Preparation and Characterization of Supercapacitor Cells Using Modified CNTs and Bimetallic MOFs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CoZn-MOF
2.3. Pretreatment of CNTs
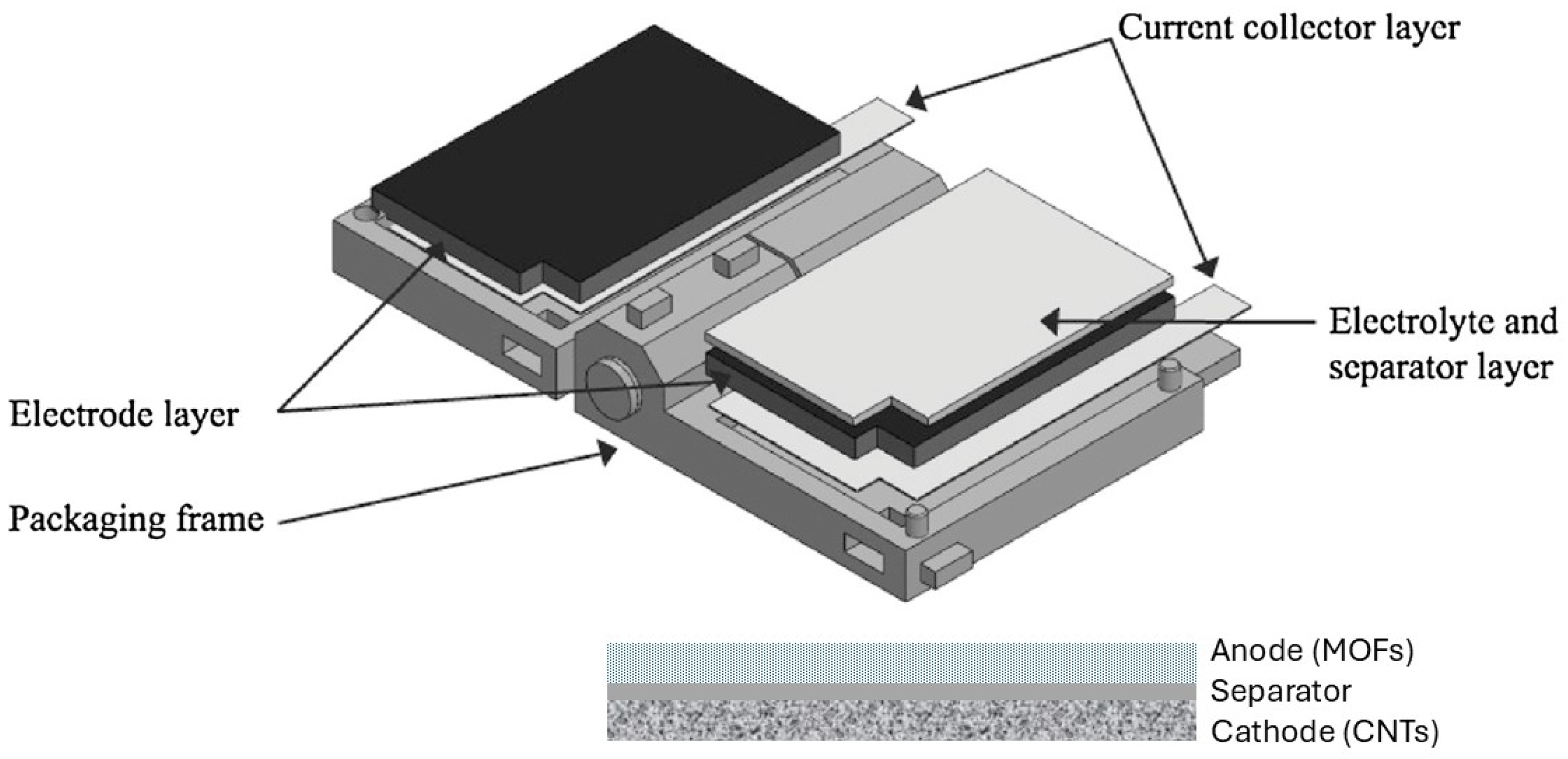
2.4. Experimental Setup
3. Results and Discussion
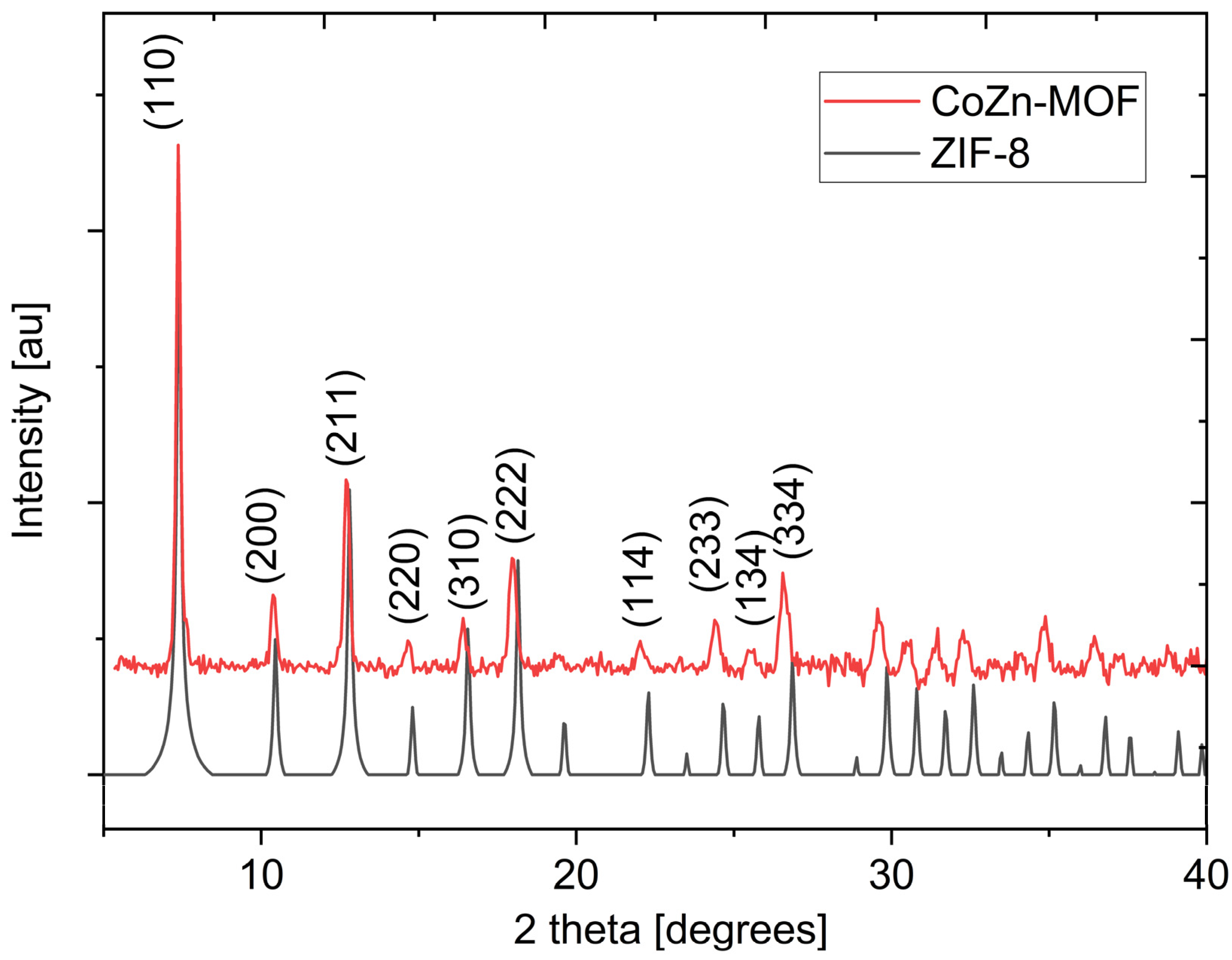
3.1. Characterization
3.2. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutta, A.; Mitra, S.; Basak, M.; Banerjee, T. A comprehensive review on batteries and supercapacitors: Development and challenges since their inception. Energy Storage 2023, 5, e339. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Gomez-Romero, P.; Kim, D.-H. Fundamentals of binary metal oxide-based supercapacitors. In Metal Oxides in Supercapacitors; Dubal, D.P., Gomez-Romero, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 79–98. [Google Scholar]
- Jalal, N.I.; Ibrahim, R.I.; Oudah, M.K. A review on Supercapacitors: Types and components. J. Phys. Conf. Ser. 2021, 1973, 012015. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Tong, Y.; Wang, X.; Zheng, J.; Wu, Y.; Li, H.; Niu, L.; Hou, Y. Exploration in materials, electrolytes and performance towards metal ion (Li, Na, K, Zn and Mg)-based hybrid capacitors: A review. Nano Energy 2021, 86, 106070. [Google Scholar] [CrossRef]
- Tanwilaisiri, A. Design and Fabrication of Supercapacitors. PhD Thesis, Design and Physical Science, Department of Design, College of Engineering, Brunel University, London, UK, 2018. [Google Scholar]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R. Integration of supercapacitors and batteries towards high-performance hybrid energy storage devices. Int. J. Energy Res. 2021, 45, 1449–1479. [Google Scholar] [CrossRef]
- Park, K.; Zheng, N.; Côté, A.; Choi, J.; Huang, R.; Uribe-Romo, F.; Chae, H.; O’Keeffe, M.; Yaghi, O. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Wang, H.; Jiao, X.; Ouyang, Y.; Xia, X.; Lei, W.; Hao, Q. ZIF-8 nanocrystals derived N-doped carbon decorated graphene sheets for symmetric supercapacitors. Electrochim. Acta 2018, 289, 494–502. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A Mater. 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Minakshi, M.; Samayamanthry, A.; Whale, J.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Shrestha, L.K. Phosphorous—Containing Activated Carbon Derived from Natural Honeydew Peel Powers Aqueous Supercapacitors. Chem. Asian J. 2024, 19, e202400622. [Google Scholar] [CrossRef]
