Chitosan–Glucan Biopolymer Design: Extraction from Champignons with Improved Antioxidant and Antimicrobial Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Methods
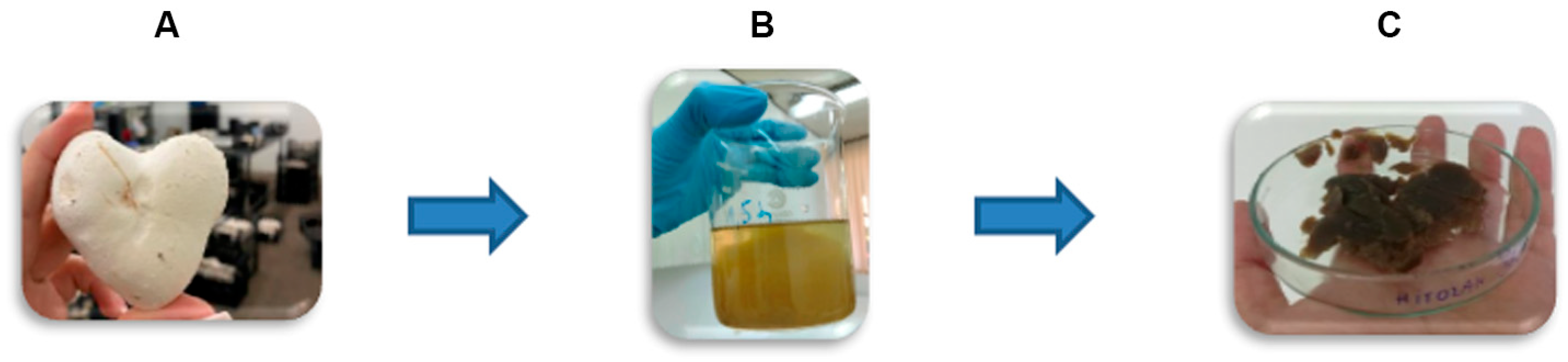
2.2.1. Extraction of Chitosan–Glucan Complex
2.2.2. Characterization of Cs-Agrif
Chemical Characterization
Rheological Characterization
Molecular Weight Determination
Fourier Transform Infrared Spectroscopy (FTIR)
1H-NMR Spectroscopy
X-Ray Diffraction (XRD)
2.2.3. Determination of Antimicrobial Activity
2.2.4. Determination of Antioxidant Potential
2.2.5. Statistical Analysis
3. Results and Discussion
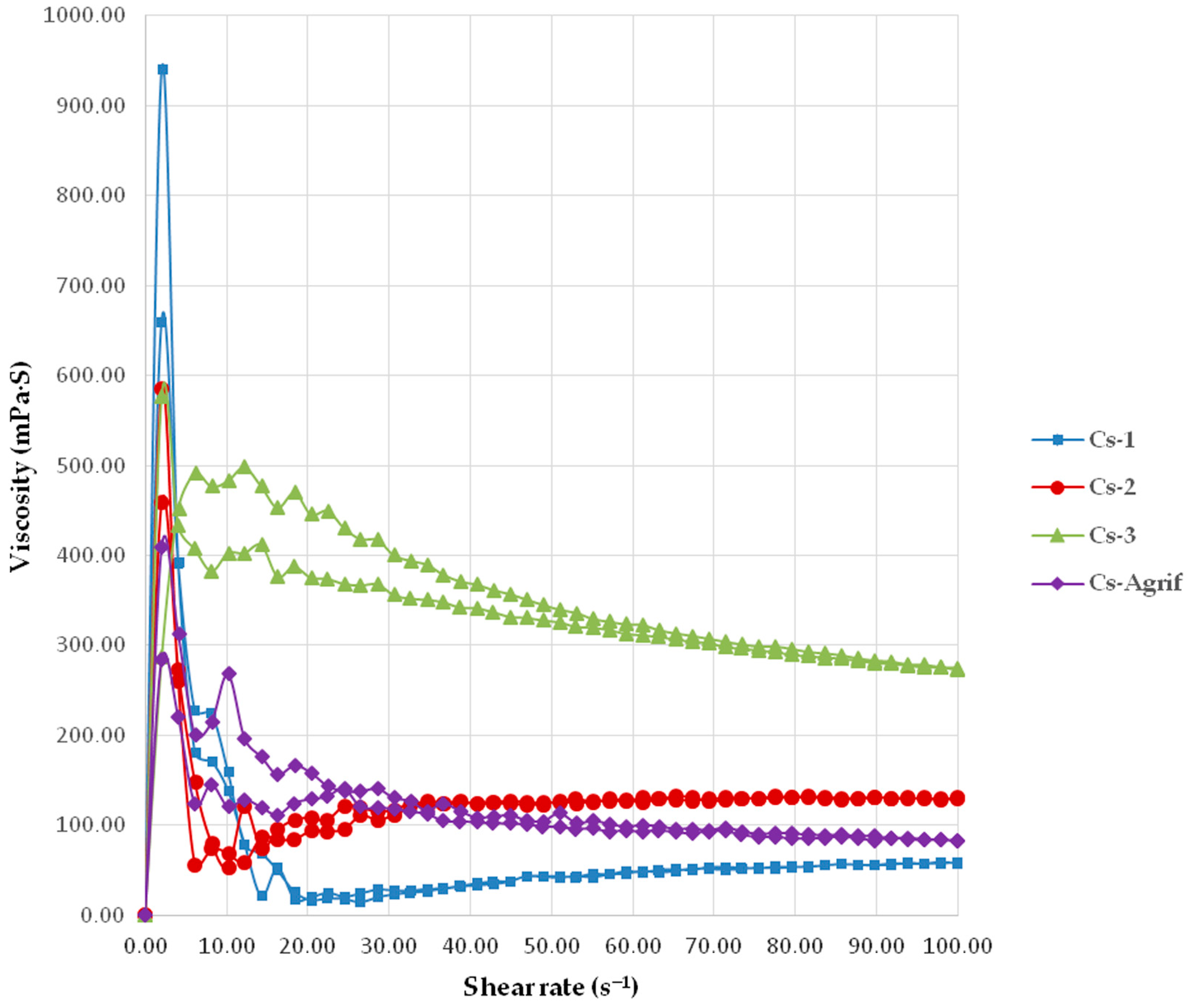
3.1. Determination of Cs-Agrif Molecular Weight and Rheological Characterization
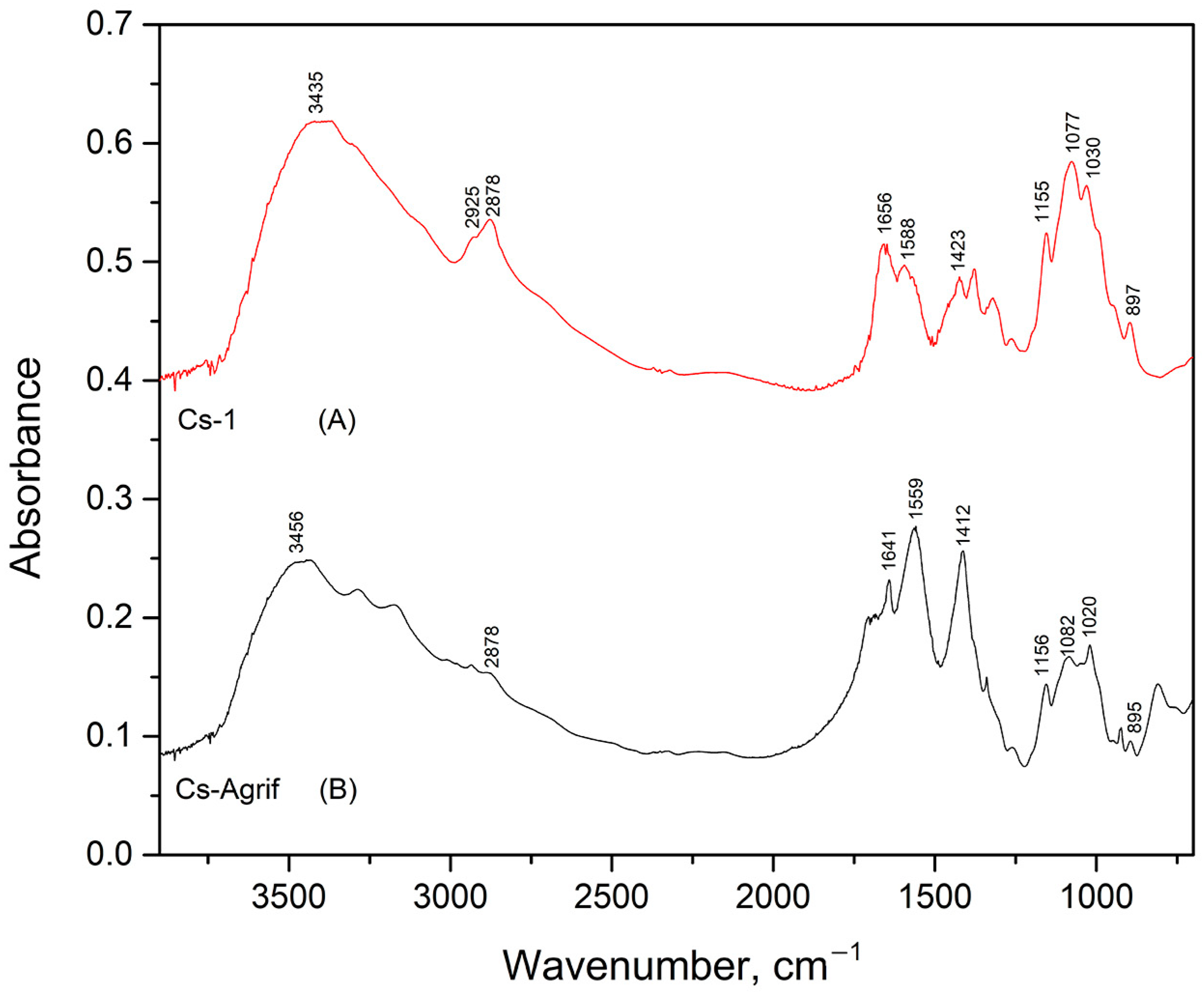
3.2. FTIR Analysis of Cs-Agrif
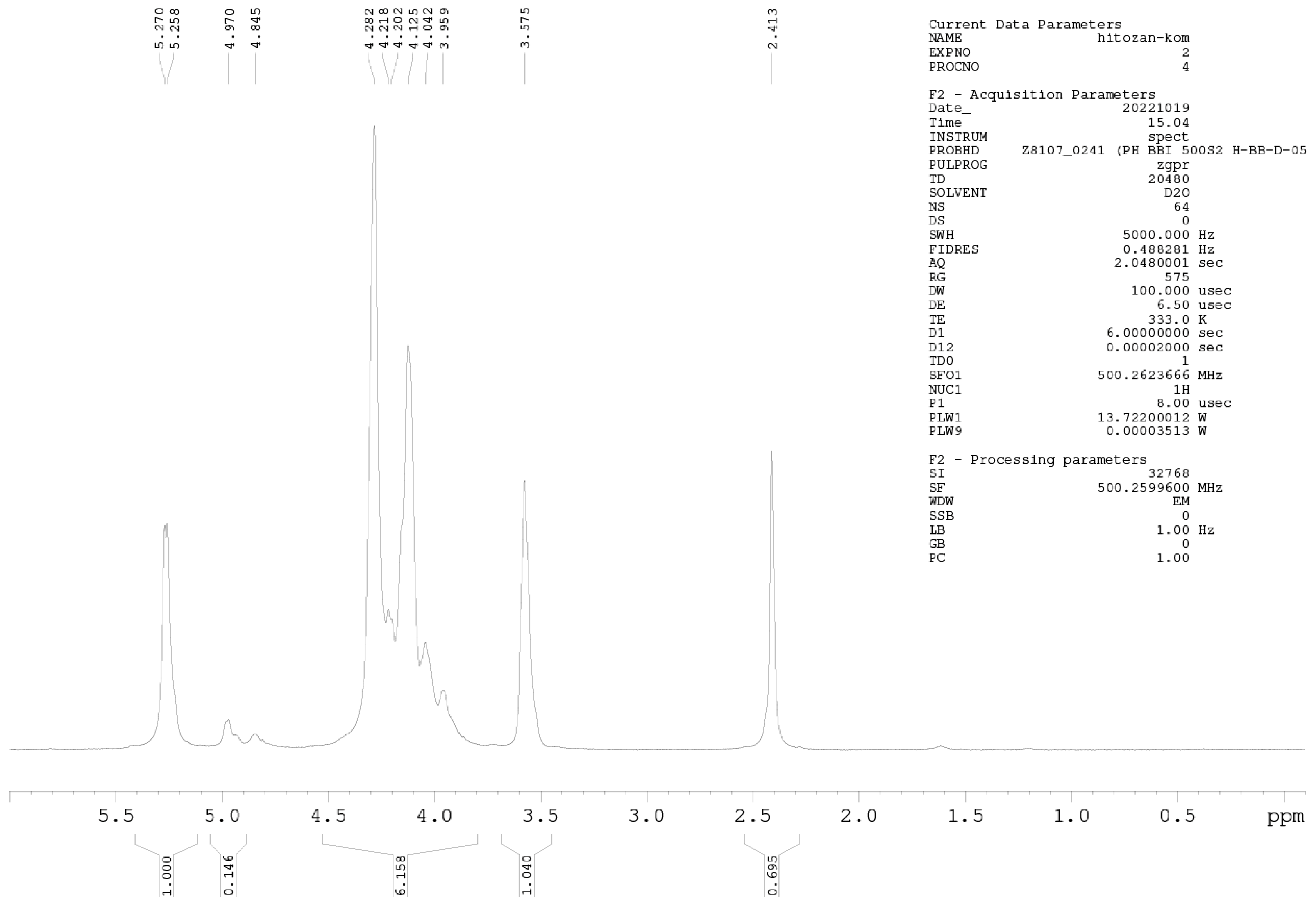
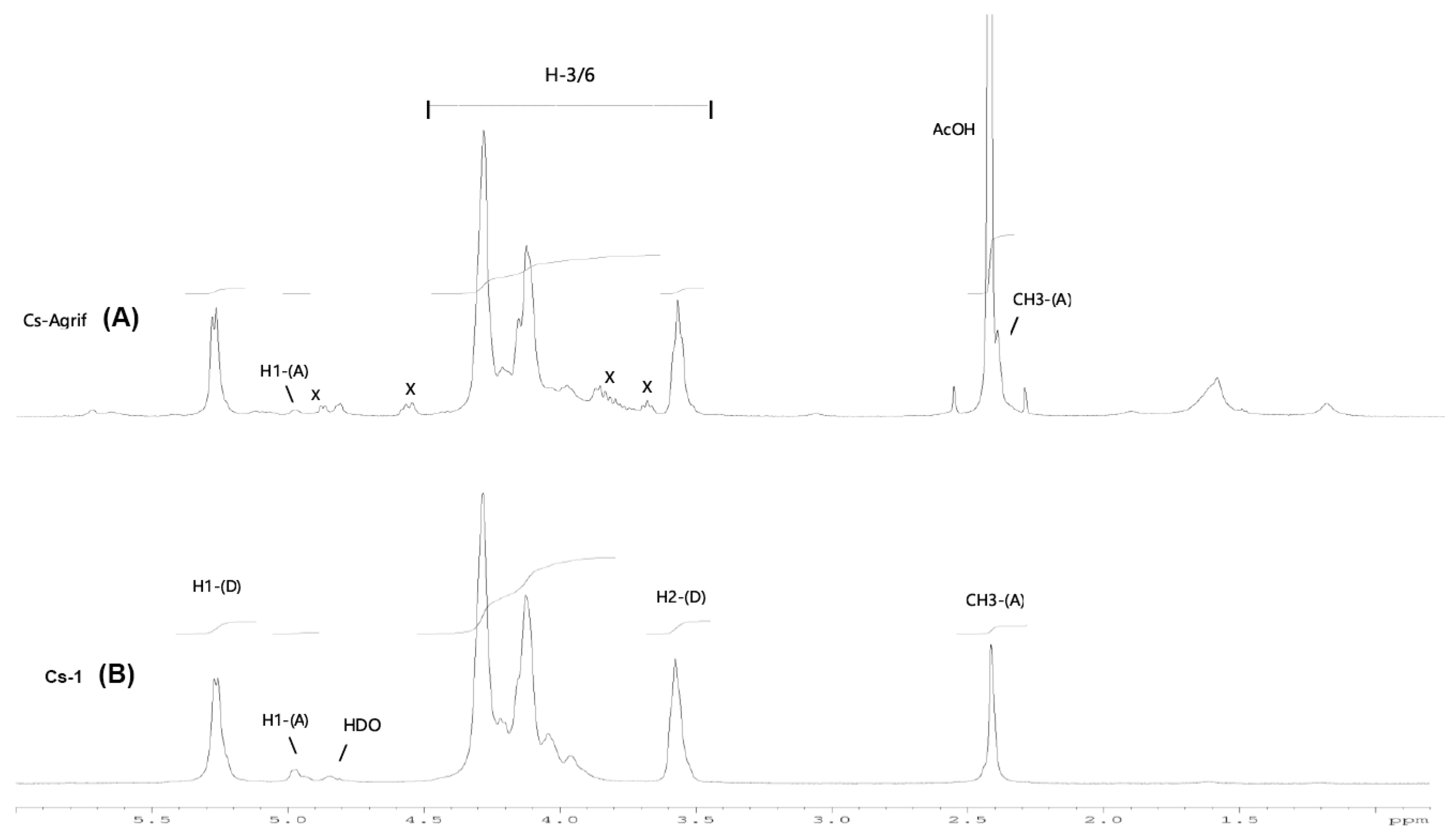
3.3. 1H-NMR Analysis of Cs-Agrif
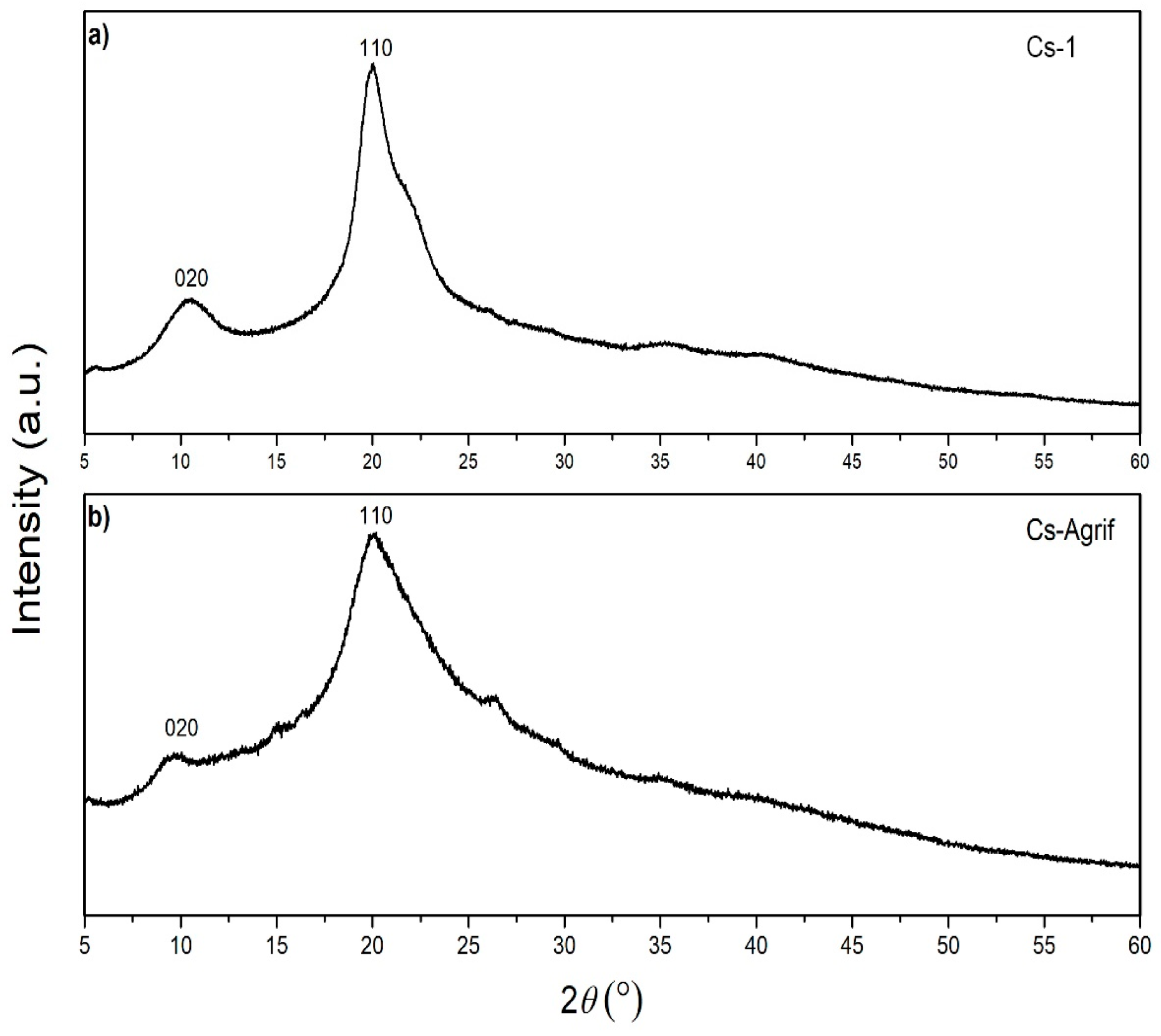
3.4. XRD Analyses of Cs-Agrif
3.5. Antimicrobial Activity of Cs-Agrif
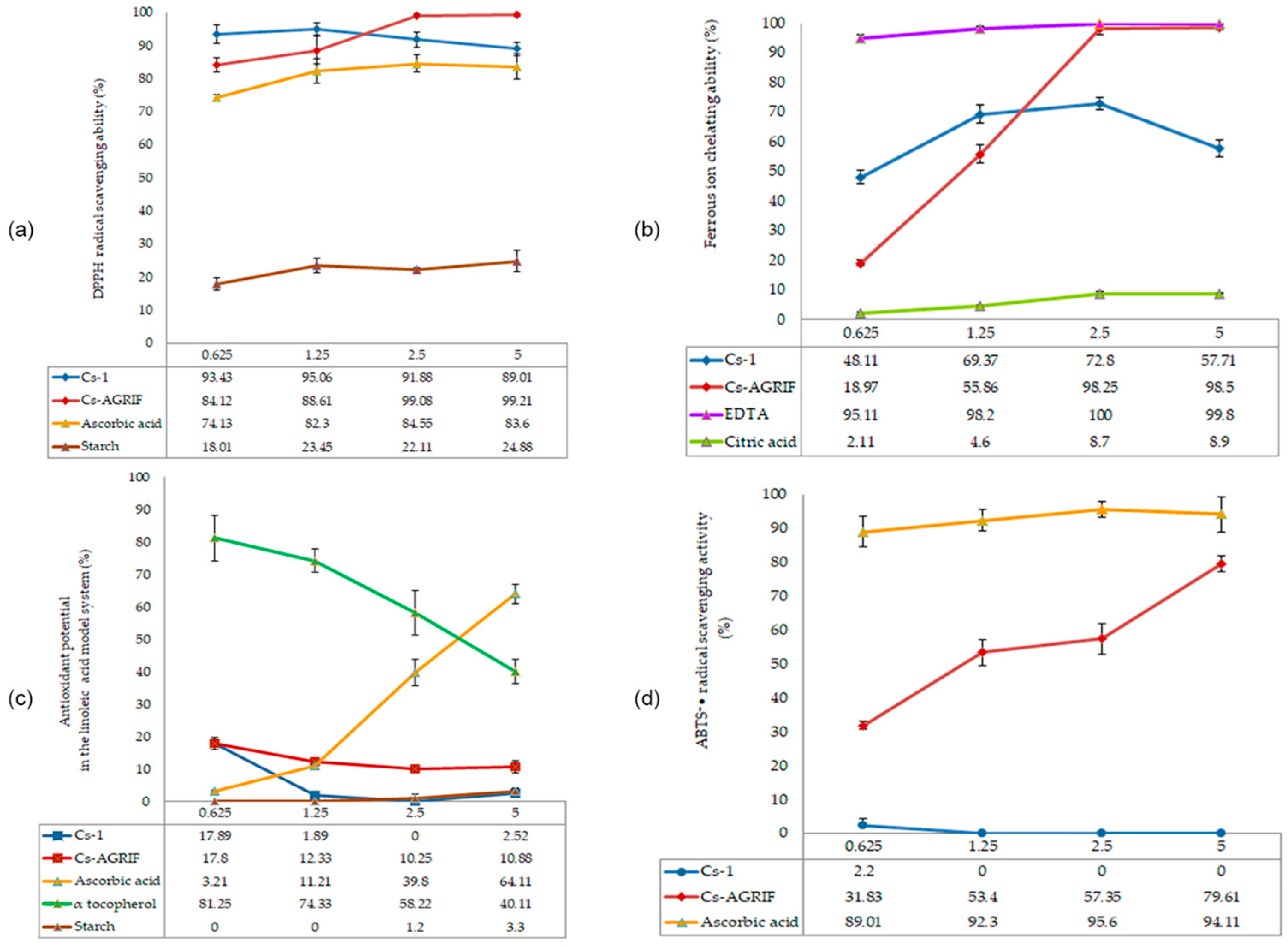
3.6. Antioxidant Activity of Cs-Agrif
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Savin, S.; Craciunescu, O.; Oancea, A.; Ilie, D.; Ciucan, T.; Antohi, L.S.; Toma, A.; Nicolescu, A.; Deleanu, C.; Oancea, F. Antioxidant, Cytotoxic and Antimicrobial Activity of Chitosan Preparations Extracted from Ganoderma Lucidum Mushroom. Chem. Biodivers. 2020, 17, e2000175. [Google Scholar] [CrossRef]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Alasvandian, S.; Shahgholi, M.; Karimipour, A. Investigating the effects of chitosan atomic ratio and drug type on mechanical properties of silica aerogel/chitosan nanocomposites using molecular dynamics approach. J. Mol. Liq. 2024, 401, 124639. [Google Scholar] [CrossRef]
- Yen, M.-T.; Tseng, Y.-H.; Li, R.-C.; Mau, J.-L. Antioxidant Properties of Fungal Chitosan from Shiitake Stipes. LWT—Food Sci. Technol. 2007, 40, 255–261. [Google Scholar] [CrossRef]
- Vargas, M.; Gonzalez-Martinez, C. Recent Patents on Food Applications of Chitosan. FNA 2010, 2, 121–128. [Google Scholar] [CrossRef]
- Latańska, I.; Rosiak, P.; Paul, P.; Sujka, W.; Kolesińska, B. Modulating the physicochemical properties of chitin and chitosan as a method of obtaining new biological properties of biodegradable materials. In Chitin and Chitosan—Physicochemical Properties and Industrial Applications; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Čalija, B.; Milić, J.; Krajišnik, D.; Račić, A. Karakteristike i primena hitozana u farmaceutskim/biomedicinskim preparatima. Arh. Farm. 2013, 63, 347–364. [Google Scholar]
