Optimising Supercritical Carbon Dioxide Extraction of Rosmarinic Acid from Rosmarinus officinalis L. and Enhancing Yield Through Soxhlet Coupling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Optimising scCO2 Extraction
2.2.1. Plant Material and Extraction Methods
2.2.2. Response Surface Methodology
2.3. Soxhlet Extraction
2.4. Determining the RA Content
2.5. Evaluating Antioxidant Activity
2.6. Scanning Electron Microscopy
3. Results
3.1. Optimising Supercritical CO2 Extraction
3.1.1. Design of the Experiments Matrix
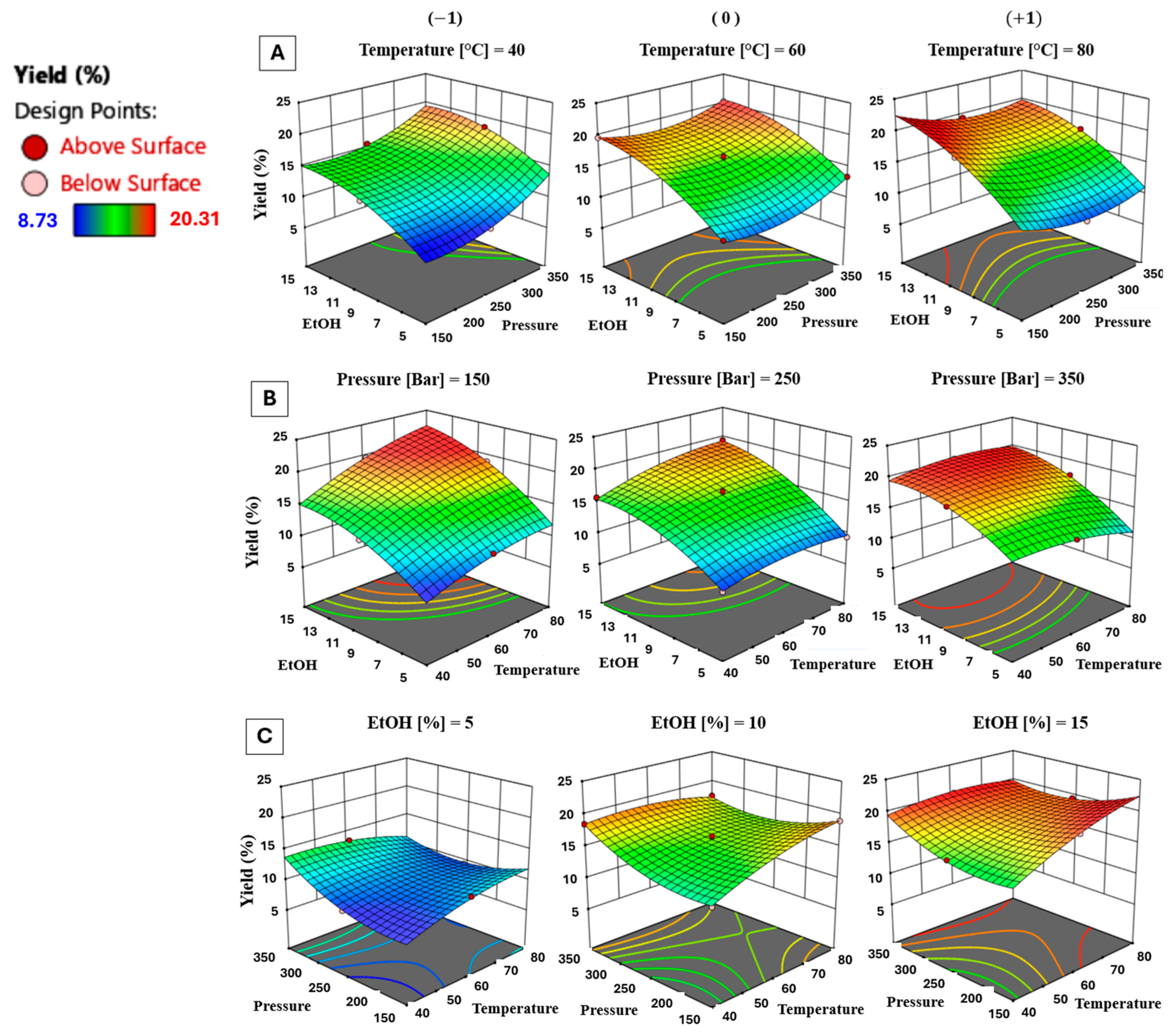
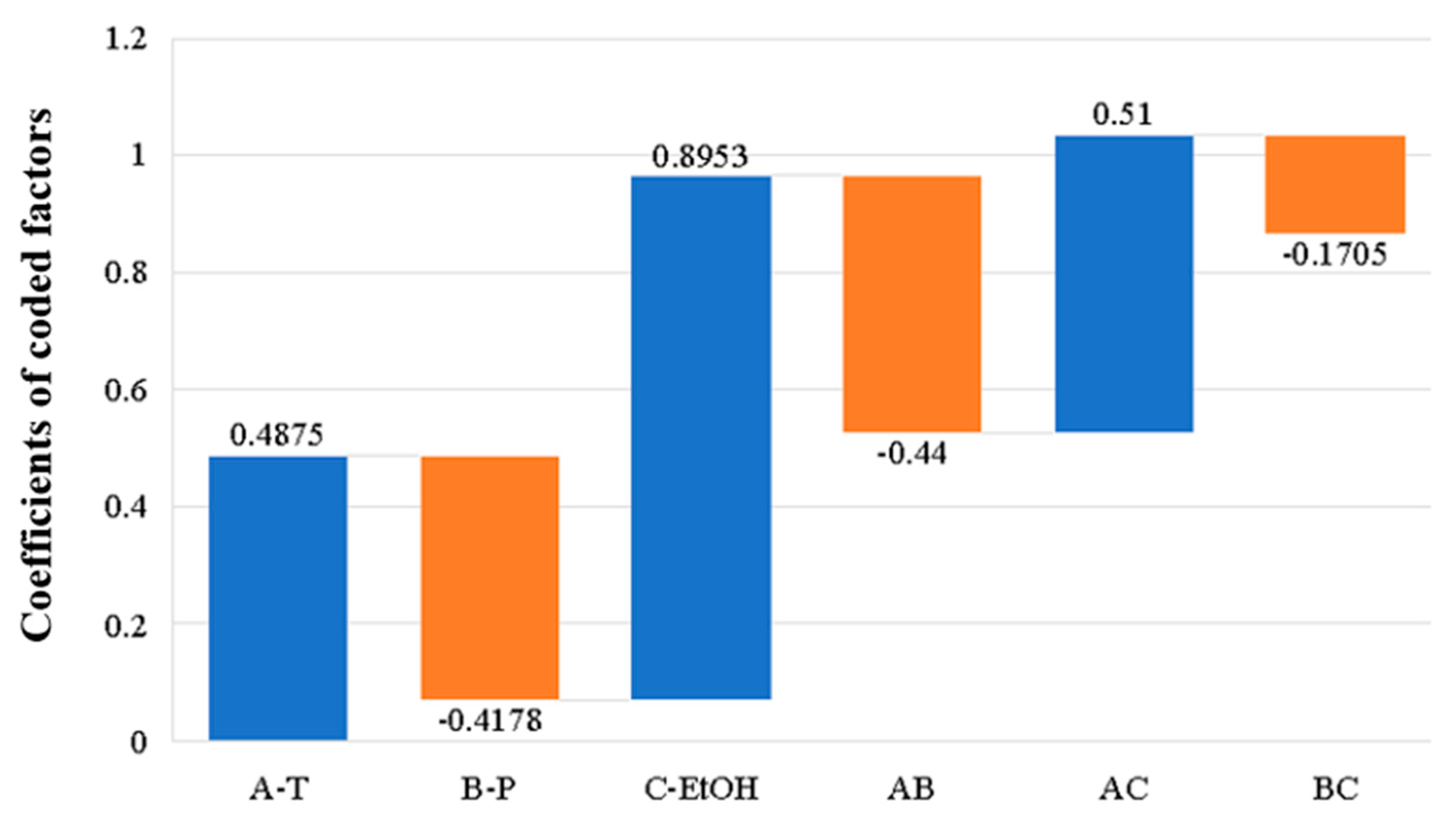
3.1.2. Effect of Process Parameters on Extraction Yield
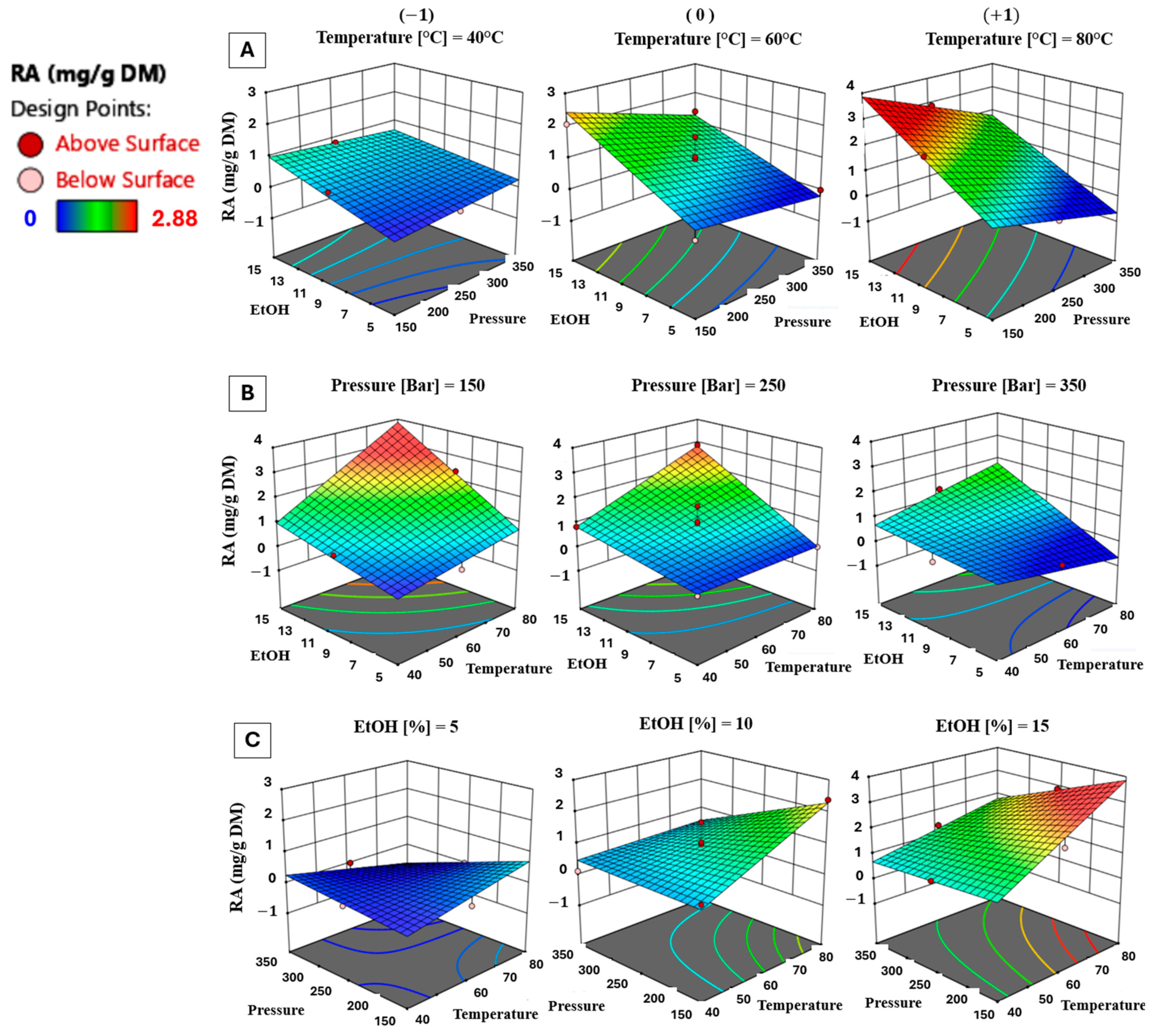
3.1.3. Effect of Process Parameters on RA Content
3.1.4. Validating Prediction Models
3.2. Scanning Electron Microscopy
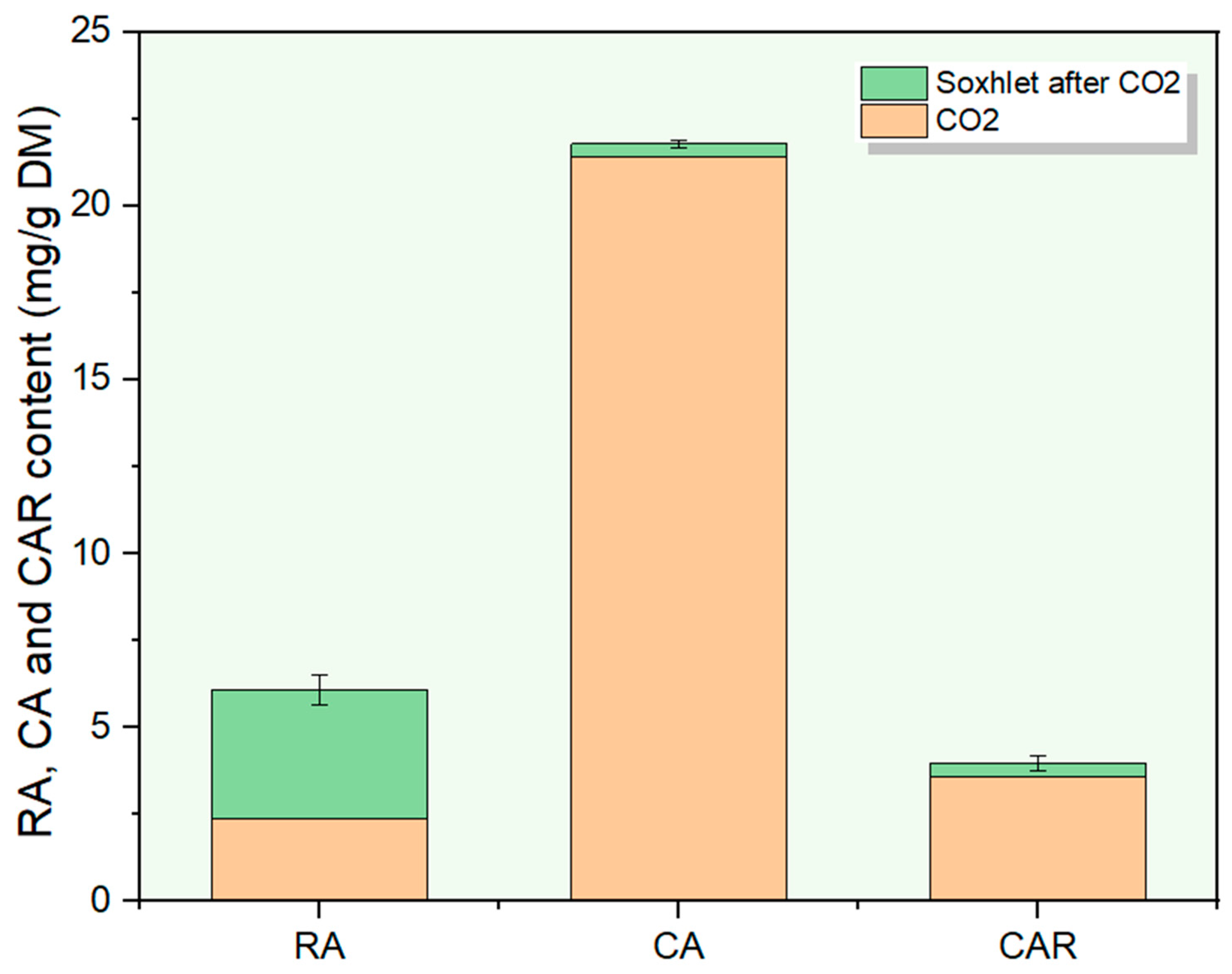
3.3. Extracting with Soxhlet
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Campillo, M.; Gabaldon, J.A.; Castillo, J.; Benavente-García, O.; Del Baño, M.J.; Alcaraz, M.; Vicente, V.; Alvarez, N.; Lozano, J.A. Rosmarinic Acid, a Photo-Protective Agent against UV and Other Ionizing Radiations. Food Chem. Toxicol. 2009, 47, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Ku, S.K.; Lee, W.; Lee, S.; Lee, T.; Song, K.S.; Bae, J.S. Barrier Protective Effects of Rosmarinic Acid on HMGB1-Induced Inflammatory Responses in Vitro and in Vivo. J. Cell. Physiol. 2013, 228, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Walker, T.S.; Schweizer, H.P.; Vivanco, J.M. Root Specific Elicitation and Antimicrobial Activity of Rosmarinic Acid in Hairy Root Cultures of Ocimum basilicum. Plant Physiol. Biochem. 2002, 40, 983–995. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic Acid—From Bench to Valuable Applications in Food Industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Dahchour, A. Anxiolytic and Antidepressive Potentials of Rosmarinic Acid: A Review with a Focus on Antioxidant and Anti-Inflammatory Effects. Pharmacol. Res. 2022, 184, 106421. [Google Scholar] [CrossRef]
- Ramalho, L.N.Z.; Pasta, Â.A.C.; Terra, V.A.; Augusto, M.J.; Sanches, S.C.; Souza-Neto, F.P.; Cecchini, R.; Gulin, F.; Ramalho, F.S. Rosmarinic Acid Attenuates Hepatic Ischemia and Reperfusion Injury in Rats. Food Chem. Toxicol. 2014, 74, 270–278. [Google Scholar] [CrossRef]
