Biosurfactant Production by Yarrowia lipolytica in Corn Steep Liquor-Based Media for Hydrocarbon Bioremediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strain and Preculture
2.3. Biosurfactant Production in Different Concentrations of Corn Steep Liquor (CSL)
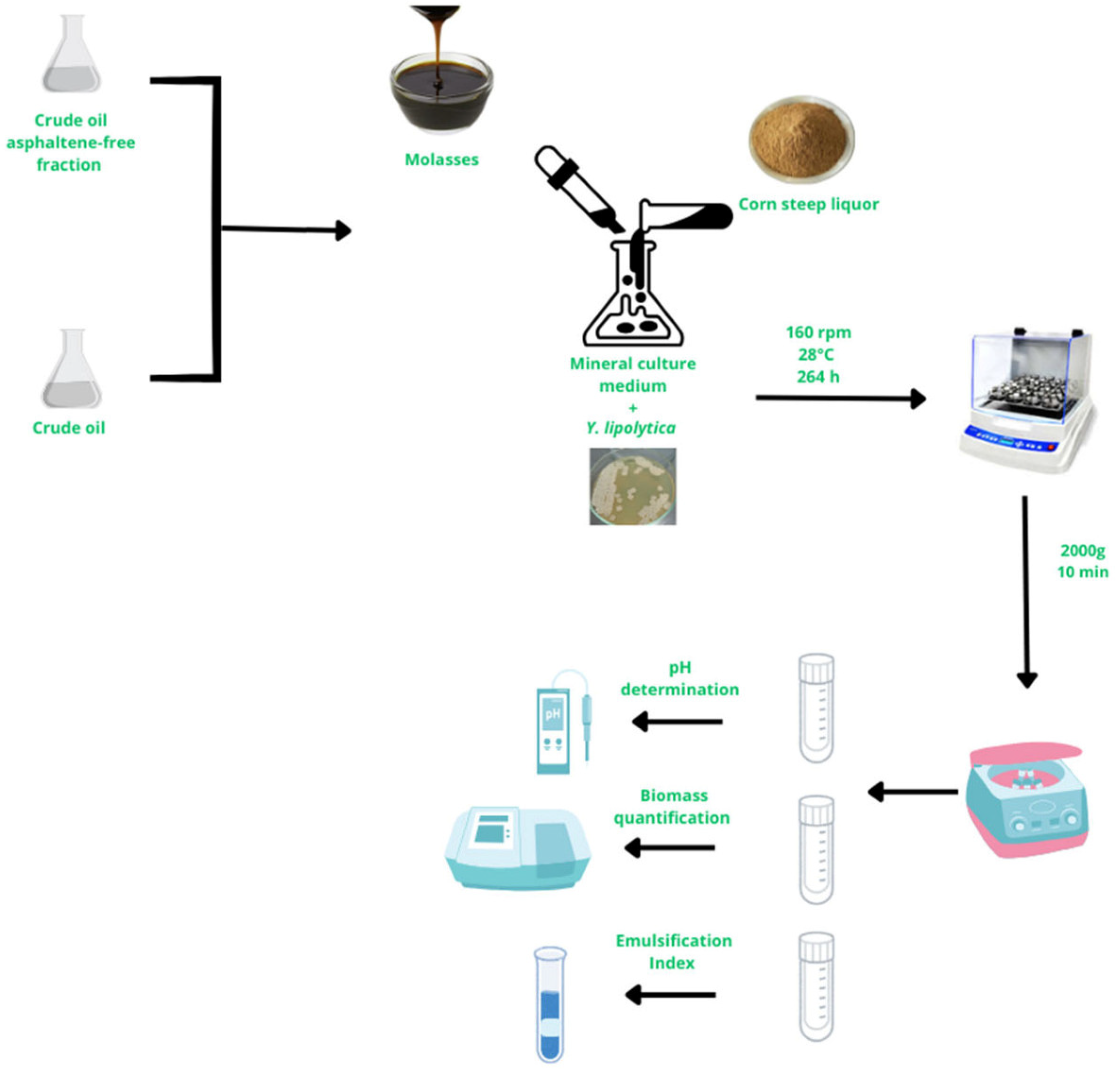
2.4. Biosurfactant Production During Biodegradation in Corn Steep Liquor-Based Media
2.5. Analytical Procedures
2.5.1. Biomass Quantification and pH Determination
2.5.2. Glucose Concentration
2.5.3. Emulsification Index
2.6. Statistical Analysis
3. Results
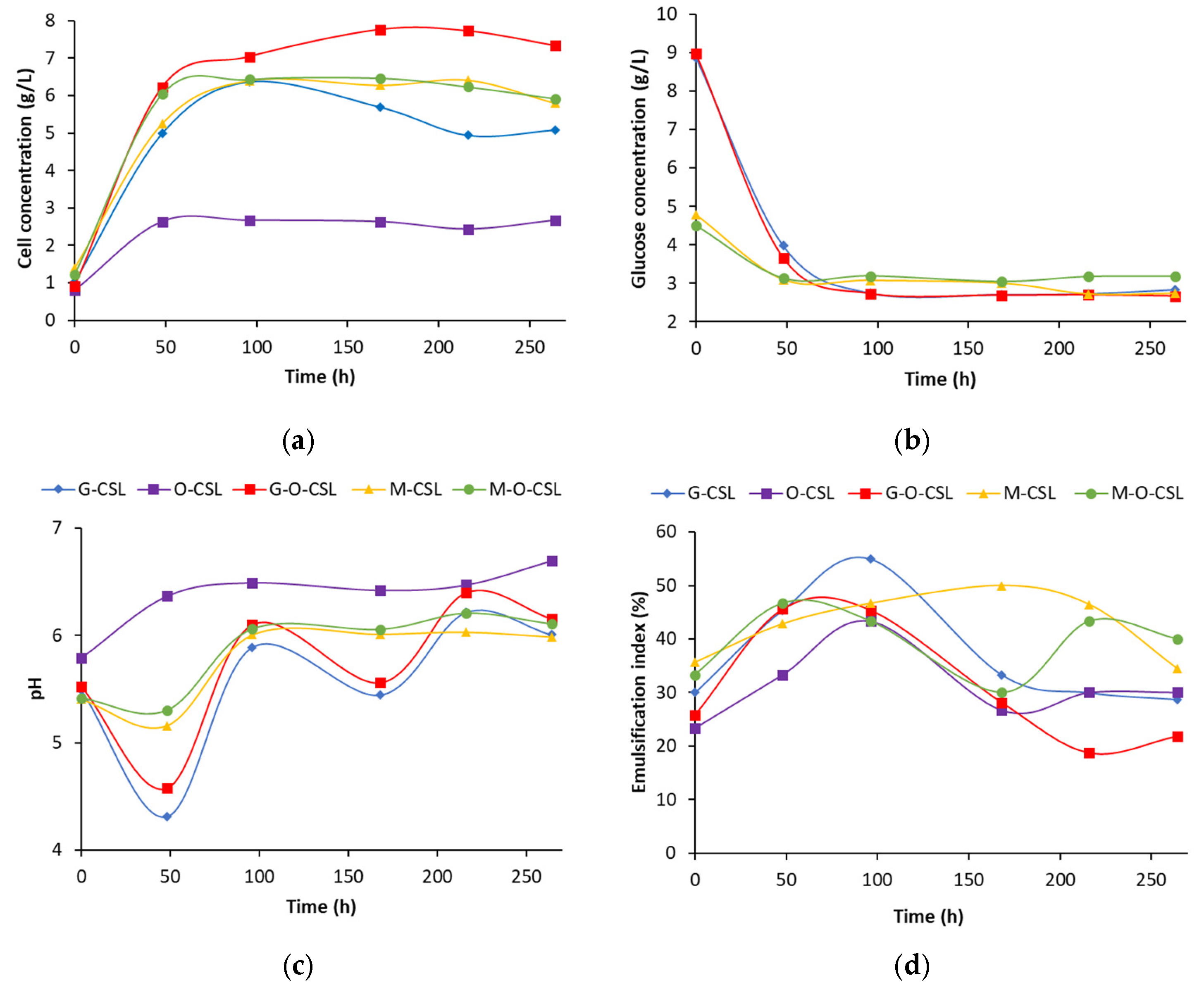
3.1. Influence of Corn Steep Liquor (CSL) Concentration on Biosurfactant Production
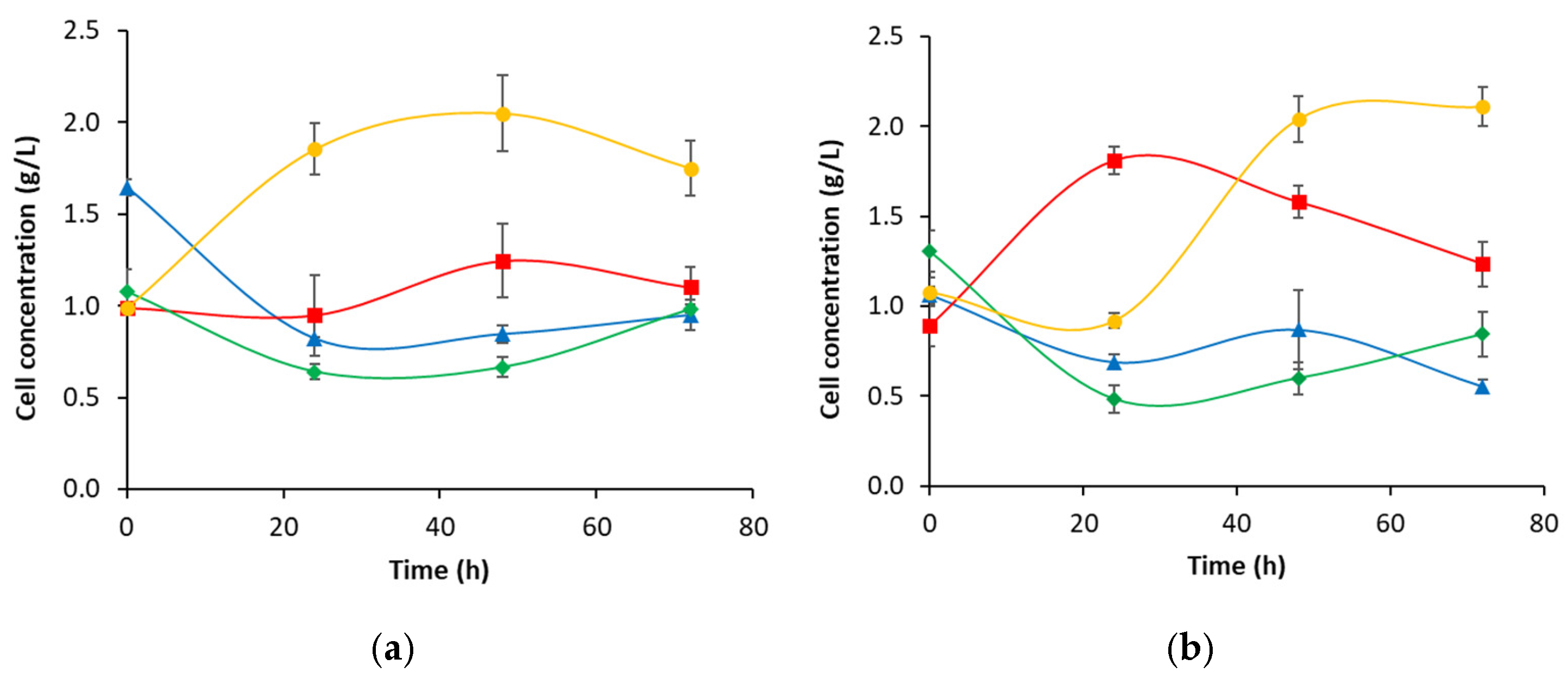
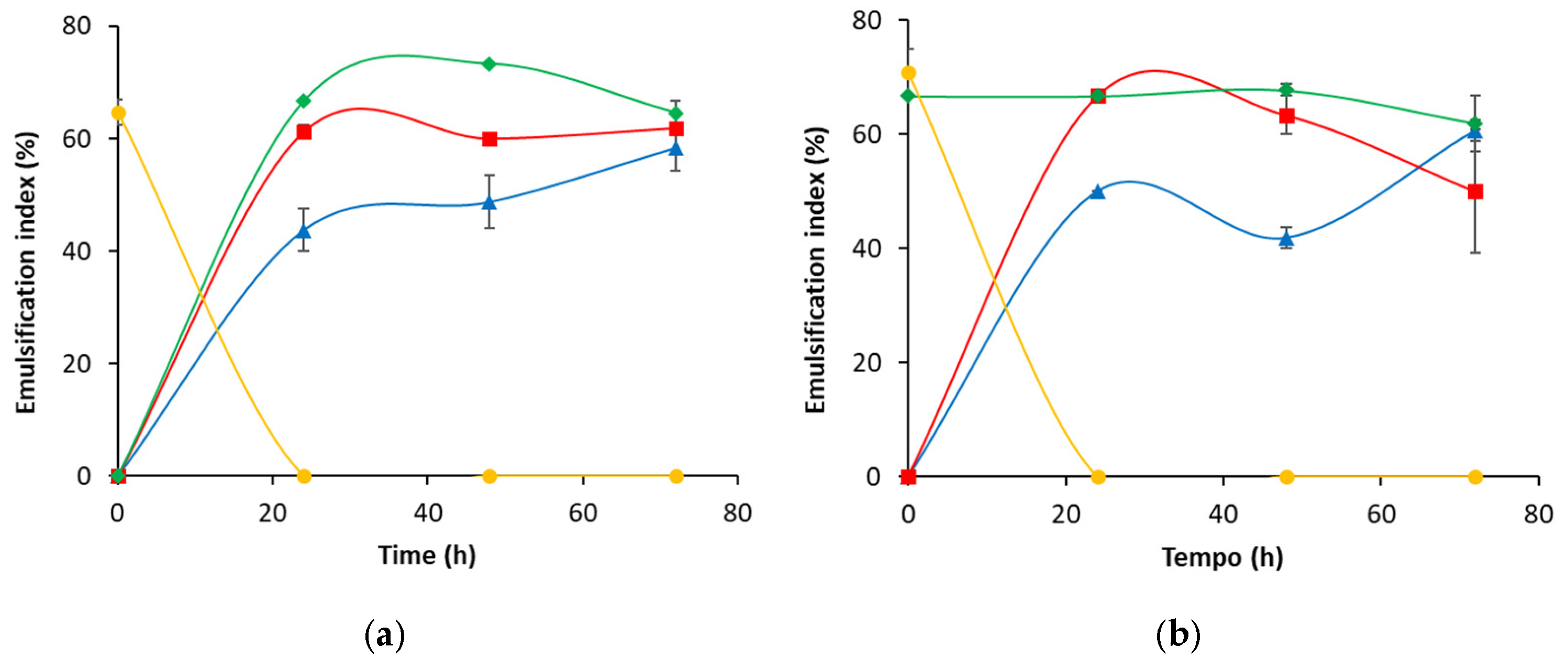
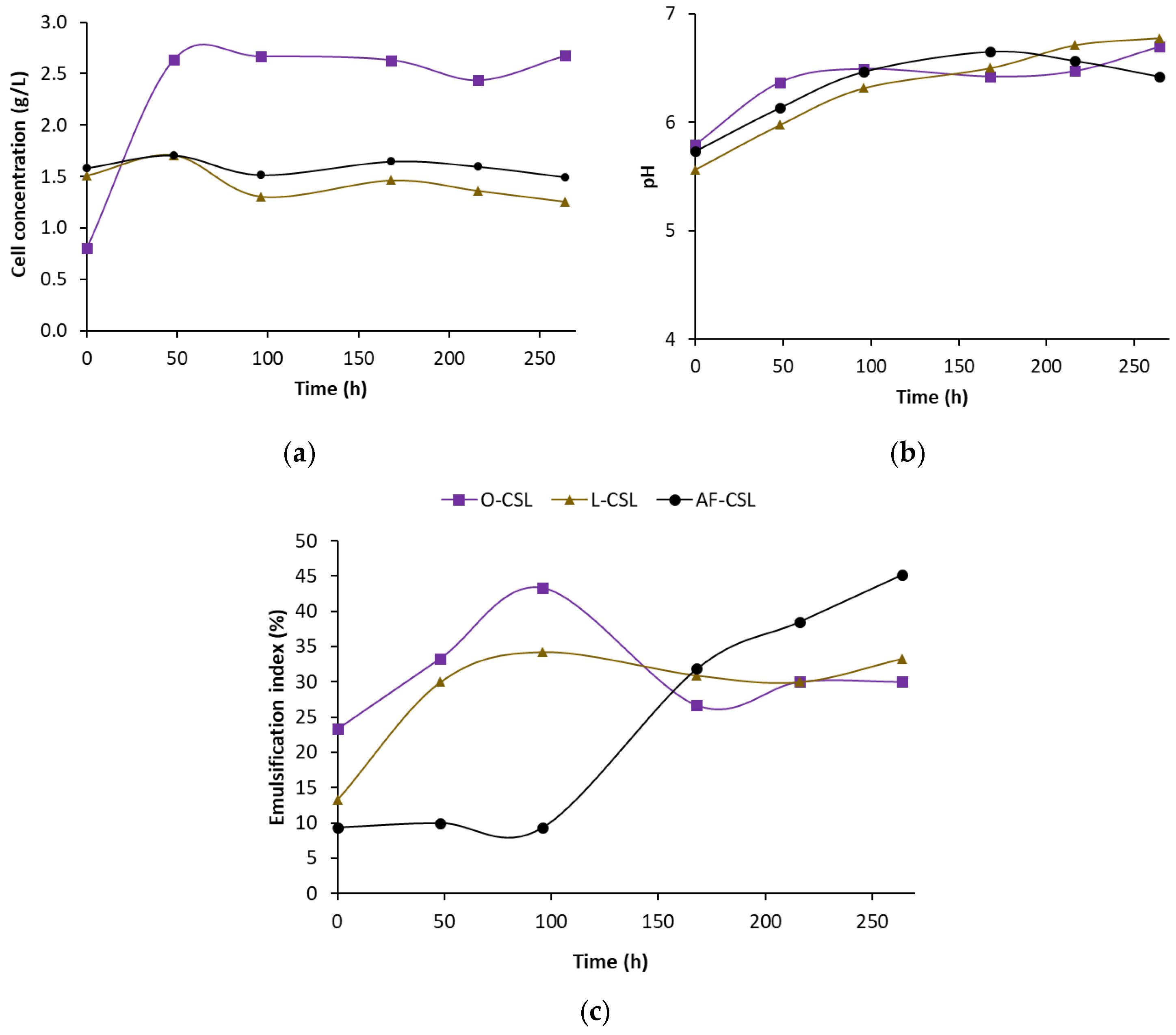
3.2. Biosurfactant Production During Crude Oil Biodegradation in Corn Steep Liquor-Based Media
3.3. Biosurfactant Production During the Biodegradation of Crude Oil Asphaltene-Free Fraction in Corn Steep Liquor-Based Media
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanungo, J.; Sahoo, T.; Swain, L.P.; Behera, I.D. Toxicity of Persistent Hydrocarbon Pollutants, Sources and Sustainable Remediation Process. In Impact of Petroleum Waste on Environmental Pollution and Its Sustainable Management Through Circular Economy; Behera, I.D., Das, A.P., Eds.; Springer: Cham, Switzerland, 2023; pp. 39–65. [Google Scholar] [CrossRef]
- ITOPF. Oil Tanker Spill Statistics 2023; ITOPF: London, UK, 2024. [Google Scholar]
- Hassanshahian, M.; Tebyanian, H.; Cappello, S. Isolation and characterization of two crude oil-degrading yeast strains, Yarrowia lipolytica PG-20 and PG-32, from the Persian Gulf. Mar. Pollut. Bull. 2012, 64, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Ji, X.J.; Huang, H. Biotechnological applications of Yarrowia lipolytica: Past, present and future. Biotechnol. Adv. 2015, 33, 1522–1546. [Google Scholar] [CrossRef] [PubMed]
- Csutak, O.; Corbu, V.M. Bioremediation of oil-contaminated soil by yeast bioaugmentation. In Advances in Yeast Biotechnology for Biofuels and Sustainability: Value-Added Products and Environmental Remediation Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 395–447. [Google Scholar] [CrossRef]
