Sustainable Bio-Preservation of Concentrated Yogurt (Labneh) Using Syzygium aromaticum L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Ethanolic Clove Flower Buds’ Extraction
2.2.2. LC-ESI-MS/MS Analysis of the Extract
2.2.3. Antimicrobial Evaluation of Ethanolic Clove Extract
Disk Diffusion Assay
Determination of Minimal Inhibitory Concentration (MIC)
2.2.4. Manufacturing of Bio-labneh with Clove Extract
2.2.5. Microbiological Quality Analyses
2.2.6. Chemical Composition of Bio-Labneh
2.2.7. Antioxidant Activity of Bio-Labneh
2.2.8. Textural Profile of Bio-Labneh
2.2.9. Determination of Volatile Compounds of Bio-Labneh by GC-Mass Spectrometry
Sensory Properties of Bio-Labneh
Statistical Analysis
3. Results and Discussion
3.1. LC-ESI-MS/MS Profiling of Ethanolic Clove Extract
3.2. Antimicrobial Activity of Clove Extract
3.2.1. Disk Diffusion Assay
3.2.2. Minimal Inhibitory Concentration
3.3. Bio-labneh and Its Properties
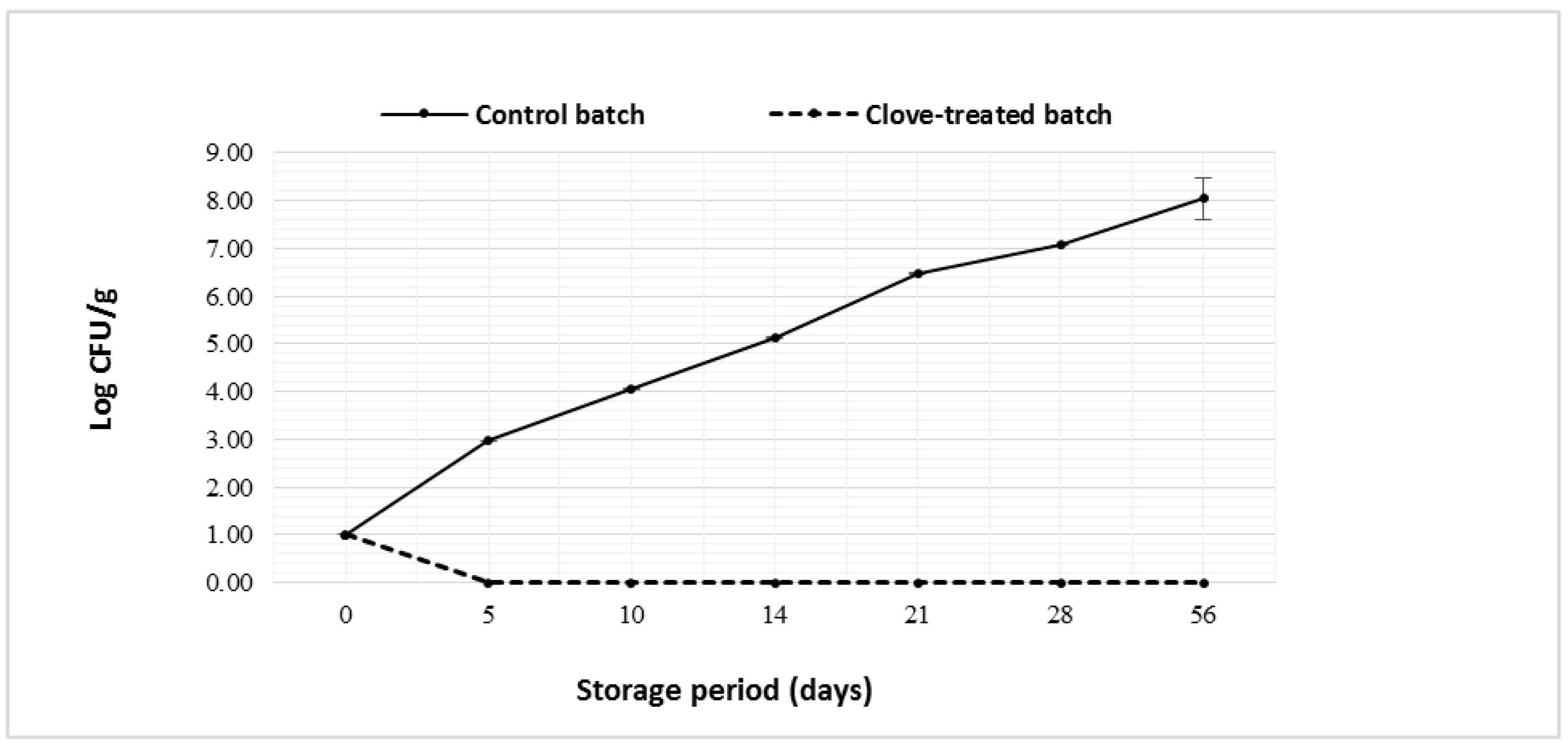
3.3.1. Microbiological Quality of Manufactured Bio-labneh Cheese
3.3.2. Chemical Composition of Bio-Labneh
3.3.3. Antioxidant Activity of Bio-Labneh
3.3.4. Texture Profile Analysis of Bio-Labneh
3.3.5. Aroma Compounds
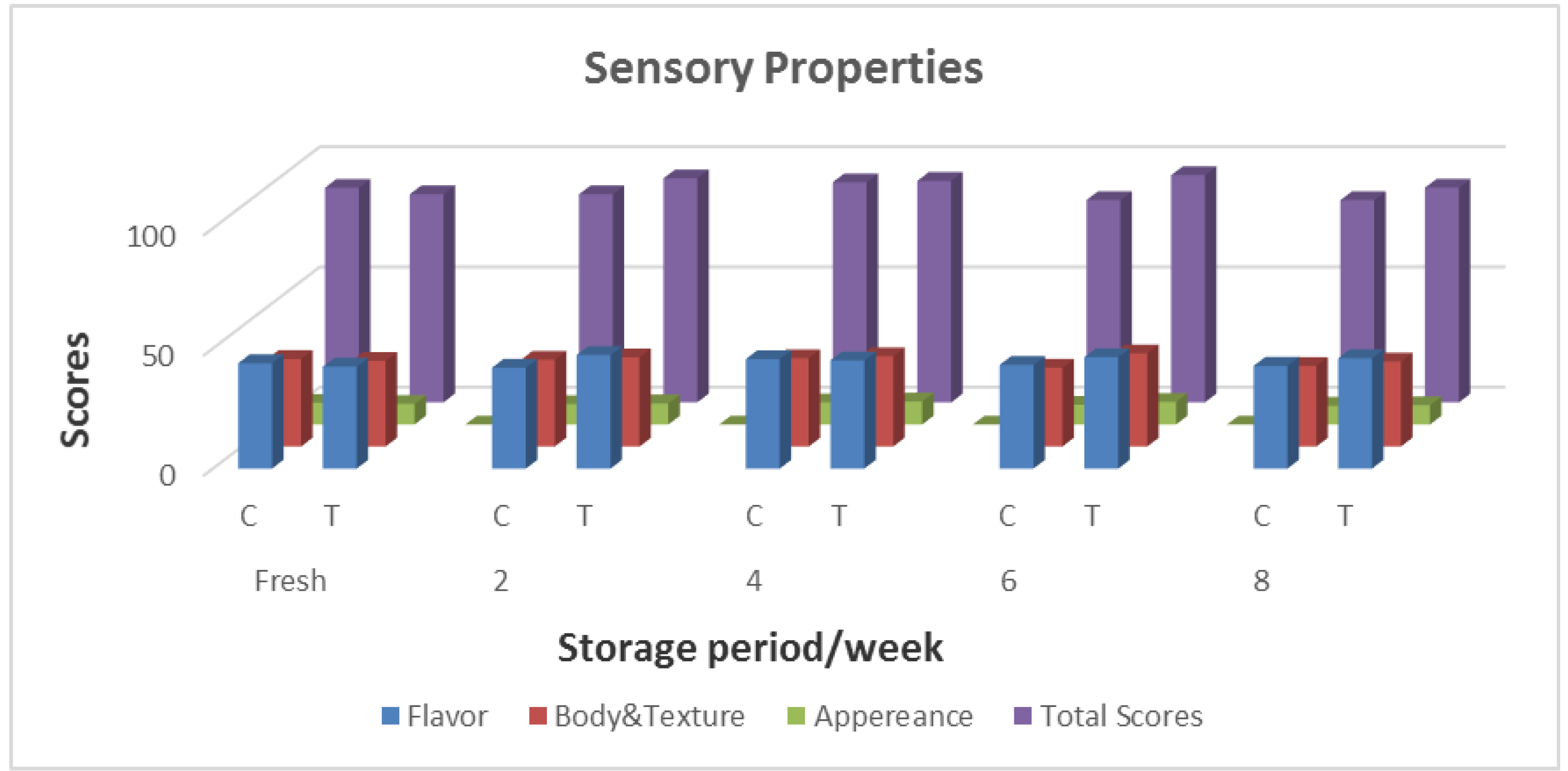
3.3.6. Sensory Attributes of Bio-Labneh
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaaki, D.; Baghdadi, O.K.; Najm, N.E.; Olabi, A. Preference mapping of commercial Labneh (strained yogurt) products in the Lebanese market. J. Dairy Sci. 2012, 95, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Thabet, H.M.; Nogaim, Q.A.; Qasha, A.S.; Abdoalaziz, O.; Alnsheme, N. Evaluation of the effects of some plant derived essential oils on shelf-life extension of Labneh. Merit Res. J. Food Sci. Technol. 2014, 2, 8–14. [Google Scholar]
- Khider, M.; Nasr, N.M.; Atallah, K.M.; Metry, W.A. Functional UF-low and full-fat Labneh supplemented with Oats (Avena sativa L.) powder and probiotic bacteria. J. Umm Al-Qura Univ. Appl. Sci. 2022, 8, 21–32. [Google Scholar] [CrossRef]
- Nsabimana, C.; Jiang, B.; Kossah, R. Manufacturing, properties and shelf life of Labneh: A review. Int. J. Dairy Technol. 2005, 58, 129–137. [Google Scholar] [CrossRef]
- Dijksterhuis, J. Fungal spores: Highly variable and stress-resistant vehicles for distribution and spoilage. Food Microbiol. 2019, 81, 2–11. [Google Scholar] [CrossRef]
