CTAB-Assisted Formation of Hierarchical Porosity in Cu-BDC-NH2 Metal–Organic Frameworks and Its Enhanced Peroxidase-like Catalysis for Xanthine Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation and Methods
2.2. Materials
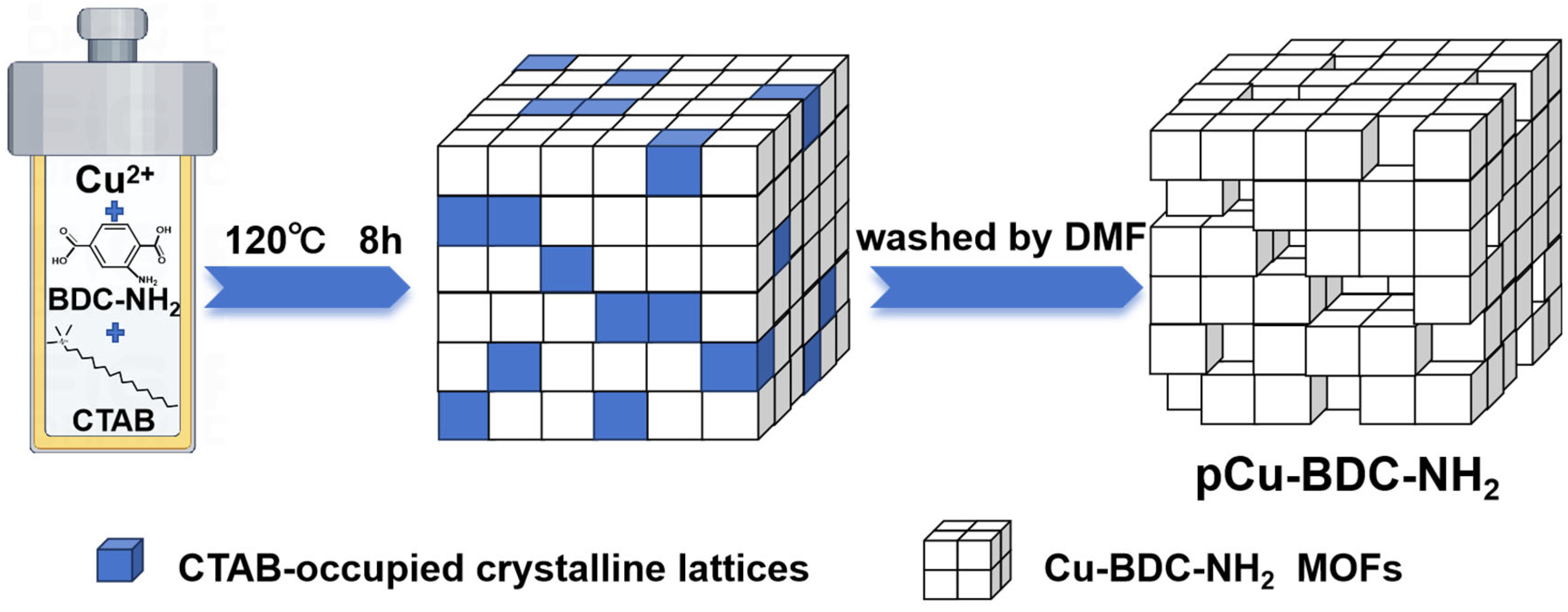
2.3. Synthesis of the Porous MOFs
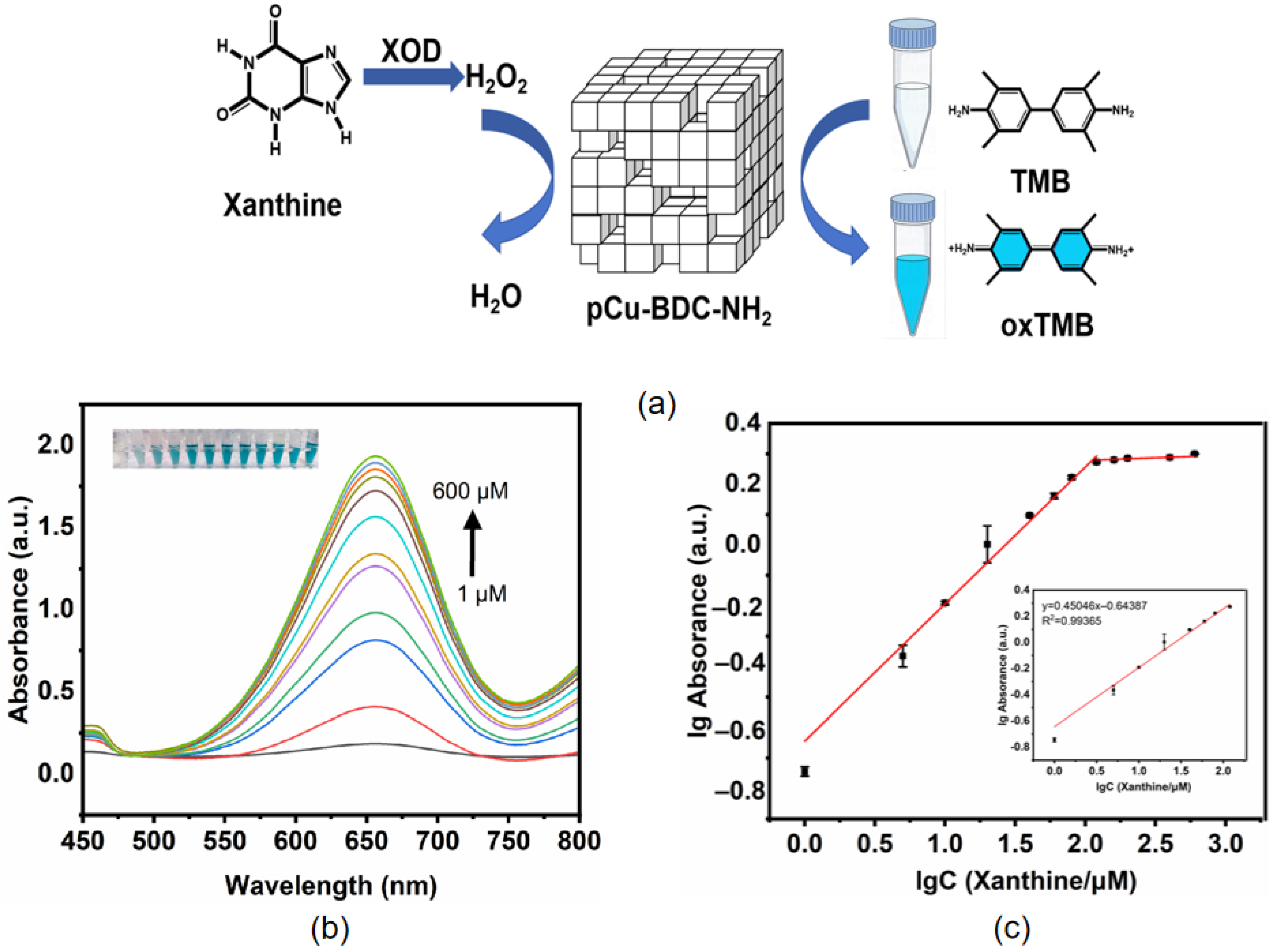
2.4. Peroxidase-like Activity of Cu-BDC-NH2 MOFs
2.5. Xanthine Concentration Sensing
3. Results and Discussion
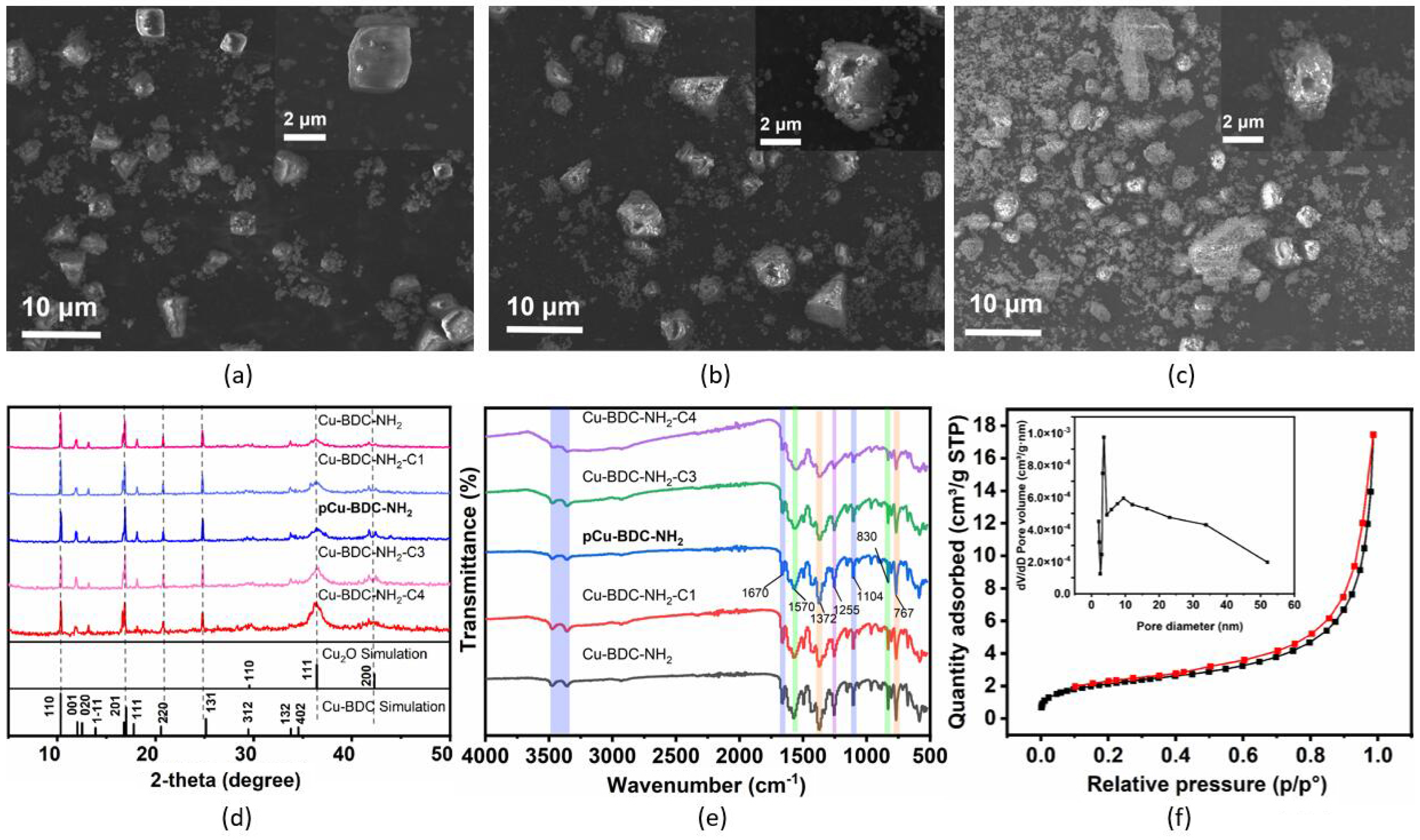
3.1. Characterization of Cu-BDC-NH2 MOFs
3.2. Hierarchical Porosity in pCu-BDC-NH2
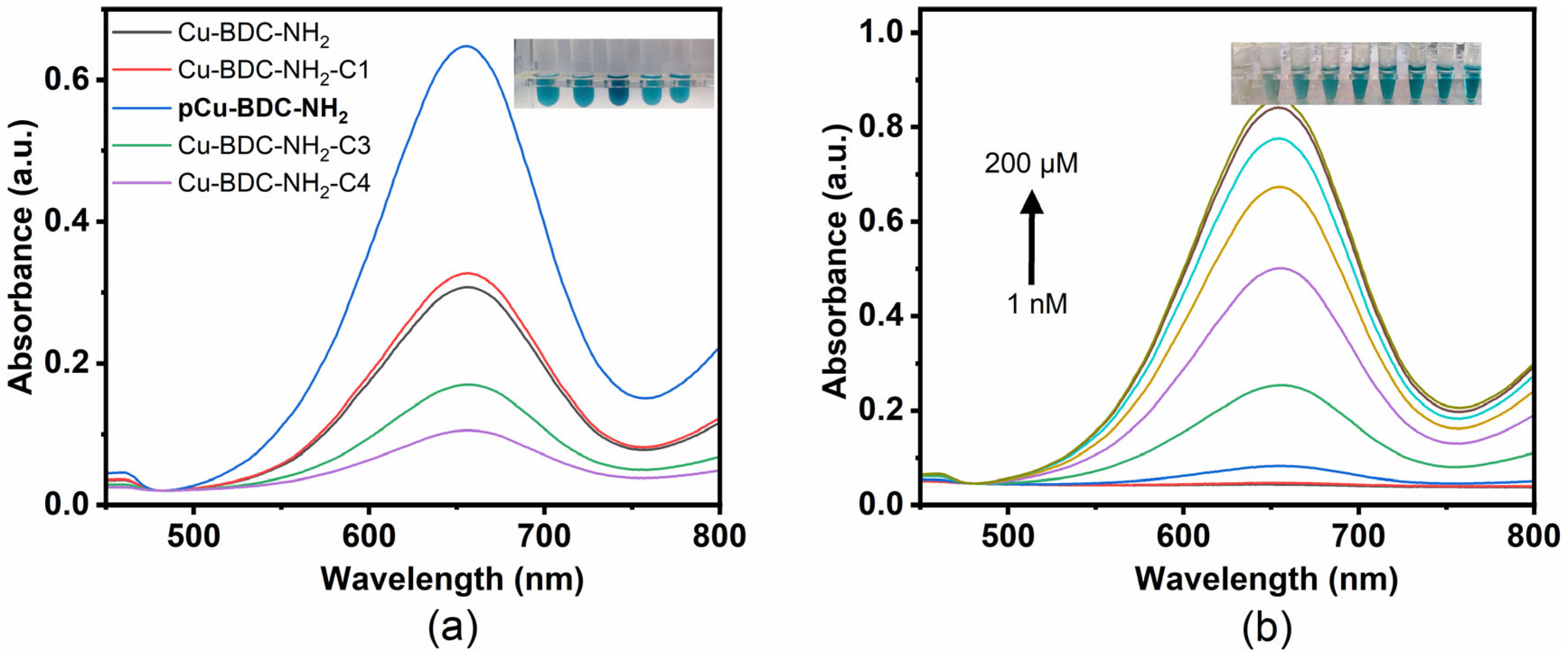
3.3. Synergetic Enhancement of Catalysis
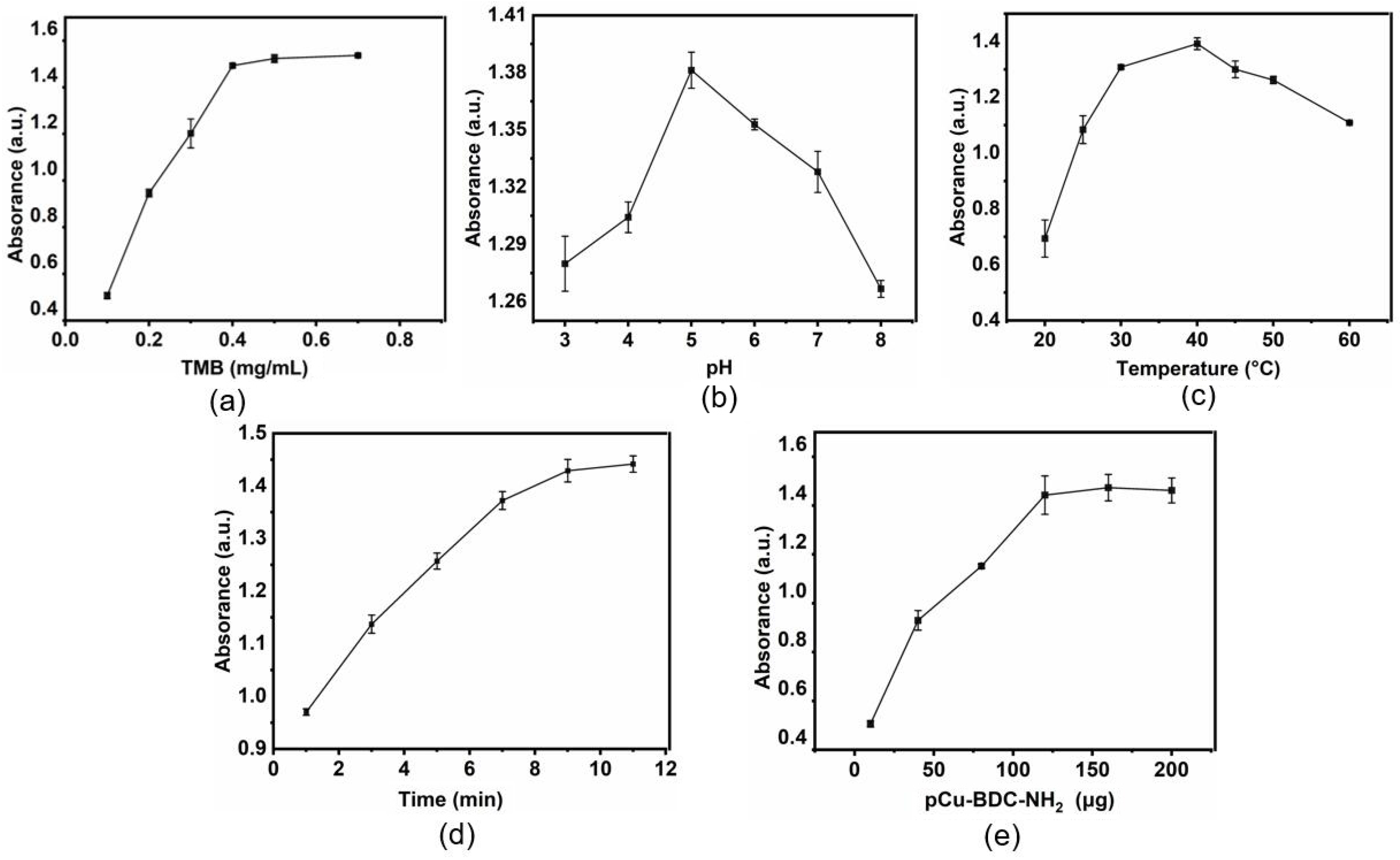
3.4. pCu-BDC-NH2 Enzyme Mimicry
3.5. pCu-BDC-NH2 Xanthine Colorimetric Monitoring
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tranchemontagne, D.J.; Mendoza-Cortés, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary Building Units, Nets and Bonding in The Chemistry of Metal–Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Long, J.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Vaitsis, C.; Sourkouni, G.; Argirusis, C. Metal Organic Frameworks (MOFs) and ultrasound: A review. Ultrason. Sonochem. 2019, 52, 106–119. [Google Scholar] [CrossRef]
- Sun, K.; Qian, Y.; Jiang, H.-L. Metal-Organic Frameworks for Photocatalytic Water Splitting and CO2 Reduction. Angew. Chem. Int. Ed. 2023, 62, e202217565. [Google Scholar] [CrossRef]