- Navas-Cáceres, O.D.; Parada, M.; Zafra, G. Development of a highly tolerant bacterial consortium for asphaltene biodegradation in soils. Environ. Sci. Pollut. Res. Int. 2023, 30, 123439–123451. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.H.; Lim, D.S.; Keum, Y.S. Biodegradation of 1-alkoxy-2,4-dichlorobenzenes by Yarrowia lipolytica KCTC 17618. Int. Biodeterior. Biodegrad. 2016, 114, 8–13. [Google Scholar] [CrossRef]
- de Oliveira Barros, V.P.; Silva, J.R.M.; Melo, V.M.M.; Terceiro, P.S.; de Oliveira, I.N.; de Freitas, J.D.; da Silva Moura, O.F.; de Araújo-Júnior, J.X.; da Silva Rodrigues, E.E.; Maraschin, M.; et al. Biosurfactants production by marine yeasts isolated from zoanthids and characterization of an emulsifier produced by Yarrowia lipolytica LMS 24B. Chemosphere 2024, 355, 141807. [Google Scholar] [CrossRef]
- Kosiorowska, K.E.; Połomska, X.; Wang, G.; Borodina, I.; Mirończuk, A.M. Efficient biodegradation of aliphatic polyester by genetically engineered strains of the yeast Yarrowia lipolytica. Int. Biodeterior. Biodegrad. 2021, 161, 105232. [Google Scholar] [CrossRef]
- Ferreira, T.F.; Martins, F.F.; Cayres, C.A.; Amaral, P.F.; Azevedo, D.D.A.; Coelho, M.A.Z. Biosurfactant Production from the Biodegradation of n-Paraffins, Isoprenoids and Aromatic Hydrocarbons from Crude Petroleum by Yarrowia lipolytica IMUFRJ 50682. Fermentation 2023, 9, 21. [Google Scholar] [CrossRef]
- Hashem, M.; Alamri, S.A.; Al-Zomyh, S.S.A.A.; Alrumman, S.A. Biodegradation and detoxification of aliphatic and aromatic hydrocarbons by new yeast strains. Ecotoxicol. Environ. Saf. 2018, 151, 28–34. [Google Scholar] [CrossRef]
- Zinjarde, S.; Apte, M.; Mohite, P.; Kumar, A.R. Yarrowia lipolytica and pollutants: Interactions and applications. Biotechnol. Adv. 2014, 32, 920–933. [Google Scholar] [CrossRef]
- Miranda, S.M.; Belo, I.; Lopes, M. Yarrowia lipolytica growth, lipids, and protease production in medium with higher alkanes and alkenes. World J. Microbiol. Biotechnol. 2024, 40, 318. [Google Scholar] [CrossRef]
- Buarque, F.S.; Sales, J.C.S.; Lobo, L.C.; Chrisman, E.C.A.N.; Ribeiro, B.D.; Coelho, M.A.Z. Asphaltenes biodegradation from heavy crude oils by the yeast Yarrowia lipolytica. Bioprocess Biosyst. Eng. 2024. [Google Scholar] [CrossRef] [PubMed]
- Csutak, O.; Corbu, V.; Stoica, I.; Ionescu, R.; Vassu, T. Biotechnological Applications of Yarrowia lipolytica CMGB32. Agric. Agric. Sci. Procedia 2015, 6, 545–553. [Google Scholar] [CrossRef]