- Al-Otaibi, M.; El Demerdash, H. Improvement of the quality and shelf life of concentrated yoghurt (labneh) by the addition of some essential oils. Afr. J. Microbiol. Research 2008, 2, 156–161. [Google Scholar]
- Fadel, H.H.M.; El-Ghorab, A.H.; Hussein, A.M.; El-Massry, K.F.; Lotfy, S.N.; Ahmed, M.Y.S.; Soliman, T.N. Correlation between chemical composition and radical scavenging activity of 10 commercial essential oils: Impact of microencapsulation on functional properties of essential oils. Arab. J. Chem. 2020, 13, 6815–6827. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Chedid, M.; Shaib, H.; Jaber, L.; El Iskandarani, Y.; Hamadeh, S.K. Microbial Quality and Production Methods of Traditional Fermented Cheeses in Lebanon. Int. J. Food Sci. 2025, 2025, 5673559. [Google Scholar] [CrossRef]
- Dey, B.K.; Mukherjee, S.S. Potential of clove and its nutritional benefits in physiological perspective: A review. Int. J. Physiol. Nutr. Phys. Educ. 2021, 6, 103–106. [Google Scholar]
- Idowu, S.; Adekoya, A.E.; Igiehon, O.O.; Idowu, A.T. Clove (Syzygium aromaticum) spices: A review on their bioactivities, current use, and potential application in dairy products. J. Food Meas. Charact. 2021, 15, 3419–3435. [Google Scholar] [CrossRef]
- Mahmoud, K.F.; Hussein, A.; Kamil, M.M.; Hegazy, N. Effect the Addition of Micro- and Nano-Capsule Cumin and Clove Oils as Antioxidants and Anti-Cancer on Rancidity and Shelf Life in Some Biscuit Products. Egypt. J. Chem. 2022, 65, 593–606. [Google Scholar] [CrossRef]
- Mahmoud, E.A.; Abd-Allab, A.A.; Salman, K.H. Utilization of Cinnamon, Clove and Thyme Essential Oils as Antimicrobial and Bioactive Compounds in Kishk Manufacturing. J. Food Sci. Suez Canal Univ. 2020, 7, 43–54. [Google Scholar]
- Assem, F.M.; El Shafei, K.; El-Sayed, H.S.; Matter, M.A.; Hanafy, M.S.; Amer, A.M. Effects of Carnation Essential Oil Extracted from Carnation Calli on Extending Shelf Life of Yoghurt. Plant Tissue Cult. Biotechnol. 2019, 29, 1–14. [Google Scholar] [CrossRef]