- Ding, B.; Chen, H.; Tan, J.; Meng, Q.; Zheng, P.; Ma, P.A.; Lin, J. ZIF-8 Nanoparticles Evoke Pyroptosis for High-Efficiency Cancer Immunotherapy. Angew. Chem. Int. Ed. 2023, 62, e202215307. [Google Scholar] [CrossRef]
- Rao, R.; Ma, S.; Gao, B.; Bi, F.; Chen, Y.; Yang, Y.; Liu, N.; Wu, M.; Zhang, X. Recent advances of metal-organic framework-based and derivative materials in the heterogeneous catalytic removal of volatile organic compounds. J. Colloid Interface Sci. 2023, 636, 55–72. [Google Scholar] [CrossRef]
- Fu, H.; Ma, H.; Zhao, S. Structural Control of Copper-Based MOF Catalysts for Electroreduction of CO2: A Review. Processes 2024, 12, 2205. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.-H. Recent advances in post-synthetic modification of metal–organic frameworks: New types and tandem reactions. Coord. Chem. Rev. 2019, 378, 500–512. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.Q.; Jiang, H.L.; Sun, L.B. Metal-Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-S.; Lu, Y.; Chen, K.; Zhao, Y.; Wang, P.; Sun, W.-Y. Metal–organic frameworks with catalytic centers: From synthesis to catalytic application. Coord. Chem. Rev. 2019, 378, 262–280. [Google Scholar] [CrossRef]
- Deng, H.; Grunder, S.; Cordova, K.E.; Valente, C.; Furukawa, H.; Hmadeh, M.; Gándara, F.; Whalley, A.C.; Liu, Z.; Asahina, S.; et al. Large-Pore Apertures in a Series of Metal-Organic Frameworks. Science 2012, 336, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.M.; Aragones-Anglada, M.; Gandara-Loe, J.; Danaf, N.A.; Lamb, D.C.; Mehta, J.P.; Vulpe, D.; Wuttke, S.; Silvestre-Albero, J.; Moghadam, P.Z.; et al. Tuning porosity in macroscopic monolithic metal-organic frameworks for exceptional natural gas storage. Nat. Commun. 2019, 10, 2345. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Chen, L.-H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B.-L. Hierarchically porous materials: Synthesis strategies and structure design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef]
- Fang, Z.; Bueken, B.; De Vos, D.E.; Fischer, R.A. Defect-Engineered Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2015, 54, 7234–7254. [Google Scholar] [CrossRef]
- Furukawa, S.; Reboul, J.; Diring, S.; Sumida, K.; Kitagawa, S. Structuring of metal–organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 2014, 43, 5700–5734. [Google Scholar] [CrossRef]
- Chen, G.; Song, R.; Wang, H.; Huang, L.; Wang, L.; Lei, J. Construction of hierarchical porous MIL-100(Fe) for horseradish peroxidase immobilization and its degradation performance of bisphenol A. J. Clean. Prod. 2024, 481, 144178. [Google Scholar] [CrossRef]
- Tuo, K.; Li, J.; Li, Y.; Liang, C.; Shao, C.; Hou, W.; Fan, C.; Li, Z.; Pu, S.; Chen, Z.; et al. Phytic Acid Functionalized Hierarchical Porous Metal-Organic Framework Microspheres for Efficient Extraction of Uranium from Seawater. Small 2024, 21, 2407272. [Google Scholar] [CrossRef]
