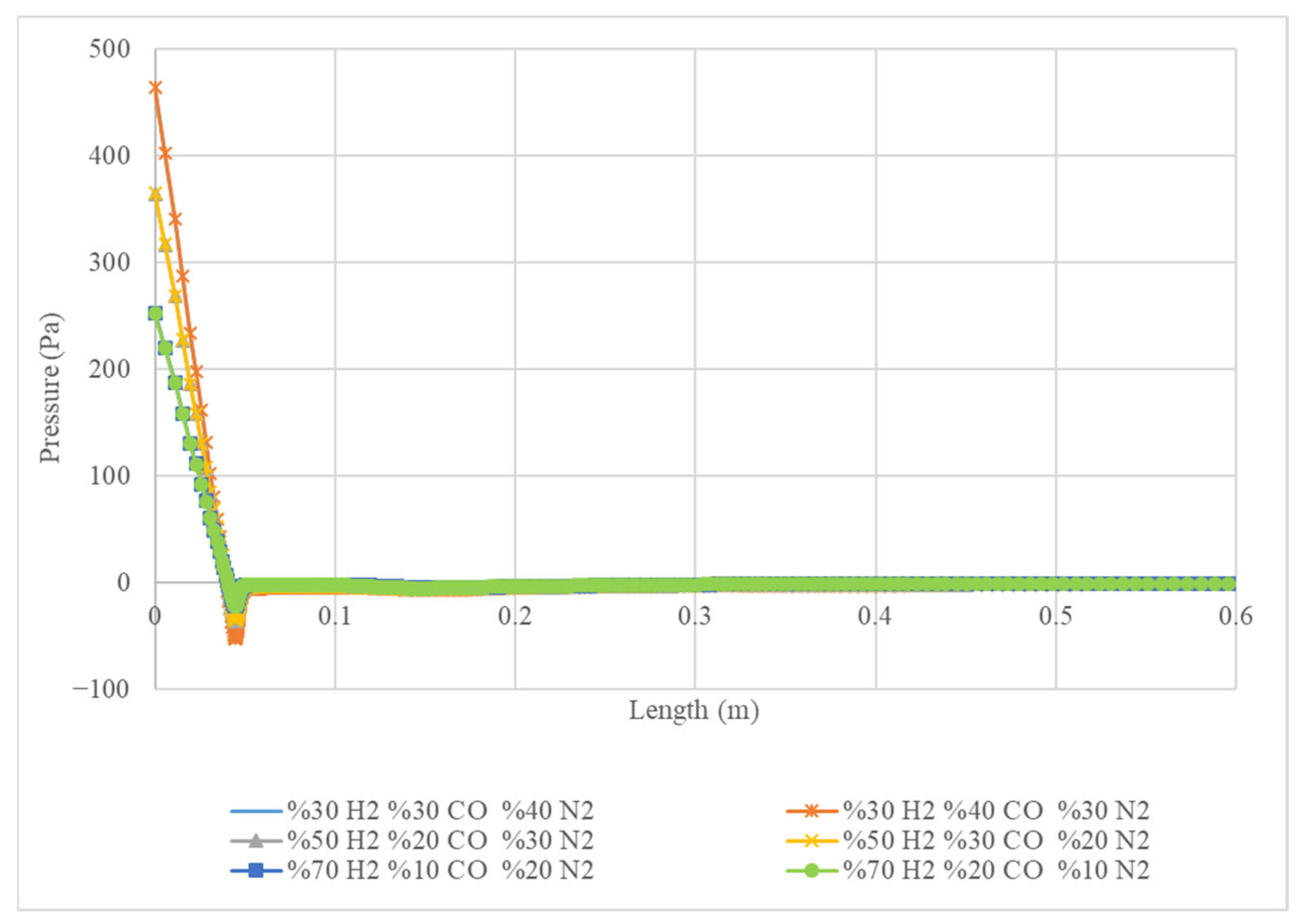
Figure 7 displays the pressure of four different gas mixtures as they flow along a specified length. The x-axis represents the length in metres, and the y-axis represents the pressure in Pascals (Pa). Each line represents a different gas mixture, with the percentages of hydrogen (H
2), carbon monoxide (CO), and nitrogen (N
2) indicated in the legend. The graph shows that the pressure drops significantly along the length of all mixtures, indicating that the gases are expanding as they flow. The initial pressure is highest for the mixture with 30% H
2, 40% CO, and 30% N
2 (orange line) and decreases the fastest. The pressure drop is less significant for the mixture with 70% H
2, 20% CO, and 10% N
2 (green line), indicating a slower expansion rate. The graph suggests that the gas composition affects the pressure drop rate, with mixtures containing higher percentages of hydrogen exhibiting a faster pressure drop.
