Bioactive Properties of Polyphenolic Extracts from Flourensia cernua Obtained by Emerging Technologies Under a Taguchi L18 Orthogonal Array
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Characterization
2.2. L18 Orthogonal Taguchi Experimental Array
2.2.1. Ultrasound-Assisted Extraction (UAE)
2.2.2. Microwave-Assisted Extraction (MAE)
2.3. Quantification of Total Hydrolysable Polyphenols (THPs)
2.4. Quantification of Total Flavonoids (TFC)
2.5. Antioxidant Activity
2.5.1. ABTS●+ Radical Cation (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid)) Assay
2.5.2. Antioxidant Activity by 1,1-Diphenyl-2-Picrylhydrazyl (DPPH●)
2.6. RP-HPLC-ESI-MS Analysis
2.7. Hemolytic Activity
2.8. Antihemolytic Activity Assay
2.9. Data Analysis
3. Results
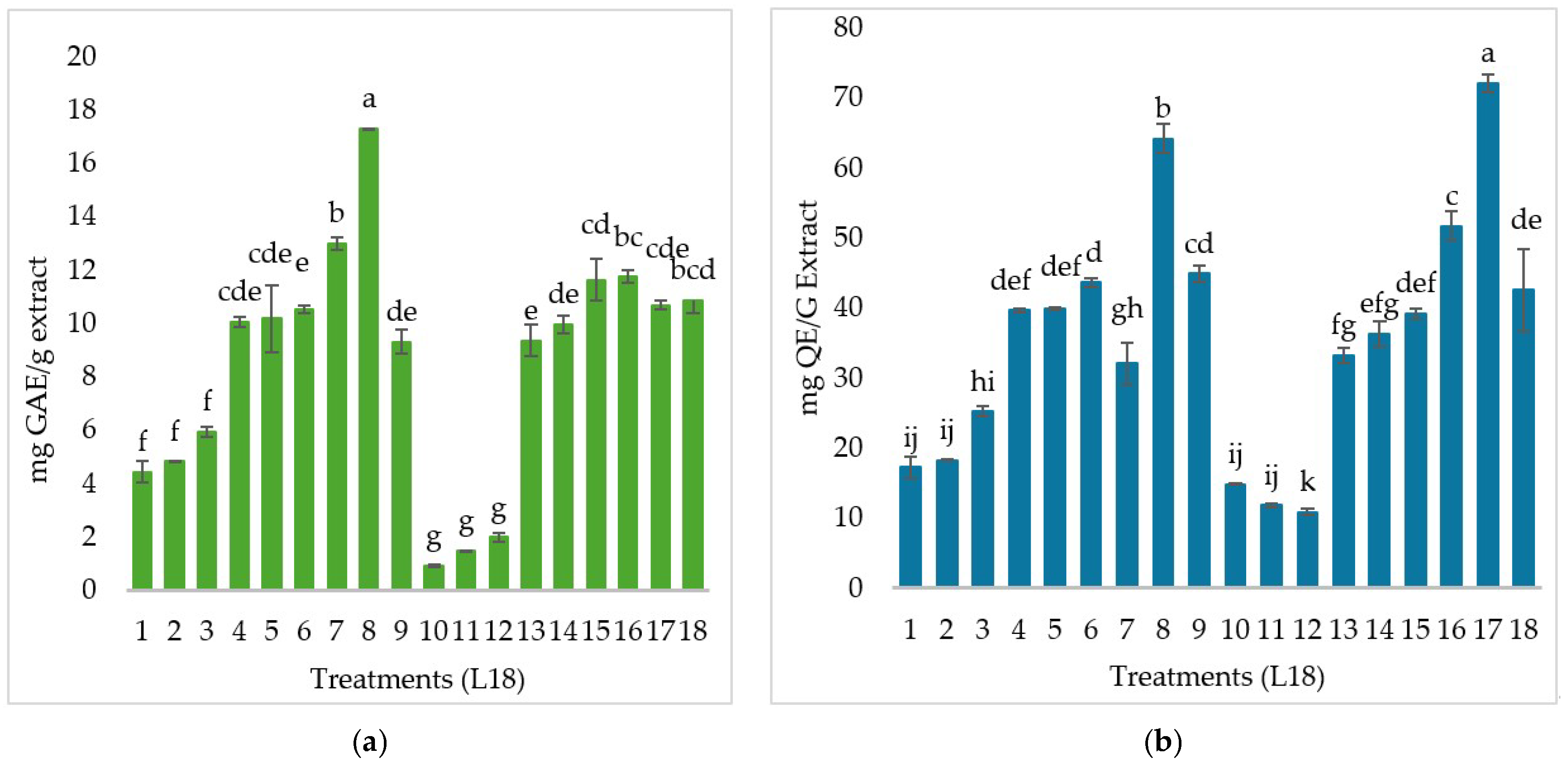
3.1. Quantification of Total Hydrolyzable Polyphenols and Total Flavonoids
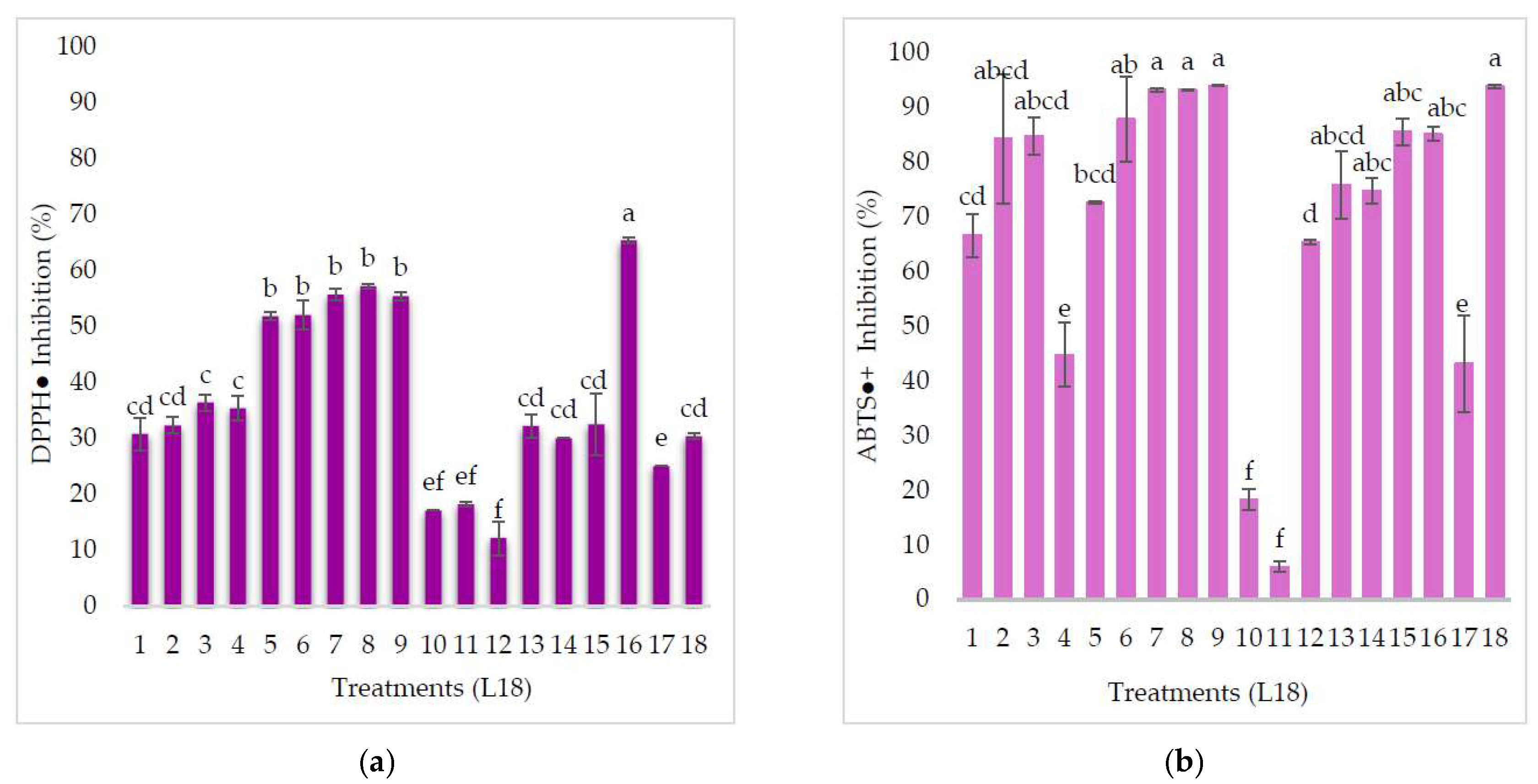
3.2. Antioxidant Activity
3.3. Identification of Secondary Metabolites Present in Flourensia cernua
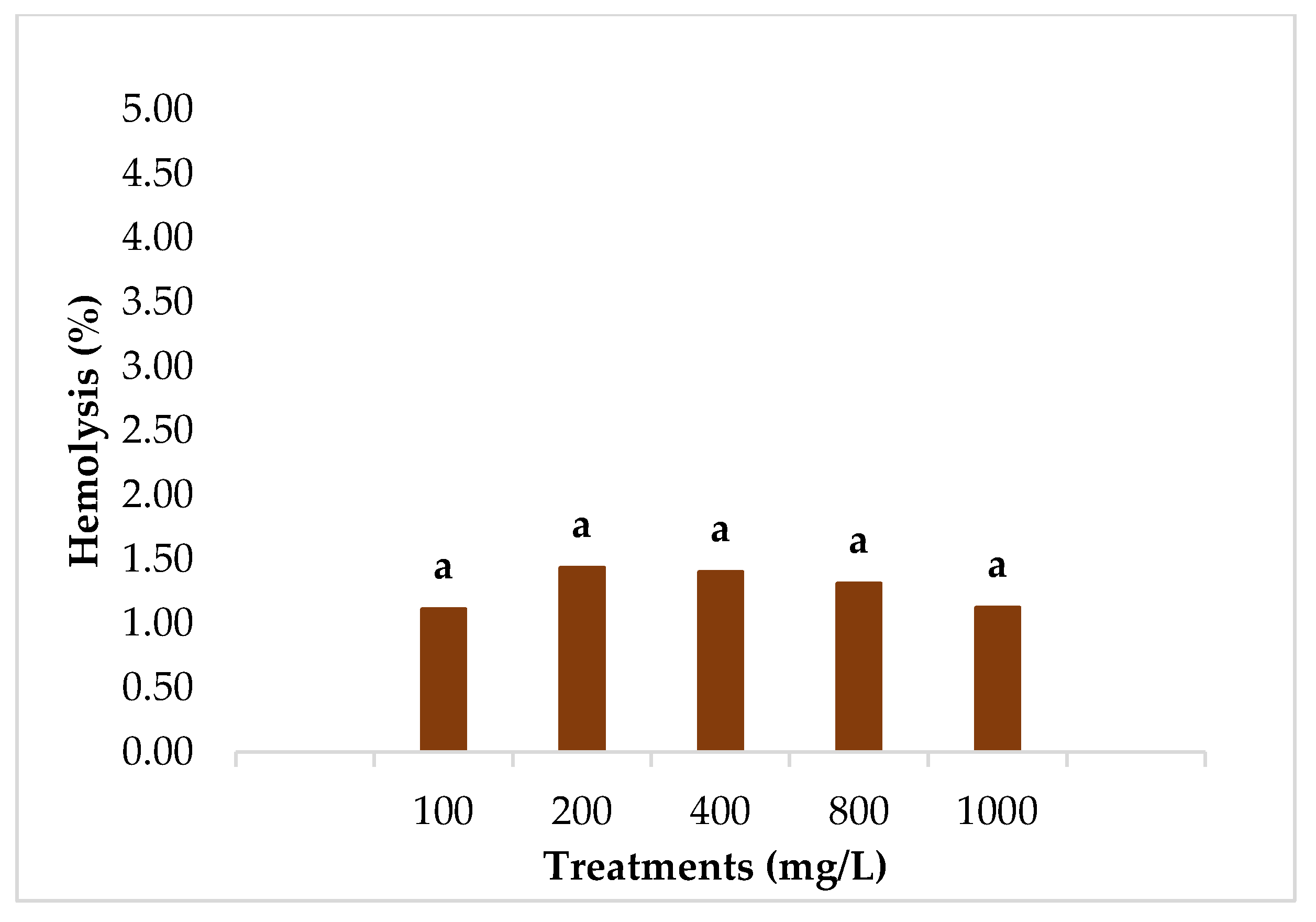
3.4. Hemolytic Activity
3.5. Antihemolytic Activity
4. Discussion
4.1. Total Hydrolyzable Phenols and Flavonoids
4.2. Antioxidant Activity
4.3. Identification of Secondary Metabolites Present in Flourensia cernua
4.4. Hemolytic Activity and Antihemolytic