- Costa, R.S.; Carneiro, T.C.B.; Cerqueira-Lima, A.T.; Queiroz, N.V.; Alcântara-Neves, N.M.; Pontes-De-Carvalho, L.C.; Velozo, E.D.S.; Oliveira, E.J.; Figueiredo, C.A. Ocimum Gratissimum Linn. and Rosmarinic Acid, Attenuate Eosinophilic Airway Inflammation in an Experimental Model of Respiratory Allergy to Blomia tropicalis. Int. Immunopharmacol. 2012, 13, 126–134. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, D.D.; Xu, Y.X.; Wang, C.; Cao, L.; Liu, Y.S.; Zhu, C.Q. Aging as a Precipitating Factor in Chronic Restraint Stress-Induced Tau Aggregation Pathology, and the Protective Effects of Rosmarinic Acid. J. Alzheimer’s Dis. 2016, 49, 829–844. [Google Scholar] [CrossRef]
- Sasaki, K.; El Omri, A.; Kondo, S.; Han, J.; Isoda, H. Rosmarinus officinalis Polyphenols Produce Anti-Depressant like Effect through Monoaminergic and Cholinergic Functions Modulation. Behav. Brain Res. 2013, 238, 86–94. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, H.R.; Woo, E.R.; Hong, S.T.; Chae, H.J.; Chae, S.W. Inhibitory Effects of Rosmarinic Acid on Adriamycin-Induced Apoptosis in H9c2 Cardiac Muscle Cells by Inhibiting Reactive Oxygen Species and the Activations of c-Jun N-Terminal Kinase and Extracellular Signal-Regulated Kinase. Biochem. Pharmacol. 2005, 70, 1066–1078. [Google Scholar] [CrossRef]
- Saiko, P.; Steinmann, M.T.; Schuster, H.; Graser, G.; Bressler, S.; Giessrigl, B.; Lackner, A.; Grusch, M.; Krupitza, G.; Bago-Horvath, Z.; et al. Epigallocatechin Gallate, Ellagic Acid, and Rosmarinic Acid Perturb DNTP Pools and Inhibit de Novo DNA Synthesis and Proliferation of Human HL-60 Promyelocytic Leukemia Cells: Synergism with Arabinofuranosylcytosine. Phytomedicine 2015, 22, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.M.; Real Hernandez, L.M.; Berhow, M.A.; De Mejia, E.G. Bioactive Compounds from Culinary Herbs Inhibit a Molecular Target for Type 2 Diabetes Management, Dipeptidyl Peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Encalada, M.A.; Hoyos, K.M.; Rehecho, S.; Berasategi, I.; de Ciriano, M.G.Í.; Ansorena, D.; Astiasarán, I.; Navarro-Blasco, Í.; Cavero, R.Y.; Calvo, M.I. Anti-Proliferative Effect of Melissa officinalis on Human Colon Cancer Cell Line. Plant Foods Hum. Nutr. 2011, 66, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Gîrd, C.E.; Costea, T.; Mitran, V. Evaluation of Cytotoxic Activity and Anticancer Potential of Indigenous Rosemary (Rosmarinus officinalis L.) and Oregano (Origanum vulgare L.) Dry Extracts on MG-63 Bone Osteosarcoma Human Cell Line. Rom. J. Morphol. Embryol. 2021, 62, 525. [Google Scholar] [CrossRef]