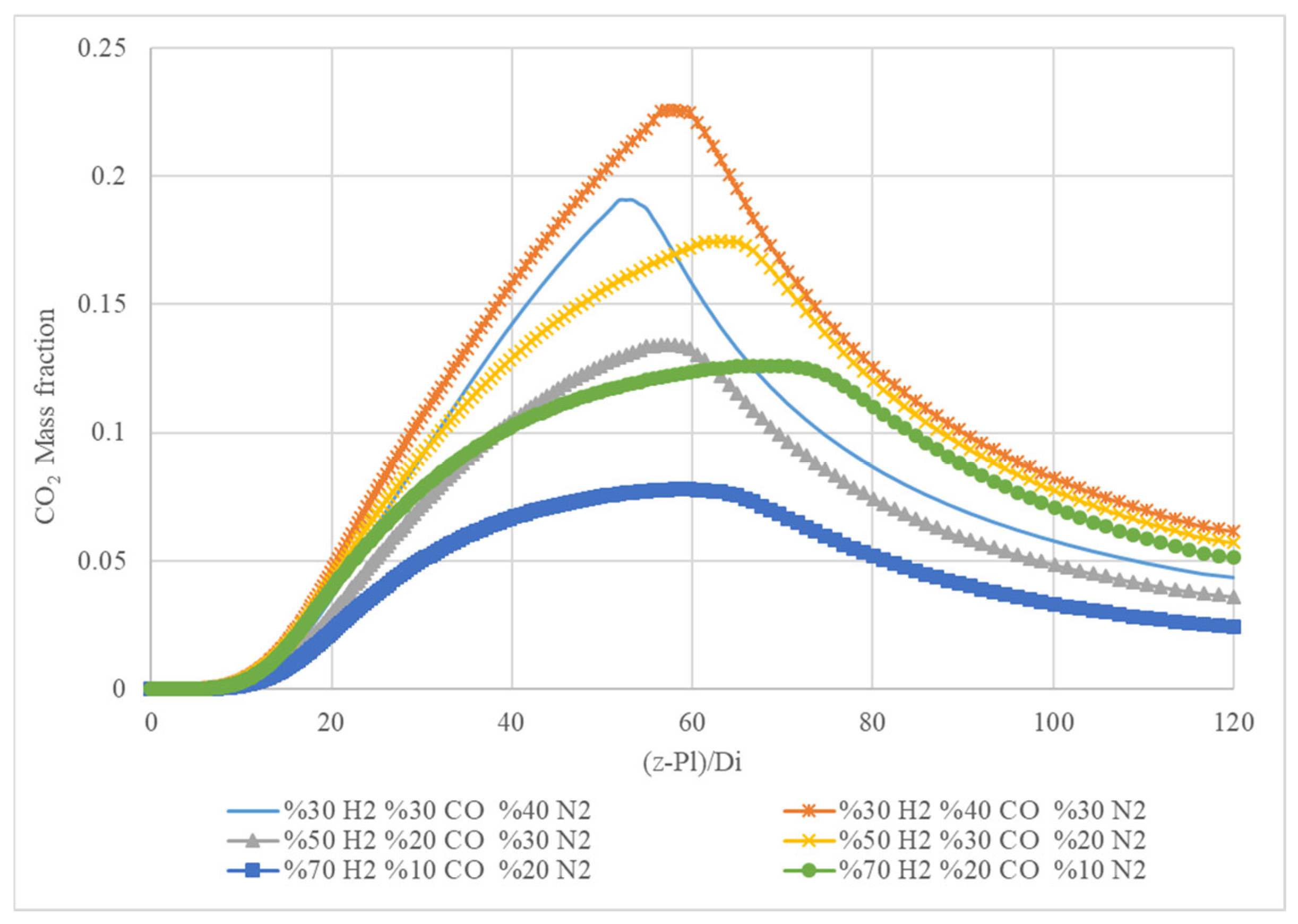
Figure 8 presents the mass fraction of carbon dioxide (CO
2) in a gas mixture as a function of the position along a specified length. The x-axis represents the position along the length in non-dimensional form, (z-Pl)/D
i, where ‘z’ is the position, ‘Pl’ is the position of the inlet, and ‘D
i’ is the diameter of the chamber. The y-axis represents the mass fraction of CO
2. The graph shows five different curves, each representing a different gas mixture. Each mixture consists of different percentages of hydrogen (H
2), carbon monoxide (CO), and nitrogen (N
2). All curves display a similar trend, showing an increase in the CO
2 mass fraction with increasing position, reaching a peak, and then decreasing. The peak values and the positions where the peaks occur vary based on the gas composition. This graph suggests that the gas composition significantly impacts the CO
2 formation and distribution along the length of the chamber. The mixtures with higher hydrogen content generally exhibit lower peak CO
2 values and shift the peak further down the length.
Figure 9 provides a graph showing the H
2O mass fraction along the axial position normalized by the inlet diameter (z−PI)/Di for different gas mixtures. The legend indicates the composition of each gas mixture, and the graph displays how the water vapour (H
2O) mass fraction evolves along the reactor’s length for these mixtures.
All the mixtures start with a low H2O mass fraction near zero at the beginning of the reactor. As the reaction progresses (moving right along the x-axis), the H2O mass fraction increases. Each mixture reaches a peak H2O mass fraction at different positions along the reactor. Mixtures with higher hydrogen content (70% H2) tend to have higher peak H2O mass fractions. For example, the green and blue dotted lines reach the highest peaks. Mixtures with higher nitrogen content tend to have lower peaks. The mixture with 30% H2, 30% CO, and 40% N2 (blue line) shows a lower peak compared to mixtures with less nitrogen. After reaching the peak, the H2O mass fraction decreases for all mixtures. The rate of decrease varies among the mixtures. Mixtures with higher H2 content show a more gradual decline compared to those with lower H2 content. Mixtures with 70% H2 (green and blue dotted lines) show the highest H2O mass fractions, indicating more efficient water production. Mixtures with 50% H2 (yellow and grey lines) show moderate peaks, demonstrating that intermediate H2 levels produce less water compared to higher H2 levels. Mixtures with 30% H2 (blue and orange lines) have the lowest H2O mass fractions, indicating less water production efficiency.