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estrada-Castillón, E.; Villarreal-Quintanilla, J.Á.; Encina-Domínguez, J.A.; Jurado-Ybarra, E.; Cuéllar-Rodríguez, L.G.; Garza-Zambrano, P.; Arévalo-Sierra, J.R.; Cantú-Ayala, C.M.; Himmelsbach, W.; Salinas-Rodríguez, M.M.; et al. Ethnobotanical biocultural diversity by rural communities in the Cuatrociénegas Valley, Coahuila, Mexico. J. Ethnobiol. Ethnomed. 2021, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Rangel, I.E.; Charles-Rodríguez, A.V.; López-Romero, J.C.; Flores-López, M.L. Plants from Arid and Semi-Arid Zones of Mexico Used to Treat Respiratory Diseases: A Review. Plants 2024, 13, 792. [Google Scholar] [CrossRef] [PubMed]
- Ríos, M.Y. Chemistry and biology of the genus Flourensia (Asteraceae). Chem. Biodivers. 2015, 12, 1595–1634. [Google Scholar] [CrossRef] [PubMed]
- De Rodríguez, D.J.; Puente-Romero, G.N.; Díaz-Jiménez, L.; Rodríguez-García, R.; Ramírez-Rodríguez, H.; Villarreal-Quintanilla, J.A.; Flores-López, M.L.; Carrillo-Lomelí, D.A.; Genisheva, Z.A. In Vitro Gastrointestinal Digestion of Microencapsulated Extracts of Flourensia cernua, F. microphylla and F. retinophylla. Ind. Crops Prod. 2019, 138, 111482. [Google Scholar] [CrossRef]
- Linares-Braham, A.; Palomo-Ligas, L.; Nery-Flores, S.D. Bioactive Compounds and Pharmacological Potential of Hojasen (Flourensia cernua): A Mini Review. Plant Sci. Today 2023, 10, 304–312. [Google Scholar] [CrossRef]