- Raafat, D.; Sahl, H. Chitosan and Its Antimicrobial Potential—A Critical Literature Survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Ivanova, D.G.; Yaneva, Z.L. Antioxidant Properties and Redox-Modulating Activity of Chitosan and Its Derivatives: Biomaterials with Application in Cancer Therapy. BioResearch Open Access 2020, 9, 64–72. [Google Scholar] [CrossRef]
- Hussain, M.; Sam, S.T.; Yaacob, N.D.; Tan, W.K. Fungal Chitosan in Focus: A Comprehensive Review on Extraction Methods and Applications. Food Res. Int. 2025, 220, 117103. [Google Scholar] [CrossRef]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Wu, T. Production and Characterization of Fungal Chitin and Chitosan. Master’s Thesis, The University of Tennessee, Knoxville, TN, USA, 2004. [Google Scholar]
- Silva, T.H.; Alves, A.; Ferreira, B.M.; Oliveira, J.M.; Reys, L.L.; Ferreira, R.J.F.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Materials of Marine Origin: A Review on Polymers and Ceramics of Biomedical Interest. Int. Mater. Rev. 2012, 57, 276–306. [Google Scholar] [CrossRef]
- Amaral, L.; Silva, D.; Couto, M.; Nunes, C.; Rocha, S.M.; Coimbra, M.A.; Coimbra, A.; Moreira, A. Safety of Chitosan Processed Wine in Shrimp Allergic Patients. Ann. Allergy Asthma Immunol. 2016, 116, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.M.; Nunes, F.M. Agaricus bisporus By-Products as a Source of Chitin-Glucan Complex Enriched Dietary Fibre with Potential Bioactivity. Appl. Sci. 2020, 10, 2232. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- Kour, H.; Kour, D.; Kour, S.; Singh, S.; Jawad Hashmi, S.A.; Yadav, A.N.; Kumar, K.; Sharma, Y.P.; Ahluwalia, A.S. Bioactive Compounds from Mushrooms: Emerging Bioresources of Food and Nutraceuticals. Food Biosci. 2022, 50, 102124. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The Antioxidant Potential of Different Edible and Medicinal Mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef]
- Martinez-Medina, G.A.; Chávez-González, M.L.; Verma, D.K.; Prado-Barragán, L.A.; Martínez-Hernández, J.L.; Flores-Gallegos, A.C.; Thakur, M.; Srivastav, P.P.; Aguilar, C.N. Bio-Funcional Components in Mushrooms, a Health Opportunity: Ergothionine and Huitlacohe as Recent Trends. J. Funct. Foods 2021, 77, 104326. [Google Scholar] [CrossRef]
- Bahndral, A.; Shams, R.; Dash, K.K.; Chaudhary, P.; Mukarram Shaikh, A.; Béla, K. Microwave Assisted Extraction of Chitosan from Agaricus Bisporus: Techno-Functional and Microstructural Properties. Carbohydr. Polym. Technol. Appl. 2025, 9, 100730. [Google Scholar] [CrossRef]
- Affandy, M.A.M.; Rovina, K. Characterization of chitosan derived from mushroom sources: Physicochemical, morphological, thermal analysis. Sustain. Chem. Pharm. 2024, 40, 101624. [Google Scholar] [CrossRef]