- Wierzchowska, K.; Zieniuk, B.; Fabiszewska, A. Use of Non-Conventional Yeast Yarrowia lipolytica in Treatment or Upgradation of Hydrophobic Industry Wastes. Waste Biomass Valorization 2022, 13, 757–779. [Google Scholar] [CrossRef]
- Celińska, E. “Fight-flight-or-freeze”—How Yarrowia lipolytica responds to stress at molecular level? Appl. Microbiol. Biotechnol. 2022, 106, 3369–3395. [Google Scholar] [CrossRef]
- Sayed, K.; Baloo, L.; Sharma, N.K. Bioremediation of Total Petroleum Hydrocarbons (TPH) by Bioaugmentation and Biostimulation in Water with Floating Oil Spill Containment Booms as Bioreactor Basin. Int. J. Environ. Res. Public Health 2021, 18, 2226. [Google Scholar] [CrossRef]
- dos Santos, F.F.; de Freitas, K.M.L.; Pereira, A.d.S.; Fontes-Sant’ana, G.C.; da Rocha-Leão, M.H.M.; Amaral, P.F.F. Butter whey and corn steep liquor as sole raw materials to obtain a bioemulsifier from Yarrowia lipolytica for food oil-in-water emulsions. Ciência Rural 2021, 51, e20200323. [Google Scholar] [CrossRef]
- Zhang, X.X.; Yu, H.Y.; Wang, Y.X.; Wu, H.G. Novel PAHs-Degrading Strain Yarrowia lipolytica TZYx3 and its Synergistic Interaction with HA. Adv. Mat. Res. 2015, 1092–1093, 1064–1067. [Google Scholar] [CrossRef]
- Santos, F.F.; Freitas, K.M.L.; da Costa Neto, J.J.G.; Fontes-Sant’Ana, G.; Rocha-Leão, M.H.M.; Amaral, P.F.F. Tiger nut (Cyperus esculentus) milk byproduct and corn steep liquor for biosurfactant production by Yarrowia lipolytica. Chem. Eng. Trans. 2018, 65, 331–336. [Google Scholar]
- de Moraes Ferreira, R.; de Almeida, M.O.Q.; Nunes Chrisman, E.C.A.; Ribeiro, B.D.; Coelho, M.A.Z. Cold extraction of post-salt oil asphaltenes and their solubilization in deep eutectic solvents. Chem. Eng. Res. Des. 2023, 198, 349–356. [Google Scholar] [CrossRef]
- Haegler, A.N.; Mendonça-Haegler, L.C. Yeasts from marine and estuarine waters with different levels of pollution in the State of Rio de Janeiro. Appl. Environ. Microbiol. 1981, 41, 173–178. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Chevalot, I.; Komaitis, M.; Marc, I.; Aggelis, G. Single cell oil production by Yarrowia lipolytica growing on an industrial derivative of animal fat in batch cultures. Appl. Microbiol. Biotechnol. 2002, 58, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Benevenuti, C.; Botelho, A.; Ribeiro, R.; Branco, M.; Pereira, A.; Vieira, A.C.; Ferreira, T.; Amaral, P. Experimental design to improve cell growth and ethanol production in syngas fermentation by Clostridium carboxidivorans. Catalysts 2020, 10, 59. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-Active Agents from Two Bacilllus Species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Lazar, Z.; Rossignol, T.; Verbeke, J.; Crutz-Le Coq, A.-M.; Nicaud, J.-M.; Robak, M. Optimized invertase expression and secretion cassette for improving Yarrowia lipolytica growth on sucrose for industrial applications. J. Ind. Microbiol. Biotechnol. 2013, 40, 1273–1283. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Bauer, C.M.; Schmitz, C.; Landell, M.F.; Maraschin, M. Biosynthesis of fatty acids and biosurfactants by the yeast Yarrowia lipolytica with emphasis on metabolic networks and bioinformatics. Biotechnol. Res. Innov. 2022, 6, e2022001. [Google Scholar] [CrossRef]
- Adedeji, J.A.; Tetteh, E.K.; Amankwa, M.O.; Asante-Sackey, D.; Ofori-Frimpong, S.; Armah, E.K.; Rathilal, S.; Mohammadi, A.H.; Chetty, M. Microbial Bioremediation and Biodegradation of Petroleum Products—A Mini Review. Appl. Sci. 2022, 12, 2212. [Google Scholar] [CrossRef]
- Liberato, V.; Benevenuti, C.; Coelho, F.; Botelho, A.; Amaral, P.; Pereira, N., Jr.; Ferreira, T. Clostridium sp. as bio-catalyst for fuels and chemicals production in a biorefinery context. Catalysts 2019, 9, 962. [Google Scholar] [CrossRef]
- Liberato, V.S.; Martins, F.F.; Maria SRibeiro, C.; Alice ZCoelho, M.; Ferreira, T.F. Two-waste culture medium to produce 1,3-propanediol through a wild Clostridium butyricum strain. Fuel 2022, 322, 124202. [Google Scholar] [CrossRef]
- Santos, D.K.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Salgueiro, A.A.; Sarubbo, L.A. Synthesis and evaluation of biosurfactant produced by Candida lipolytica using animal fat and corn steep liquor. J. Pet. Sci. Eng. 2013, 105, 43–50. [Google Scholar]
- Madzak, C. Yarrowia lipolytica strains and their biotechnological applications: How natural biodiversity and metabolic engineering could contribute to cell factories improvement. J. Fungi 2021, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.; Hussain, M.S.; Gambill, L.; Blenner, M. Alternative substrate metabolism in Yarrowia lipolytica. Front. Microbiol. 2018, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Qamar, S.A.; Pacifico, S. Cleaner production of biosurfactants via bio-waste valorization: A comprehensive review of characteristics, challenges, and opportunities in bio-sector applications. J. Environ. Chem. Eng. 2023, 11, 111555. [Google Scholar] [CrossRef]