- Álvarez-Pérez, O.B.; Ventura-Sobrevilla, J.M.; Ascacio-Valdés, J.A.; Rojas, R.; Verma, D.K.; Aguilar, C.N. Valorization of Flourensia cernua DC as Source of Antioxidants and Antifungal Bioactives. Ind. Crops Prod. 2020, 152, 112422. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Salas-Méndez, E.J.; Rodríguez-García, R.; Hernández-Castillo, F.D.; Díaz-Jiménez, M.L.; Sáenz-Galindo, A.; González-Morale, S.; Flores-López, M.L.; Villarreal-Quintanilla, J.A.; Peña-Ramos, F.M.; et al. Antifungal activity in vitro of ethanol and aqueous extracts of leaves and branches of Flourensia spp. against postharvest fungi. Ind. Crops Prod. 2017, 107, 499–508. [Google Scholar] [CrossRef]
- De Rodríguez, D.J.; Angulo-Sánchez, J.L.; Hernández-Castillo, F.D. An overview of the antimicrobial properties of Mexican medicinal plants. In Advances in Phytomedicine, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 325–377. [Google Scholar] [CrossRef]
- Ruiz-Martínez, J.; Aguirre-Joya, J.A.; Rojas, R.; Vicente, A.; Aguilar-González, M.A.; Rodríguez-Herrera, R.; Alvarez-Perez, O.B.; Torres-León, C.; Aguilar, C.N. Candelilla wax edible coating with Flourensia cernua bioactives to prolong the quality of tomato fruits. Foods 2020, 9, 1303. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Hernández-Castillo, D.; Angulo-Sánchez, J.L.; Rodríguez-García, R.; Villarreal-Quintanilla, J.A.; Lira-Saldivar, R.H. Antifungal activity in vitro of Flourensia spp. extracts on Alternaria sp., Rhizoctonia solani and Fusarium oxysporum. Ind Crops Prod. 2007, 25, 111–116. [Google Scholar] [CrossRef]
- Aguirre-García, Y.L.; Castillo-Manzanares, A.; Palomo-Ligas, L.; Ascacio-Valdés, J.A.; Campos-Múzquiz, L.G.; Esparza-González, S.C.; Rodríguez-Herrera, R.; Nery-Flores, S.D. Toxicity evaluation of a polyphenolic extract from Flourensia cernua DC through Artemia lethality assay, hemolytic activity, and acute oral test. J. Toxicol. 2024, 2024, 2970470. [Google Scholar] [CrossRef]
- Peralta Bello, J.E. Evaluación de la Actividad de Extractos de Hojasén Flourensia cernua D.C. in vitro en el Control de las Bacterias Fitopatógenas: Xanthomonas campestris pv. phaseoli (Smith) Dye, Erwinia carotovora pv. atroseptica (Van Hall) Dye y Pseudomonas cichorii (Swingle) Stapp. Bachelor’s Thesis, Universidad Autónoma Agraria Antonio Narro, Saltillo, Coahuila, México, 2006. [Google Scholar]
- Zavala, C.D.; Carrillo, I.M.L.; Alvarado, S.B.; Sánchez, C.H.A.O. Evaluación de la toxicidad aguda de un extracto alcohólico de hojas de hojasén (Flourensia cernua). Rev. Mex. Cienc. Farm. 2010, 41, 50–54. Available online: https://www.redalyc.org/pdf/579/57916078007.pdf (accessed on 10 November 2025).