- Mesa Ospina, N.; Ospina Alvarez, S.P.; Escobar Sierra, D.M.; Rojas Vahos, D.F.; Zapata Ocampo, P.A.; Ossa Orozco, C.P. Isolation of Chitosan from Ganoderma Lucidum Mushroom for Biomedical Applications. J. Mater. Sci. Mater. Med. 2015, 26, 135. [Google Scholar] [CrossRef] [PubMed]
- Sousa, I.C.G.; Teixeira, S.C.; Souza, M.V.d., Jr.; Conde, M.B.M.; Bailon, G.R.; Cardoso, S.H.S.; Araújo, L.D.; Oliveira, E.B.d.; Ferreira, S.O.; Oliveira, T.V.d.; et al. Sustainable Extraction and Multimodal Characterization of Fungal Chitosan from Agaricus bisporus. Foods 2025, 14, 2785. [Google Scholar] [CrossRef]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P.; Kozak, L. The Effect of Selenium on Phenolics and Flavonoids in Selected Edible White Rot Fungi. LWT—Food Sci. Technol. 2015, 63, 726–731. [Google Scholar] [CrossRef]
- Račić, A.; Čalija, B.; Milić, J.; Milašinović, N.; Krajišnik, D. Development of Polysaccharide-Based Mucoadhesive Ophthalmic Lubricating Vehicles: The Effect of Different Polymers on Physicochemical Properties and Functionality. J. Drug Deliv. Sci. Technol. 2019, 49, 50–57. [Google Scholar] [CrossRef]
- Abdelaal, M.Y.; Abdel-Razik, E.A.; Abdel-Bary, E.M.; El-Sherbiny, I.M. Chitosan-based Interpolymeric pH-responsive Hydrogels for in Vitro Drug Release. J. Appl. Polym. Sci. 2007, 103, 2864–2874. [Google Scholar] [CrossRef]
- Liu, C.; Wang, G.; Sui, W.; An, L.; Si, C. Preparation and Characterization of Chitosan by a Novel Deacetylation Approach Using Glycerol as Green Reaction Solvent. ACS Sustain. Chem. Eng. 2017, 5, 4690–4698. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J. Determination of Degree of Deacetylation of Chitosan—Comparision of Methods. Prog. Chem. Appl. Chitin Its Deriv. 2012, 2012, 5–20. [Google Scholar]
- Stojanova, M.; Pantic, M.; Klaus, A.; Mihajlovic, D.; Miletic, D.; Sobajic, S.; Stojanova, M.T.; Niksic, M. Bio Soups—New Functional Dehydrated Soups Enriched with Lyophilised Fuscoporia torulosa Extracts. Int. J. Food Sci. Technol. 2023, 58, 3628–3636. [Google Scholar] [CrossRef]
- Duvnjak, D.; Pantić, M.; Pavlović, V.; Nedović, V.; Lević, S.; Matijašević, D.; Sknepnek, A.; Nikšić, M. Advances in Batch Culture Fermented Coriolus Versicolor Medicinal Mushroom for the Production of Antibacterial Compounds. Innov. Food Sci. Emerg. Technol. 2016, 34, 1–8. [Google Scholar] [CrossRef]