- Gao, R.; Li, Z.; Zhou, X.; Bao, W.; Cheng, S.; Zheng, L. Enhanced lipid production by Yarrowia lipolytica cultured with synthetic and waste-derived high-content volatile fatty acids under alkaline conditions. Biotechnol. Biofuels 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Mortensen, M.; Poudel, B.; Cotter, C.; Myers, R.; Okekeogbu, I.O.; Ryu, S.; Khomami, B.; Giannone, R.J.; Laursen, S.; et al. Proteomes reveal metabolic capabilities of Yarrowia lipolytica for biological upcycling of polyethylene into high-value chemicals. mSystems 2023, 8, e0074123. [Google Scholar] [CrossRef]
- Bouchedja, D.N.; Danthine, S.; Kar, T.; Fickers, P.; Sassi, H.; Boudjellal, A.; Blecker, C.; Delvigne, F. PH level has a strong impact on population dynamics of the yeast Yarrowia lipolytica and oil micro-droplets in multiphasic bioreactor. FEMS Microbiol. Lett. 2018, 365, fny173. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Farias, C.B.B.; Campos-Takaki, G.M. Co-utilization of canola oil and glucose on the production of a surfactant by Candida lipolytica. Curr. Microbiol. 2007, 54, 68–73. [Google Scholar] [CrossRef]
- Amaral, P.F.F.; da Silva, J.M.; Lehocky, M.; Barros-Timmons, A.M.V.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A.P. Production and characterization of a bioemulsifier from Yarrowia lipolytica. Process Biochem. 2006, 41, 1894–1898. [Google Scholar] [CrossRef]
- Li, J.; Zhu, K.; Miao, L.; Rong, L.; Zhao, Y.; Li, S.; Ma, L.; Li, J.; Zhang, C.; Xiao, D.; et al. Simultaneous Improvement of Limonene Production and Tolerance in Yarrowia lipolytica through Tolerance Engineering and Evolutionary Engineering. ACS Synth. Biol. 2021, 10, 884–896. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Tanveer, S.; Alia, S.; Anees, M.; Sultan, A.; Iqbal, M.; Yousaf, S. Role of nutrients in bacterial biosurfactant production and effect of biosurfactant production on petroleum hydrocarbon biodegradation. Ecol. Eng. 2017, 104, 158–164. [Google Scholar] [CrossRef]
- Zhou, H.; Huang, X.; Liang, Y.; Li, Y.; Xie, Q.; Zhang, C.; You, S. Enhanced bioremediation of hydraulic fracturing flowback and produced water using an indigenous biosurfactant-producing bacteria Acinetobacter sp. Y2. Chem. Eng. J. 2020, 397, 125348. [Google Scholar] [CrossRef]