- De León-Zapata, M.A.; Sáenz-Galindo, A.; Rojas-Molina, R.; Rodríguez-Herrera, R.; Jasso-Cantú, D.; Aguilar, C.N. Edible candelilla wax coating with fermented extract of tarbush improves the shelf life and quality of apples. Food Packag. Shelf Life 2015, 3, 70–75. [Google Scholar] [CrossRef]
- Ganchev, D. Antisporulation action of tarbush plant (Flourensia cernua) towards conidiospores of plant pathogens. Malays. J. Sustain. Agric. 2022, 6, 81–84. [Google Scholar] [CrossRef]
- Mata, R.; Bye, R.; Linares, E.; Macías, M.; Rivero-Cruz, I.; Pérez, O.; Timmermann, B.N. Phytotoxic compounds from Flourensia cernua. Phytochemistry 2003, 64, 285–291. [Google Scholar] [CrossRef]
- Aranda-Ledesma, N.E.; González-Hernández, M.D.; Rojas, R.; Paz-González, A.D.; Rivera, G.; Luna-Sosa, B.; Martínez-Ávila, G.C.G. Essential oil and polyphenolic compounds of Flourensia cernua leaves: Chemical profiling and functional properties. Agronomy 2022, 12, 2274. [Google Scholar] [CrossRef]
- Silva, G.C.C.; Machado, M.A.; Sakumoto, K.; Inumaro, R.S.; Gonçalves, J.E.; Mandim, F.; Vaz, J.; do Valle, J.S.; Faria, M.G.I.; Ruiz, S.P.; et al. Cellular antioxidant, anti-inflammatory, and antiproliferative activities from the flowers, leaves and fruits of Gallesia integrifolia Spreng. Harms. Molecules 2023, 28, 5406. [Google Scholar] [CrossRef]
- López-Benítez, A.; López-Betancourt, S.R.; Vázquez-Badillo, M.E.; Rodríguez-Herrera, S.A. Inhibición del crecimiento micelial de Fusarium oxysporum Schlechtend. f. sp. lycopersici (Sacc.) Snyder y Hanses, Rhizoctonia solani Kühn y Verticillium dahliae Kleb. Rev. Mex. Fitopatol. 2005, 23, 183–190. Available online: https://www.redalyc.org/pdf/612/61223212.pdf (accessed on 10 November 2025).
- Pérez-Gutiérrez, R.M.; Martínez-Jerónimo, F.F.; Contreras-Soto, J.G.; Muñiz-Ramírez, A.; Estrella-Mendoza, M.F. Optimization of Ultrasonic-Assisted Extraction of Polyphenols from a Polyherbal Formulation of Cinnamomum verum, Origanum majorana, and Origanum vulgare and Their Antidiabetic Capacity in Zebrafish (Danio rerio). Heliyon 2022, 8, e08682. [Google Scholar] [CrossRef]
- Rojas, T.; Gómez, S.; Fuentes-Campos, M.E.; Contreras-López, E.; Muñoz-Jáuregui, A.M. Extracción asistida por ultrasonido de compuestos fenólicos de la cáscara de Sanky (Corryocactus brevistylus). Rev. Soc. Quím. Perú 2018, 85, 258–267. [Google Scholar] [CrossRef]
- Arya, P.; Kumar, P. Comparison of Ultrasound and Microwave-Assisted Extraction of Diosgenin from Trigonella foenum-graecum Seed. Ultrason. Sonochem. 2021, 74, 105572. [Google Scholar] [CrossRef]
- Aranda-Ledesma, N.E.; Aguilar-Zárate, P.; Bautista-Hernández, I.; Rojas, R.; Robledo-Jiménez, C.L.; Martínez-Ávila, G.C.G. Optimization of Ultrasound-Assisted Extraction for Bioactive Compounds from Flourensia cernua and Jatropha dioica and Evaluation of Their Functional Properties. Horticulturae 2024, 10, 709. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 9th ed.; John Wiley & Sons: New York, NY, USA, 2017; Available online: https://books.google.com.mx/books?id=Py7bDgAAQBAJ (accessed on 10 November 2025).