- Campos, D.A.; Madureira, A.R.; Gomes, A.M.; Sarmento, B.; Pintado, M.M. Optimization of the Production of Solid Witepsol Nanoparticles Loaded with Rosmarinic Acid. Colloids Surf. B Biointerfaces 2014, 115, 109–117. [Google Scholar] [CrossRef]
- Madureira, A.R.; Campos, D.; Gullon, B.; Marques, C.; Rodríguez-Alcalá, L.M.; Calhau, C.; Alonso, J.L.; Sarmento, B.; Gomes, A.M.; Pintado, M. Fermentation of Bioactive Solid Lipid Nanoparticles by Human Gut Microflora. Food Funct. 2016, 7, 516–529. [Google Scholar] [CrossRef]
- Himed-Idir, H.; Mouhoubi, K.; Siar, E.-H.; Boudries, H.; Mansouri, H.; Adjeroud, N.; Madani, K.; Boulekbache-Makhlouf, L. Effect of Rosemary (Rosmarinus officinalis L.) Supplementation on Fresh Cheese: Physicochemical Properties, Antioxidant Potential, and Sensory Attributes. J. Food Process. Preserv. 2021, 45, e15057. [Google Scholar] [CrossRef]
- Ferraro, V.; Madureira, A.R.; Sarmento, B.; Gomes, A.; Pintado, M.E. Study of the Interactions between Rosmarinic Acid and Bovine Milk Whey Protein α-Lactalbumin, β-Lactoglobulin and Lactoferrin. Food Res. Int. 2015, 77, 450–459. [Google Scholar] [CrossRef]
- Oliveira, G.D.A.R.; De Oliveira, A.E.; Da Conceição, E.C.; Leles, M.I.G. Multiresponse Optimization of an Extraction Procedure of Carnosol and Rosmarinic and Carnosic Acids from Rosemary. Food Chem. 2016, 211, 465–473. [Google Scholar] [CrossRef]
- Bettaieb Rebey, I.; Kefi, S.; Bourgou, S.; Ouerghemmi, I.; Ksouri, R.; Tounsi, M.S.; Marzouk, B. Ripening Stage and Extraction Method Effects on Physical Properties, Polyphenol Composition and Antioxidant Activities of Cumin (Cuminum cyminum L.) Seeds. Plant Foods Hum. Nutr. 2014, 69, 358–364. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Ponce-Alquicira, E.; Jaramillo-Flores, M.E.; Guerrero Legarreta, I. Antioxidant Effect Rosemary (Rosmarinus officinalis L.) and Oregano (Origanum vulgare L.) Extracts on TBARS and Colour of Model Raw Pork Batters. Meat Sci. 2009, 81, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Brunton, N.P.; Brennan, C.S. Handbook of Plant Food Phytochemicals Sources, Stability and Extraction; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Abdul Aziz, A.H.; Putra, N.R.; Kong, H.; Che Yunus, M.A. Supercritical Carbon Dioxide Extraction of Sinensetin, Isosinensetin, and Rosmarinic Acid from Orthosiphon Stamineus Leaves: Optimization and Modeling. Arab. J. Sci. Eng. 2020, 45, 7467–7476. [Google Scholar] [CrossRef]