- Savic, M.; Anedjelkovic, I.; Duvnjak, D.; Matijasevic, D.; Avramovic, A.; Niksic, M. The Fungistatic Activity of Organic Selenium and Its Application to the Production of Cultivated Mushrooms Agaricus bisporus and Pleurotus spp. Arch. Biol. Sci. 2012, 64, 1455–1463. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A Validated 1H NMR Method for the Determination of the Degree of Deacetylation of Chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Nikšić, M.; Vrvić, M.M.; Todorović, N.; Jakovljević, D.; Van Griensven, L.J.L.D. Antioxidative Activities and Chemical Characterization of Polysaccharide Extracts from the Widely Used Mushrooms Ganoderma Applanatum, Ganoderma Lucidum, Lentinus Edodes and Trametes Versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; Van Griensven, L.J.L.D. Antioxidative and Immunomodulating Activities of Polysaccharide Extracts of the Medicinal Mushrooms Agaricus Bisporus, Agaricus Brasiliensis, Ganoderma Lucidum and Phellinus Linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, O.M.; Sultan, Y.Y.; Seif, M.M.; Marrez, D.A. Bio-Evaluation of Crustacean and Fungal Nano-Chitosan for Applying as Food Ingredient. Toxicol. Rep. 2018, 5, 348–356. [Google Scholar] [CrossRef]
- Yen, M.-T.; Mau, J.-L. Physico-Chemical Characterization of Fungal Chitosan from Shiitake Stipes. LWT—Food Sci. Technol. 2007, 40, 472–479. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of Chitin from Prawn Shells and Conversion to Low Molecular Mass Chitosan. Food Hydrocoll. 2013, 31, 166–171. [Google Scholar] [CrossRef]
- Yanat, M.; Colijn, I.; de Boer, K.; Schroën, K. Comparison of the Degree of Acetylation of Chitin Nanocrystals Measured by Various Analysis Methods. Polymers 2023, 15, 294. [Google Scholar] [CrossRef]
- Hromis, N.; Lazic, V.; Popovic, S.; Suput, D.; Bulut, S. Antioxidative Activity of Chitosan and Chitosan Based Biopolymer Film. Food Feed Res. 2017, 44, 91–100. [Google Scholar] [CrossRef]
- Ssekatawa, K.; Byarugaba, D.K.; Wampande, E.M.; Moja, T.N.; Nxumalo, E.; Maaza, M.; Sackey, J.; Ejobi, F.; Kirabira, J.B. Isolation and Characterization of Chitosan from Ugandan Edible Mushrooms, Nile Perch Scales and Banana Weevils for Biomedical Applications. Sci. Rep. 2021, 11, 4116. [Google Scholar] [CrossRef]
- Hemmami, H.; Ben Amor, I.; Zeghoud, S.; Ben Amor, A.; Laouini, S.E.; Alsalme, A.; Cornu, D.; Bechelany, M.; Barhoum, A. Chitosan Extraction from Amanita Phalloides: Yield, Crystallinity, Degree of Deacetylation, Azo Dye Removal and Antibacterial Properties. Front. Chem. 2024, 12, 1353524. [Google Scholar] [CrossRef]
- Marx, N.; Fernández, L.; Barceló, F.; Spikes, H. Shear Thinning and Hydrodynamic Friction of Viscosity Modifier-Containing Oils. Part I: Shear Thinning Behaviour. Tribol. Lett. 2018, 66, 92. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Suresh, H.; Ho, V.; Zhou, J. Rheological Characteristics of Soluble Fibres during Chemically Simulated Digestion and Their Suitability for Gastroparesis Patients. Nutrients 2020, 12, 2479. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef]