- Naranjo-Palacios, F.; Ríos-Lira, A.J.; Pantoja-Pacheco, Y.V.; Tapia-Esquivias, M. Diseños ortogonales de Taguchi fraccionados. Ing. Investig. Tecnol. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Kurata, K.; Shimada, K.; Takamatsu, H. Application of the Taguchi Method to Explore a Robust Condition of Tumor-Treating Field Treatment. PLoS ONE 2022, 17, e0262133. [Google Scholar] [CrossRef] [PubMed]
- Kherbachi, S.; Kheniche, M.; Tacherfiout, M. Antihemolytic Activity of Hydroalcoholic Leaves and Bark Extracts from Rhamnus alaternus against AAPH-Induced Hemolysis on Human Erythrocytes. Int. J. Plant Based Pharm. 2022, 2, 210–219. Available online: https://ijpbp.com (accessed on 10 November 2025). [CrossRef]
- Tabet-Zatla, A.; Hammoudi, A.; El-Hiti, G.A.; Fellah, M.; Mohammed, D.Z.; Pérard, J. In Vitro Study of the Antihemolytic and Antioxidant Potential of Two Essential Oils from Salvia officinalis L. and Curcuma longa L. against Glucantime® Toxicity. J. Eng. Res. 2025, in press. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Martínez-Ávila, G.C.G.; Belmares-Cerda, R.E.; Aguilar, C.N. Ultrasound-Assisted Extraction of Polyphenols from Native Plants in the Mexican Desert. Ultrason. Sonochem. 2015, 22, 474–481. [Google Scholar] [CrossRef]
- Muñoz-Acevedo, A.; Vargas-Rueda, S.J.; Guerra, E.X.; Cervantes-Díaz, M. Determinación del contenido total de flavonoides presentes en residuos agroindustriales de frutas tropicales. Rev. Agunkuyâa 2021, 11, 28–35. [Google Scholar] [CrossRef]
- Valero-Mendoza, A.G.; Meléndez-Rentería, N.P.; Chávez-González, M.L.; Flores-Gallegos, A.C. The whole pomegranate (Punica granatum L.): Biological properties and important findings—A review. Food Chem. Adv. 2023, 2, 100153. [Google Scholar] [CrossRef]
- Estrada-Gil, L.; Contreras-Esquivel, J.C.; Flores-Gallegos, C.; Zugasti-Cruz, A.; Govea-Salas, M.; Mata-Gómez, M.A.; Ascacio-Valdés, J.A. Recovery of bioactive ellagitannins by ultrasound/microwave-assisted extraction from Mexican rambutan peel (Nephelium lappaceum L.). Molecules 2022, 27, 1592. [Google Scholar] [CrossRef]
- De León-Medina, J.C.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Aguilar, C.N.; Ascacio-Valdés, J.A. Fungal biodegradation of ellagitannins extracted from rambutan peel. Food Bioprod. Process. 2023, 141, 81–90. [Google Scholar] [CrossRef]
- Nasiri, S.N.; Aghajanloo, B.; Nasiri, N.; Nazarnezhad, S. Cytotoxicity and biocompatibility of green biomaterials. In Green Biomaterials in Tissue Engineering; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2025; Volume 1497, pp. 175–205. [Google Scholar] [CrossRef]
- Alonso-Montemayor, F.J.; Neira-Velázquez, M.G.; Zugasti-Cruz, A.; Sáenz-Galindo, A. Minimum inhibitory but maximum non-hemolytic concentration of Larrea tridentata and Origanum vulgare extracts. Afinidad 2023, 80, 1–9. [Google Scholar] [CrossRef]
- Pacheco Coello, F.J. Primer análisis comparativo de la actividad antioxidante, hemolítica y antihemolítica de extractos acuosos de cinco especies del género Hibiscus presentes en Latinoamérica. Cienc. Ambiente Clima 2023, 6, 9–32. [Google Scholar] [CrossRef]
- Lopes, T.A.M.; Godoy, A.C.; Sinosaki, N.B.M.; Chiavelli, L.U.R.; Silveira, R.; Figueiredo, B.H.; Santos, O.O. Green extraction optimization of bioactive compounds from rosemary. J. Braz. Chem. Soc. 2020, 31, 2603–2610. [Google Scholar] [CrossRef]
- Amarowicz, R.; Carle, R.; Dongowski, G.; Durazzo, A.; Galensa, R.; Kammerer, D.; Maiani, G.; Piskula, M.K. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S151–S183. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Estell, R.E.; Fredrickson, E.L.; James, D.K. Effect of light intensity and wavelength on concentration of plant secondary metabolites in leaves of Flourensia cernua. Biochem. Syst. Ecol. 2016, 65, 108–114. [Google Scholar] [CrossRef]
- Henkel, S.; Misuraca, M.C.; Troselj, P.; Davidson, J.; Hunter, C.A. Polarisation effects on the solvation properties of alcohols. Chem. Sci. 2018, 9, 88–99. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef]
- Galvan D’Alessandro, L.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound-assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Nishad, J.; Saha, S.; Kaur, C. Enzyme- and ultrasound-assisted extractions of polyphenols from Citrus sinensis (cv. Malta) peel: A comparative study. J. Food Process. Preserv. 2019, 43, e14046. [Google Scholar] [CrossRef]
- Pérez, R.M.; Vargas, R.; Martínez, F.J.; García, E.V.; Hernández, B. Actividad antioxidante de los alcaloides de Bocconia arborea: Estudio sobre seis métodos de análisis. Ars. Pharm. 2003, 44, 5–21. Available online: https://www.ugr.es/~ars/abstract/44-5-03.pdf (accessed on 10 November 2025).