- Pandey, V.K.; Srivastava, S.; Ashish; Dash, K.K.; Singh, R.; Dar, A.H.; Singh, T.; Farooqui, A.; Shaikh, A.M.; Kovacs, B. Bioactive properties of clove (Syzygium aromaticum) essential oil nanoemulsion: A comprehensive review. Heliyon 2024, 10, e22437. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, W.B.; El Bous, M.M.; El Said, M.; El Baz, H. In vitro Evaluation of Syzygium aromaticum L. Ethanol Extract as Biocontrol Agent against Postharvest Tomato and Potato Diseases. Egypt J. Bot. 2019, 59, 81–94. [Google Scholar]
- El-Ssayad, M.F.; Abd-Rabou, A.A.; El-Sayed, H.S. Utilization of lactobacilli extracts as antibacterial agent and to induce colorectal cancer cells apoptosis: Application in white soft cheese. Food Biosci. 2023, 56, 103252. [Google Scholar] [CrossRef]
- Andrews, J.M. BSAC standardized disc susceptibility testing method (version 4). J. Antimicrob. Chemother. 2005, 56, 60–76. [Google Scholar] [CrossRef]
- EUCAST. European Committee for Antimicrobial Susceptibility Testing of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [Google Scholar] [CrossRef]
- El-Saye, S.M.; El-Saye, H.S.; Salama, H.H.; El-Nor, S.A. Improving the nutritional value and extending shelf life of labneh by adding Moringa oleifera oil. Int. J. Dairy Sci. 2017, 12, 81–92. [Google Scholar] [CrossRef]
- Food and Drug Administration. Bacteriological Analytical Manual, 9th ed.; AOAC International: Arlington, VA, USA, 2002. [Google Scholar]
- Association of Official Analytical Chemist. Official Methods of Analysis, 21st ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Ali, M.A.; El-Tawab, Y.A.A.; Ebrahim, H.M. Effect of some Herbs Essential Oils on Labneh. J. Food Dairy Sci. 2019, 10, 101–106. [Google Scholar] [CrossRef]
- Abbas, H.M.; Assem, F.M.; Zaky, W.M.; Kassem, J.M.; Omer, E.A. Antioxidant, Rheological and Sensorial Properties of Ultra-filtrated Soft Cheese Supplemented with Basil Essential Oil. Int. J. Dairy Sci. 2017, 12, 301–309. [Google Scholar] [CrossRef]
- Erkaya, T.; Şengül, M. Comparison of volatile compounds in yoghurts made from cows’, buffaloes’, ewes’ and goats’ milks. Int. J. Dairy Technol. 2011, 64, 240–246. [Google Scholar] [CrossRef]
- Elisha, E.; Ajobiewe, H.F.; Ibrahim, A.E.; Alau, K.K.; Umeji, L.C.; Salami, A.; Udefuna, P.A.; Yashim, A.N.; Ajobiewe, J. Antimicrobial Activity of Clove Plant Flower Bud Extract (Syzygium aromaticum) on Salmonella typhi. Sch. J. Appl. Med. Sci. 2022, 10, 698–708. [Google Scholar] [CrossRef]