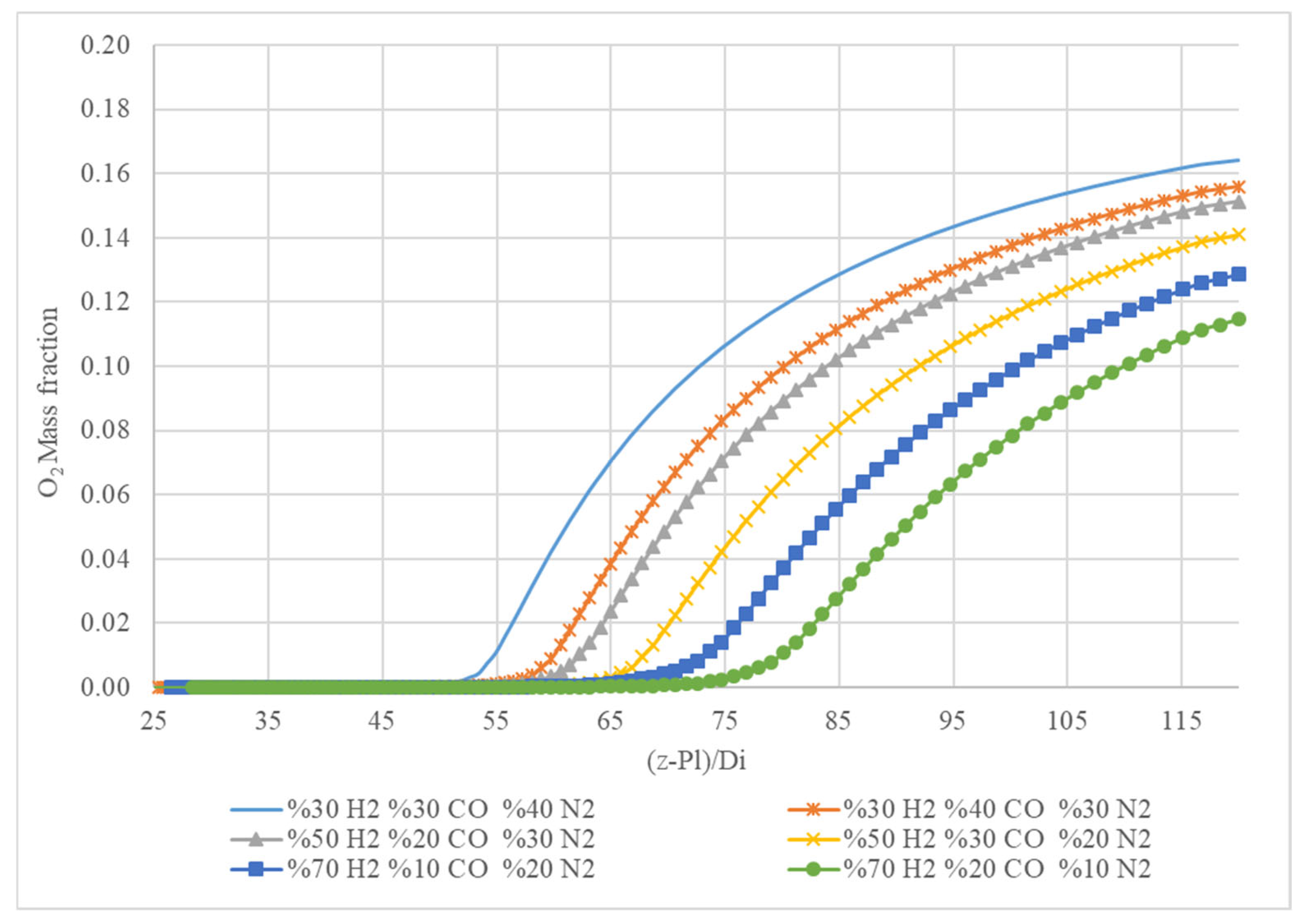
Figure 10 shows a graph of the O
2 mass fraction along the axial position normalized by the inlet diameter (z−PI)/D
i for different gas mixtures. The legend indicates the composition of each gas mixture, and the graph displays how the oxygen (O
2) mass fraction evolves along the reactor’s length for these mixtures.
All mixtures start with a very low O2 mass fraction near zero at the beginning of the reactor. As the reaction progresses (moving right along the x-axis), the O2 mass fraction increases for all mixtures. The increase starts after a certain point along the axial length, indicating the onset of oxygen consumption reactions. Mixtures with lower hydrogen content (30% H2, blue and orange lines) reach higher O2 mass fractions compared to those with higher hydrogen content. Mixtures with higher hydrogen content (70% H2, green and blue squares) reach lower O2 mass fractions, indicating more efficient consumption of oxygen in the presence of higher hydrogen levels. The mixture with 70% H2 and 20% CO (green line) shows the lowest final O2 mass fraction, suggesting the most efficient consumption of oxygen. Mixtures with 50% H2 (yellow and grey lines) show intermediate O2 mass fractions. Mixtures with 30% H2 (blue and orange lines) reach the highest O2 mass fractions, indicating less efficient consumption of oxygen compared to mixtures with higher hydrogen content.