- Yuan, B.; Ritzoulis, C.; Chen, J. Rheological investigations of beta glucan functionality: Interactions with mucin. Food Hydrocoll. 2018, 87, 180–186. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Román-Doval, S.R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its Application in Agriculture in Context of Molecular Weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef] [PubMed]
- Crognale, S.; Russo, C.; Petruccioli, M.; D’Annibale, A. Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation 2022, 8, 76. [Google Scholar] [CrossRef]
- Tanasale, M.F.J.D.P.; Bijang, C.M.; Rumpakwara, E. Preparation of Chitosan with Various Molecular Weight and Its Effect on Depolymerization of Chitosan with Hydrogen Peroxide Using Conventional Technique. IJCTR 2019, 12, 112–120. [Google Scholar] [CrossRef]
- Wang, W.; Du, Y.; Qiu, Y.; Wang, X.; Hu, Y.; Yang, J.; Cai, J.; Kennedy, J.F. A New Green Technology for Direct Production of Low Molecular Weight Chitosan. Carbohydr. Polym. 2008, 74, 127–132. [Google Scholar] [CrossRef]
- Pantic, M.; Lazic, V.; Kozarski, M. Mushroom chitosan: A promising biopolymer in the food industry and agriculture. Hrana Ishr. 2023, 64, 1–11. [Google Scholar] [CrossRef]
- Vilar Junior, J.C.; Ribeaux, D.R.; Alves Da Silva, C.A.; De Campos-Takaki, G.M. Physicochemical and Antibacterial Properties of Chitosan Extracted from Waste Shrimp Shells. Int. J. Microbiol. 2016, 2016, 5127515. [Google Scholar] [CrossRef]
- Focher, B.; Beltrame, P.L.; Naggi, A.; Torri, G. Alkaline N-Deacetylation of Chitin Enhanced by Flash Treatments. Reaction Kinetics and Structure Modifications. Carbohydr. Polym. 1990, 12, 405–418. [Google Scholar] [CrossRef]
- Matijašević, D.; Pantić, M.; Rašković, B.; Pavlović, V.; Duvnjak, D.; Sknepnek, A.; Nikšić, M. The Antibacterial Activity of Coriolus versicolor Methanol Extract and Its Effect on Ultrastructural Changes of Staphylococcus aureus and Salmonella Enteritidis. Front. Microbiol. 2016, 7, 1226. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhang, T.; He, W.; He, Y. Antibacterial Ability and Feature of Polyvinyl Alcohol/Chitosan/Montmorillonite/Copper Nanoparticle Composite Gel Beads. Processes 2025, 13, 3518. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Mendis, A.; Thambiliyagodage, C.; Ekanayake, G.; Liyanaarachchi, H.; Jayanetti, M.; Vigneswaran, S. Fabrication of Naturally Derived Chitosan and Ilmenite Sand-Based TiO2/Fe2O3/Fe-N-Doped Graphitic Carbon Composite for Photocatalytic Degradation of Methylene Blue under Sunlight. Molecules 2023, 28, 3154. [Google Scholar] [CrossRef]