- Huang, Z.; Chiew, Y.C.; Lu, W.D.; Kawi, S. Solubility of Aspirin in Supercritical Carbon Dioxide/Alcohol Mixtures. Fluid Phase Equilib. 2005, 237, 9–15. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Idham, Z.; Qomariyah, L.; Che Yunus, M.A. Extraction Rate of Valuable Compounds from Peanut Skin Waste by Ethanol-Assisted Supercritical Carbon Dioxide: Modelling and Optimization. Malays. J. Fundam. Appl. Sci. 2022, 18, 157–170. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory Effects of Rosemary Extracts, Carnosic Acid and Rosmarinic Acid on the Growth of Various Human Cancer Cell Lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic Acid: New Aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Chang, C.H.; Chyau, C.C.; Hsieh, C.L.; Wu, Y.Y.; Ker, Y.B.; Tsen, H.Y.; Peng, R.Y. Relevance of Phenolic Diterpene Constituents to Antioxidant Activity of Supercritical CO2 Extract from the Leaves of Rosemary. Nat. Prod. Res. 2008, 22, 76–90. [Google Scholar] [CrossRef]
- Ayyildiz, S.S.; Pelvan, E.; Karadeniz, B. Optimization of Accelerated Solvent Extraction, Ultrasound Assisted and Supercritical Fluid Extraction to Obtain Carnosol, Carnosic Acid and Rosmarinic Acid from Rosemary. Sustain. Chem. Pharm. 2024, 37, 101422. [Google Scholar] [CrossRef]
- Vieitez, I.; Maceiras, L.; Jachmanián, I.; Alborés, S. Antioxidant and Antibacterial Activity of Different Extracts from Herbs Obtained by Maceration or Supercritical Technology. J. Supercrit. Fluids 2018, 133, 58–64. [Google Scholar] [CrossRef]
- Bahri, S.; Ali, R.B.; Gasmi, K.; Mlika, M.; Fazaa, S.; Ksouri, R.; Serairi, R.; Jameleddine, S.; Shlyonsky, V. Prophylactic and Curative Effect of Rosemary Leaves Extract in a Bleomycin Model of Pulmonary Fibrosis. Pharm. Biol. 2017, 55, 462–471. [Google Scholar] [CrossRef]
- Haddou, S.; Mounime, K.; Loukili, E.H.; Ou-yahia, D.; Hbika, A.; Idrissi, M.Y.; Legssyer, A.; Lgaz, H.; Asehraou, A.; Touzani, R.; et al. Investigating the Biological Activities of Moroccan Cannabis sativa L. Seed Extracts: Antimicrobial, Anti-Inflammatory, and Antioxidant Effects with Molecular Docking Analysis. Moroc. J. Chem. 2023, 11, 1116–1136. [Google Scholar] [CrossRef]