- Siswadi, S.; Saragih, G.S. Phytochemical analysis of bioactive compounds in ethanolic extract of Sterculia quadrifida R.Br. AIP Conf. Proc. 2021, 2353, 030098. [Google Scholar] [CrossRef]
- Medina, M.E.; Iuga, C.; Alvarez-Idaboy, J.R. Antioxidant activity of propyl gallate in aqueous and lipid media: A theoretical study. Phys. Chem. Chem. Phys. 2013, 15, 13137–13146. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef]
- Kusumah, D.; Wakui, M.; Murakami, M.; Xie, X.; Yukihito, K.; Maeda, I. Linoleic acid, α-linolenic acid, and monolinolenins as antibacterial substances in the heat-processed soybean fermented with Rhizopus oligosporus. Biosci. Biotechnol. Biochem. 2020, 84, 1285–1290. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Sofia, P.K.; Prasad, R.; Vijay, V.K.; Srivastava, A.K. Evaluation of antibacterial activity of Indian spices against common foodborne pathogens. Int. J. Food Sci. Technol. 2007, 42, 910–915. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Doghish, A.S.; El-Dakroury, W.A.; Hassanin, M.M.H.; Al-Askar, A.A.; AbdElgawad, H.; Hashem, A.H. Antimicrobial, Antibiofilm, and Anticancer Activities of Syzygium aromaticum Essential Oil Nanoemulsion. Molecules 2023, 28, 5812. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Liu, T.; Hu, Q.P.; Cao, X.M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.-N.; Tang, G.-Y.; Li, H.-B. Antibacterial and Antifungal Activities of Spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakamura, A.; Fujino, N.; Sawaguchi, Y.; Sato, M.; Kuda, T.; Kimura, B. Evaluation of the antibacterial activity of allyl isothiocyanate, clove oil, eugenol and carvacrol against spoilage lactic acid bacteria. LWT 2021, 145, 111263. [Google Scholar] [CrossRef]
- Hoque, M.M.; Inatsu, M.; Juneja, V.; Kawamoto, S. Antimicrobial activity of Cloves and Cinnamon extracts against food borne pathogens and spoilage bacteria, and inactivation of Listeria monocytogenes in ground chicken meat with their essential oils. Rep. Nat’l. Food Res. Inst. 2008, 72, 9–21. [Google Scholar]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef]
- El-Awady, A.A.; Yossif, H.; Abo-Srea, M.M.; Shalabi, O.M.A.K. Impact of Mint and Clove Extract (Nano-formulations) on Functional Yoghurt Properties. J. Food Dairy Sci. 2023, 14, 181–188. [Google Scholar] [CrossRef]