- Minakshi, M.; Mujeeb, A.; Whale, J.; Evans, R.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Shrestha Kumar, L. Synthesis of Porous Carbon Honeycomb Structures Derived from Hemp for Hybrid Supercapacitors with Improved Electrochemistry. ChemPlusChem 2024, e202400408. [Google Scholar] [CrossRef]
- Liang, Y.; Yao, W.; Duan, J.; Chu, M.; Sun, S.; Li, X. Nickel cobalt bimetallic metal-organic frameworks with a layer-and-channel structure for high-performance supercapacitors. J. Energy Storage 2021, 33, 102149. [Google Scholar] [CrossRef]
- Brousse, T.; Comte, A.L.; Bélanger, D. To be or not to be pseudocapacitive. J. Electrochem. Soc. 2014, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Electrochemical capacitors: Materials, technologies and performance. Energy Storage Mater. 2021, 36, 31–55. [Google Scholar] [CrossRef]
- Gonzalez, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Sundriyal, S.; Kaur, H.; Bhardwaj, S.; Kumar, B.; Mishra, S.; Kim, K.; Deep, A. Metal-organic frameworks and their composites as efficient electrodes for supercapacitor applications. Coord. Chem. Rev. 2018, 369, 15–38. [Google Scholar] [CrossRef]
- Vaitsis, C.; Mechili, M.; Argirusis, N.; Kanellou, E.; Pandis, P.; Sourkouni, G.; Zorpas, A.; Argirusis, C. Ultrasound-assisted preparation methods of nanoparticles for energy related applications. In Nanotechnology and the Environment; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Chen, D.; Wei, L.; Li, J.; Wu, Q. Nanoporous materials derived from metal-organic framework for supercapacitor application. J. Energy Storage 2020, 31, 101525. [Google Scholar] [CrossRef]
- Vangari, M.; Pryor, T.; Li, J. Supercapacitors: Review of materials and fabrication methods. J. Energy Eng. 2013, 139, 72–79. [Google Scholar] [CrossRef]
- Zhong, C.; Duan, Y.; Cao, X.; Yang, W.; Wu, X.; Xia, X.; Fan, Z. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable thin film supercapacitors using single-walled carbon nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef]