- De León-Zapata, M.A.; Pastrana-Castro, L.; Rua-Rodríguez, M.L.; Álvarez-Pérez, O.B.; Rodríguez-Herrera, R.; Aguilar, C.N. Experimental protocol for the recovery and evaluation of bioactive compounds of tarbush against postharvest fruit fungi. Food Chem. 2016, 198, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Rojano, A.; Gaviria, C.A.; Ochoa, C.I.; Sánchez, N.; Medina, C.; Lobo, M. Propiedades antioxidantes de los frutos de agraz o mortiño (Vaccinium meridionale Swartz). In Perspectivas del Cultivo de Agraz o Mortiño en la Zona Altoandina de Colombia; Gente Nueva Editorial: Bogotá, Colombia, 2009; pp. 95–112. Available online: https://repository.agrosavia.co/bitstream/handle/20.500.12324/20444/81392_56989.pdf (accessed on 10 November 2025).
- ASTM F756-17(2025); Standard Practice for Assessment of Hemolytic Properties of Materials. ASTM International: West Conshohocken, PA, USA, 2025.
- Belmares, R.; Garza, Y.; Rodríguez, R.; Contreras-Esquivel, J.C.; Aguilar, C.N. Composition and fungal degradation of tannins present in semiarid plants. Electron. J. Environ. Agric. Food Chem. 2009, 8, 312–318. [Google Scholar]
- Álvarez, R.E.; Jiménez, G.O.J.; Posada, A.C.M.; Rojano, B.A.; Gil, G.G.H.; García, P.C.M.; Durango, R.D.L. Actividad antioxidante y contenido fenólico de los extractos provenientes de las bayas de dos especies del género Vismia (Guttiferae). Vitae 2008, 15, 165–172. Available online: https://www.scielo.org.co/pdf/vitae/v15n1/v15n1a20.pdf (accessed on 10 November 2025).
- Cai, Y.-Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–Radical Scavenging Activity Relationships of Phenolic Compounds from Traditional Chinese Medicinal Plants. Life Sci. 2006, 78, 2872–2878. [Google Scholar] [CrossRef]
- Chen, Z.; Kong, S.; Song, F.; Li, L.; Jiang, H. Pharmacokinetic Study of Luteolin, Apigenin, Chrysoeriol, and Diosmetin after Oral Administration of Flos chrysanthemi Extract in Rats. Fitoterapia 2012, 83, 1616–1622. [Google Scholar] [CrossRef]
- Hmidani, A.; Bouhlali, E.T.; Ajebli, M.; Khouya, T.; Benlyas, M.; Alem, C. In Vitro Investigation of Antioxidant and Antihemolytic Activities of Three Lamiaceae Species from Morocco. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 27. [Google Scholar] [CrossRef]





| Treatment | Concentration | Temperature, °C | Time, min |
|---|---|---|---|
| Ultrasound | 0 | 30 | 5 |
| Ultrasound | 0 | 40 | 10 |
| Ultrasound | 0 | 50 | 20 |
| Ultrasound | 30 | 30 | 5 |
| Ultrasound | 30 | 40 | 10 |
| Ultrasound | 30 | 50 | 20 |
| Ultrasound | 50 | 30 | 10 |
| Ultrasound | 50 | 40 | 20 |
| Ultrasound | 50 | 50 | 5 |
| Microwave | 0 | 30 | 20 |
| Microwave | 0 | 40 | 5 |
| Microwave | 0 | 50 | 10 |
| Microwave | 30 | 30 | 10 |
| Microwave | 30 | 40 | 20 |
| Microwave | 30 | 50 | 5 |
| Microwave | 50 | 30 | 20 |
| Microwave | 50 | 40 | 5 |
| Microwave | 50 | 50 | 10 |
| RT (min) | Mass | Compound | |
|---|---|---|---|
| 12.5 | 341 | Caffeic acid 4-O-glucoside | Hydroxycinnamic acids |
| 15.141 | 352.9 | 1-Caffeoylquinic acid | Hydroxycinnamic acids |
| 37.492 | 352.9 | 3-Caffeoylquinic acid | Hydroxycinnamic acids |
| 40.072 | 592.9 | Apigenin 6,8-di-C-glucoside | Flavones |
| 42.566 | 310.9 | Caffeoyl tartaric acid | Hydroxycinnamic acids |