- Rifky, M.; Serkayev, K.; Samadiy, M. Essential Oils Incorporation Technology to Improve Shelf life And Stability of Yoghurt. Food Technol. 2022, 2022, 45–51. [Google Scholar] [CrossRef]
- Mohamed, S.H.S.; Zaky, W.M.; Kassem, J.M.; Abbas, H.M.; Salem, M.M.E.; Ahl, H.A.H.S.-A. Impact of Antimicrobial Properties of Some Essential Oils on Cheese Yoghurt Quality. World Appl. Sci. J. 2013, 27, 497–507. [Google Scholar]
- El-Aziz, M.A.; Salama, H.H.; Sayed, R.S. Plant extracts and essential oils in the dairy industry: A review. Food Raw Mater. 2023, 11, 321–337. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Potential application of spice and herb extracts as natural preservatives in cheese. J. Med. Food 2011, 14, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Fouad, M.T.; Khair, A.G.A.-E.; El-Sayed, S.M.; Shazly, A.B.; El-Sayed, H.S. Bio-Labneh fortified with functional microcapsules filled with chickpea flour and probiotics. Biocatal. Agric. Biotechnol. 2022, 42, 102345. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Reis, M.G.; Wa, Y.; Alfante, R.; Chanyi, R.M.; Altermann, E.; Day, L. Differences in Aroma Metabolite Profile, Microstructure, and Rheological Properties of Fermented Milk Using Different Cultures. Foods 2023, 12, 1875. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Elaaser, M.; El-Shafei, K.; Mabrouk, A.M.; Kassem, J.M.; Assem, F.; Tawfik, N.F.; Effat, B.A.M. A Comprehensive Study of Carbonated Probiotic UF-Labneh Cheese as a Novel Product. Egypt. J. Food Sci. 2022, 50, 127–143. [Google Scholar] [CrossRef]
- Hamad, M.N.-E.F.; El-Bushuty, D.H.A.; Abdallah, A.M.H. Chemical, Rheological, Microbiological and Organoleptic Properties of Labneh Manufactured by Using Some Vegetable Oils. Acad. J. Life Sci. 2021, 7, 28–38. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Jin, R.; Zhang, H.; Zhou, T.; Sun, T. Characterization of volatile compounds in fermented milk using solid-phase microextraction methods coupled with gas chromatography-mass spectrometry. J. Dairy Sci. 2017, 100, 2488–2500. [Google Scholar] [CrossRef]
- Zaky, W.M.; Kassem, J.M.; Abbas, H.M.; Mohamed, S.H.S. Evaluation of Salt- Free Labneh Quality Prepared Using Dill and Caraway Essential Oils. Life Sci. J. 2013, 10, 3379–3386. [Google Scholar]