- Vaitsis, C.; Sourkouni, G.; Argirusis, C. Sonochemical Synthesis of MOFs. In Metal-Organic Frameworks for Biomedical Applications; Mozafari, M., Ed.; Woodhead Publishing: Sawston, UK, 2020; pp. 223–244. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.-H. Recent advances in post-synthetic modification of metal–organic frameworks: New types and tandem reactions. Coord. Chem. Rev. 2019, 378, 500–512. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, X.; Yao, Y.; Hong, G. Recent advances of electrically conductive metal-organic frameworks in electrochemical applications. Mater. Today Nano 2021, 13, 100105. [Google Scholar] [CrossRef]
- Vaitsis, C.; Kanellou, E.; Angelara, C.; Pandis, P.; Argirusis, N.; Sourkouni, G.; Zorpas, A.; Karantonis, A.; Argirusis, C. Chapter 18—MOFs–metal oxides/sulfides/phosphides nanocomposites for supercapacitors. In Metal-Organic Framework-Based Nanomaterials for Energy Conversion and Storage; Nguyen, T.A., Ram, G.Y., Gupta, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–412. [Google Scholar]
- Osorio, A.G.; Silveira, I.C.L.; Bueno, V.L.; Begmann, C.P. H2SO4/HNO3/HCl—Functionalization and its effect on dispersion of carbon nanotubes in aqueous media. Appl. Surf. Sci. 2008, 255, 2485–2489. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Mobin, S.M. Robust nanocomposite of nitrogen-doped reduced graphene oxide and MnO2 nanorods for high-performance supercapacitors and nonenzymatic peroxide sensors. ACS Sustain. Chem. Eng. 2018, 6, 10489–10504. [Google Scholar] [CrossRef]
- Platek, A.; Nita, C.; Ghimbeu, C.M.; Frąckowiak, E.; Fic, K. Electrochemical capacitors operating in aqueous electrolyte with volumetric characteristics improved by sustainable templating of electrode materials. Electrochim. Acta 2020, 338, 135788. [Google Scholar] [CrossRef]
- Raymundo-Pinero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon 2006, 44, 2498–2507. [Google Scholar] [CrossRef]
- Nordin, A.; Ismail, A.F.; Misdan, N.; Mohd Nazri, N.A. Modified ZIF-8 mixed matrix membrane for CO2/CH4 separation. AIP Conf. Proc. 2017, 1891, 020091. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, B.; Sun, Y.; Zhang, X.; Yang, H.; Wang, J. Carbon nanotubes@metal–organic frameworks as Mn-based symmetrical supercapacitor electrodes for enhanced charge storage. RSC Adv. 2015, 5, 58100–58106. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Wang, H.; Yan, H. A review of performance optimization of MOF-derived metal oxide as electrode materials for supercapacitors. Int. J. Energy Res. 2019, 43, 697–716. [Google Scholar] [CrossRef]