- Ma, L.; Huang, C.; Yao, Y.; Fu, M.; Han, F.; Li, Q.; Wu, M.; Zhang, H.; Xu, L.; Ma, H. Self-assembled MOF microspheres with hierarchical porous structure for efficient uranium adsorption. Sep. Purif. Technol. 2023, 314, 123526. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Song, L.; Chen, J.; Yang, Y.; Wang, Y. Enhanced adsorption performance of gaseous toluene on defective UiO-66 metal organic framework: Equilibrium and kinetic studies. J. Hazard. Mater. 2019, 365, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.; Tyagi, B.; Shukla, R.S.; Bajaj, H.C. Esterification of palmitic acid with methanol over template-assisted mesoporous sulfated zirconia solid acid catalyst. Appl. Catal. B Environ. 2015, 172, 108–115. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, B.; Li, J.; Qian, L.; Liu, L.; Liu, Z.; Fang, P.; He, C. Dependence of dye molecules adsorption behaviors on pore characteristics of mesostructured MOFs fabricated by surfactant template. ACS Appl. Mater. Interfaces 2019, 11, 31441–31451. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, T.; Zheng, H.; Fan, F.; Gu, Z.; He, W.; Zhang, B.; Shao, L.; Chen, H.; Li, Y.; et al. Fabrication of mesoporous MOF nanosheets via surfactant-template method for C–S coupling reactions. Microporous Mesoporous Mater. 2020, 303, 110254. [Google Scholar] [CrossRef]