- Carpintero, M.; Marcet, I.; Cortizo, C.; Guerrero, P.; De La Caba, K.; Rendueles, M.; Díaz, M. Chitosan Modification with Octenyl Succinic Anhydride (OSA): Effect of the Degree of Substitution on the Structural, Mechanical and Barrier Properties in the Synthetized Bioplastics. Food Hydrocoll. 2025, 171, 111838. [Google Scholar] [CrossRef]
- Mamad, D.M.; Muhammad, D.S.; Aziz, S.B.; Muheddin, D.Q.; Ahmed, B.Y.; Abdalkarim, K.A.; Al-Asbahi, B.A.; Ahmed, A.A.A.; Abdullah, O.G. Improvement the Optoelectronic Properties of Chitosan Biopolymer Using Natural Dye of Black Olive as a Novel Approach: FTIR, XRD and UV–Vis Spectroscopic Studies. Opt. Mater. 2025, 164, 117057. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. ISBN 978-981-15-0262-0. [Google Scholar]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Khubiev, O.M.; Egorov, A.R.; Kirichuk, A.A.; Khrustalev, V.N.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan-Based Antibacterial Films for Biomedical and Food Applications. Int. J. Mol. Sci. 2023, 24, 10738. [Google Scholar] [CrossRef]
- Minh, N.C.; Van Hoa, N.; Trung, T.S. Preparation, Properties, and Application of Low-Molecular-Weight Chitosan. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 453–471. ISBN 978-0-12-817970-3. [Google Scholar]
- Guan, G.; Azad, M.A.K.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological Effects and Applications of Chitosan and Chito-Oligosaccharides. Front. Physiol. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Gani, A.; Khanday, F.A.; Masoodi, F.A. Biological and Pharmaceutical Activities of Mushroom β-Glucan Discussed as a Potential Functional Food Ingredient. Bioact. Carbohydr. Diet. Fibre 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial Activity of Phenolic Compounds Identified in Wild Mushrooms, SAR Analysis and Docking Studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; van Griensven, L.; Jakovljevic, D.; Todorovic, N.; Wan-Mohtar, W.A.A.Q.I.; Vunduk, J. Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: Nature of the application. Food Sci. Hum. Wellness 2023, 12, 378–396. [Google Scholar] [CrossRef]
- de Graaff, P.; Govers, C.; Wichers, H.J.; Debets, R. Consumption of β-glucans to spice up T cell treatment of tumors: A review. Expert Opin. Biol. Ther. 2018, 18, 1023–1040. [Google Scholar] [CrossRef]
- Fadhil, A.; Mousa, E. Antimicrobal Activities of Chitosan Produces from Agaricus Bisporus Stalks. Plant Arch. 2020, 20, 109–114. [Google Scholar]