- Ritoré, E.; Coquelet, B.; Arnaiz, C.; Morillo, J.; Usero, J. Guidelines for surfactant selection to treat petroleum hydrocarbon-contaminated soils. Environ. Sci. Pollut. Res. 2022, 29, 7639–7651. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, Y.; Zang, T.; Wei, J.; Wu, H.; Wei, C.; Qiu, G.; Li, F. A biosurfactant-producing Pseudomonas aeruginosa S5 isolated from coking wastewater and its application for bioremediation of polycyclic aromatic hydrocarbons. Bioresour. Technol. 2019, 281, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Rasekh, B.; Maal, K.B.; Karbasiun, F.; Yazdian, F.; Emami-Karvani, Z.; Peighami, R. A novel biosurfactant producing Kocuria rosea ABR6 as potential strain in oil sludge recovery and lubrication. AMB Express 2021, 11, 131. [Google Scholar] [CrossRef]
- Femina Carolin, C.; Kumar, P.S.; Joshiba, G.J.; Madhesh, P.; Ramamurthy, R. Sustainable strategy for the enhancement of hazardous aromatic amine degradation using lipopeptide biosurfactant isolated from Brevibacterium casei. J. Hazard. Mater. 2021, 408, 124943. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, Y.; Zhang, N.; Liu, F.; Ding, M.; Yuan, Y. Construction of Yarrowia lipolytica and microbial consortia for degradation of n-hexadecane. J. Environ. Chem. Eng. 2024, 12, 113209. [Google Scholar] [CrossRef]
- Perera, M.; Wijayarathna, D.; Wijesundera, S.; Chinthaka, M.; Seneviratne, G.; Jayasena, S. Biofilm mediated synergistic degradation of hexadecane by a naturally formed community comprising Aspergillus flavus complex and Bacillus cereus group. BMC Microbiol. 2019, 19, 84. [Google Scholar] [CrossRef]
- Xia, M.; Fu, D.; Chakraborty, R.; Singh, R.P.; Terry, N. Enhanced crude oil depletion by constructed bacterial consortium comprising bioemulsifier producer and petroleum hydrocarbon degraders. Bioresour. Technol. 2019, 282, 456–463. [Google Scholar] [CrossRef]
- Chen, W.; Kong, Y.; Li, J.; Sun, Y.; Min, J.; Hu, X. Enhanced biodegradation of crude oil by constructed bacterial consortium comprising salt-tolerant petroleum degraders and biosurfactant producers. Int. Biodeterior. Biodegrad. 2020, 154, 105047. [Google Scholar] [CrossRef]
- Sun, S.; Wang, H.; Yan, K.; Lou, J.; Ding, J.; Snyder, S.A.; Wu, L.; Xu, J. Metabolic interactions in a bacterial co-culture accelerate phenanthrene degradation. J. Hazard. Mater. 2021, 403, 123825. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Formicola, F.; Sbaffoni, S.; Franzetti, A.; Vaccari, M. Insights into rhamnolipid amendment towards enhancing microbial electrochemical treatment of petroleum hydrocarbon contaminated soil. Chemosphere 2022, 307, 136126. [Google Scholar] [CrossRef] [PubMed]