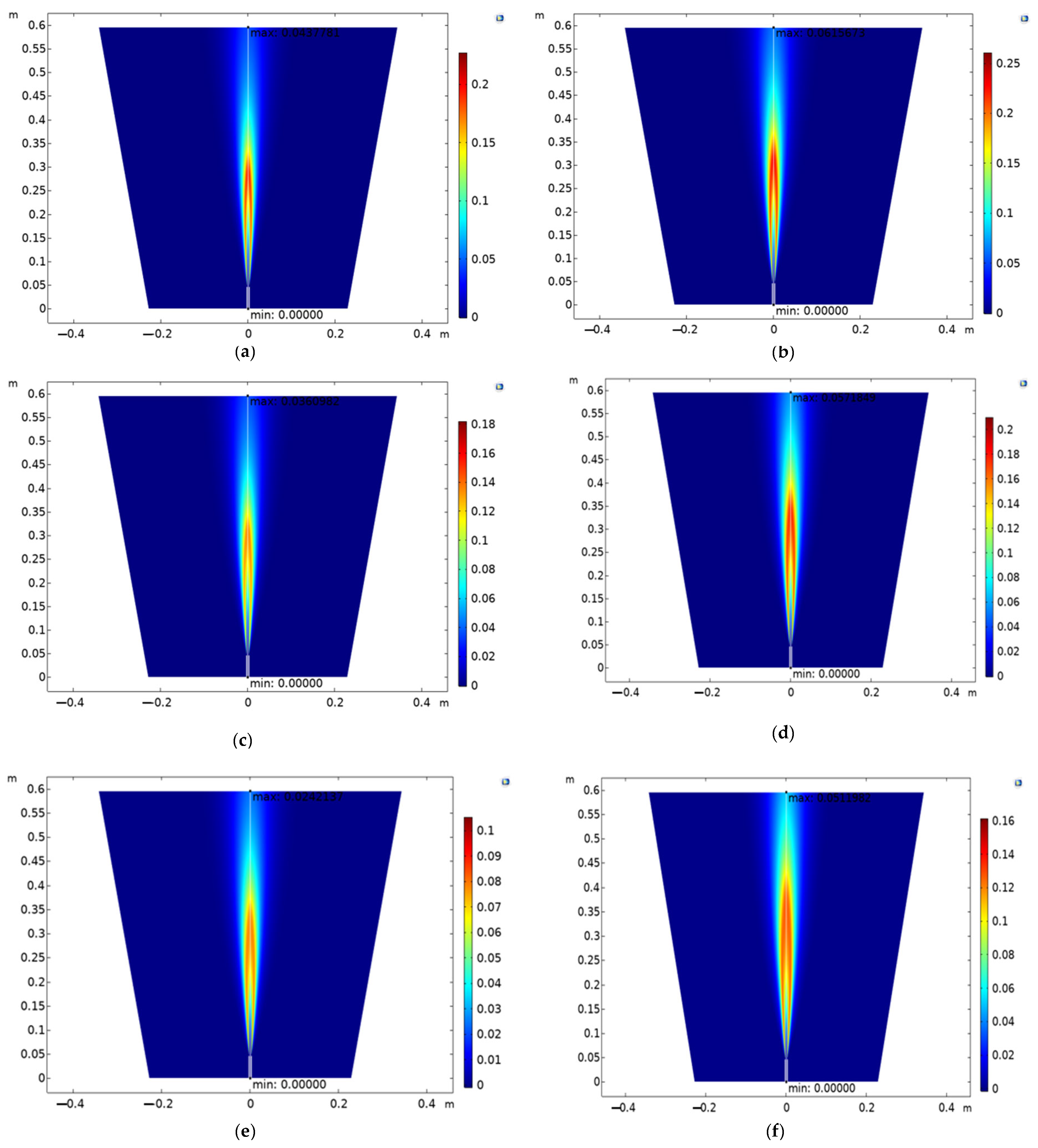
The colour bar (
Figure 11) next to each subplot represents the concentration of CO
2. The scale ranges from 0 (blue) to the maximum concentration (red) shown in the colour bar. The plots illustrate the vertical spatial distribution of CO
2 concentration in the simulation domain. The x-axis represents the horizontal distance (m) and the y-axis represents the vertical distance (m). Areas with red or warmer colours indicate higher concentrations of CO
2. Areas with blue or cooler colours indicate lower concentrations of CO
2.
Figure 11a shows a balanced mixture of H
2 and CO, with a higher concentration of N
2, leading to a specific CO
2 distribution.
Figure 11b increases the CO percentage while decreasing N
2, which alters the CO
2 concentration pattern.
Figure 11c,d have higher H
2 percentages, impacting the combustion process and CO
2 formation.
Figure 11e,f have the highest H
2 concentrations, significantly affecting CO
2 emissions due to the reduced CO and N2 components.
The colour bar (
Figure 12) next to each subplot represents the concentration of O
2. The scale ranges from 0 (blue) to the maximum concentration (red) shown in the colour bar. The plots illustrate the vertical spatial distribution of O
2 concentration in the simulation domain. The x-axis represents the horizontal distance (m), and the y-axis represents the vertical distance (m). Areas with red or warmer colours indicate higher concentrations of O
2. Areas with blue or cooler colours indicate lower concentrations of O
2.
Figure 12a shows a balanced mixture of H
2 and CO, with a higher concentration of N
2, leading to a specific O
2 distribution.
Figure 12b increases the CO percentage while decreasing N
2, which alters the O
2 concentration pattern.
Figure 12c,d have higher H
2 percentages, impacting the combustion process and O
2 consumption.
Figure 12e,f have the highest H
2 concentrations, significantly affecting the O
2 distribution due to the reduced CO and N
2 components.
The colour bar (
Figure 13) next to each subplot represents the concentration of H
2O. The scale ranges from 0 (blue) to the maximum concentration (red) shown in the colour bar. The plots illustrate the vertical spatial distribution of H
2O concentration in the simulation domain. The x-axis represents the horizontal distance (m), and the y-axis represents the vertical distance (m). Areas with red or warmer colours indicate higher concentrations of H
2O. Areas with blue or cooler colours indicate lower concentrations of H
2O.
Figure 13a shows a balanced mixture of H
2 and CO, with a higher concentration of N2, leading to a specific H
2O distribution.
Figure 13b increases the CO percentage while decreasing N
2, which alters the H
2O concentration pattern.
Figure 13c,d have higher H
2 percentages, impacting the combustion process and H
2O formation.
Figure 13e,f have the highest H
2 concentrations, significantly affecting the H
2O distribution due to the reduced CO and N
2 components.
These subplots help in understanding how varying the gas mixture composition influences the resultant H2O concentrations, which is crucial for optimizing combustion processes and understanding the water vapour emissions from different fuel mixtures.