- Boufetacha, M.; Ayad, A.; Hbika, A.; Boussetta, N.; Benali, M.; Gharibi, E. Study of Essential Oil Extraction from Moroccan Rosmarinus officinalis L. by Distillation Based on Quantum Chemical Calculation and Molecular Dynamics Simulation. J. Essent. Oil Bear. Plants 2024, 27, 640–658. [Google Scholar] [CrossRef]
- Niphadkar, S.S.; Rathod, V.K. Optimization of Ethanol Modified Supercritical Fluid Extraction (SFE) of Acetyl 11 Keto β Boswellic Acid (AKBA) from Boswellia serrata Using Box–Behnken Experimental Design. Biocatal. Agric. Biotechnol. 2018, 13, 304–310. [Google Scholar] [CrossRef]
- Tunna, T.S.; Sarker, M.Z.I.; Ghafoor, K.; Ferdosh, S.; Jaffri, J.M.; Al-Juhaimi, F.Y.; Ali, M.E.; Akanda, M.J.H.; Awal, M.S.; Ahmed, Q.U. Enrichment, in Vitro, and Quantification Study of Antidiabetic Compounds from Neglected Weed Mimosa pudica Using Supercritical CO2 and CO2-Soxhlet. Sep. Sci. Technol. 2018, 53, 243–260. [Google Scholar] [CrossRef]
- Panadare, D.; Dialani, G.; Rathod, V. Extraction of Volatile and Non-Volatile Components from Custard Apple Seed Powder Using Supercritical CO2 Extraction System and Its Inventory Analysis. Process Biochem. 2021, 100, 224–230. [Google Scholar] [CrossRef]
- Klein, E.J.; Náthia-Neves, G.; Vardanega, R.; Meireles, M.A.A.; da Silva, E.A.; Vieira, M.G.A. Supercritical CO2 Extraction of α-/β-Amyrin from Uvaia (Eugenia pyriformis Cambess.): Effects of Pressure and Co-Solvent Addition. J. Supercrit. Fluids 2019, 153, 104595. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Danguien, M.; Bily, A.; Chemat, F. Ultrasound versus Microwave as Green Processes for Extraction of Rosmarinic, Carnosic and Ursolic Acids from Rosemary. Ultrason. Sonochem. 2015, 27, 102–109. [Google Scholar] [CrossRef]
- Peev, G.; Penchev, P.; Peshev, D.; Angelov, G. Solvent Extraction of Rosmarinic Acid from Lemon Balm and Concentration of Extracts by Nanofiltration: Effect of Plant Pre-Treatment by Supercritical Carbon Dioxide. Chem. Eng. Res. Des. 2011, 89, 2236–2243. [Google Scholar] [CrossRef]
- Chadni, M.; Isidore, E.; Lagalle, F.; Langlait, M.; Dosso, A.; Ioannou, I. Optimization of the Supercritical Extraction of Rosmarinic Acid from Clary Sage Residue and the Antioxidant Activity of the Extracts. J. Supercrit. Fluids 2023, 193, 105830. [Google Scholar] [CrossRef]
- Boufetacha, M.; Ayad, A.; Thiebault, N.; Boussetta, N.; Gharibi, E.; Benali, M. Selective Extraction of Carnosic Acid, Carnosol, and Rosmarinic Acid from Rosmarinus officinalis L. Using Supercritical Fluid and Their Antioxidant Activity. J. Supercrit. Fluids 2024, 212, 106344. [Google Scholar] [CrossRef]