- Amali, R.K.A.; Lim, H.N.; Ibrahim, I.; Zainal, Z.; Ahmad, S.A.A. Silver nanoparticles-loaded copper (II)-terephthalate framework nanocomposite as a screen-printed carbon electrode modifier for amperometric nitrate detection. J. Electroanal. Chem. 2022, 918, 116440. [Google Scholar] [CrossRef]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabrés i Xamena, F.X.; Gascon, J. Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2015, 14, 48–55. [Google Scholar] [CrossRef]
- Carrington, E.J.; McAnally, C.A.; Fletcher, A.J.; Thompson, S.P.; Warren, M.; Brammer, L. Solvent-switchable continuous-breathing behaviour in a diamondoid metal–organic framework and its influence on CO2 versus CH4 selectivity. Nat. Chem. 2017, 9, 882–889. [Google Scholar] [CrossRef]
- Gupta, N.K.; Kim, S.; Bae, J.; Soo Kim, K. Fabrication of Cu(BDC)0.5(BDC-NH2)0.5 metal-organic framework for superior H2S removal at room temperature. Chem. Eng. J. 2021, 411, 128536. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Z.; Wang, Y.; Zhang, J.; Zheng, X.; Rao, D.; Han, X.; Zhong, C.; Hu, W.; Deng, Y. Enhanced light harvesting and electron-hole separation for efficient photocatalytic hydrogen evolution over Cu7S4-enwrapped Cu2O nanocubes. Appl. Catal. B 2019, 246, 202–210. [Google Scholar] [CrossRef]
- Nayak, A.; Viegas, S.; Dasari, H.; Sundarabal, N. Cu-BDC and Cu2O Derived from Cu-BDC for the Removal and Oxidation of Asphaltenes: A Comparative Study. ACS Omega 2022, 7, 34966–34973. [Google Scholar] [CrossRef]