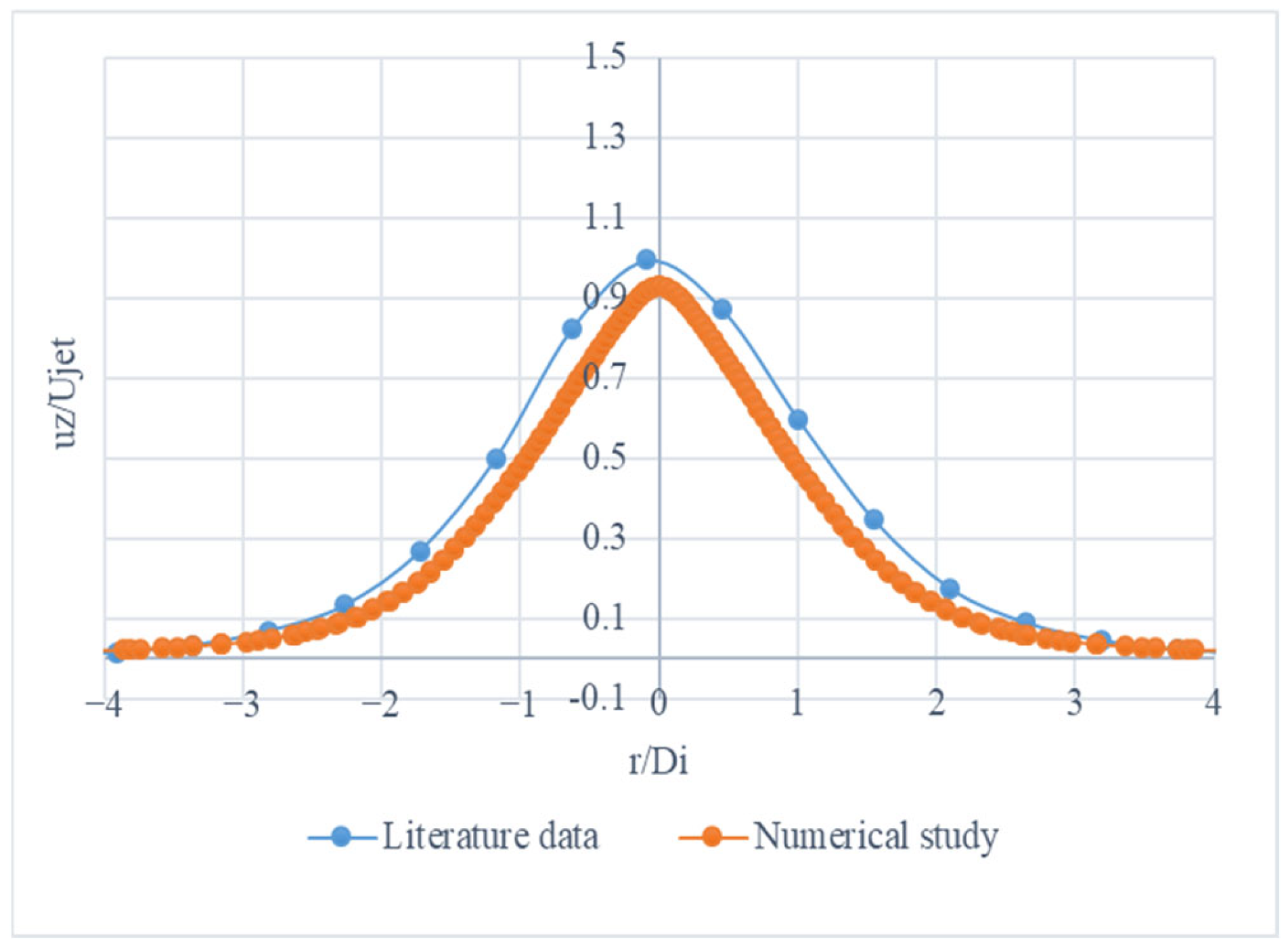
Figure 14 appears to be a graph depicting the relationship between the ratio r/D
i (the ratio of the distance from the centre to the distance from the edge) and the normalized intensity or reflectivity (u
z/u
jet) for different gas mixtures.
The curves exhibit a characteristic peak shape, indicating that the reflectivity or intensity reaches a maximum at a certain ratio of r/Di. This type of graph is commonly used in optical or spectroscopic analysis to understand the behaviour of different gas mixtures under various conditions.
Figure 14 provides a visual representation of how the normalized intensity or reflectivity changes as the ratio r/D
i is varied for different gas mixture compositions. This information can be useful in applications such as gas sensing, combustion analysis, or other processes where the optical properties of gas mixtures are important.