| RT | Name | Area | Height | Nature | Activity | Reference |
|---|---|---|---|---|---|---|
| 2.54655 | Chaulmoogric Acid | 23,252,350 | 868,137.5 | Cyclopentenyl fatty acid | Antioxidant, Antimicrobial, anti-inflammatory | [27] |
| 7.54475 | Hexadecanedioic acid | 3,556,171 | 153,683.8 | Fatty acid | ||
| 3.155767 | Propyl gallate | 12,997,020 | 637,667.1 | Phenolic acid derivative | Antioxidant | [28] |
| 4.486117 | Protocatechuic acid | 1,147,789 | 53,354.38 | Phenolic acid | Antioxidant, anti-inflammatory, antibacterial, antiviral, radical-scavenging, gastroprotective, anticancer, anti-aging, neurological effective and immune-modulatory activities | [29] |
| 6.5758 | Quercetin | 3,431,954 | 152,700.2 | Flavonol | ||
| 7.54475 | Kaempferol | 2,636,916 | 114,283.4 | Flavonol | Anti-inflammatory | [30] |
| 8.249967 | Linoleic acid | 3,794,725 | 232,804.7 | Fatty acid | antibacterial | [31] |
| 19.53632 | Trans-cinnamic acid | 20,571,800 | 819,391.6 | Aromatic carboxylic acid | Antioxidant, anti-inflammatory, anticancer, antimutagenic, anti-glycemic, neuroprotective, and antibacterial activities. | [32] |
| 26.77547 | Sinapine | 11,317,340 | 651,271.3 | Alkaloid |
| Test Organisms | Inhibition Diameter (mm) * | |
|---|---|---|
| Ethanolic Extract | Positive Control | |
| Bacillus cereus | 7.33 ± 0.34 F | 16.50 ± 0.00 C |
| Staphylococcus aureus | 6.33 ± 0.34 HI | 16.50 ± 0.00 C |
| Listeria monocytogenes | 6.83 ± 0.17 FGH | 15.0 ± 0.00 D |
| Escherichia coli | 6.17 ± 0.17 I | 18.83 ± 0.17 B |
| Salmonella enterica | 7.0 ± 0.00 FG | 20.0 ± 0.00 A |
| Pseudomonas aeruginosa | ND | ND |
| Penicillium verrocosum | 20.33 ± 0.34 A | 12.0 ± 0.00 E |
| Aspergillus flavus | 20.33 ± 0.34 A | 17.0 ± 0.00 C |
| Saccharomyces cerviceae | 6.67 ± 0.34 GHI | 12.0 ± 0.00 E |
| Indicator Strain | MIC Value (mg/mL) |
|---|---|
| Bacillus cereus | 1.00 ± 0.00 C |
| Staphylococcus aureus | 2.50 ± 0.00 B |
| Listeria monocytogenes | 2.50 ± 0.00 B |
| Escherichia coli | 2.50 ± 0.00 B |
| Salmonella enterica | 2.50 ± 0.00 B |
| Pseudomonas aeruginosa | 6.50 ± 0.00 A |
| Penicillium verrocosum | 0.25 ± 0.00 D |
| Aspergillus flavus | 0.125 ± 0.00 E |
| Saccharomyces cerevisiae | 1.00 ± 0.00 C |
| Parameters | Samples | Storage Period/Weeks | ||||