- Putrino, F.M.; Tedesco, M.; Bodini, R.B.; de Oliveira, A.L. Study of Supercritical Carbon Dioxide Pretreatment Processes on Green Coconut Fiber to Enhance Enzymatic Hydrolysis of Cellulose. Bioresour. Technol. 2020, 309, 123387. [Google Scholar] [CrossRef] [PubMed]
- Allaf, T.; Tomao, V.; Ruiz, K.; Bachari, K.; ElMaataoui, M.; Chemat, F. Deodorization by Instant Controlled Pressure Drop Autovaporization of Rosemary Leaves Prior to Solvent Extraction of Antioxidants. LWT Food Sci. Technol. 2013, 51, 111–119. [Google Scholar] [CrossRef]
- Zeroual, A.; Hassan Sakar, E.; Mahjoubi, F.; Chaouch, M.; Chaqroune, A.; Taleb, M. Effects of Extraction Technique and Solvent on Phytochemicals, Antioxidant, and Antimicrobial Activities of Cultivated and Wild Rosemary (Rosmarinus officinalis L.) from Taounate Region (Northern Morocco). Biointerface Res. Appl. Chem. 2022, 12, 8441–8452. [Google Scholar] [CrossRef]
- Oussaid, A.; Azzouzi, M.; Ibn Mansour, A.; Azouagh, M.; Koudad, M.; Oussaid, A. Assessment of the Chemical/Biological Activities of Extracts and Essential Oil of Rosmarinus officinalis L. from the Oriental Region of Morocco. Moroc. J. Chem. 2020, 8, 732–744. [Google Scholar] [CrossRef]
- Wellwood, C.R.L.; Cole, R.A. Relevance of Carnosic Acid Concentrations to the Selection of Rosemary, Rosmarinus officinalis L.), Accessions for Optimization of Antioxidant Yield. J. Agric. Food Chem. 2004, 52, 6101–6107. [Google Scholar] [CrossRef]
- Hirondart, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Bily, A.; Chemat, F. Comparison between Pressurized Liquid Extraction and Conventional Soxhlet Extraction for Rosemary Antioxidants, Yield, Composition, and Environmental Footprint. Foods 2020, 9, 584. [Google Scholar] [CrossRef]
- Vasile, C.; Tudorachi, N.; Zaharescu, T.; Darie-Nita, R.N.; Cheaburu-Yilmaz, C.N. Study on Thermal Behavior of Some Biocompatible and Biodegradable Materials Based on Plasticized PLA, Chitosan, and Rosemary Ethanolic Extract. Int. J. Polym. Sci. 2020, 2020, 4269792. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, H.N.T.; Huang, M.Y.; Lin, K.H.; Pham, D.C.; Tran, Y.B.; Su, C.H. Optimization of Aqueous Enzyme-Assisted Extraction of Rosmarinic Acid from Rosemary (Rosmarinus officinalis L.) Leaves and the Antioxidant Activity of the Extract. J. Food Process. Preserv. 2021, 45, e15221. [Google Scholar] [CrossRef]