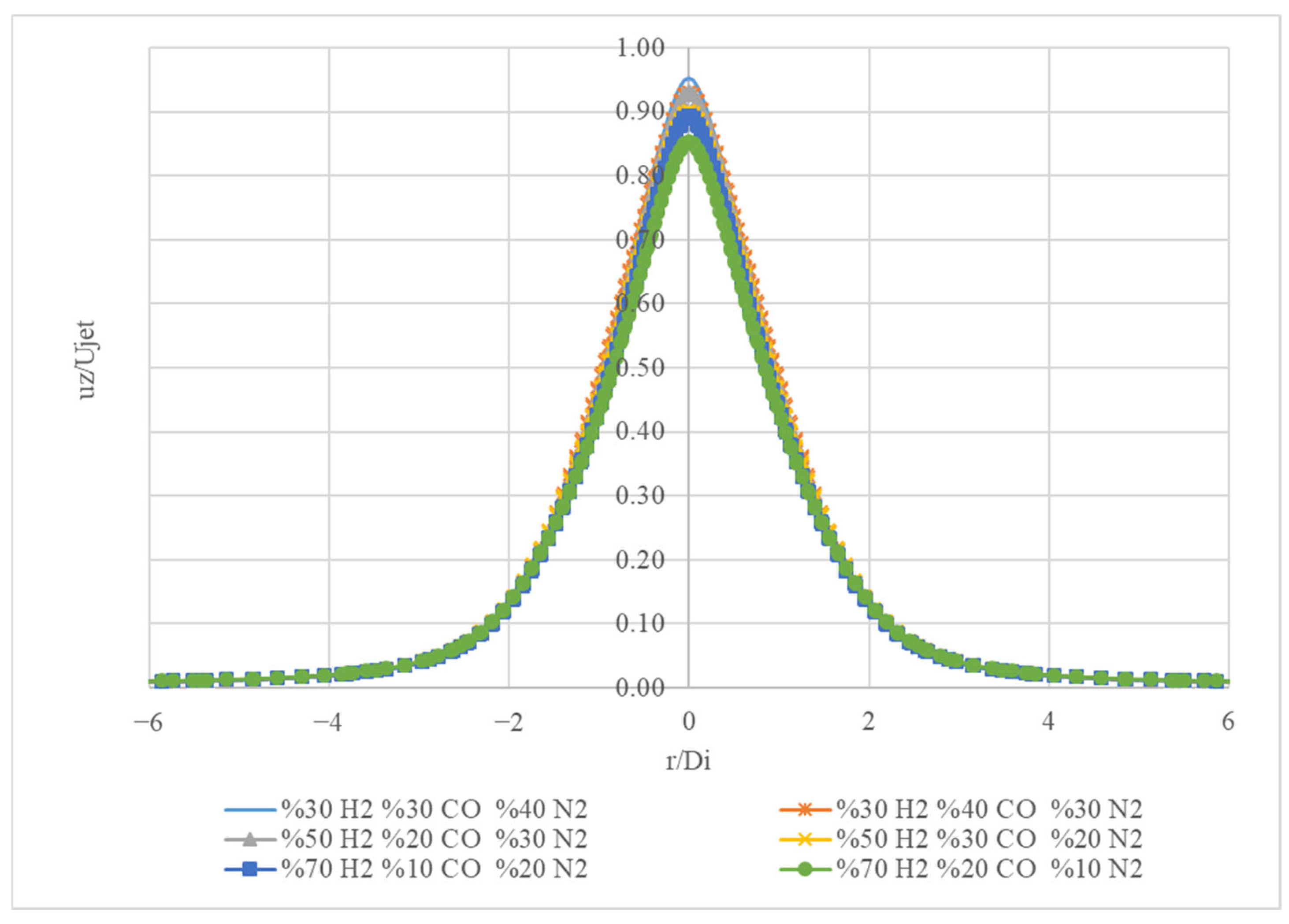
Figure 14 presents the normalized axial velocity (u
z/U
jet) profiles for different gas mixtures as a function of the radial position (r/D
i).
The mixture (30% H2, 30% CO, 40% N2) exhibits a peak normalized axial velocity of approximately 0.95 at r/Di = 0. It shows a symmetrical profile, decreasing to a normalized velocity of 0.1 at r/Di values near −3 and 3. The mixture (50% H2, 20% CO, 30% N2) reaches a peak normalized axial velocity of around 0.90 at r/Di = 0. It also shows a symmetrical profile, declining to a normalized velocity of 0.1 at r/Di values close to −3 and 3. The mixture (70% H2, 10% CO, 20% N2) has a peak normalized axial velocity of about 0.85 at r/Di = 0. It exhibits a symmetrical shape similar to the other curves, falling to a normalized velocity of 0.1 at r/Di values near −3 and 3. The mixture (30% H2, 40% CO, 30% N2) has a peak normalized axial velocity of approximately 0.90 at r/Di = 0. It displays a symmetrical profile, diminishing to a normalized velocity of 0.1 at r/Di values near −3 and 3. The mixture (50% H2, 30% CO, 20% N2) has a peak normalized axial velocity of approximately 0.85 at r/Di = 0. It exhibits a symmetrical profile, decreasing to a normalized velocity of 0.1 at r/Di values near −3 and 3. The mixture (70% H2, 20% CO, 10% N2) has a peak normalized axial velocity of approximately 0.80 at r/Di = 0. It displays a symmetrical profile, decreasing to a normalized velocity of 0.1 at r/Di values near −3 and 3.
The gas mixtures with the highest peak normalized axial velocities are 30% H2, 30% CO, and 40% N2, and 30% H2, 40% CO, and 30% N2, with both reaching approximately 0.95 and 0.90, respectively. All the gas mixtures show a symmetrical profile, with the peak velocity occurring at r/Di = 0. This indicates that the velocity distribution is symmetrical around the centre. As the proportion of H2 increases and the proportion of CO decreases, the peak normalized axial velocity tends to decrease. This trend is consistent across all the mixtures.
Figure 14 shows the normalized axial velocity profiles of different gas mixtures. It highlights how the composition of the gas mixture can affect the peak velocity and overall velocity distribution. Mixtures with higher CO ratios tend to have higher peak velocities. Additionally, the velocity distribution for all gas mixtures is symmetrical around the centre.