- Vaitsis, C.; Sourkouni, G.; Argirusis, C. Metal Organic Frameworks (MOFs) and ultrasound: A review. Ultrason. Sonochemistry 2019, 52, 106–119. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, H.; Mei, H.; Sun, D. Recent progress in metal-organic framework-based supercapacitor electrode materials. Coord. Chem. Rev. 2020, 420, 213438. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs)—Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-X.; Liao, P.-Q.; Lin, R.-B.; Wei, Y.-S.; Zeng, M.-H.; Chen, X.-M. Metal cluster-based functional porous coordination polymers. Coord. Chem. Rev. 2015, 293–318, 263–278. [Google Scholar] [CrossRef]
- Bigdeli, F.; Ghasempour, H.; Tehrani, A.; Morsali, A.; Hosseini-Monfared, H. Ultrasound-assisted synthesis of nano-structured Zinc(II)-based metal-organic frameworks as precursors for the synthesis of ZnO nano-structures. Ultrason. Sonochemistry 2017, 37, 29–36. [Google Scholar] [CrossRef]
- Abuzalat, O.; Wong, D.; Elayed, M.; Park, S.; Kim, S. Sonochemical fabrication of Cu(II) and Zn(II) metal-organic framework films on metal substrates. Ultrason. Sonochemistry 2018, 45, 180–188. [Google Scholar] [CrossRef]
- Schneuwly, A.; Gallay, R. Properties and applications of supercapacitors from the state-of-the-art to future trends. In Proceedings of the PCIM 2000 Europe, Nuremberg, Germany, 6–8 June 2000; pp. 1–10. [Google Scholar]
- Neisi, Z.; Ansari-Asl, Z.; Dezfuli, A.S. Polyaniline/Cu(II) Metal-Organic Frameworks Composite for High Performance Supercapacitor Electrode. J. Inorg. Organomet. Polym. 2019, 29, 1838–1847. [Google Scholar] [CrossRef]
- Sharma, S.; Chand, P. Supercapacitor and electrochemical techniques: A brief review. Results Chem. 2023, 5, 100885. [Google Scholar] [CrossRef]
- Tanwilaisiri, A.; Xu, Y.; Zhang, R.; Harrison, D.; Fyson, J.; Areir, M. Design and fabrication of modular supercapacitors using 3D printing. J. Energy Storage 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Halper, M.S.; Ellenbogen, J.C. Supercapacitors: A Brief Overview. The MITRE Corporation, March 2006, pp. 1–34. Available online: https://www.mitre.org/sites/default/files/pdf/06_0667.pdf (accessed on 9 April 2024).