|---|---|---|---|---|---|---|
| Fresh | 2 | 4 | 6 | 8 | ||
| TS % | C | 25.27 Ab ± 0.03 | 25.29 Ab ± 0.04 | 25.98 Ba ± 0.02 | 26.43 Aa ± 0.03 | 26.74 Aa ± 0.55 |
| T | 25.42 Ab ± 0.47 | 25.85 Ab ± 0.04 | 26.20 Aab ± 0.03 | 26.63 Aab ± 0.03 | 27.15 Aa ± 5.75 | |
| Fat % | C | 0.75 Ac ± 0.25 | 0.79 Ac ± 0.28 | 0.85 Ab ± 0.38 | 0.89 Ab ± 0.20 | 0.91 Aa ± 0.20 |
| T | 0.76 Ac ± 0.50 | 0.75 Ac ± 0.25 | 0.84 Ac ± 0.38 | 0.85 Ab ± 0.18 | 0.89 Aa ± 0.20 | |
| TP % | C | 11.68 Aa ± 0.19 | 11.95 aa ± 0.05 | 12.75 Aa ± 0.29 | 13.01 Aa ± 0.13 | 13.17 Aa ± 0.17 |
| T | 11.735 Aa ± 0.11 | 11.97 Aa ± 0.04 | 12.45 Aa ± 0.28 | 12.90 Aa ± 0.12 | 13.24 Aa ± 0.14 | |
| pH | C | 4.81 Bb ± 0.01 | 4. 85 Aa ± 0.01 | 4.56 Ac ± 0.02 | 4.44 Ad ± 0.01 | 4.35 Be ± 0.02 |
| T | 4.86 Aa ± 0.005 | 4.71 Bb ± 0.01 | 4.58 Ac ± 0.03 | 4.48 Ad ± 0.02 | 4.40 Ad ± 0.02 | |
| Parameters | Samples | Storage Period/Weeks | ||||
|---|---|---|---|---|---|---|
| Fresh | 2 | 4 | 6 | 8 | ||
| Hardness (N) | C | 11.5 Ad ± 0.15 | 14.30 Ac ± 0.30 | 17.86 Ab ± 0.11 | 19.11 Ba ± 0.18 | 19.27 Ba ± 0.19 |
| T | 7.88 Bd ± 0.04 | 12.00 Bc ± 0.15 | 17.55 Ab ± 0.18 | 20.22 Aa ± 0.16 | 21.05 Aa ± 0.31 | |
| Cohesiveness (B/A area) | C | 0.613 Ba ± 0.01 | 0.617 Ba ± 0.01 | 0.522 Bc ± 0.0 | 0.569 Ab ± 0.01 | 0.576 Ab ± 0.01 |
| T | 0.704 Ab ± 0.01 | 0.624 Ac ± 0.01 | 0.770 Aa ± 0.01 | 0.328 Bd ± 0.00 | 0.352 Bd ± 0.03 | |
| Springiness (mm) | C | 0.769 Bb ± 0.01 | 0.780 Aa ± 0.01 | 0.698 Bc ± 0.01 | 0.714 Ac ± 0.01 | 0.721 Ac ± 0.01 |
| T | 0.860 Aa ± 0.01 | 0.730 Bc ± 0.01 | 0.743 Ab ± 0.01 | 0.538 Bd ± 0.01 | 0.535 Bd ± 0.01 | |
| Gumminess (N) | C | 7.050 Bd ± 0.19 | 8.820 Ac ± 0.23 | 9.320 Bb ± 0.29 | 10.87 Aa ± 0.27 | 10.973 Aa ± 0.12 |
| T | 5.547 Ad ± 0.03 | 7.49 Bb ± 0.16 | 13.51 Aa ± 0.27 | 6.960 Bc ± 0.13 | 6.85 Bc ± 0.21 | |
| Chewiness (N/m) | C | 5.421 Ac ± 0.18 | 6.88 Ab ± 0.32 | 6.50 Bb ± 0.25 | 7.761 Aa ± 0.24 | 7.966 Aa ± 0.47 |
| T | 4.77 Bc ± 0.08 | 5.47 Bb ± 0.15 | 10.04 Aa ± 0.24 | 3.744 Bd ± 0.14 | 3.70 Bd ± 0.10 | |
| Aroma Compounds (Area%) | Storage Period/Weeks | |||||
|---|---|---|---|---|---|---|
| Fresh | 4 Weeks | 8 Weeks | ||||
| C | T | C | T | C | T | |
| Benzene | 15.44 | 2.87 | 4.76 | 18.61 | 4.64 | 4.59 |
| 2,3-Pentanedione | 3.21 | 3.34 | ||||