- Amarowicz, R.; Shahidi, F. Antioxidant Activity of Broad Bean Seed Extract and Its Phenolic Composition. J. Funct. Foods 2017, 38, 656–662. [Google Scholar] [CrossRef]
- Mtunzi, F.M.; Ejidike, I.P.; Ledwaba, I.; Ahmed, A.; Pakade, V.E.; Klink, M.J.; Modise, S.J. Solvent–Solvent Fractionations of Combretum erythrophyllum (Burch.) Leave Extract: Studies of Their Antibacterial, Antifungal, Antioxidant and Cytotoxicity Potentials. Asian Pac. J. Trop. Med. 2017, 10, 670–679. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant Activities of Rosemary (Rosmarinus officinalis L.) Extract, Blackseed (Nigella sativa L.) Essential Oil, Carnosic Acid, Rosmarinic Acid and Sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Outaleb, T.; Hazzit, M.; Ferhat, Z.; Baaliouamer, A.; Yekkour, A.; Zitouni, A.; Sabaou, N. Composition, Antioxidant and Antimicrobial Activities of Algerian Rosmarinus officinalis L. Extracts. J. Essent. Oil Bear. Plants 2015, 18, 654–665. [Google Scholar] [CrossRef]
- Hosseini, H.; Bolourian, S.; Yaghoubi Hamgini, E.; Ghanuni Mahababadi, E. Optimization of Heat- and Ultrasound-Assisted Extraction of Polyphenols from Dried Rosemary Leaves Using Response Surface Methodology. J. Food Process. Preserv. 2018, 42, e13778. [Google Scholar] [CrossRef]
- Hbika, A.; Daoudi, N.E.; Bouyanzer, A.; Bouhrim, M.; Mohti, H.; Loukili, E.H.; Mechchate, H.; Al-Salahi, R.; Nasr, F.A.; Bnouham, M.; et al. Artemisia absinthium L. Aqueous and Ethyl Acetate Extracts: Antioxidant Effect and Potential Activity In Vitro and In Vivo against Pancreatic α-Amylase and Intestinal α-Glucosidase. Pharmaceutics 2022, 14, 481. [Google Scholar] [CrossRef] [PubMed]



| Factors | Factors Level * | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Temperature (°C) | 40 | 60 | 80 |
| Pressure (bar) | 150 | 250 | 350 |
| % EtOH (w/w) | 5 | 10 | 15 |
| Variable Values | Responses | ||||
|---|---|---|---|---|---|
| Run | Temperature (°C) | Pressure (bar) | % EtOH (w/w) | Yield (%) | RA Content (mg/g DM) |
| 1 | 40 | 250 | 5 | 8.73 | 0.00 |
| 2 | 60 | 350 | 5 | 13.41 | 0.00 |
| 3 | 40 | 150 | 10 | 13.02 | 0.57 |
| 4 | 60 | 150 | 15 | 19.60 | 2.06 |
| 5 | 60 | 250 | 10 | 15.88 | 1.68 |
| 6 | 80 | 250 | 15 | 19.39 | 2.88 |
| 7 | 60 | 350 | 15 | 20.31 | 1.38 |
| 8 | 60 | 150 | 5 | 10.97 | 0.00 |
| 9 | 80 | 150 | 10 | 18.98 | 2.38 |
| 10 | 80 | 350 | 10 | 17.52 | 0.17 |
| 11 | 40 | 250 | 15 | 15.67 | 0.84 |
| 12 | 60 | 250 | 10 | 16.65 | 0.98 |