- Parthipan, P.; Preetham, E.; Machuca, L.L.; Rahman, P.K.S.M.; Murugan, K.; Rajasekar, A. Biosurfactant and Degradative Enzymes Mediated Crude Oil Degradation by Bacterium Bacillus subtilis A1. Front. Microbiol. 2017, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Chand, P.; Dutta, S.; Mukherji, S. Slurry phase biodegradation of heavy oily sludge and evidence of asphaltene biotransformation. J. Environ. Manag. 2022, 324, 116315. [Google Scholar] [CrossRef] [PubMed]
- Swathi, K.V.; Muneeswari, R.; Ramani, K.; Sekaran, G. Biodegradation of petroleum refining industry oil sludge by microbial-assisted biocarrier matrix: Process optimization using response surface methodology. Biodegradation 2020, 31, 385–405. [Google Scholar] [CrossRef] [PubMed]
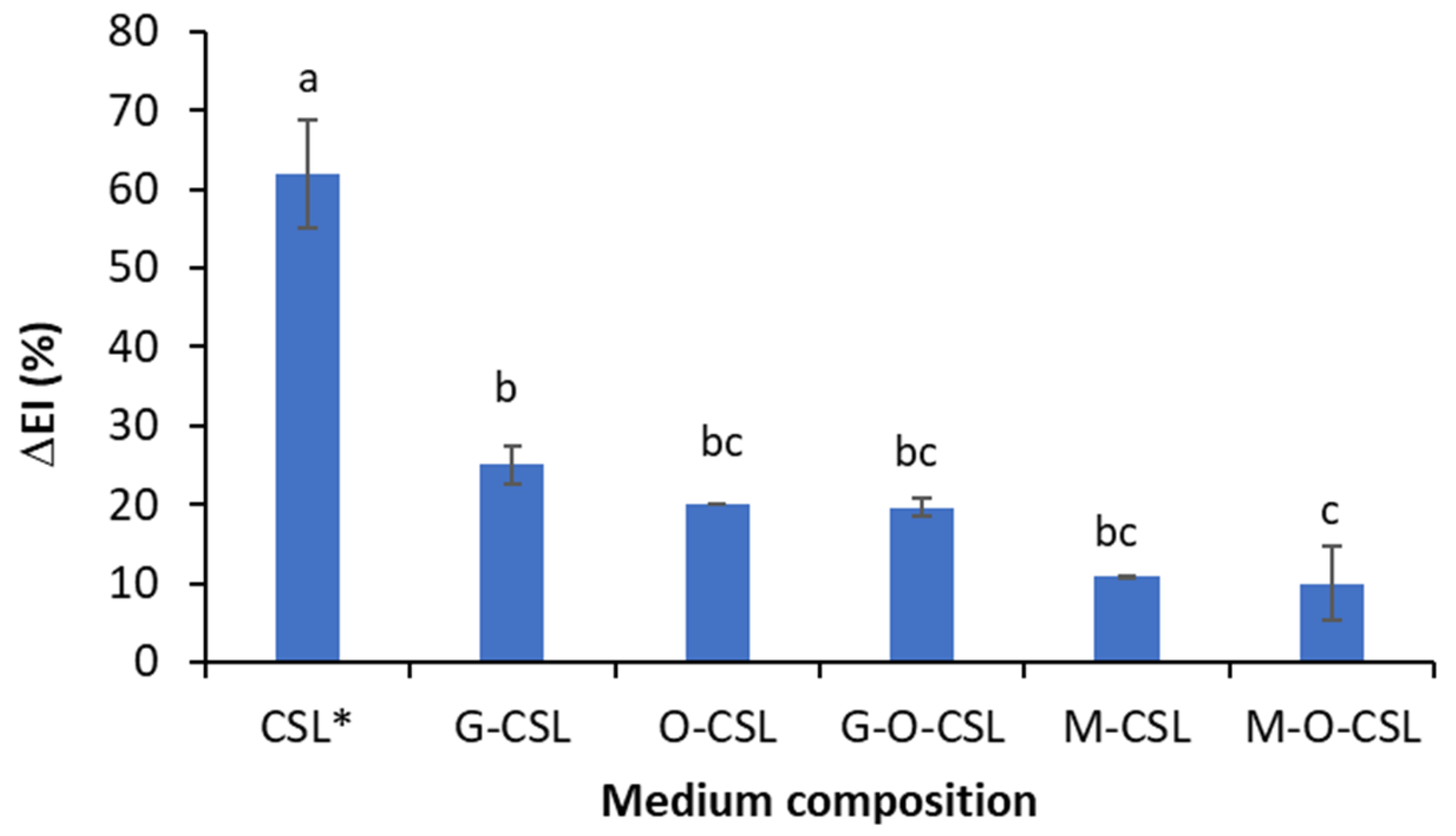
| Medium | G (% w/v) | M (% w/v) | O (% v/v) | L (% v/v) | AF (% v/v) | CSL (% w/v) |
|---|---|---|---|---|---|---|
| G-CSL | 1 | - | - | - | - | 1 |
| O-CSL | - | - | 1 | - | - | 1 |
| G-O-CSL | 1 | - | 1 | - | - | 1 |
| M-CSL | - | 1.7 | - | - | - | 1 |
| M-O-CSL | - | 1.7 | 1 | - | - | 1 |
| L-CSL | - | - | - | 1 | - | 1 |
| AF-CSL | - | - | - | - | 1 | 1 |
| Time (h) | pH (Medium Without Ammonium Sulfate) | |||
| CSL Concentration | ||||
| 5 g/L | 10 g/L | 20 g/L | 30 g/L | |
| 0 | 4.14 ± 0.06 | 4.11 ± 0.02 | 4.11 ± 0.00 | 4.07 ± 0.02 |
| 24 | 7.41 ± 0.11 | 7.33 ± 0.08 | 7.63 ± 0.04 | 7.02 ± 0.13 |
| 48 | 7.94 ± 0.02 | 7.70 ± 0.01 | 7.81 ± 0.12 | 7.70 ± 0.01 |
| 72 | 7.93 ± 0.09 | 7.79 ± 0.04 | 7.87 ± 0.04 | 7.98 ±0.05 |
| Time (h) | pH (Medium With Ammonium Sulfate) | |||
| CSL Concentration | ||||
| 5 g/L | 10 g/L | 20 g/L | 30 g/L | |
| 0 | 4.00 ± 0.00 | 3.99 ± 0.01 | 4.00 ± 0.02 | 4.11 ± 0.06 |
| 24 | 7.37 ± 0.00 | 7.64 ± 0.04 | 7.43 ± 0.02 | 7.13 ± 0.01 |
| 48 | 7.53 ± 0.04 | 7.91 ± 0.00 | 7.60 ± 0.04 | 7.72 ± 0.02 |
| 72 | 7.76 ± 0.07 | 8.06 ± 0.04 | 7.77 ± 0.04 | 7.93 ± 0.04 |
| Study | Condition | Findings | Implications |
|---|---|---|---|
| Santos et al. [19] | CSL (1–5 g/L) | Improved biosurfactant production when CSL was combined with ammonium sulfate; Highest EI: 58% | Demonstrates potential of CSL and ammonium sulfate as effective nutrient combinations |