- Chien, R.-C.; Yen, M.-T.; Mau, J.-L. Antimicrobial and Antitumor Activities of Chitosan from Shiitake Stipes, Compared to Commercial Chitosan from Crab Shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef]
- Ban, Z.; Horev, B.; Rutenberg, R.; Danay, O.; Bilbao, C.; McHugh, T.; Rodov, V.; Poverenov, E. Efficient Production of Fungal Chitosan Utilizing an Advanced Freeze-Thawing Method; Quality and Activity Studies. Food Hydrocoll. 2018, 81, 380–388. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P.; Guo, Z. Cationic Chitosan Derivatives as Potential Antifungals: A Review of Structural Optimization and Applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef]
- Shahadha, A.; Khaleel, I.; Rashid, N. Evaluation of the Efficiency of Chitosan Produces from the Stalks of Agaricus Bisporus against Aspergillus Flavus and Reducing Aflatoxin B1. Iraqi J. Mark. Res. Consum. Prot. 2024, 16, 266–275. [Google Scholar] [CrossRef]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal Activity of Chitosan Nanoparticles and Correlation with Their Physical Properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Qian, Z.-J.; Vo, T.-S.; Ryu, B.; Ngo, D.-N.; Kim, S.-K. Antioxidant Activity of Gallate-Chitooligosaccharides in Mouse Macrophage RAW264.7 Cells. Carbohydr. Polym. 2011, 84, 1282–1288. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active Chitosan–Polyvinyl Alcohol Films with Natural Extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Tomida, H.; Fujii, T.; Furutani, N.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Anraku, M. Antioxidant Properties of Some Different Molecular Weight Chitosans. Carbohydr. Res. 2009, 344, 1690–1696. [Google Scholar] [CrossRef]
- Zrnic-Ciric, M.; Dabetic, N.; Todorovic, V.; Djuris, J.; Vidovic, B. Beta-Glucan Content and Antioxidant Activities of Mushroom-Derived Food Supplements. J. Serbian Chem. Soc. 2020, 85, 439–451. [Google Scholar] [CrossRef]
- Nandi, A.K.; Samanta, S.; Maity, S.; Sen, I.K.; Khatua, S.; Devi, K.S.P.; Acharya, K.; Maiti, T.K.; Islam, S.S. Antioxidant and Immunostimulant β-Glucan from Edible Mushroom Russula albonigra (Krombh.) Fr. Carbohydr. Polym. 2014, 99, 774–782. [Google Scholar] [CrossRef]
- Seniuk, O.; Kurochko, N.; Cuzminski, Z.; Tyshko, R. Evaluation of the Chitin-Glucan-Melanin Complex from Fomes Fomentarius for Stopping Bleeding and Providing First Aid for Laceration and Burns in Combat Conditions. EUREKA Life Sci. 2024, 3, 39–53. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Liu, H.; Bao, J.; Du, Y.; Zhou, X.; Kennedy, J.F. Effect of Ultrasonic Treatment on the Biochemphysical Properties of Chitosan. Carbohydr. Polym. 2006, 64, 553–559. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Cs-Agrif | Cs-1 |
|---|---|---|