- Keypour, H.; Kouhdareh, J.; Rabiei, K.; Karakaya, İ.; Karimi-Nami, R.; Alavinia, S. Pd nanoparticles decorated on a porous Co(BDC-NH2) MOF as an effective heterogeneous catalyst for dye reduction. Nanoscale Adv. 2023, 5, 5570–5579. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tian, P.; Ding, D.; Yang, Z.; Wang, W.; Xin, H.; Xu, J.; Han, Y.-F. Revealing the active species of Cu-based catalysts for heterogeneous Fenton reaction. Appl. Catal. B 2019, 258, 117985. [Google Scholar] [CrossRef]
- Li, S.; Liang, L.; Tian, L.; Wu, J.; Zhu, Y.; Qin, Y.; Zhao, S.; Ye, F. Enhanced peroxidase-like activity of MOF nanozymes by co-catalysis for colorimetric detection of cholesterol. J. Mater. Chem. B 2023, 11, 7913–7919. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Chen, F.; Cheng, D.; Zou, S.; Wang, J.; Shen, M.; Wang, Y. MOFs-derived Cu/Carbon membrane as bi-functional catalyst: Improving catalytic activity in detection and degradation of PFOA via regulation of reactive Cu species. Chem. Eng. J. 2024, 481, 148467. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.-N.; Yang, B.; Liu, Z.; Zhang, X.; Liu, Q. Iron Doped CuSn(OH)6 Microspheres as a Peroxidase-Mimicking Artificial Enzyme for H2O2 Colorimetric Detection. ACS Sustain. Chem. Eng. 2018, 6, 14383–14393. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, W.; Gong, M.; Ma, J.; Liu, G.; Mei, T.; Luo, X. Xanthine oxidase immobilized cellulose membrane-based colorimetric biosensor for screening and detecting the bioactivity of xanthine oxidase inhibitors. Int. J. Biol. Macromol. 2024, 275, 133450. [Google Scholar] [CrossRef]