| Present study | CSL (≥10 g/L) | No significant difference in biosurfactant production with or without ammonium sulfate; Highest EI: 73% | Suggests CSL alone provides sufficient nitrogen for higher concentrations, reducing reliance on additional supplements |
| Santos et al. [19] | Butter whey + CSL | Comparable EI achieved with butter whey and CSL within 24 h, faster than butter whey + CSL + ammonium sulfate; Highest EI: 67% | Highlights potential negative impact of ammonium sulfate in media with certain carbon sources like butter whey |
| Santos et al. [33] | Animal fat + CSL | Surface tension reduction (50 to 28 mN/m) and highest EI with petroleum (100%) and motor oil (94%) | Versatility of biosurfactant produced with CSL, including enhanced oil recovery and bioremediation |
| Present study | Crude oil/asphaltene-free fraction + CSL | Highest EI achieved (35.8% after 264 h) in asphaltene-free fraction medium; limonene inhibited cell growth | Suggests asphaltene-free fractions are promising substrates, with further optimization needed for solvent compatibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, J.M.; Macedo, B.P.; Sodré, T.S.; Cayres, C.A.; Ferreira, R.M.; Fraga, J.L.; Coelho, M.A.Z.; Amaral, P.F.F.; Ferreira, T.F. Biosurfactant Production by Yarrowia lipolytica in Corn Steep Liquor-Based Media for Hydrocarbon Bioremediation. Processes 2025, 13, 412. https://doi.org/10.3390/pr13020412
Lopes JM, Macedo BP, Sodré TS, Cayres CA, Ferreira RM, Fraga JL, Coelho MAZ, Amaral PFF, Ferreira TF. Biosurfactant Production by Yarrowia lipolytica in Corn Steep Liquor-Based Media for Hydrocarbon Bioremediation. Processes. 2025; 13(2):412. https://doi.org/10.3390/pr13020412
Chicago/Turabian StyleLopes, Juliana M., Bruno P. Macedo, Thuane S. Sodré, Caroline A. Cayres, Rachel M. Ferreira, Jully L. Fraga, Maria Alice Z. Coelho, Priscilla F. F. Amaral, and Tatiana F. Ferreira. 2025. "Biosurfactant Production by Yarrowia lipolytica in Corn Steep Liquor-Based Media for Hydrocarbon Bioremediation" Processes 13, no. 2: 412. https://doi.org/10.3390/pr13020412
APA StyleLopes, J. M., Macedo, B. P., Sodré, T. S., Cayres, C. A., Ferreira, R. M., Fraga, J. L., Coelho, M. A. Z., Amaral, P. F. F., & Ferreira, T. F. (2025). Biosurfactant Production by Yarrowia lipolytica in Corn Steep Liquor-Based Media for Hydrocarbon Bioremediation. Processes, 13(2), 412. https://doi.org/10.3390/pr13020412