| Color | Dark brown | Faint beige to beige |
| Yield AIM (%) | 15.70 ± 3.00 | n.d. |
| Yield chitosan (%) | 6.52 ± 0.92 | n.d. |
| Total glucan content (%) | 25.36 ± 0.37 | – |
| β-glucan content (%) | 22.97 ± 0.40 | – |
| Total phenols (mg g−1 dw) | 29.63 ± 0.38 | 13.48 ± 0.29 |
| η (cm3 g−1) | 39.10 ± 0.00 | 257.4 ± 0.00 |
| MW(kDa) | 45.70 ± 5.20 | 347.30 ± 58.70 |
| DD (according to 1H-NMR) (%) | 92.7 | 86.1 |
| Sample | Enterococcus faecalis ATCC 29219 | Escherichia coli ATCC 25922 | Candida albicans ATCC 10231 |
|---|---|---|---|
| Cs-1 | 0 (−) | 0 (−) | 12.5 ± 0.5 (++++) |
| Cs-Agrif | 11.3 ± 0.8 | 9.2 ± 0.6 (+++) | 8.2 ± 0.7 (++) |
| Ciprofloxacin | 29.0 ± 0.0 (+++++) | 29.5 ± 0.5 (+++++) | / |
| Nystatin | / | / | 25.3 ± 0.3 (+++++) |
| Sample | Enterococcus faecalis ATCC 29219 | Escherichia coli ATCC 25922 | Candida albicans ATCC 10231 | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MFC | |
| Cs-1 | 0.625 ± 0.000 | 1.25 ± 0.00 | 0.625 ± 0.000 | 0.625 ± 0.000 | ˂0.078 ± 0.000 | ˂0.078 ± 0.000 |
| Cs-Agrif | 0.625 ± 0.000 | 0.625 ± 0.000 | 2.5 ± 0.0 | 2.5 ± 0.0 | ˂0.078 ± 0.000 | ˂0.078 ± 0.000 |
| Sample | 5 Days | % of Reduction |
|---|---|---|
| Control | 71.00 ± 0.00 a | / |
| Cs-1 | 53.67 ± 9.67 | 24.41 |
| Cs-Agrif | 40.12 ± 8.18 b | 43.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorov, J.; Pantić, M.; Kozarski, M.; Lazić, V.; Todorović, N.; Obradović, M.; Daković, A.; Krajišnik, D.; Milašinović, N.; Mirković, M. Chitosan–Glucan Biopolymer Design: Extraction from Champignons with Improved Antioxidant and Antimicrobial Features. Processes 2025, 13, 3937. https://doi.org/10.3390/pr13123937
Todorov J, Pantić M, Kozarski M, Lazić V, Todorović N, Obradović M, Daković A, Krajišnik D, Milašinović N, Mirković M. Chitosan–Glucan Biopolymer Design: Extraction from Champignons with Improved Antioxidant and Antimicrobial Features. Processes. 2025; 13(12):3937. https://doi.org/10.3390/pr13123937
Chicago/Turabian StyleTodorov, Jelisaveta, Milena Pantić, Maja Kozarski, Vesna Lazić, Nina Todorović, Milena Obradović, Aleksandra Daković, Danina Krajišnik, Nikola Milašinović, and Miljana Mirković. 2025. "Chitosan–Glucan Biopolymer Design: Extraction from Champignons with Improved Antioxidant and Antimicrobial Features" Processes 13, no. 12: 3937. https://doi.org/10.3390/pr13123937
APA StyleTodorov, J., Pantić, M., Kozarski, M., Lazić, V., Todorović, N., Obradović, M., Daković, A., Krajišnik, D., Milašinović, N., & Mirković, M. (2025). Chitosan–Glucan Biopolymer Design: Extraction from Champignons with Improved Antioxidant and Antimicrobial Features. Processes, 13(12), 3937. https://doi.org/10.3390/pr13123937