- Bagheri, N.; Khataee, A.; Hassanzadeh, J.; Habibi, B. Visual detection of peroxide-based explosives using novel mimetic Ag nanoparticle/ZnMOF nanocomposite. J. Hazard. Mater. 2018, 360, 233–242. [Google Scholar] [CrossRef]
- Lu, M.; Wang, Z.; Xie, W.; Zhang, Z.; Su, L.; Chen, Z.; Xiong, Y. Cu-MOF derived CuO@g-C3N4 nanozyme for cascade catalytic colorimetric sensing. Anal. Bioanal. Chem. 2023, 415, 5949–5960. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Z.-Z.; Hong, C.-Y.; Huang, Z.-Y. Colorimetric detection of hypoxanthine in aquatic products based on the enzyme mimic of cobalt-doped carbon nitride. New J. Chem. 2021, 45, 18307–18314. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Liang, X.-H.; Zhong, J.; Guo, L.; Fu, F. Colorimetric determination of xanthine in urine based on peroxidase-like activity of WO3 nanosheets. Talanta 2019, 204, 278–284. [Google Scholar] [CrossRef]
- Wu, X.; Chen, T.; Wang, J.; Yang, G. Few-layered MoSe2 nanosheets as an efficient peroxidase nanozyme for highly sensitive colorimetric detection of H2O2 and xanthine. J. Mater. Chem. B 2018, 6, 105–111. [Google Scholar] [CrossRef]
- Li, Z.; Cao, L.; Sui, J.; Wang, L.; Lin, H.; Wang, K. Bimetallic Fe/Ni metal organic framework-based hypoxanthine biosensor for early monitoring of freshness changes of aquatic products. Food Chem. 2024, 447, 138902. [Google Scholar] [CrossRef]


| Nanozyme | Linear Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|