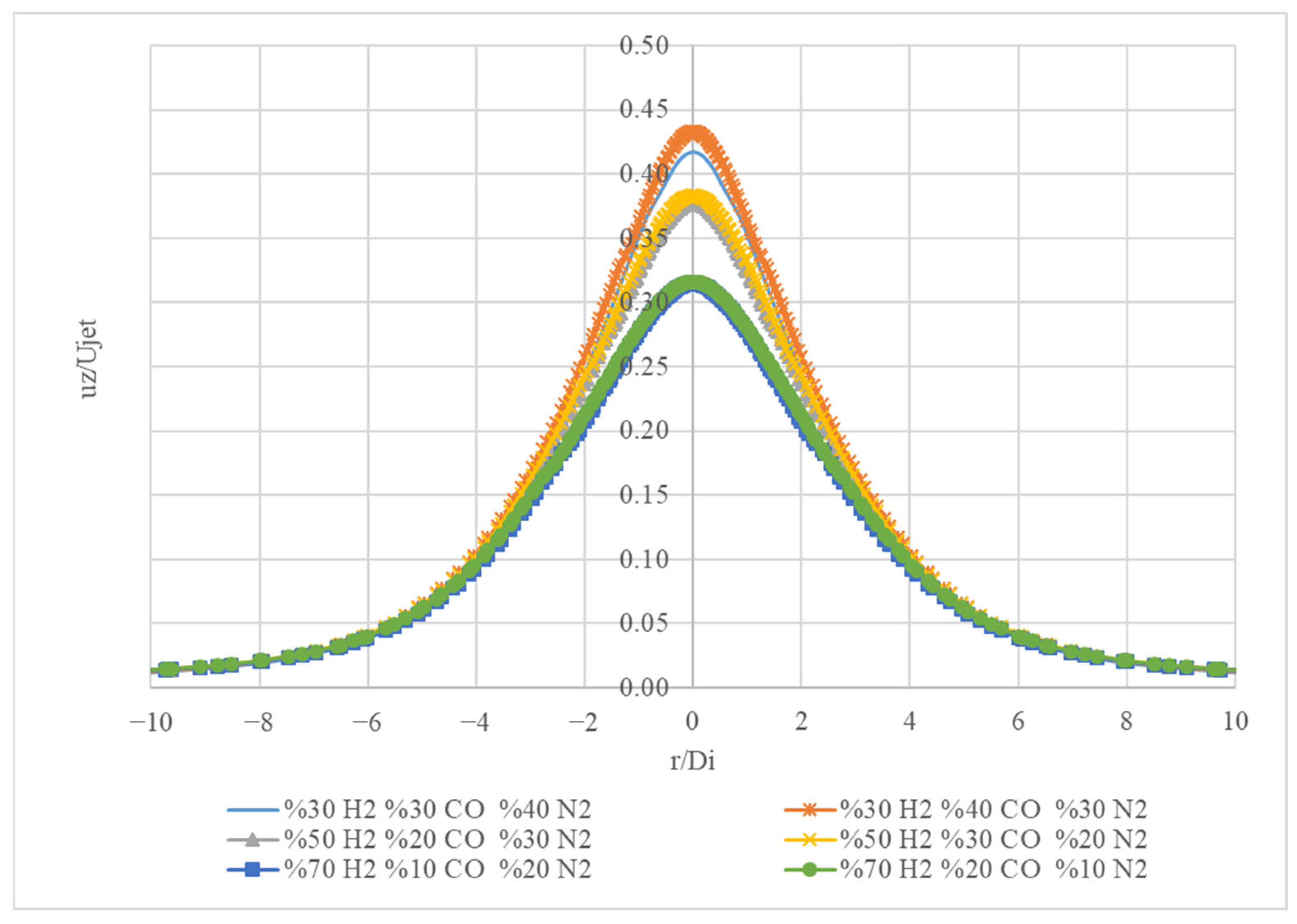
Figure 15 shows a graph with four different curves, each representing the normalized intensity of a specific gas mixture. The x-axis represents the ratio of the distance from the centre (r) to the distance from the edge (D
i). The y-axis represents the normalized intensity or reflectivity (u
z/U
jet).
The curves all exhibit a bell-shaped curve, reaching a peak at a specific value of r/Di. This indicates that the intensity reaches a maximum at a certain point within the gas mixture. This type of graph is commonly used in optical or spectroscopic analysis to understand the behaviour of different gas mixtures under various conditions.
Figure 15 shows the normalized intensity (u
z/U
jet) of four different gas mixtures as a function of the ratio of the distance from the centre to the distance from the edge (r/D
i).
The mixture (30% H2, 30% CO, 40% N2) reaches a peak intensity of approximately 0.42 at a r/Di of around 0.5. The mixture (50% H2, 20% CO, 30% N2) has a peak intensity of around 0.38 at a r/Di of about 0.5. The mixture (70% H2, 10% CO, 20% N2) shows a peak intensity of approximately 0.33 at a r/Di of roughly 0.5. The mixture (30% H2, 40% CO, 30% N2) has a peak intensity of approximately 0.44 at a r/Di of around 0.5.
The gas mixture with the highest peak intensity is 30% H2, 40% CO, and 30% N2, reaching a maximum of 0.44. This indicates that this mixture has the highest reflectivity or intensity at a certain point within the gas mixture. As the proportion of H2 increases and the proportion of CO decreases, the peak intensity decreases. The peak intensity of each mixture occurs at roughly the same r/Di value, indicating that the position of maximum intensity is similar for these different gas mixtures.
Overall, the graph demonstrates how the composition of the gas mixture affects its normalized intensity. It shows that different gas mixtures exhibit distinct intensity profiles, with variations in peak intensity and the shape of the curve.