| 44.427 | 563.0 | Apigenin arabinoside-glucoside | Flavones |
| 47.288 | 562.9 | Apigenin galactoside-arabinoside | Flavones |
| 51.291 | 293 | Caffeoyl aspartic acid | Hydroxycinnamic acids |
| 53.25 | 514.9 | 1,3-Dicaffeoylquinic acid | Hydroxycinnamic acids |
| 54.054 | 514.9 | 1,5-Dicaffeoylquinic acid | Hydroxycinnamic acids |
| 56.805 | 514.9 | 3,4-Dicaffeoylquinic acid | Hydroxycinnamic acids |
| 58.596 | 317 | Myricetin | Flavonols |
| 59.901 | 313 | Cirsimaritin | Methoxyflavones |
| RT (min) | Mass | Compound | |
|---|---|---|---|
| 7.66 | 355 | Ferulic acid 4-O-glucoside | Methoxycinnamic acids |
| 11.398 | 340.9 | Caffeic acid 4-O-glucoside | Hydroxycinnamic acids |
| 12.196 | 341.0 | Caffeic acid 4-O-glucoside | Hydroxycinnamic acids |
| 15.218 | 352.9 | 1-Caffeoylquinic acid | Hydroxycinnamic acids |
| 38.994 | 593 | Apigenin 6,8-di-C-glucoside | Flavones |
| 39.977 | 593 | Luteolin 7-O-rutinoside | Flavones |
| 43.186 | 311 | Caffeoyl tartaric acid | Hydroxycinnamic acids |
| 44.626 | 563 | Apigenin arabinoside-glucoside | Flavones |
| 45.148 | 563 | Apigenin galactoside-arabinoside | Flavones |
| 46.042 | 563 | Apigenin 7-O-apiosyl-glucoside | Flavones |
| 47.019 | 562.9 | Theaflavin | Theaflavins |
| 48.092 | 364.9 | Secoisolariciresinol | Lignans |
| 52.099 | 338.9 | Esculin | Hydroxycoumarins |
| 53.872 | 514.9 | 1,3-Dicaffeoylquinic acid | Hydroxycinnamic acids |
| 54.948 | 514.9 | 1,5-Dicaffeoylquinic acid | Hydroxycinnamic acids |
| 58.757 | 317 | Myricetin | Flavonols |
| 59.93 | 313.1 | Cirsimaritin | Methoxyflavones |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero-Mendoza, A.G.; Nuncio, A.; Govea Salas, M.; Zugasti-Cruz, A.; Ríos-González, L.J.; Ascacio-Valdés, J.A.; Morales-Martínez, T.K.; Cruz-Requena, M.; Medina-Morales, M.A. Bioactive Properties of Polyphenolic Extracts from Flourensia cernua Obtained by Emerging Technologies Under a Taguchi L18 Orthogonal Array. Processes 2025, 13, 3725. https://doi.org/10.3390/pr13113725
Valero-Mendoza AG, Nuncio A, Govea Salas M, Zugasti-Cruz A, Ríos-González LJ, Ascacio-Valdés JA, Morales-Martínez TK, Cruz-Requena M, Medina-Morales MA. Bioactive Properties of Polyphenolic Extracts from Flourensia cernua Obtained by Emerging Technologies Under a Taguchi L18 Orthogonal Array. Processes. 2025; 13(11):3725. https://doi.org/10.3390/pr13113725
Chicago/Turabian StyleValero-Mendoza, Andrea G., Alberto Nuncio, Mayela Govea Salas, Alejandro Zugasti-Cruz, Leopoldo J. Ríos-González, Juan A. Ascacio-Valdés, Thelma K. Morales-Martínez, Marisol Cruz-Requena, and Miguel A. Medina-Morales. 2025. "Bioactive Properties of Polyphenolic Extracts from Flourensia cernua Obtained by Emerging Technologies Under a Taguchi L18 Orthogonal Array" Processes 13, no. 11: 3725. https://doi.org/10.3390/pr13113725
APA StyleValero-Mendoza, A. G., Nuncio, A., Govea Salas, M., Zugasti-Cruz, A., Ríos-González, L. J., Ascacio-Valdés, J. A., Morales-Martínez, T. K., Cruz-Requena, M., & Medina-Morales, M. A. (2025). Bioactive Properties of Polyphenolic Extracts from Flourensia cernua Obtained by Emerging Technologies Under a Taguchi L18 Orthogonal Array. Processes, 13(11), 3725. https://doi.org/10.3390/pr13113725