- Chen, Z.; Wang, D.; Chua, D.; Poh, C.K.; Chen, P.; Lin, J.; Lou, D.; Chen, L.; Wu, Y. High-performance supercapacitors based on hierarchically porous graphite particles. Adv. Energy Mater. 2011, 1, 551–556. [Google Scholar] [CrossRef]
- Hen, J.; Li, W.; Wang, D.; Yang, S.; Wen, J. Electrochemical characterization of carbon nanotubes as electrode in electrochemical double-layer capacitors. Carbon 2022, 40, 1193–1197. [Google Scholar] [CrossRef]
- Pachfule, P.; Das, R.; Poddar, P.; Banerjee, R. Solvothermal Synthesis, Structure, and Properties of Metal Organic Framework Isomers Derived from a Partially Fluorinated Link. Chem. Mater. 2011, 23, 1215–1222. [Google Scholar] [CrossRef]
- Forster, P.M.; Stock, N.; Cheetham, A. A high-throughput investigation of the role of pH, temperature, concentration, and time on the synthesis of hybrid inorganic-organic materials. Chem. Commun. 2005, 44, 7608–7611. [Google Scholar] [CrossRef] [PubMed]
- Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Rapid Room-Temperature Synthesis and Characterization of Nanocrystals of a Prototypical Zeolitic Imidazolate Framework. Chem. Mater. 2009, 21, 1410–1412. [Google Scholar] [CrossRef]
- Kim, B.K.; Sy, S.; Yu, A.; Zhang, J. Electrochemical supercapacitors for energy storage and conversion. In Handbook of Clean Energy Systems; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–25. [Google Scholar] [CrossRef]
- Thanh, M.T.; Thien, T.V.; Chau, V.T.T.; Du, P.D.; Hung, N.P.; Khieu, D.Q. Synthesis of Iron Doped Zeolite Imidazolate Framework-8 and Its Remazol Deep Black RGB Dye Adsorption Ability. J. Chem. 2017, 1–18. [Google Scholar] [CrossRef]
- Nik Abdul Hadi, M. Nordin; Ahmad Fauzi Ismail; Noorhana Yahya, Zeolitic imidazole framework 8 decorated graphene oxide (ZIF-8/GO) mixed matrix membrane (MMM) for CO2/CH4 separation. J. Teknol. 2017, 2017, 5045973. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Jorio, A.; Filho, A.S.; Dresselhaus, G.; Saito, R. Raman spectroscopy on one isolated carbon nanotube. Phys. B 2002, 323, 15–20. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristic Group Frequencies: Tables and Charts, 2nd ed.; John Wiley & Sons: Chichester, UK, 1994. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argirusis, C.; Angelara, C.; Argirusis, N.; Karantonis, A.; Pandis, P.P.; Sourkouni, G. Preparation and Characterization of Supercapacitor Cells Using Modified CNTs and Bimetallic MOFs. Processes 2024, 12, 2778. https://doi.org/10.3390/pr12122778
Argirusis C, Angelara C, Argirusis N, Karantonis A, Pandis PP, Sourkouni G. Preparation and Characterization of Supercapacitor Cells Using Modified CNTs and Bimetallic MOFs. Processes. 2024; 12(12):2778. https://doi.org/10.3390/pr12122778
Chicago/Turabian StyleArgirusis, Christos, Christina Angelara, Nikolaos Argirusis, Antonis Karantonis, Pavlos P. Pandis, and Georgia Sourkouni. 2024. "Preparation and Characterization of Supercapacitor Cells Using Modified CNTs and Bimetallic MOFs" Processes 12, no. 12: 2778. https://doi.org/10.3390/pr12122778
APA StyleArgirusis, C., Angelara, C., Argirusis, N., Karantonis, A., Pandis, P. P., & Sourkouni, G. (2024). Preparation and Characterization of Supercapacitor Cells Using Modified CNTs and Bimetallic MOFs. Processes, 12(12), 2778. https://doi.org/10.3390/pr12122778