| CuO@g-C3N4XOD | 1–120 | 0.20 | [38] |
| Co-doped-g-C3N4 | 7–450 | 5.40 | [39] |
| WO3 nanosheets | 25–200 | 1.24 | [40] |
| MoSe2nanosheets | 10–320 | 1.96 | [41] |
| Fe3Ni7MOF | 3–70 | 1.39 | [42] |
| pCu-BDC-NH2 | 1–120 | 0.11 | our work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.; He, J.; Zhou, F.; Xu, R.; Gao, Y.; Marks, R.S.; Li, J. CTAB-Assisted Formation of Hierarchical Porosity in Cu-BDC-NH2 Metal–Organic Frameworks and Its Enhanced Peroxidase-like Catalysis for Xanthine Sensing. Processes 2025, 13, 387. https://doi.org/10.3390/pr13020387
Tan C, He J, Zhou F, Xu R, Gao Y, Marks RS, Li J. CTAB-Assisted Formation of Hierarchical Porosity in Cu-BDC-NH2 Metal–Organic Frameworks and Its Enhanced Peroxidase-like Catalysis for Xanthine Sensing. Processes. 2025; 13(2):387. https://doi.org/10.3390/pr13020387
Chicago/Turabian StyleTan, Chao, Junjie He, Fei Zhou, Ruicheng Xu, Yilei Gao, Robert S. Marks, and Junji Li. 2025. "CTAB-Assisted Formation of Hierarchical Porosity in Cu-BDC-NH2 Metal–Organic Frameworks and Its Enhanced Peroxidase-like Catalysis for Xanthine Sensing" Processes 13, no. 2: 387. https://doi.org/10.3390/pr13020387
APA StyleTan, C., He, J., Zhou, F., Xu, R., Gao, Y., Marks, R. S., & Li, J. (2025). CTAB-Assisted Formation of Hierarchical Porosity in Cu-BDC-NH2 Metal–Organic Frameworks and Its Enhanced Peroxidase-like Catalysis for Xanthine Sensing. Processes, 13(2), 387. https://doi.org/10.3390/pr13020387