| Dimethylsilanedio | 16.29 | 9.82 | 20.27 | 0.49 | ||
| Methylbenzene | 5.96 | 2.43 | ||||
| 2-Hexanone | 2.13 | 1.42 | ||||
| 3-Heptanone | 2.93 | 1.47 | ||||
| Styrene | 6.66 | 9.3 | 10.79 | 7.31 | 4.99 | 15.32 |
| Styrene | 3.86 | 4.28 | 3.24 | 3.86 | ||
| 5-Methyl-3-hexanol | 1.43 | 1.3 | ||||
| Cyclopentyl 4-ethylbenzoate | 9.29 | 7.6 | ||||
| Benzaldehyde | 11.02 | 1.76 | 0.74 | |||
| Heptanal | 2.84 | 2.12 | 1.41 | 2.57 | 1.26 | |
| p-Cymene | 0.75 | 1.82 | 3.74 | 2.95 | 5.13 | |
| 1,6-Dihydrocarveol | 4.14 | 2.72 | 1.77 | 2.72 | 2.28 | 5.15 |
| γ-Terpinene | 0.62 | 0.76 | 1.41 | 1.36 | 2.19 | |
| 2-Propyl-1-pentanol | 0.95 | 0.95 | 14.26 | |||
| 2-Nonanone | 0.93 | 0.91 | ||||
| α-Cubebene | 0.89 | |||||
| Eugenol | 11.58 | 2.65 | ||||
| Copaene | 3.4 | |||||
| β-Caryophyllene | 36.02 | 3.8 | ||||
| Aromandendrene | 1.25 | |||||
| α-Humulene | 5.6 | 0.35 | ||||
| Acetyleugenol | 1.74 | 0.42 | ||||
| δ-Cadinene | 1.98 | |||||
| Toluene | 13.04 | 10.84 | 18.72 | |||
| Methyl tartronic acid | 20.94 | |||||
| Ethyl Benzene | 26.05 | 11.63 | 0.93 | |||
| Palmitic acid | 1.47 | 5.15 | 1.5 | |||
| Methyl N-hydroxy benzene carboximidoate | 9.36 | 14.02 | 5.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-ssayad, M.F.; Assem, F.M.; El-Gawad, M.A.M.A.; Mohamed, S.H.S.; Kassem, J.M.; Alsaleem, K.A. Sustainable Bio-Preservation of Concentrated Yogurt (Labneh) Using Syzygium aromaticum L. Processes 2025, 13, 413. https://doi.org/10.3390/pr13020413
El-ssayad MF, Assem FM, El-Gawad MAMA, Mohamed SHS, Kassem JM, Alsaleem KA. Sustainable Bio-Preservation of Concentrated Yogurt (Labneh) Using Syzygium aromaticum L. Processes. 2025; 13(2):413. https://doi.org/10.3390/pr13020413
Chicago/Turabian StyleEl-ssayad, Mohamed F., Fayza M. Assem, Mona A. M. Abd El-Gawad, Sahar H. S. Mohamed, Jihan M. Kassem, and Khalid A. Alsaleem. 2025. "Sustainable Bio-Preservation of Concentrated Yogurt (Labneh) Using Syzygium aromaticum L." Processes 13, no. 2: 413. https://doi.org/10.3390/pr13020413
APA StyleEl-ssayad, M. F., Assem, F. M., El-Gawad, M. A. M. A., Mohamed, S. H. S., Kassem, J. M., & Alsaleem, K. A. (2025). Sustainable Bio-Preservation of Concentrated Yogurt (Labneh) Using Syzygium aromaticum L. Processes, 13(2), 413. https://doi.org/10.3390/pr13020413