| 13 | 60 | 250 | 10 | 15.75 | 1.05 |
| 14 | 40 | 350 | 10 | 18.48 | 0.12 |
| 15 | 80 | 250 | 5 | 9.34 | 0.00 |
| F-Value | p-Value | |
|---|---|---|
| Model | 122.99 | |
| Temperature (A) | 61.50 | 0.0005 |
| Pressure (B) | 36.10 | 0.0018 |
| EtOH (C) | 748.45 | <0.0001 |
| A*B | 67.62 | 0.0004 |
| A*C | 13.69 | 0.0140 |
| B*C | 4.24 | 0.0946 |
| A*A | 18.50 | 0.0077 |
| B*B | 71.50 | 0.0004 |
| C*C | 73.12 | 0.0004 |
| Lack of fit | 0.5773 | |
| R2 | 0.9955 | |
| Adjusted R2 | 0.9874 | |
| Predicted R2 | 0.9612 | |
| Adeq precision | 34.2945 |
| F-Value | p-Value | |
|---|---|---|
| Model | 13.41 | 0.0009 |
| Temperature (A) | 13.14 | 0.0067 |
| Pressure (B) | 9.65 | 0.0145 |
| EtOH (C) | 44.32 | 0.0002 |
| A*B | 5.35 | 0.0494 |
| A*C | 7.19 | 0.0279 |
| B*C | 0.8038 | 0.3961 |
| Lack of fit | 0.9644 | |
| R2 | 0.9096 | |
| Adjusted R2 | 0.8417 | |
| Predicted R2 | 0.7012 | |
| Adeq precision | 11.6817 | |
| Extraction Method | Yield (%) | Total Polyphenol Content (g/100 g DM) | RA Content (mg/g DM) | CAR Content (mg/g DM) | CA Content (mg/g DM) | IC50 (μg/mL) |
|---|---|---|---|---|---|---|
| Soxhlet | 19.42 ± 0.28 | 4.78 ± 0.16 | 4.19 ± 0.33 | 8.65 ± 2.98 | 16.67 ± 0.94 | 214.55 ± 6.33 |
| Soxhlet after scCO2 | 7.03 ± 1.06 | 2.12 ± 0.15 | 3.7 ± 0.42 | 0.38 ± 0.20 | 0.38 ± 0.10 | 256.05 ± 18.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boufetacha, M.; Gharibi, E.; Benali, M. Optimising Supercritical Carbon Dioxide Extraction of Rosmarinic Acid from Rosmarinus officinalis L. and Enhancing Yield Through Soxhlet Coupling. Processes 2025, 13, 655. https://doi.org/10.3390/pr13030655
Boufetacha M, Gharibi E, Benali M. Optimising Supercritical Carbon Dioxide Extraction of Rosmarinic Acid from Rosmarinus officinalis L. and Enhancing Yield Through Soxhlet Coupling. Processes. 2025; 13(3):655. https://doi.org/10.3390/pr13030655
Chicago/Turabian StyleBoufetacha, Meryem, Elkhadir Gharibi, and Mohammed Benali. 2025. "Optimising Supercritical Carbon Dioxide Extraction of Rosmarinic Acid from Rosmarinus officinalis L. and Enhancing Yield Through Soxhlet Coupling" Processes 13, no. 3: 655. https://doi.org/10.3390/pr13030655
APA StyleBoufetacha, M., Gharibi, E., & Benali, M. (2025). Optimising Supercritical Carbon Dioxide Extraction of Rosmarinic Acid from Rosmarinus officinalis L. and Enhancing Yield Through Soxhlet Coupling. Processes, 13(3), 655. https://doi.org/10.3390/pr13030655