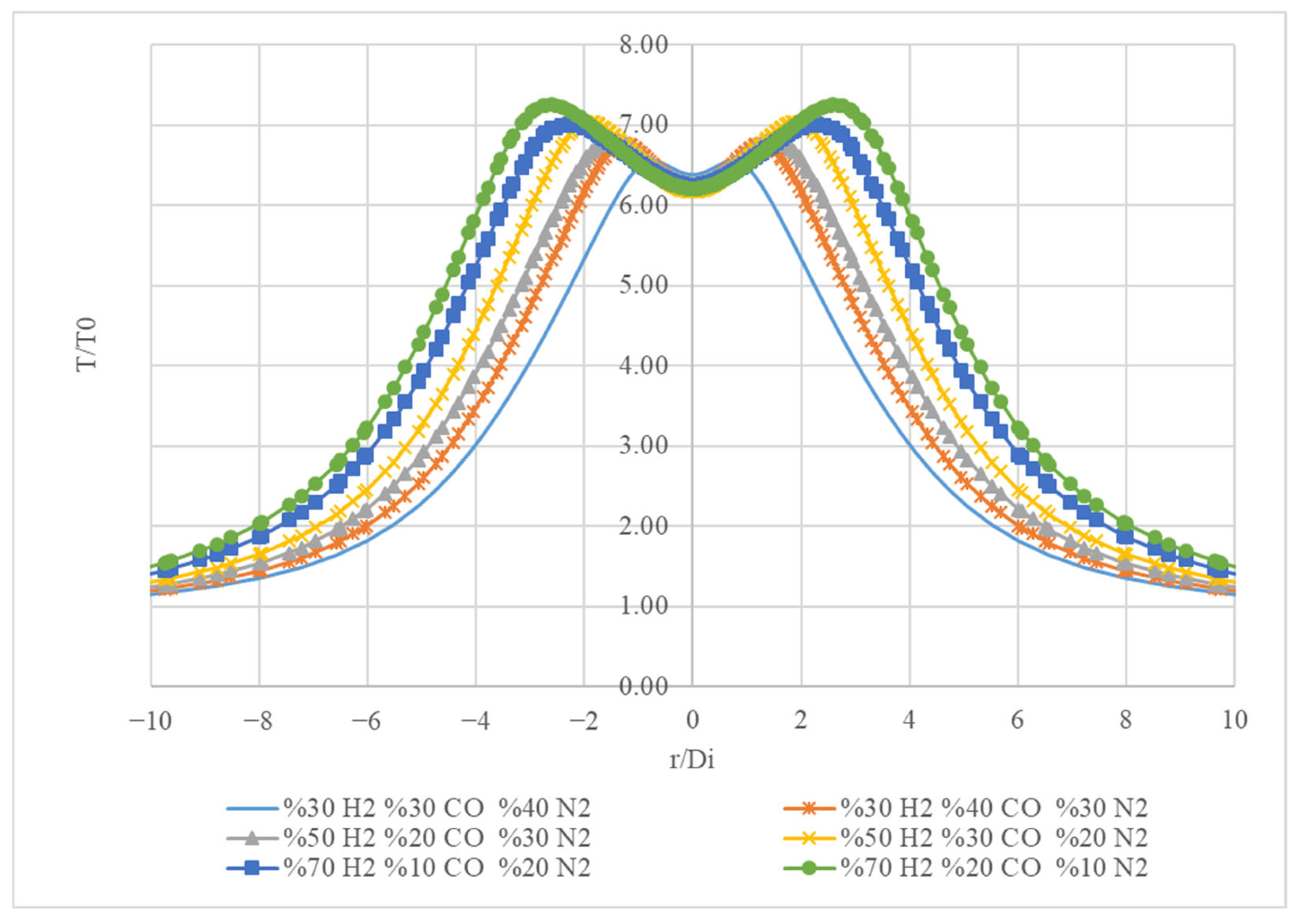
Figure 16 presents temperature profiles (T/T
0) as a function of the radial distance (r/D
i) for three different gas compositions. On the x-axis (r/D
i), the radial distance is plotted from −6 to 6, indicating the distance from the centre of the system. On the y-axis (T/T
0), the temperature is normalized to a reference temperature (T
0), ranging from 0 to 8.
For the mixture (30% H2, 30% CO, 40% N2), the blue curve shows the highest peak temperature, reaching approximately 6.54 at r/Di = −0.8 The temperature decreases symmetrically as you move away from the centre, indicating a strong thermal gradient. For the mixture 50% H2, 20% CO, and 30% N2 (orange curve) the peak temperature is slightly lower, around 6.6. The shape of the curve is similar to the blue curve but with a reduced peak, indicating that increasing the hydrogen concentration affects the thermal profile. For the mixture of 70% H2, 10% CO, and 20% N2 (green curve) the curve has the highest peak temperature of approximately 7.24.
Figure 17 illustrates how varying the composition of gases affects the temperature profile in a radial system. The decreasing peak temperatures with increasing hydrogen concentration suggest that the thermal properties of the gas mixture are significantly influenced by its composition. This information is crucial for applications in thermodynamics, combustion processes, and chemical engineering, where understanding temperature distributions is essential for optimizing performance and efficiency.
It is important that the emission values of CO
2 gases reach low levels. Power companies have come to recognize the importance of thoroughly examining the combustion performance of synthetic gas to enhance the availability and reliability of syngas turbines. Extensive studies have been conducted to investigate syngas combustion in order to address these issues. Notable participants in this research include power companies and major heavy industries, such as General Electric, Mitsubishi Heavy Industries, Korea Electric Power Corporation, and the Central Research Institute of Japan. The electric power sector has engaged in both experimental and computational studies to evaluate the combustion performance of synthetic gas and hydrogen [
36,
37,
38]. In this study, results regarding the changes in the emission values of gases resulting from combustion are given, and information that can be useful for industrial users in their designs and analyses is presented.
The heat released during the reaction causes temperature increases in the system. The magnitude of these temperature increases depends on the reaction rate and the thermal properties of the environment. Enthalpy of formation and the molar heat capacities given in
Table 2 (based on [
35]) express the resistance of substances to temperature changes. Because the products have a lower heat of formation than the reactants, both reactions are exothermic and emit heat. The molar heat capacity of water is higher than that of hydrogen and oxygen, indicating that water is more resistant to temperature changes. Reaction rates tend to increase with temperature, which leads to more heat release and, thus, temperature peaks. In addition, changes in the mass ratios of the components in the system can affect the progress of the reaction and, therefore, the temperature distribution. For example, an increase in the concentration of hydrogen or carbon monoxide can increase the reaction rate and cause higher temperature peaks.
Furthermore, given that steam possesses the highest heat capacity among the gases listed in
Table 2, it exerts a greater influence on flame temperature and reaction rate compared to N
2 and CO
2. Consequently, steam dilution results in higher CO emissions. This observation further supports the notion that the diminished reaction rate due to dilution is a primary factor contributing to the significant increase in CO emissions when steam is injected at a low heat input. Therefore, it is advisable to opt for N
2 or CO
2 dilution rather than steam dilution to mitigate CO emissions.